Abstract
This experiment was carried out to evaluate the effect of dietary supplementation of three different tropical seaweed species on rumen fermentation, methane emission, antioxidant status, immunity and milk production in lactating Murrah buffaloes. Twenty-four lactating Murrah buffaloes were divided into four groups of six each in an experiment based on randomized block design (RBD) and were fed to meet their nutrient requirements (ICAR 2013). Animals in control (CON) group were fed basal diet without any supplemental seaweed, however, Kappaphycus alvarezii (KA), Gracilaria salicornia (GS) and Turbinaria conoides (TC) were supplemented at 1% of the dietary dry matter in KA, GS and TC groups, respectively. Intake and apparent digestibility of nutrients, plasma concentrations of selected blood metabolites, and thyroid hormones were similar among the groups. Supplementation of KA and GS, but not TC increased (P < 0.001) the proportion of propionate in rumen fluid with a concurrent decrease (P < 0.001) in acetate: propionate. The enteric methane emission was lower (P < 0.05) in KA, GS groups and the maximum values were observed in groups CON and TC. Total antioxidant capacity (TAC) and immune response (cell-mediated and humoral) were higher (P < 0.001), and MDA was lower (P < 0.001) in seaweed-fed groups as compared to CON; the higher response was observed in group KA, followed by TC and GS. Plasma concentration of cortisol was lower (P < 0.001) in group KA as compared to other groups. Milk yield and the 6% fat-corrected milk (FCM) yield (kg day−1) were higher (P = 0.009) in KA, followed GS, corresponding values in group TC were not significantly different than those of group CON. Thus, supplementation of the tropical red seaweed improved antioxidant status, cellular and humoral immunity, and milk yield; the greater response was obtained when KA was used as feed supplement. It is concluded that supplementation of K. alvarezii at 1% of dietary DM of lactating Murrah buffaloes would improve antioxidant status, immunity and milk yield with reduction in enteric methane emission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seaweeds are macroscopic algae that grow in the marine environment and are available in a variety of forms, sizes, colors, and compositions. Feeding of macro-algae to ruminants is a common practice in coastal areas due to their ability to improve lactation yield (Singh et al. 2017; Sharma et al. 2022), antioxidant (Allen et al. 2001; Maheswari et al. 2021) and immune status (Anderson et al. 2023) and reduce enteric methane (CH4) production (Reyes et al. 2023). However, injudicious use of seaweeds in the diet may also pose certain negative effects on performance of ruminants through decrease in fiber degradability in the rumen (Wang et al. 2008) and toxicity of heavy metals like arsenic and lead (Cabrita et al. 2017). Thus the potential of seaweeds as a major feed component in ruminant ration may rather be limited. On the other hand, many species of macroalgae contain a variety of bioactive substances that have antibacterial, antiviral, antioxidant, and anti-inflammatory characteristics, all of which improve productivity, health and welfare of animals (Bach et al. 2008). The good amount of macro and micronutrients and many biologically active compounds of seaweeds, with health promoting benefits, indicate that they might be used as functional nutritional supplements (Makkar et al. 2016).
The red seaweeds, Kappaphycus alvarezii and Gracilaria salicornia and brown seaweed, Turbinaria conoides (TC) are the most commonly cultivated species in India (Maheswari et al. 2021). These seaweeds are mostly used as sources of phycocolloids, which have got wider applications in food processing industry (Bixler and Porse 2011) and also as livestock feed (Sharma et al. 2022). The few available studies indicate that supplementation of red seaweeds such as K. alvarezii improved the growth performance (Munde 2018), and lactation yield (Sharma et al. 2022) in ruminants. Although both K. alvarezii and G. salicornia are high in carrageenan and sulphated polysaccharides, it was proved that G. salicornia had a stronger anti-methanogenic effect than K. alvarezii (Munde 2018). This indicates that functional characteristics of seaweeds differ by species, dose, habitat and bioactive compound status. On the other side, T. conoides has functional benefits due to its high amount of phlorotannins, fucoidans, and alginate and microminerals (Munde et al. 2018; Yengkhom et al. 2019; Maheswari et al. 2021). Given the diversity of nutrient make-up and functional qualities of tropical seaweed, a direct comparison across species would be desired, and it would be more appropriate to evaluate three of the most promising Indian seaweed species as feed supplements for ruminants using lactating Murrah buffalo as a model. We hypothesized that response of supplementary feeding of tropical seaweeds on performance of lactating Murrah buffalo may vary according to seaweed species. The specific objective of this experiment was to study the effect of supplementation of three different tropical seaweeds on lactation performance, methane emission, anti-oxidant profile and immunity of lactating Murrah buffaloes.
Materials and methods
The experiment was conducted at the Livestock Research Centre, Animal Nutrition Division, ICAR-National Dairy Research Institute (NDRI), Karnal, Haryana, India. The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC) with approval no. 45-IAEC-19–2. The thrashed seaweed powders were procured from Aquagri Processing Pvt Ltd, Madurai, Tamil Nadu. Fresh seaweeds were sun-dried to a moisture content of about 10%, thrashed by hand on a thrashing bed, and then ground in a hammer mill to produce the thrashed powder.
Experimental animals and design
This experiment was conducted following randomized block design (RBD). Twenty-four lactating Murrah buffaloes (547.5 ± 18.58 kg body weight (BW), 23.4 ± 5.75 days in milk (DIM), 10.31 ± 0.18 kg day−1 of milk yield) were selected from the Institute dairy herd. Based upon their milk yield and body weight, buffaloes were divided into 6 blocks of four animals each. Four dietary treatments namely, control (CON), Kappaphycus alvarezii (KA), Gracilaria salicornia (GS), and Turbinaria conoides (TC) were randomly assigned to experimental units within a block so that each treatment appeared within each block.
Before the actual collection started, an adaptation period of 14-days was followed during which all cows were fed CON diet and the average output during the period was considered as base line data (0-day). All the animals were fed to meet their nutrient requirements as per ICAR (2013). The animals in CON group were fed a basal diet without any seaweed, whereas diets of the animals in treatment groups namely, KA, GS, and TC were supplemented with K. alvarezii, G. salicornia and T. conoides at 1% of the dietary dry matter (DM), respectively. The experiment was conducted for 90 days.
Diet and shelter management
All experimental animals were fed with roughage (freshly cut oat fodder and wheat straw) and concentrate mixture to meet their requirements as per ICAR (2013) standards. The concentrate mixture was formulated using maize, wheat, mustard oil cake, groundnut oil cake, de-oiled rice bran (DORB), molasses, mineral mixture, and urea (Table 1). The red seaweeds K. alvarezii, G. salicornia and T. conoides were added 2.5% in concentrate mixture of KA, GS and TC groups respectively by replacing the deoiled rice bran and molasses in order to make the diets iso-nitrogenous and iso-caloric. Weighed amount of concentrate and roughage was offered daily to all the experimental buffaloes based on their body weight and milk yield. Concentrates were offered twice daily at 5:30 AM and 17:30 PM, just before milking. The chaffed oat fodder (1.5 inches) was fed daily at 10.30 AM and chaffed wheat straw (0.5 inches) was offered 20% in excess of previous days’ intake at 12.00 h and was made available for remainder of the day. Clean and fresh potable water was offered at ad libitum to all the animals twice daily at 9:30 AM and 17:30 PM.
Experimental animals were kept in a cross-ventilated animal shed (floor space = 6.04 m2 per animal) with the facilities of individual animal feeding; so that one buffalo did not have access to feed of other ones. The shed was washed two times daily to remove feces and dirt. Clean and hygienic conditions were maintained in the shed. All the animals were dewormed using Fenbendazole at 10 mg kg−1 BW before the start of the experiment.
Sampling and measurement
A timeline for data recording and sampling of different parameters studied in this experiment is presented in Fig. 1.
Body weight, body condition score, and dry matter intake
During the experimental period the fortnightly body weight changes of all the animals were recorded. After overnight fasting they were measured on a digital scale (range 0–1000 kg, accuracy 0.1 kg). The body condition score (BCS) of all the buffaloes were recorded on a scale of 1–5 based on the method of Ferguson et al. (1994). All animals were offered with measured quantity of feed and residues left by animals during a 24 h feeding cycle were recorded. Samples of feed and residues were taken on three consecutive days, dry matter (DM) content was determined to find average daily DM intake (DMI) of the experimental groups.
Milk yield and milk composition
Milking was done twice daily at 6:00 A.M. and 6:00 P.M. Milk produced from each cow during each milking was recorded and pooled for a day to determine the milk yield of an individual cow. Milk samples (about 1/100th of total yield) were collected at fortnightly interval separately from individual animals and composited in equal volumes for each buffalo on each sampling day. Collected samples were analyzed for total solids (TS), fat, solid not fat (SNF), protein and lactose with the help of Automated Milk Analyzer (Lactostar, Funke Gerber, Berlin). The 6% fat corrected milk (FCM) was calculated from the actual milk yield according to Rice et al. (1970)
Metabolism trial
A metabolism trial of 6-day collection period was conducted towards the end of experiment period (day 80–86 of experiment) to determine the intake and digestibility of nutrients, and nitrogen (N) balance. Animals were weighed and transferred to metabolism cages 3 days prior to the start of the trial for proper adaptation. During the collection period, daily feed offered, residue left, feces, urine voided and milk yield were recorded and representative samples were collected and stored for further analysis. Suitable aliquots of fecal (1/1000th) and urine (1/100th) samples were preserved in 1:4 H2SO4 to prevent the loss of ammonia and processed for N estimation. Milk samples were collected twice (morning and evening) on a sampling day in plastic vials and stored at -20 °C for further analysis. Dry matter (DM) of collected feed and fecal samples were determined by drying to constant weight at 100 ± 2 °C by keeping the samples in hot air oven for 24 h, and dried samples were ground to fine powder (to pass through a sieve of 1 mm) and stored in an airtight jar for further analysis. These samples were subjected for proximate analyses as per standard method of AOAC (2005).
In order to determine the proportion of consumed nutrient that was apparently absorbed apparent total tract digestibility (ATTD) of nutrients was calculated as: ATTD (%) = [Nutrient intake (g day−1) − Fecal output (g day−1)]/Nutrient intake (g day−1) × 100.
The cell wall constituents such as neutral detergent fiber (NDF), acid detergent fiber (ADF), cellulose, hemicellulose and lignin were estimated as per Van Soest et al. (1991). Acid detergent and neutral detergent insoluble nitrogen (ADIN and NDIN) were estimated as per method of Licitra et al. (1996) and were multiplied by 6.25 to calculate ADICP and NDICP. Different protein fractions of feed and fodder offered were determined following Cornell Net Carbohydrate Protein (CNCP) system as per method of Sniffen et al. (1992) in order to derive metabolizable protein (MP) content of diet. The total digestible nutrients (TDN) were calculated from metabolism trial digestibility data using formula: TDN% = % Digestible crude protein (%DCP) + [% Digestible ether extract (%DEE\() \times\)2.25] + % Digestible crude fibre (%DCF) + % Digestible nitrogen free extract (%DNFE), and it was used to calculate the digestible energy (DE) following the formulae given by NRC (2001):
Preparation of seaweed extract
Exactly 1 g of ground sample was put into an Erlenmeyer flask and about 40 mL of 70% methanol was added and incubated in a shaker incubator for 24 h. Then the samples were filtered using Whatman filter paper no. 42 with repeated washing using methanol; filtrate obtained was concentrated in rotary evaporator. Finally, crude extract of concentration 1 mg mL−1 was prepared using methanol and was stored in an amber-coloured bottle for further analysis. Total phenolic content (mg GAE g−1) of methanolic extract of seaweeds was determined as per the method of Vijayabaskar and Shiyamala (2012) which is based upon the principle that Folin–Ciocalteu reagent (FCR) in the presence of phenolics results in the production of molybdenum–tungsten blue that is measured spectrophotometrically. About 0.2 mL of crude extract (1 mg mL−1) and standards of different concentrations were taken in a different test tube and volume was made to 3 mL with distilled water. Then, 0.5 mL of Folin- Ciocalteu reagent was added, after incubation for 3 min, 2 mL of 7% sodium carbonate was added to all the tubes. The reaction mixture was incubated for 90 min in dark and the absorbance was measured at 720 nm. Total antioxidant activity was measured by FRAP assay of Benzie and Strain (1999). At low pH, antioxidant present in the sample causes reduction of ferric tripyridyl triazine complex to ferrous form which had an intense blue color. The intensity of the blue color was measured at 593 nm and the change in absorbance was therefore directly related to the total reducing power of the electron-donating antioxidants present in the samples.
Rumen liquor sampling and analysis
Rumen liquor was collected (50th day) from the experimental animals at 06:00 A.M. before feeding by using a stomach tube to estimate rumen fermentation metabolites. Rumen fluid samples were strained through four layers of cheesecloth and pH was determined immediately after collection using a digital pH meter (Eutech pH tester 30, Thermo Fisher Sci. Inc., USA; pH range: 1.00–14.00, resolution 0.01 pH, accuracy ± 0.01 pH). Samples for ammonia nitrogen (NH3-N) and volatile fatty acid (VFA) analysis were stored at -20℃ after acidifying them with 20% H2SO4 and 25% metaphosphoric acid, respectively. The ruminal ammonia nitrogen was estimated by the procedure of Weatherburn (1967). Estimation of VFA was done by using a Nucon-5765 gas chromatograph (AIMIL, India) equipped with a double flame ionization detector and the glass column (4ft length and 1/8-inch diameter) packed with Chromosorb 101 as per method described by Cottyn and Boucque (1968).
Methane emission
The methane (CH4) emission from each experimental buffalo was measured during day 51 to day 56 using sulphur hexafluoride (SF6) tracer technique. On day 49 a permeation tube (PT) filled with pure SF6 gas under liquid nitrogen, which can release SF6 at a constant rate was inserted into the rumen through the mouth using stomach tube. Then, the buffalo was fitted with sampling device to collect air sample from around the mouth and nostrils. The air sample was then assayed for concentrations of CH4 and SF6. Concentration of SF6 in the canisters was analyzed by gas chromatography (Nucon 5700, Nucon Engineers, India), fitted with an electron capture detector (250 ºC) and 3.3 mm molecular sieve column with an internal diameter of 0.32 mm. Another gas chromatograph instrument was fitted with a flame-ionization detector (100 ºC) and stainless-steel column packed with Porapak-Q to determine CH4 concentration. The column and injector temperatures were 50 and 40ºC in both the instruments. All samples were analyzed in duplicate except standards, which were analyzed in triplicate. Nitrogen was used as the carrier gas. Methane emission rate was then calculated from the known release rate of SF6 and concentration of CH4 and SF6 in collected exhaled air sample using the formula:
where, SCH4 and BCH4 are methane concentrations in sample and background canisters (ppm), SSF6 and BSF6 represent the concentrations of SF6 in sample and background’s canister’s (ppt), MCH4 and MSF6 are molecular weight of methane and SF6 (g), respectively and QSF6 represents release rate of SF6 (mg day−1).
Sampling and analyses of blood
The blood sample was collected early in the morning before feeding from the experimental animals via jugular venipuncture on the 0th, 30th, 60th and 90th (monthly interval) day of the experiment for analyses of blood biochemical, hormonal and antioxidant parameters. Blood samples (10 mL) were collected into heparin-coated Vacutainer tubes (Vakli-8, India) and serum was separated by centrifuging the samples at 1107 × g for 20 min and then collected and stored at -20 °C in storage vials for further analysis. In addition, 2 mL of blood was collected separately in Eppendorf tube with 0.3 mL of acid citrate dextrose (ACD) solution and centrifuged at 700 × g for 15 min for hemolysate preparation. The plasma and buffy coat were removed and erythrocytes were washed with isotonic washing solution (PBS, pH 7.4) 3–4 times and then packed cells were re-suspended in the same solution to give a 33% erythrocyte suspension.
The blood biochemical parameters glucose (Trinder 1969a), total protein (Doumas 1975), albumin (Doumas and Watson 1971), globulin (G = total protein - albumin), triglycerides (Trinder 1969b), cholesterol (Trinder 1969c), non-esterified fatty acids (Shipe et al. 1980) were estimated with the help of commercial kits (Span Diagnostics, India).
Superoxide dismutase (SOD) was estimated by the method described by Madesh and Balasubramanian (1998) using RBC hemolysate on the same day of collection of blood. In addition, total antioxidant capacity (TAC) was measured by FRAP assay of Benzie and Strain (1999) and malondialdehyde (MDA) which is a biomarker for lipid peroxidation was estimated by following the method of Niehaus and Samuelsson (1968) modified by Kaushal and Kansal (2012).
Tri-iodothyronine (T3) was assayed in plasma samples by using bovine T3 ELISA kit (Catalog No. E0215Bo), thyroxine (T4) was assayed using bovine T4 ELISA kit (Catalog No. E0216Bo), cortisol was measured by using cortisol ELISA kit (Catalog No. E0110Bo) of Bioassay Technology Laboratory, Shanghai, China.
Immune response
Hemagglutination antibody (HA) titer was measured against the chicken red blood cells (CRBC) to find out the humoral immune response. For this, buffalos were injected with 1 mL of 10% CRBC suspension. Then the blood samples were collected on the 0th (before), 7th, 14th, 21st and 28th day of post-injection. At the end, HA test was performed with 100 µL serum sample and 100 µL CRBC in U-bottom HA plate.
At the end of the trial, cell-mediated immune (CMI) response was assessed by delayed-type hypersensitivity (DTH) skin test against phytohaemagglutinin-P (PHA-P) as given by Masucci et al. (2011). One day prior to the injection, both sides of the neck were shaved. Next day, a 1-cm2 area at the middle the shaved area was marked and the skin thickness was recorded using a Vernier calliper. After sterilization with 70% alcohol, 0.1 mL of 1.5 mg mL−1 of PHA-P (50 µg (100 µL)−1 PBS, Sigma Chemical) on one side and 0.1 mL of sterile PBS on the other side of the neck was injected intra-dermally. The skinfold thickness was measured before administration of PHA-P (0 h), and thereafter at 6, 12, 24 and 48 h post-injection with a Vernier caliper. Finally, the cell-mediated immune response was assessed by comparing the percent increase in skin thickness to baseline reading (0 h).
Bovine total immunoglobulin assay
Total plasma immunoglobulin was measured using an ELISA kit (Bioassay Technology Laboratory, China).
Statistical Analysis
Before analysis all data were tested for normality using the UNIVARIATE procedure (Shapiro–Wilk test). Data that did not meet the assumptions of homoscedasticity and normality of residuals were square root transformed. Regression analysis was done by plotting data on methane emission (g day−1, g kg−1 DMI) on y-axis, and proportion of propionate (%) and A/P ratio on x-axis.
The data that were collected periodically (BW, DMI, milk yield and FCM, milk composition biochemical parameters, plasma hormones, antioxidant activity, and immunoglobulin) were analysed using the general linear model procedure of Statistical Package for the Social Sciences (SPSS for Windows, V21.0; SPSS Inc., USA). The following model was used:
where Yijk is the dependent variable, µ is the overall mean of the population, Ti is the mean effect of the ith treatments, Dj(i) the the random effect of the ith buffalo within treatment, Pk the mean effect of day of sampling with day as a repeated factor (k = 0, 30, 60, 90 and 120 days of dietary treatment), (T × P)ik is the interaction between effects of treatment and day of sampling, and eijk is the unexplained residual element assumed to be independent and normally distributed. Base line data (0 day) were included as co-variate in the statistical analysis using an autoregressive co-variate structure. The means were compared using least squares means adjusted by the Tukey’s post-hoc procedure.
Data on other parameters collected during metabolic trial (intake and digestibility of nutrients and balance of N), VFA and methane analysis were analysed using one way analysis of variance (ANOVA) with the following model:
where Yij = each observation, µ = overall mean, Ti = effect of ith treatment, ɛij = residual error.
The individual animal was used as the experimental unit for all data. The pair-wise comparison of means was carried out using “Tukey’s post-hoc test”. A probability value of P < 0.05 was considered significant although mean differences with P < 0.10 were considered as trends and results are reported accordingly.
Results
Nutrient composition of feed, fodder and seaweed
The chemical composition of oat fodder, wheat straw, concentrates, and seaweeds namely K. alvarezii (KA), G. salicornia (GS), and T. conoides (TC) are presented in Table 2. The crude protein (CP), ether extract (EE), and crude fiber (CF) contents of KA, GS, and TC were similar, but GS had a greater amount of total ash (TA) content (74.60 percent on a DM basis) than other two seaweeds. Total phenolics content and FRAP-value were higher in TC as compared to two other seaweeds.
Feed intake, digestibility and nitrogen balance
The consumption of feed (kg DM day−1), dry matter intake (DMI), and intake of nutrients such as dry matter (DM), organic matter (OM), crude protein (CP), ether extract (EE), neutral detergent fiber (NDF), acid detergent fiber (ADF), total digestible nutrients (TDN), non-fibrous carbohydrates (NFC), and metabolizable energy (ME) were found to be comparable among the groups (Table 3). Similarly, there was no significant variation in the apparent digestibility of the various nutrients such as OM, CP, EE, and NDF among the groups. The nitrogen (N) intake (g day−1), faecal outgo (g day−1), N absorbed (g day−1), urinary N losses (g day−1), and N retention (g day−1) were found to be similar among the groups (Table 4). Efficiency of utilization of N for milk synthesis (either as g N deposited (100 g)−1 N absorbed N or g N deposited (100 g)−1N intake) was also found to be similar among the groups.
Intake of ME (Mcal day−1), and the portion ME being utilized for maintenance and gain were similar (P > 0.05) among the groups. There was a tendency (P = 0.072) of increased portioning of ME towards milk yield in group KA (Table 4). The ME deposited in the milk in terms of % ME available and % ME intake were found to be similar among the groups.
Rumen fermentation and methane emission
The pH and ammonia nitrogen (NH3-N) level of rumen fluid was found to be similar among the groups (Table 5). The concentration of VFA in rumen fluid was also not affected by seaweed supplementation. However, there was an increase (P = < 0.001) in proportion of propionate in rumen fluid with concurrent decrease (P = < 0.001) in A:P ratio on supplementation of KA and GS and the higher response being observed in KA group. The concentration of acetate tended to decrease (P = 0.084) in all the seaweed-supplemented groups as compared to CON group whereas, butyrate was unaffected by any of the supplemented seaweeds. Methane production expressed either as g day−1, g kg−1 milk or g kg−1 DMI was lower (P < 0.001) in buffaloes fed supplementary KA and GS, and the most evident anti-methanogenic activity was shown by GS as compared to the other groups. The regression analysis between propionate (%), A/P ratio and methane emission (g day−1, g kg−1 DMI) revealed a negative relationship (Y = -17.68X + 649.7, R2 = 0.6071, P = < 0.0001; Y = -1.03X + 39.50, R2 = 0.5792, P = < 0.0001) between propionate concentration and methane emission; whereas a positive relationship (Y = 82.28X + 15.60, R2 = 0.5026, P = 0.0001; Y = 5.04X + 1.88, R2 = 0.5314, P = < 0.0001) was observed between A/P ratio and methane emission (Fig. 2).
Blood biochemical profile
Supplementation of seaweeds did not show any adverse effect on the blood biochemical parameters such as blood glucose, total protein, albumin, globulin, triglycerides, cholesterol and non-esterified fatty acids (NEFA) and all the estimated parameters were within the normal range (Table 6). Neither seaweed supplementation nor stages of lactation did elicit any effect on these parameters.
Antioxidant, hormonal and immune status
There was a tendency (P = 0.09) of increased SOD activity (U mg−1 Hb) in groups KA, TC and GS as compared to CON (Table 6). However, no significant effect of period or period x treatment interaction was observed. Plasma concentration of total antioxidant capacity (TAC mmol L−1) was significantly (P < 0.01) higher in seaweed-supplemented groups, however, there was no species difference among the seaweed in eliciting their antioxidant activity (Fig. 3). The plasma concentration of MDA was significantly (P < 0.01) lower in KA and GS group as compared to CON group (Fig. 3).
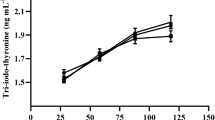
The plasma concentration of triiodo thyronine (T3) and thyroxine (T4) was unaffected by seaweed supplementation. The results of plasma cortisol estimates revealed that cortisol level was reduced (P < 0.05) in all the seaweed-supplemented groups as compared to CON group, however, the lowest value was reported in KA, followed by GS and TC (Fig. 4).
The absolute skin thickness was greater (P < 0.001) in KA, followed by GS, however, values were similar between CON and TC group. The highest HA titer in response to CRBC was seen 14 days after inoculation, following which it gradually declined. In comparison to CON group, the humoral immune response assessed as titer in reaction to chicken RBC was greater (P < 0.001) in KA and GS supplemented groups, indeed the response was greater in KA. Plasma concentration of immunoglobulin was higher (P < 0.001) in animals fed diet supplemented with KA, followed by GS and TC.
Bodyweight changes and body condition score
From perusals of the data, it is evident that supplementation of different species of seaweeds did not manifest any significant impact on fortnightly changes in body weight and body condition score (BCS) in lactating Murrah buffaloes. All the groups had similar body weight and BCS at various fortnights; neither the treatment, period, nor the period × treatment interaction was significant (Table 7).
Milk yield and composition
Milk yield and 6% FCM yield were higher (P < 0.05) in KA group followed by GS group as compared to CON and TC groups (Table 7 and Fig. 5). Average daily milk yield and FCM increased up to 4th fortnight of the experiment, and then it gradually declined, a lactation curve that is typical to Murrah buffalo was observed. The average FCR (DMI kg−1 milk) was found be improved (P < 0.01) in KA, GS groups. The composition of milk (fat, SNF, protein, lactose and total solids) was not influenced by supplementation of seaweeds.
Discussion
Chemical composition of feed and seaweed
The chemical composition of oat fodder, wheat straw and concentrate mixture used in the present study had similar nutritional compositions that were reported by ICAR (2013). The nutritional composition of all three seaweeds (CP, EE, CF, NDF, and ADF %) was found to be identical to earlier findings (Ahmad et al. 2016; Maheswari et al. 2021). The total ash (TA) and acid insoluble ash (AIA) levels of all three seaweeds were found to be greater. GS contained a higher amount of TA (74.60 percent on DM basis) as compared to other two seaweeds. This finding corroborates well with previous studies indicating higher ash content of G. salicornia (Maheswari et al. 2021; Anderson et al. 2023).
Intake and utilization of nutrients
The present study findings revealed that the supplementation of KA, GS and TC did not have any adverse effect on feed intake in lactating Murrah buffalos. This finding was in agreement with several other previous seaweed-supplemented studies in ruminants (Singh et al. 2017; Maheswari et al. 2021; Sharma et al. 2022; Anderson et al. 2023).
Apparent digestibility of nutrients like OM, CP, EE, and NDF was unaffected by the seaweed supplementation. However, in this experiment there was an increased tendency of DM and ADF digestibility in all three seaweed-supplemented groups, whereas negative trend on NFC digestibility was observed in KA and TC supplemented groups. It is fair to conclude that feeding Kappaphycus, Gracilaria or Turbinaria at a lower (% DM) concentration in the diet of lactating buffaloes in present study didn’t have any adverse effect on digestibility of nutrients. This is in agreement with previous findings that indicate no adverse impact of seaweed on digestibility of nutrients (Antaya et al. 2015, 2019; Belanche et al. 2016; Singh et al. 2017). The results of present study revealed that the supplementation of tropical seaweeds did not cause any significant changes in nitrogen (N) intake, outgo and balance and the efficiency of converting dietary N into milk nitrogen in lactating Murrah buffaloes. Similarly, intake and utilization of ME remained similar among the groups and all the diets supplied adequate amount of energy and protein to meet requirements of lactating buffaloes (ICAR 2013).
Rumen fermentation parameters
In the present experiment, supplementation of seaweeds did not cause any significant change in the rumen pH, NH3-N and total volatile fatty acids (TVFA) concentration of lactating Murrah buffaloes fed different tropical seaweeds. These results were corroborating well with previous reports (Kinley and Fredeen 2015; Kinley et al. 2020) which indicate that supplementation of tropical seaweeds did not influence either of these parameters. However, there are some reports (Hong et al. 2015; Belanche et al. 2016; Maia et al. 2016; Gülzari et al. 2019) which indicate that supplementation of seaweed resulted in decreased rumen fermentation leading to reduced gas and VFA production. This discrepancy can be explained on the basis of species and doses of seaweeds. Most studies indicate that rumen fermentation is inhibited when seaweeds are supplemented at a higher level (reviewed by Abbott et al. 2020). In this experiment, proportion of seaweed in the total ration did not exceed 1%. Results clearly demonstrate that supplementation of either KA, GS or TC at 1% of the diet would not have any adverse impact on rumen fermentation. This agrees with the finding of Munde (2018) who reported that supplementation of either KA or GS at 1% of the diet did not affect rumen fermentation in steers.
Supplementation of KA and GS resulted in increased proportion of propionate in the rumen fluid leading to a decreased A/P ratio. Similarly, supplementation of tropical red seaweed resulted in increased proportion of propionate in the rumen (Machado et al. 2014, 2016b; Belanche et al. 2016; Stefenoni et al. 2021). Results of the present experiment suggest that supplementation of tropical red seaweeds may divert part of the hydrogen towards production of propionate and thereby reducing the amount of hydrogen available for methanogenesis. However, how tropical red seaweed shift of rumen fermentation towards propionate is not precisely known, however, it could link to bromoform content of tropical red seaweed. Machado et al. (2016b) reported that when the level of bromoform (CHBr3) was 5 μM it caused a reduction in production of acetate by 44%. This would be the possible mechanism by which KA and GS caused a shift towards propionate production because both these seaweeds are rich sources of halogenated compounds (Leedham et al. 2013; Keng et al. 2021).
Methane production
In this experiment, supplementation of all three seaweeds was found to decrease enteric methane emission (g day−1, g kg−1 milk, g kg−1 DMI) and the greater response was observed in KA and GS group followed by the TC. A similar reduction in methane emission in several in-vivo experiments with seaweed supplementation in cattle (Machado et al. 2014; Roque et al. 2019; Katwal et al. 2021; Stefenoni et al. 2021), and also in many in-vitro experiments (Wang et al. 2008; Machado et al. 2016a; Ahmed et al. 2022). In this experiment, we observed a negative relationship between proportion of propionate and A:P on methane emission.. The antimethanogenic activity found in our study could well correlated with the higher proportion of propionic acid that found in ruminal fluid of seaweed-fed groups. As part of the hydrogen produced in the rumen might be diverted towards production of propionate in seaweed fed groups, and thereby net amount of hydrogen available for methanogenesis is reduced leading to decreased production of methane. The decreased methane emission due to feeding of tropical seaweed could also be attributed to presence of halogenated compounds in red seaweeds (Machado et al. 2016a; Stefenoni et al. 2021). In this experiment, proportion of seaweed was less than 1% of the diet DM and no decrease in apparent digestibility or rumen concentration of TVFA was observed. To date the red seaweed Asparagopsis taxiformis has been studied most widely for its anti-methanogenic activity (Machado et al. 2014; 2016a, b; Kinley et al. 2020) and it was also reported to contain several halogenated compounds like bromoform (1723 μg g−1 dry weight), dibromochloromethane (15.8 μg g−1 dry weight), bromo chloroacetic acid (9.8 μg g−1 dry weight) and dibromo acetic acid (0.9 μg g−1 dry weight) (Machado et al. 2016a). The two red seaweeds, K. alvarezii and G. salicornia that were used in this experiment were also reported to be a good source of bromoforms and emission rates of bromoform (CHBr3) for Kappaphycus and Gracilaria were 479–558 and 1037–1272 pmol g−1 fresh weight h−1, respectively (Keng et al. 2021, 2023). These halogenated compounds exert their antimethanogenic action via inhibition of vitamin B12 dependent methyl transferase which catalyses the terminal step in enzymatic pathway of methanogenesis. Additionally, these halogenated compounds may act as terminal electron acceptor (Abbott et al. 2020).
In this study, enteric methane emission was reduced by 23, 30 and 11% in groups KA, GS and TC respectively. The modest response obtained in this experiment could be explained on the basis of higher content of halogenated compounds in red seaweeds. On the other hand, brown seaweeds contain a negligible amount of bromoforms, so higher methane emission in TC group as compared other red seaweed supplemented groups (KA and GS) was in the expected line. Among the two tropical red seaweeds, Gracilaria contained more bromoform content than Kappaphycus (Keng et al. 2021) which might be the reason behind apparently lower methane emission of animals consuming G. salicornia as compared to those fed diet containing K. alvarezii.
General metabolic and hormonal profile
In the present experiment, supplementation of seaweeds showed no significant influence on plasma concentrations of T3 and T4 hormones. Similarly, previous studies conducted in buffaloes (Maheswari et al. 2021) and lactating cattle (Antaya et al. 2015, 2019; Hong et al. 2015; Sorge et al. 2016) with macro-algal supplementation did not find any negative impact on thyroid hormones.
In the present study, a reduction in plasma concentration of cortisol was observed in buffaloes fed diets supplemented with tropical seaweeds and higher response was evident in the K. alvarezii fed group. This is in agreement with Maheswari et al. (2021) who indicated that supplementation of tropical seaweed-based formulation containing Kappaphycus, Gracilaria and Turbinaria reduced circulatory concentration of cortisol in lactating Murrah buffalo. In present study, the superiority of Kappaphycus over other two seaweeds in alleviating stress could be linked to the free radical scavenging ability of Kappaphycus attributed to its higher levels of carrageenans, that contained one or more-OH and -SO-H groups (Matanjun et al. 2010). Thus, supplementation of Kappaphycus alvarezii can reduce the stress levels in early lactating animals.
Antioxidant activity
In this study antioxidant status of the lactating Murrah buffaloes were assessed by measuring superoxide dismutase (SOD), lipid peroxidation (LPO) and total antioxidant capacity (TAC). In this study, SOD activity was similar in all the groups, however a greater tendency of SOD activity was noticed in seaweed-fed groups. Similarly, an increased activity of SOD was observed on supplementation of goats with seaweed extract under transportation stress (Kannan et al. 2007). The red seaweeds have the ability to strengthen the antioxidant status through enhanced activity of SOD was reported earlier in calves (Anderson et al. 2023) and lactating buffaloes (Maheswari et al. 2021). Supplementation of brown seaweed to the goat kids, improved antioxidant status by improved SOD activity (Angulo et al. 2020).
In this experiment, supplementation of K. alvarezii and G. salicornia resulted in reduced plasma MDA concentration, however, no such positive response was observed when T. conoides was supplemented. Results were largely similar to an earlier study (Maheswari et al. 2021) which indicated that supplementation of a tropical seaweed-based formulation containing Kappaphycus, Gracilaria and Turbinaria (1:1:1) reduced lipid peroxidation in lactating buffaloes. A comparison of both studies would indicate that most of these protective actions were rendered by the two tropical red seaweeds rather than one brown seaweed. Superiority of the two red seaweeds could be linked to their higher content of sulphated polysaccharides (Makkar and Chakraborty 2017). In this experiment, supplementation of T. conoides did not cause any significant change in plasma concentration of MDA. In disagreement with present results, reduced lipid peroxidation on brown seaweed supplementation was observed in sheep exposed to prolonged heat stress (Saker et al. 2004). However, studies on stress-relieving response of brown seaweeds in livestock pertains mostly to one temperate brown seaweed species i.e., Ascophyllum nodosum. The discrepancy in response to insult caused by reactive oxygen species (ROS) was indicative of potential difference between A. nodosum and T. conoides which need to be studied further with different brown seaweed species from different habitats.
In this experiment, total antioxidant capacity (TAC) was increased on seaweed supplementation in Murrah buffalo diet. Similarly, supplementation of macro-algae increased the antioxidant capacity of animals exposed to stress induced by either heat or due to grazing on toxicants (Saker et al. 2001, 2004). Dietary supplementation of a tropical seaweed-based formulation resulted in increased TAC in buffaloes (Maheswari et al. 2021). The antioxidant nature of tropical red seaweeds (K. alvarezii and G. salicornia) was due to the fact that they contain good number of sulphated polysaccharides, polyphenolic compounds, vitamin E, and minerals such as iron (Fe), manganese (Mn), copper (Cu) and selenium (Se) which acts as cofactors for some antioxidant enzymes (Kumar et al. 2011). Sulfated polysaccharides such as carrageenan have the capacity to scavenge free radicals due to the presence of singular or numerous sulphate groups (Arunkumar et al. 2021). While red seaweeds are high in sulphated polysaccharides, they lack phlorotannins, which are found only in brown seaweeds and acts as an excellent source of antioxidant defence system (Vijayabaskar et al. 2012). Other bioactive compounds found in brown seaweeds with potential antioxidant activity include pigments (fucoxanthin, astaxanthin, carotenoids) and polyphenols (phenolic acid, flavonoids, tannins). Thus, it is evident that red (Kappaphycus, Gracilaria) and brown seaweeds (Turbinaria) used in present study render protection against oxidative damage through different mechanisms. Irrespective of the treatment, plasma levels of SOD and TAC increased and that of MDA decreased with increase of days in milk. Similarly, Maheswari et al. (2021) also reported an improved antioxidant status of lactating Murrah buffaloes with advancement of lactation.
Immune parameters
In present study, supplementation of both the red seaweeds improved the immune response of lactating Murrah buffaloes, however, supplementation of the brown seaweed T. conoides did not have any significant effect on cell-mediated immunity (CMI) and humoral immune response. Similar to our results, supplementation of three different brown seaweeds did not cause any significant change in the phagocytic index in fish (Thepot et al. 2021). However, other reports indicate positive response of brown seaweed supplementation on cellular immunity in livestock (Hwang et al. 2014; Bussy et al. 2019). Feeding of brown seaweed A. nodosum enhanced phagocytic activity in calves grazing on endophyte-infected tall fescue (Allen et al. 2001) and in lambs exposed to chronic heat stress (Saker et al. 2004). Discrepancy in obtained response could be due to considerable variation in functional properties of different species of brown seaweeds, habitat, and may also be dependent on the physiological status of the animals. In this experiment, CMI response was improved due to supplementation of both the red seaweeds, but the greater response was obtained with K. alvarezii. Present findings corroborate well with a previous report (Maheswari et al. 2021) which indicate that supplementation of tropical seaweed-based formulation (mostly red seaweed based) improved CMI response in lactating Murrah buffaloes. Similarly, supplementation of K. alvarezii improved cellular immune response in hens (Qadri et al. 2019). Both the red seaweeds (K. alvarezii and G. salicornia) that were studied contained appreciable quantity of bioactive molecules such as sulfated polysaccharides, phenolics, α-tocopherol, and pigments (Makkar et al. 2016), which are capable of augmenting cell-mediated immune function (Fike et al. 2001). Thus, it is logical to assume that supplementary feeding of red seaweeds would increase supply of antioxidant bioactive compounds that will increase the power of phagocytes to kill pathogens. Further, red seaweed contains sulphated polysaccharides that are potent immunological stimulators through the activation of dectin-1 receptors of immune cells (Maqsood et al. 2011). In this study, superior cellular immune response in red seaweed supplemented buffaloes was also accompanied by increased plasma concentration of immunoglobulin, the higher response being observed when K. alvarezii was used as a supplement.
In this study, supplementation with both res seaweeds enhanced the humoral immune response in lactating Murrah buffaloes, while the brown seaweed did not cause any favorable response. Similarly, an improved humoral immunity was also reported in buffaloes (Maheswari et al. 2021), sheep (Cabrita et al. 2017) and chicken (Qadri et al. 2019) on supplementation of red seaweed-based formulation. It could be result of appreciable amount of lectin present in K. alvarezii which might facilitate enhanced hemagglutination activity (Hung et al. 2009). The bioactive molecules of seaweeds have the ability to stimulate the release of cytokines (IL‐2 and IL‐4) and chemokines which show positive effect on humoral and cellular immune functions (Leonard et al. 2011). The inclusion of Gracilaria spp. at 5% in broiler chicken diet improved the immunity with enhanced circulatory immunoglobulins (Al-Khalaifah et al. 2022). In nutshell, supplementation of K. alvarezii and G. salicornia improved both cellular and humoral immune response of lactating Murrah buffaloes, the greater response being observed when K. alvarezii was used as supplement. The superiority of Kappaphycus over Gracilaria in boosting immunity can at least be partially explained on the basis of greater antioxidant activity of K. alvarezii than G. salicornia (Ganesan et al. 2008).
Milk yield and composition
In the present study, supplementation of Kappaphycus and Gracilaria shown to improve the milk yield and 6% FCM yield in Murrah buffaloes. However, the brown seaweed, T. conoides supplementation did not have any significant effect on milk yield. Although, 6% FCM yield was shown improvement in TC group. The fact is, most of the earlier studies related to the effect of supplementation of seaweeds on milk production were focussed on one single species of the temperate brown seaweed, A. nodosum and the results reported were inconsistent. The majority of the studies indicated that brown seaweed supplementation eighter had no effect or decreased milk yield (Antaya et al. 2019; Katwal et al. 2021; Thorsteinsson et al. 2023), but the results of the present experiment were clearly demonstrated that brown seaweed (T. conoides) may have the potential to cause some improvement in milk yield, although response was not robust enough. On the other hand, there are some reports which indicates an increased milk production on brown seaweed supplementation (Singh et al. 2017).
Interestingly, supplementation of both of the tropical red seaweeds caused significant increase in milk yield and FCM yield. These findings agree well with the study of Maheswari et al. (2021) indicating that supplementation of a tropical seaweed-based formulation (mostly K. alvarezii and G. salicornia) improved milk and FCM yield in lactating Murrah buffaloes. The positive response that we have observed in this study with respect to milk production in red seaweed supplemented groups could be linked to improved ruminal concentration of propionate in KA and GS groups, which acts as a potent precursor for intra-mammary synthesis of lactose (Kleiber et al. 1953). A similar increase in milk production mediated through increased concentration of propionate was reported earlier (Xue et al. 2019). Increased milk yield could also be related to decreased methane emission in KA and GS supplemented groups. In general, 2–12% of the gross energy (GE) intake will be lost as methane in ruminants, and in particular loss will be higher than 6% in dairy animals (Tamminga et al. 2007). Thus, it would be logical to assume that the reduced CH4 emission due to feeding of red seaweeds might have contributed to improved milk yield. Along with these, improved antioxidant and immune status of buffaloes fed red seaweeds (KA and GS) have improved overall welfare and which might have enabled the buffaloes to divert more energy and nutrients towards milk production.
During early lactation, high energy demand causes physiological imbalances leading to disruption of endocrine homeostasis and balance of pro-oxidants and anti-oxidants. Oxidative stress-induced inflammatory changes in dairy cattle often associated with rise in circulatory concentrations of pro-inflammatory cytokines, which may adversely affect the energy partitioning and lactational performance (Kuhla 2020). Under such conditions, there will be shift in energy partitioning towards maintenance than production. Both the red seaweeds namely K. alvarezii (Paul et al. 2021; Zhang et al. 2022) and Gracilaria spp. (Antony and Chakraborty 2020) can strengthen the immune system by reducing circulatory concentration of pro-inflammatory cytokines. Thus, they can reverse some of the adverse changes associated with nutrient partitioning observed during early lactation. An improved immune system as observed during the present study would ensure that a lesser amount of energy and nutrients are diverted towards maintenance and more is available for milk production.
In this experiment the greater response with respect to milk yield was obtained when KA was supplemented, which is clearly evident on direct comparison between the two red seaweeds that the immune response (humoral immunity and cell-mediated immunity), and plasma concentration of immunoglobulin was higher, and that of cortisol was lower in buffaloes fed KA as compared to those fed with GS. Improved immune function and ability to combat stress might have resulted in enhanced milk production in buffaloes fed KA as compared to GS. In this experiment, composition of milk was uninfluenced by feeding of any of the three tropical seaweeds. On the contrary, Bendary et al. (2013) reported that supplementation of seaweed to lactating HF cows increased fat, lactose, SNF and total solid contents of milk. However, results of present experiment show that incorporation of tropical seaweeds at 2.5% of the concentrate mixture in lactating Murrah buffaloes would have no adverse impact on milk composition. This agrees well with several previous reports (Karatzia et al. 2012; Antaya et al. 2015, 2019; Roque et al. 2019; Maheswari et al. 2021).
Conclusion
Supplementation of Turbinaria conoides improved antioxidant status, and reduced methane emission and stress with an apparent increase in 6% FCM milk yield, however, the responses were not as pronounced as in animals fed supplementary Kappaphycus alvarezii and Gracilaria salicornia. Supplementation of both the tropical red seaweeds significantly increased the proportion of propionate, decreased enteric methane emission, improved antioxidant, immunity status and reduced the stress level by lowering cortisol, that resulted in increased milk and 6% FCM yield. The results clearly demonstrate the superiority of the two tropical red seaweeds over the brown seaweed as a feed supplement for lactating Murrah buffalo. A comparison between the two seaweeds indicates that antioxidant status was similar between the groups, however, immune response was higher and plasma level of cortisol was lower in buffaloes fed K. alvarezii as compared to those fed G. salicornia. Buffaloes fed K. alvarezii produced more milk and FCM with improved FCR as compared those fed G. salicornia. It is concluded that supplementation of K. alvarezii at 1% of dietary DM of lactating Murrah buffaloes would be beneficial in terms of improved antioxidant and immune status, and milk yield with a reduction in enteric methane emission.
Data availability
The data that support our findings of the present study are available from the corresponding author upon reasonable request.
References
Abbott DW, Aasen IM, Beauchemin KA, Grondahl F, Gruninger R, Hayes M Xing X (2020) Seaweed and seaweed bioactives for mitigation of enteric methane: Challenges and opportunities. Animals 10:2432
Ahmad F, Sulaiman MR, Saimon W, Yee CF, Matanjun P (2016) Proximate compositions and total phenolic contents of selected edible seaweed from Semporna, Sabah, Malaysia. Borneo Sci 31:85–96
Ahmed E, Batbekh B, Fukuma N, Hanada M, Nishida T (2022) Evaluation of different brown seaweeds as feed and feed additives regarding rumen fermentation and methane mitigation. Fermentation 8:504
Al-Khalaifah HS, Al-Nasser A, Surrayai T (2022) Effects from dietary addition of Sargassum sp., Spirulina sp., or Gracilaria sp. powder on immune status in broiler chickens. Front Vet Sci 9:928235
Allen G, Pond KR, Saker KE, Fontenot JJP, Bargley CP, Ivy RL, Evans RR, Schmidt RE, Fike JH, Zhang X, Ayad JY, Brown CP, Miller MF, Montgomery JL, Mahan J, Wester DB, Melton C (2001) Tasco: Influence of a brown seaweed on antioxidants in forages and livestock- A review. J Anim Sci 79:E21–E31
Anderson P, Malik R, Ojha L, Naliyapara A-M, HB, (2023) Investigations on modulating effect of three tropical red seaweed by-products on growth performance, immune response, antioxidant status and endocrine variables in crossbred calves. J Appl Phycol 35:445–457
Angulo C, Chavez-Infante L, Reyes-Becerril M, Angulo M, Romero-Geraldo R, Llinas-Cervantes X, Cepeda-Palacios R (2020) Immunostimulatory and antioxidant effects of supplemental feeding with macroalga Sargassum spp. on goat kids. Trop Anim Health Prod 52:2023–2033
Antaya NT, Ghelichkhan M, Pereira AB, Soder KJ, Brito AF (2019) Production, milk iodine, and nutrient utilization in Jersey cows supplemented with the brown seaweed Ascophyllum nodosum (kelp meal) during the grazing season. J Dairy Sci 102:8040–8058
Antaya NT, Soder KJ, Kraft J, Whitehouse NL, Guindon NE, Erickson PS, Conroy AB, Brito AF (2015) Incremental amounts of Ascophyllum nodosum meal do not improve animal performance but do increase milk iodine output in early lactation dairy cows fed high-forage diets. J Dairy Sci 98:1991–2004
Antony T, Chakraborty K (2020) Anti-inflammatory polyether triterpenoids from the marine macroalga Gracilaria salicornia: Newly described natural leads attenuate pro-inflammatory 5-lipoxygenase and cyclooxygenase-2. Algal Res 47:101791
AOAC (2005) Official Methods of Analysis, 18th edn. Association of Official Analytical Chemists, Washington, DC
Arunkumar K, Raja R, Kumar VS, Joseph A, Shilpa T, Carvalho IS (2021) Antioxidant and cytotoxic activities of sulfated polysaccharides from five different edible seaweeds. J Food Meas Charact 15:567–576
Bach SJ, Wang Y, McAllister TA (2008) Effect of feeding sun-dried seaweed (Ascophyllum nodosum) on fecal shedding of Escherichia coli O157:H7 by feedlot cattle and on growth performance of lambs. Anim Feed Sci Technol 142:17–32
Belanche A, Ramos-Morales E, Newbold CJ (2016) In vitro screening of natural feed additives from crustaceans, diatoms, seaweeds and plant extracts to manipulate rumen fermentation. J Sci Food Agric 96:3069–3078
Bendary MM, Bassiouni MI, Ali MF, Gaafar HM, Shamas AS (2013) Effect of premix and seaweed additives on productive performance of lactating Friesian cows. Int Res J Agric Sci Soil Sci 3:174–181
Benzie IF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth Enzymol 299:15–27
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Bussy F, Salmon H, Delaval J, Berri M, Pi NC (2019) Immunomodulating effect of a seaweed extract from Ulva armoricana in pig: Specific IgG and total IgA in colostrum, milk, and blood. Vet Anim Sci 7:100051
Cabrita AR, Correia A, Rodrigues AR, Cortez PP, Vilanova M, Fonseca AJ (2017) Assessing in vivo digestibility and effects on immune system of sheep fed alfalfa hay supplemented with a fixed amount of Ulva rigida and Gracilaria vermiculophylla. J Appl Phycol 29:1057–1067
Cottyn BG, Boucque CV (1968) Rapid method for the gas-chromatographic determination of volatile fatty acids in rumen fluid. J Agric Food Chem 16:105–107
Doumas BT (1975) Standards for total serum protein assays—a collaborative study. Clin Chem 21:1159–6116
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31:87–96
Ferguson JD, Galligan DT, Thomsen N (1994) Principal descriptors of body condition score in Holstein cows. J Dairy Sci 77:2695–2703
Fike JH, Allen VG, Schmidt RE, Zhang X, Fontenot JP, Bagley CP, Ivy RL, Evans RR, Coelho RW, Wester DB (2001) Tasco-Forage: I. Influence of a seaweed extract on antioxidant activity in tall fescue and in ruminants. J Anim Sci 79:1011–1021
Ganesan P, Kumar CS, Bhaskar N (2008) Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol 99:2717–2723
Gülzari ŞÖ, Lind V, Aasen IM, Steinshamn H (2019) Effect of supplementing sheep diets with macroalgae species on in vivo nutrient digestibility, rumen fermentation and blood amino acid profile. Animal 13:2792–2801
Hong ZS, Kim EJ, Jin YC, Lee JS, Choi YJ, Lee HG (2015) Effects of supplementing brown seaweed by-products in the diet of Holstein cows during transition on ruminal fermentation, growth performance and endocrine responses. Asian-Australas J Anim Sci 28:1296–1302
Hung LD, Hori K, Nang HQ, Kha T, Hoa LT (2009) Seasonal changes in growth rate, carrageenan yield and lectin content in the red alga Kappaphycus alvarezii cultivated in Camranh Bay. Vietnam J Appl Phycol 21:265–272
Hwang JA, Islam MM, Ahmed ST, Mun HS, Kim GM, Kim YJ, Yang CJ (2014) Sea mustard (Undaria pinnatifida) improves growth, immunity, fatty acid profile and reduces cholesterol in Hanwoo steers. Asian-Australas J Anim Sci 27:1114
ICAR (2013) Nutrient requirements of cattle and buffalo. Indian Council of Agricultural Research, New Delhi
Kannan G, Saker KE, Terrill TH, Kouakou B, Galipalli S, Gelaye S (2007) Effect of seaweed extract supplementation in goats exposed to simulated preslaughter stress. Small Rumin Res 73:221–227
Karatzia M, Christaki E, Bonos E, Karatzias C, Florou-Paneri P (2012) The influence of dietary Ascophyllum nodosum on haematologic parameters of dairy cows. Ital J Anim Sci 11:31
Katwal S, Pandya P, Trivedi MM, Sorathiya KK, Shah SV (2021) Antimethanogenic effects of soybean straw and seaweed (Sargassum johnstonii) based total mixed ration in crossbred cows. Indian J Dairy Sci 74:498–503
Kaushal D, Kansal VK (2012) Probiotic dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum alleviates age-inflicted oxidative stress and improves expression of biomarkers of ageing in mice. Mol Biol Rep 39:1791–1799
Keng FS, Phang SM, Abd Rahman N, Yeong HY, Malin G, Elvidge EL, Sturges W (2021) Halocarbon emissions by selected tropical seaweeds exposed to different temperatures. Phytochemistry 190:112869
Keng FS-L, Phang S-M, Rahman NA, Yeong H-Y, Malin G, Elvidge EL, Sturges W, Lee C-W (2023) Emission of volatile halocarbons from the farming of commercially important tropical seaweeds. J Appl Phycol 35:3007–3020
Kinley RD, Fredeen AH (2015) In vitro evaluation of feeding North Atlantic stormtoss seaweeds on ruminal digestion. J Appl Phycol 27:2387–2393
Kinley RD, Martinez-Fernandez G, Matthews MK, de Nys R, Tomkins MM, NW, (2020) Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J Clean Prod 259:120836
Kleiber M, Black AL, Brown MA, Tolbert BM (1953) Propionate as a precursor of milk constituents in the intact dairy cow. J Biol Chem 203:339–346
Kuhla B (2020) Pro-inflammatory cytokines and hypothalamic inflammation: implications for insufficient feed intake of transition dairy cows. Animal 14:s65-77
Kumar M, Kumari P, Trivedi N, Shukla MK, Gupta V, Reddy CR, Jha B (2011) Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J Appl Phycol 23:797–810
Leedham EC, Hughes C, Keng FS, Phang SM, Malin G, Sturges WT (2013) Emission of atmospherically significant halocarbons by naturally occurring and farmed tropical macroalgae. Biogeosciences 10:3615–3633
Leonard SG, Sweeney T, Bahar B, Lynch BP, O’Doherty JV (2011) Effects of dietary seaweed extract supplementation in sows and post-weaned pigs on performance, intestinal morphology, intestinal microflora and immune status. Br J Nutr 106:688–699
Licitra G, Hernandez TM, Van Soest PJ (1996) Standardizations of procedures for nitrogen fractionation of ruminant feeds. Anim Feed Sci Technol 57:347–358
Machado L, Magnusson M, Paul NA, de Nys R, Tomkins N (2014) Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS ONE 9:e85289
Machado L, Magnusson M, Paul NA, Kinley R, de Nys R, Tomkins N (2016a) Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J Appl Phycol 28:3117–3126
Machado L, Magnusson M, Paul NA, Kinley R, de Nys R, Tomkins N (2016b) Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J Appl Phycol 28:1443–1452
Madesh M, Balasubramanian KA (1998) Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys 35:184–188
Maheswari M, Das A, Datta M, Tyagi AK (2021) Supplementation of tropical seaweed-based formulations improves antioxidant status, immunity and milk production in lactating Murrah buffaloes. J Appl Phycol 33:2629–2643
Maia MR, Fonseca AJ, Oliveira HM, Mendonça C, Cabrita AR (2016) The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Sci Rep 6:32321
Makkar F, Chakraborty K (2017) Antidiabetic and anti-inflammatory potential of sulphated polygalactans from red seaweeds Kappaphycus alvarezii and Gracilaria opuntia. Int J Food Prop 20:1326–1337
Makkar HPS, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, Lebas F, Ankers P (2016) Seaweeds for livestock diets: A review. Anim Feed Sci Technol 212:1–17
Maqsood S, Singh P, Samoon MH, Munir K (2011) Emerging role of immunostimulants in combating the disease outbreak in aquaculture. Int Aquat Res 3:147–163
Masucci F, De Rosa G, Grasso F, Napolitano F, Esposito G, Di Francia A (2011) Performance and immune response of buffalo calves supplemented with probiotic. Livest Sci 137:24–30
Matanjun P, Mohamed S, Muhammad K, Mustapha NM (2010) Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. J Med Food 13:792–800
Munde VK (2018) Evaluation of Red Seaweed Meal as Ruminant Feed Supplement. PhD thesis, ICAR-Indian Veterinary Research Institute, Izatnagar, India
Niehaus WG Jr, Samuelsson, (1968) Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130
NRC (2001) Nutrient requirements of dairy cattle, 7th edn. National Academy Press, Washington, DC
Paul SS, Vantharam Venkata HG, Raju MV, Rama Rao SV, Nori SS, Suryanarayan S, Kumar V, Perveen Z, Prasad CS (2021) Dietary supplementation of extracts of red sea weed (Kappaphycus alvarezii) improves growth, intestinal morphology, expression of intestinal genes and immune responses in broiler chickens. J Sci Food Agric 101:997–1008
Qadri SS, Biswas A, Mandal AB, Kumawat M, Saxena R, Nasir AM (2019) Production performance, immune response and carcass traits of broiler chickens fed diet incorporated with Kappaphycus alvarezii. J Appl Phycol 31:753–760
Reyes DC, Meredith J, Puro L, Berry K, Kersbergen R, Soder KJ, Quigley C, Donihue M, Cox D, Price NN, Brito AF (2023) Maine organic dairy producers’ receptiveness to seaweed supplementation and effect of Chondrus crispus on enteric methane emissions in lactating cows. Front Vet Sci 10:1153097
Rice VA, Andrews FN, Warnwick K, Legates JE (1970) Breeding and improvement of farm animals, 6th edn. Tata Mc Graw Hill Publishing Company Ltd, Bombay
Roque BM, Salwen JK, Kinley R, Kebreab E (2019) Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J Clean Prod 234:132–138
Saker KE, Allen VG, Fontenot JP, Bagley CP, Ivy RL, Evans RR, Wester DB (2001) Tasco-Forage: II. Monocyte immune cell response and performance of beef steers grazing tall fescue treated with a seaweed extract. J Anim Sci 79:1022–1031
Saker KE, Fike JH, Veit H, Ward DL (2004) Brown seaweed-(TascoTM) treated conserved forage enhances antioxidant status and immune function in heat-stressed wether lambs. J Anim Physiol Anim Nutr 88:122–130
Sharma A, Datt C, Kumar J, Dudi K (2022) Supplementary effect of K. alvarezii based seaweed product on milk production, its composition and organoleptic appraisal in crossbred cows. Indian J Dairy Sci 75:156–161
Shipe WF, Senyk GF, Fountain KB (1980) Modified copper soap solvent extraction method for measuring free fatty acid in milk. J Dairy Sci 63:193–198
Singh BK, Chopra RC, Rai SN, Verma MP, Mohanta RK (2017) Nutritional evaluation of seaweed on nutrient digestibility, nitrogen balance, milk production and composition in Sahiwal cows. Proc Nat Acad Sci India B 87:437–443
Sniffen CJ, O’ Connor JD, Van Soest PJ, Fox DG, Russell JB, (1992) A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J Anim Sci 70:3562–3577
Sorge US, Henriksen M, Bastan A, Cremers N, Olsen K, Crooker BA (2016) Iodine concentrations in serum, milk, and tears after feeding Ascophyllum nodosum to dairy cows—A pilot study. J Dairy Sci 99:8472–8476
Stefenoni HA, Räisänen SE, Cueva SF, Wasson DE, Lage CF, Melgar A, Fetter ME, Smith P, Hennessy M, Vecchiarelli B, Bender J (2021) Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J Dairy Sci 104:4157–4173
Tamminga S, Bannink A, Dijkstra J, Zom RL (2007) Feeding strategies to reduce methane loss in cattle. Report 24, Animal Sciences Group, Wageningen UR
Thepot V, Campbell AH, Paul NA, Rimmer MA (2021) Seaweed dietary supplements enhance the innate immune response of the mottled rabbitfish, Siganus fuscescens. Fish Shellfish Immunol 113:176–184
Thorsteinsson M, Weisbjerg MR, Lund P, Bruhn A, Hellwing ALF, Nielsen MO (2023) Effects of dietary inclusion of 3 Nordic brown macroalgae on enteric methane emission and productivity of dairy cows. J Dairy Sci 106:6921–6937
Trinder P (1969a) Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22:158–161
Trinder P (1969b) Enzymatic calorimetric determination of triglycerides by GOP-PAP method. Ann Clin Biochem 6:24–27
Trinder P (1969c) A simple turbidimetric method for the determination of serum cholesterol. Ann Clin Biochem 6:165–166
Van Soest PV, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Vijayabaskar P, Shiyamala V (2012) Antioxidant properties of seaweed polyphenol from Turbinaria ornata (Turner) J. Agardh, 1848. Asian Pac J Trop Biomed 2:90-S98
Wang Y, Xu Z, Bach SJ, McAllister TA (2008) Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on in vitro ruminal digestion of mixed forage or barley grain. Anim Feed Sci Technol 145:375–395
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974
Xue F, Sun F, Jiang L, Hua D, Wang Y, Nan X, Zhao Y, Xiong B (2019) Effects of partial replacment of dietary forage using kelp powder (Thallus laminariae) on ruminal fermentation and lactation performances of dairy cows. Animals 9:852
Yengkhom R, Singh P, Muwel N, Raje K, Handique B, Venkateswaran K (2019) Supplementation of brown seaweed (Turbinaria conoides) powder and its effect on blood metabolites and mineral profile in adult goats. Indian J Anim Nutr 36:103–106
Zhang Q, Yang R, Lim PE, Chin Y, Zhou S, Gao Y, Tang Q (2022) Sun-dried and air-dried Kappaphycus alvarezii attenuates 5-fluorouracil-induced intestinal mucositis in mice. Nutr Cancer 74:2113–2121
Acknowledgements
Authors are thankful to the Director, ICAR-NDRI, Karnal, Haryana (India) for providing all the facilities required to conduct the experiment and also M/S Aquagri Processing Pvt Ltd, Madurai, Tamil Nadu (India) for providing the seaweeds to carry out the experiment.
Funding
No funding information to disclose.
Author information
Authors and Affiliations
Contributions
GBD and AD performed the experiment, laboratory analysis and extracted the data; AD and AKT conceived and designed the study; SK, NT and GBD did the methane analysis and rumen fermentation studies, PBR made the graphs and Tables and AD and PBR wrote manuscript by interpreting the results. All authors read the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All the protocols and procedures used in this experiment were approved by the Institutional Animal Ethical Committee (IAEC), ICAR-National Dairy Research Institute, Karnal, Haryana (India) and CPCSEA, Ministry of Fisheries, Animal Husbandry and Dairying, New Delhi (India). The animal care procedures were approved with approval number 45-IAEC-19–2.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dahiphale, G.B., Das, A., Reddy, P.B. et al. Beneficial effects of dietary supplementation of tropical seaweeds on rumen fermentation, antioxidant status, immunity and milk yield of lactating Murrah buffaloes. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03344-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03344-5