Abstract
Dead wood is a key substrate for forest biodiversity, hosting a rich and often threatened biodiversity of wood-living species. However, the relationship between the occurrence of dead wood and associated species is modified by several environmental factors. Here we review the present state of knowledge on how dead wood on different spatial and temporal scales affects saproxylic biodiversity. We searched for peer-reviewed studies on saproxylic species that compared dead wood distribution on at least two spatial or temporal scales. We scanned close to 300 articles, of which 34 fit our criteria. 20 studies were directed towards the current amount of dead wood at different scale levels and how this relates to the abundance or occurrence of saproxylic species, embracing scales from 10 m to 10 km. 14 studies compared time-lagged effects of dead wood, covering time-lags from 25 years to more than 200 years. The reviewed articles focused mainly on European forest and addressed invertebrates (mostly beetles), alone or in combination with fungi (27 articles), fungi (six articles), or lichens (one article). Although the significance of dead wood for forest biodiversity is firmly established, the reviewed studies show that we still have limited knowledge of the relationship between saproxylic biodiversity and spatial and temporal scales. Based on the reviewed studies, we conclude that there is large variation in response to spatial and temporal dead wood patterns between different taxa and sub-groups. Still, several of the reviewed papers indicate that time-lagged effects deserve more attention, especially on a landscape scale and for specialized or red-listed species. Further work is required before firm management recommendations can be suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological assemblages do not live in isolation from their surroundings, and they represent the summed influence of years of changing conditions. However, there is quite limited empirical evidence that shows which specific, quantitatively measurable variables, at which spatial and temporal scales, shape the assemblages. Such information is urgently needed to formulate sound management and conservation actions. Better knowledge on the effect of previous conditions and of current factors at different spatial scales would have significant consequences for management and conservation planning (Lindenmayer et al. 2008).
In forest ecosystems worldwide, an essential habitat for biodiversity is dead wood. Dead and decaying trees are extremely important to flora and fauna, with hundreds of different microhabitats, and thousands of associated species (Stokland et al. 2012). Many of the species associated with dead-wood are listed as threatened or near threatened in national Red lists (Kålås et al. 2006; Rassi et al. 2001; Gärdenfors 2010). In managed forests, the amount and distribution of dead wood have often reduced dramatically. For example, in boreal Northern Europe, the amount of dead wood has been reduced to only 10–15 % of that normally found in pristine forests (Jonsson et al. 2005; Siitonen 2001). Additionally, the dead wood often occurs patchily in the landscape, concentrated in protected areas. Changes in the amount, quality and spatial and temporal distribution of dead wood have also been documented in temperate forest (e.g. Moretti and Barbalat 2004; Sirami et al. 2008) and in the tropics (Grove 2001).
Dead wood is a locally dynamic and transient substrate. Consequently, associated individuals must be able to compensate local extinctions on dead wood entities with repeated colonization events to ensure survival at the landscape level (Grove 2002; Jonsson et al. 2005). However, the spatial distribution and scarcity of dead wood substrates may restrict efficient colonization if not matched by the dispersal abilities of the relevant species. Knowledge of the relevant scale for dispersal and colonization of different saproxylic species is, thus, essential for successful management. For instance, should management efforts like retention of trees during harvests or forest protection be concentrated to areas near existing sites that currently have high levels of dead wood to ensure successful colonization, or will the dead wood also be reached and utilized if it is distributed more evenly across the landscape?
The past development of the dead wood resource may be of decisive importance for current populations of dead-wood associated species. There may be lag-phases [“relaxation” or “transition” phases (Kuussaari et al. 2009; Johansson et al. 2013)] in response to habitat changes; large current occurrence of dead-wood species may not reflect long-term viability but instead be remnant populations from former, more favorable conditions. Such populations may be destined to decline or even become locally extinct.
Alternatively, historical paucity of the resource may have caused species to become locally extinct, even if current amount of resource is high enough. The mechanisms of dead wood continuity can either be that specific microhabitats are developed in old-growth forest, or that the time period necessary for colonization of all ‘belonging’ species exceeds the turnover time for managed forests (Nordén and Appelqvist 2001). A large-scale perspective on habitat continuity in the form of landscape connectivity (sensu Hanski 2005) has proven to be of high importance in semi-natural grasslands (Münzbergova et al. 2013; Cousins et al. 2007) and for species assemblages in old trees (Ranius et al. 2008). The interplay between the spatial and the temporal dimension is likely to be a key concern for the understanding of dead-wood species.
Any effect that is related to spatial or temporal scale—such as the effect of patch isolation on colonization ability—is likely to be species dependent. Different organisms have widely different abilities to survive in a specific landscape over a given period of time, depending on e.g. habitat demands, reproductive mode and dispersal abilities. Simulations suggest that some responses of species to spatial patterns, such as an abrupt reduction in population size below a certain critical threshold of remaining habitat, are closely and predictably related to characteristics of the species (e.g. dispersal, reproduction, area/edge sensitivity) and landscape (e.g. fragmentation, matrix quality, rate of change) (Swift and Hannon 2010). Although we might lack details for species-specific analyses, looking for trends in certain functional groups (like habitat specialists), might be helpful in understanding more of the complex interactions between species and habitat patterns on a landscape scale and across generations. Such knowledge would help in the continuous development of better management guidelines.
A large amount of studies have been published on dead wood and associated species during the past 20 years, and knowledge on different aspects of dead wood in relation to biodiversity has been summarized in different reviews (Bunnell and Houde 2010; Davies et al. 2008; Grove 2002; Jonsson et al. 2005; Junninen and Komonen 2011; Lassauce et al. 2011; Müller and Bütler 2010; Paillet et al. 2010). Still, there is lack of compilation and analyses of studies that focus on the importance of spatial and temporal scales for species dependent on dead wood. We aim to fill this knowledge gap, since issues of scale are of large importance for practical management.
In this paper we review and synthesize information in the published scientific literature on the relationship between saproxylic species and the spatial and temporal scale of the distribution of dead wood. We included all studies comparing dead wood distribution on at least two spatial scales (usually stand and landscape), or at least two temporal scales. When selecting the studies, we followed the definition of saproxylic species given by Alexander (2008): Saproxylic organisms are species which are involved in or dependent on the process of fungal decay of wood, or on the products of that decay, and which are associated with living as well as dead trees. We include studies of insects, fungi, lichens, and bryophytes—but we do not include studies of species nesting in hollow trees (like birds, bats or marsupials).
We address the following questions:
-
What is the present state of knowledge on the importance of dead wood on different spatial and temporal scales for saproxylic biodiversity?
-
Do different taxa or functional groups respond differently to dead wood at different spatial and temporal scales in the accumulated literature?
-
Is the knowledge-base large enough to allow recommendations from a management perspective?
Materials and methods
Focusing the questions
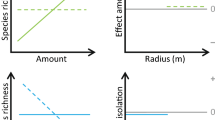
We divided the scale problem into different aspects represented by a two-by-two array of spatial scale (local vs. landscape-scale) by temporal scale (current vs. historical) (Fig. 1). In a hypothetical landscape, the saproxylic species and assemblages can be influenced by the variation in dead wood in four distinctive ways, assuming a constant pool of species that can colonize a site.
A conceptual model of how local and landscape scale, as well as time (temporal scale), can influence current species patterns in a focal patch. In a, the main influence is from current local habitat characteristics; while in b the main influence is from habitat characteristics at the current landscape-scale. In the two right side figures, the response is time-lagged (symbolized by dashed lines of habitat qualities): In c, the previous local habitat characteristics exert the main influence on the current species pattern, and in d, the previous landscape-scale habitat characteristics are most influential. In reality, these effects may act simultaneously
Firstly, local availability of suitable dead wood microhabitat is a prerequisite for local reproductive occurrence of the associated species. Local in this sense is taken to mean stand scale, commonly a focus in research and practical forest management. This situation corresponds to Fig. 1a.
Secondly, the local temporal continuity of the dead wood resource may influence species’ occurrences, both since former large amounts of dead wood may cause delayed extinction effects, and since there may be time-lagged effects in colonization of species in response to current larger amounts. This corresponds to the situation Fig. 1c.
Thirdly (Fig. 1b), the current species richness of saproxylic organisms may be influenced by the variation of habitat that currently occurs in the neighboring landscape. If this is the case, large parts of the saproxylic assemblage variation within the focal patch can be explained by the present habitat availability in the surroundings of the focal patch, but most likely in combination with focal patch characteristics. The relative importance of the habitat patches in the surrounding and the local patch can differ, according to the source-sink concept (Thomas and Kunin 1999).
Fourthly, the current assemblage in the focal patch may be affected by changes in the surrounding landscape a long time ago (Fig. 1d). Conceptually, this is close to the extinction debt and associated metapopulation models (Hanski and Ovaskainen 2002). In those models, focal patches may remain largely unchanged (such as protected areas) but their surroundings may cease to produce colonizers that are vital to maintain local populations. Final extinctions are realized long after the landscape has changed.
Naturally, these four broadly divided “effect categories” are not mutually exclusive but are likely to operate simultaneously. When reviewing the studies, we have also sought for possible combined effects that originate from different spatial and temporal scales. These combined scale effects are, quite naturally, often very complex to show and analyse.
Measured responses in studies of spatial and temporal effects like those described above would depend on species, since species have different habitat demands, different life history traits including dispersal abilities, and different response times to environmental changes.
Article selection
We included only studies published in peer-reviewed journals. The aim was to find all studies that matched the criteria of: (1) including saproxylic species, (2) comparing dead wood distribution on at least two spatial scales, or (3) comparing dead wood distribution on at least two temporal scales. We conducted a search in the ISI Web of Knowledge for papers in English that matched either of the words (in title, abstract or keywords) saproxylic, wood-inhabiting, epixylic and at the same time either of the words landscape* or scale*. This search was conducted on July 20, 2012 and contained 235 papers. In addition, we searched the reference lists for additional papers that matched the above three criteria. In total, we scanned 278 studies, and of these 34 fit our criteria. Many excluded papers described saproxylic species richness in managed versus unmanaged forest in general. Also, studies comparing very small scales, like single trees and their immediate surroundings (Buse et al. 2007; Sirami et al. 2008; Sverdrup-Thygeson et al. 2010) were excluded. Papers in which saproxylic species were not separated from other species in analyses (e.g. Janssen et al. 2009) or where two different species sampling methods were used for the two scales (e.g. Siitonen 1994) were also not included.
Article overview
Most of the 34 included studies originated from Europe, but there were also 4 from North America (Table 1). The majority of the studies addressed beetles and other invertebrates, alone (see Table 1) or in combination with fungi (Jonsell and Nordlander 2002; Komonen et al. 2000; Laaksonen et al. 2008; Kehler and Bondrup-Nielsen 1999; Rukke and Midtgaard 1998), while six addressed fungi (Berglund et al. 2011; Berglund and Jonsson 2008; Paltto et al. 2006; Penttilä et al. 2006; Sverdrup-Thygeson and Lindenmayer 2003; Nordén et al. 2013), and one focused on saproxylic fungi and lichens (Ranius et al. 2008). Species richness was the most common response variable, but some studies measured assemblages, occurrence or abundance of specific species, especially red-listed species or species assumed to be specialized to rare microhabitats or environmental conditions.
In a few studies the scale issue was the main focus (e.g. Franc et al. 2007; Paltto et al. 2006; Ranius et al. 2008), while in others it was covered more briefly and indirectly (e.g. Moretti and Barbalat 2004; Buse 2012). We divided the studies in two main groups, following our criteria above and in Fig. 1: Those that compare local and landscape current effects of dead wood, i.e. spatial effects (Fig. 1a, b), and those that consider time-lagged local and landscape-scale effects, i.e. temporal effects (Fig. 1c, d). 20 of the studies in our material were directed towards the current amount of dead wood at different scale levels and how this relates to the abundance or occurrence of saproxylic species (Table 1). These studies embrace scale levels from 10 m (Jackson et al. 2012) to 10 km (e.g. Franc et al. 2007; Gibb et al. 2006). 14 studies compare time-lagged effects of dead wood, including time-lags from 25 years as the shortest (Andersson et al. 2012) and ≈200 years as the longest time span (Ranius et al. 2008).
In the reviewed studies, several different methods were applied for quantifying the amounts and distribution of dead wood. Some studies used buffer radius metrics (sensu Calabrese and Fagan 2004), and related the response variable (in a site of fixed size) to amount or connectivity of habitat within a buffer of surrounding area, comparing two or more spatial scales. The most common between-scale comparison was stand scale to landscape scale (the latter typically covered a few kilometres) (Franc et al. 2007), but several studies used continuous measurement of scales (e.g. Ranius et al. 2011a; Bergman et al. 2012). A few studies compared effects of surroundings on response variables in sites of different sizes in a nested set-up where the larger scales are pooled from the smaller units (Kehler and Bondrup-Nielsen 1999; Rukke and Midtgaard 1998; Økland et al. 1996; Jackson et al. 2012).
Two main approaches were used to study the effect of the temporal dimension in the reviewed papers. No studies had actually collected data in different points in time. Rather they either applied retrospective methods, either by obtaining data from historical maps, or by using space-for-time substitution to compare sites that have different known history (Table 2).
A common set-up in the studies of time-lagged responses (Fig. 1c, d) was to relate the response variable to previous amount of habitat in the surrounding area versus current amount of habitat in the surrounding area, where previous amount was interpreted from historical data. Our material included six studies that used historical data (maps) to estimate the effect of previous amounts of dead wood (or a proxy for this) on current occurrences of saproxylic species (Table 2). Four of these studied fungal species that were either red-listed or assumed to indicate ecological continuity (Sverdrup-Thygeson and Lindenmayer 2003; Ranius et al. 2008; Paltto et al. 2006; Nordén et al. 2013).
Another common method for studies of time-lagged effects was using space-for-time substitution and comparing sites that had been isolated for different lengths of time or were situated in regions differing in land use history (Abrahamsson et al. 2009; Berglund and Jonsson 2008; Komonen et al. 2000; Jonsell and Nordlander 2002; Kouki et al. 2012; Lindbladh et al. 2007; Siitonen 1994; Penttilä et al. 2006; Laaksonen et al. 2008). The basic aim was to find out if long-term management of the surrounding matrix is reflected in the current composition of saproxylic assemblages and, furthermore, in the potential to restore species. The studies in this category typically aimed to include dead wood as a covariate or to keep the amount constant when revealing the effects of management and logging at the landscape scale.
Effects of current dead wood amount and distribution on different scales (Fig. 1a, b)
Single species
Five studies analysed the occurrence patterns of single saproxylic species. Kehler and Bondrup-Nielsen (1999) and Rukke and Midtgaard (1998) both studied the occurrence of a single fungivorous beetle living in basidiomes (sporocarps) of fungi and its dependence on habitat amount in surroundings. The predictor variables changed as they analysed the effects on increasingly larger spatial scales from within single logs, between logs, and finally between woodland patches. The studies both found that the intermediate scale—between trees—stand out as important for the species in focus, but Kehler and Bondrup-Nielsen found effects of isolation at all scales, between fungal basidiomes on a log (0–2 m), between clusters of basidiomes in a forest (1–71 m) and between woodlots in an agricultural matrix (25–2,000 m). Saint-Germain and Drapeau (2011) studied the effect of residual habitat in surroundings (from 40 m to 2 km) for four species of Cerambycidae in aspens. Two of the four species responded most clearly to the largest spatial scale; 2,000 m, one species to 1,200 m and one to 800 m scale (Saint-Germain and Drapeau 2011).
Bergman et al. (2012) studied the multi-scale responses of several oak-dependent beetles in a large dataset of 33,000 large or hollow oaks. They found that scale response was highly species-specific in the 16 beetles (out of 35) that showed a clear relationship with oak density. The characteristic scale of response of species richness of oak beetles was 2,284 m. Some species responded to both local and landscape scale.
A study of a territorial and dispersal-limited beetle, Odontotaenius disjunctus, also points to the influences of factors acting at multiple scales (Jackson et al. 2012). They found that while forest cover best predicted occurrence at a scale of 225 ha, it accounted for very little of the variance, and the occurrence of the beetle was most sensitive to environmental factors measured at the smallest scale (“log section scale”—which was the scale of the beetles’ territories).
Effects on species richness or composition
A few studies have considered the question of assemblage-level response to current dead-wood characteristics. Økland et al. (1996) found that the relationships between ecological and species diversity variables were improved by increasing the spatial scale, with the strongest being found at the largest scale. For parasitoids of saproxylic hosts, the spatial response peaked at 0.5–1 km for all the studied species (Gibb et al. 2008). The study used proportion of old forest in increments of 100 m (from 200 to 1 km) or 1 km (from 2 to 10 km) as a proxy for dead wood. Common for both studies, local (stand scale) availability of dead wood was found to be of limited importance.
A study from deciduous forest in Switzerland addressing the effect of forest fires on saproxylic beetles in three families found that species diversity and composition were related to various ecological variables at two levels of spatial scale (Moretti and Barbalat 2004). Specifically for dead wood, they found no effect at the small scale of 0.25 ha. At a large scale of 6.25 ha, they concluded that the mosaic of different forest habitats and successional stages created by forest, was important.
Differential effects on assemblages of generalists and of specialized species
We found six studies comparing the spatial effects of current dead wood habitat on species belonging to different groups regarding commonness. In two related studies of saproxylic oak beetles in Southern Sweden, main predictors of species richness of both all species and red-listed species were first found to be two landscape variables (Franc et al. 2007). In a follow-up study, Götmark et al. (2011) expanded the range on the axes of the predictors by adding more study sites. This partly changed the results: local species richness was then predicted by a combination of local, landscape and regional factors. Local amount of dead wood turned out as the main predictor of total species richness, while the landscape dead wood proxy was the main predictor of species richness of red-listed species. Thus, species richness of red-listed saproxylic species depended more on landscape factors than total species richness did. As an exception to this main trend, however, Brunet and Isacsson (2009) found beech-associated red-listed species to respond more both to local and landscape dead wood variables than common species.
A study of early-successional beetles in experimental logs in Sweden found that dead wood availability within 100 m of the study sites was rarely important for beetle abundance (Gibb et al. 2006). In contrast, a large area of suitable stand types within both 1 and 10 km resulted in greater abundances for several common and habitat-specific species. The authors also tested whether assemblage similarities were lower on sites that were far from each other. They found that this was indeed the case for red-listed species. This could be an indication of red-listed species having more localized distributions. Local factors were also important in a study of early post-fire saproxylic beetles in a recently burned landscape in boreal Quebec, Canada, where different but weak associations with distance to possible source habitats were found for the different trophic groups (Boulanger et al. 2010). The authors point to the fact that this study took place in a pristine ecosystem subject to large and frequent wildfires, and therefore the high regional pool of individuals may have overwhelmed any effect of dispersal from specific source habitat.
An experimental work by Lindbladh et al. (2007) is the only one in our material that failed to demonstrate any landscape-scale effect, but this conclusion was partly relaxed in a follow-up study using different sampling methods (Abrahamsson et al. 2009). Both studies investigated species richness of beetles that occupy man-made high stumps in southern Sweden. Contrary to many of the other studies, they covered a more restricted landscape gradient. This may partly explain why the result was different from the other studies.
Effects of connectivity of dead wood on local and landscape scale
Another important aspect of the scale effects of dead wood is the spatial arrangement within different scales, e.g. in the form of local or landscape-scale connectivity (sensu Hanski 2005) of dead wood. Our material included four independent studies (two articles from the same study) on this, with mixed responses.
Schiegg (2000a, b) considered connectivity of dead wood on a local scale, by mapping the position of all dead wood pieces, in plots with a radius of 200 m in mixed Fagus sylvatica, Abies alba forest in Switzerland. By analysing data with three connectivity indices she found that species richness of insects was higher in plots with high connectivity, and there were also effects on species composition as well as specific species responses. In this study, the spatial arrangement came out as more important than the total volume of dead wood.
At a landscape scale, high levels of habitat connectivity have been shown to have a positive effect on species richness and species occurrence. Ranius et al. (2011a) surveyed all oaks deemed suitable as habitat for seven saproxylic beetles and two pseudoscorpions, all red-listed, within five landscapes in southern Sweden and included oaks in 4 km buffers surrounding each landscape in the analyses. Positive correlations with connectivity were found for eight of the nine species. Models generating best fit varied between 135 and 2,857 m depending on species. The most threatened species were affected by large-scale occurrence of oaks, indicating that they need conservation efforts at larger spatial scales. Similar positive correlations with connectivity on a large scale were found in a study of aspen-specialist beetles, although there was a wide variability in spatial scale of response in individual species (Ranius et al. 2011b). The spatial scale at which species richness had its strongest response to habitat was rather small, only 93 m.
In a study of saproxylic aspen-associated beetles, habitat connectedness was not retained in analyses as a significant predictor of neither occupancy nor species richness—possibly due to time-lagged effects not captured in the study (Sahlin and Schroeder 2010).
Time-lagged effects of dead wood amount and distribution (Fig. 1c, d)
Effects on species richness in general
One study considers time-lagged effects on overall species richness of saproxylic species. Andersson et al. (2012) compared 25 year old clearcuts with and without removal of stumps to see if this contrast influenced present fauna of flying saproxylic beetles. They found little support for time-lagged local effects of stump harvesting on beetles flying in the stands. Instead the beetle community was more influenced by the characteristics of the surrounding forests.
Effects on red-listed or rare species
Also, a few studies consider effects of previous dead wood distribution on single red-listed species. Two studies from central Europe address red-listed beetles and how they respond to historical dead wood-related parameters in the landscape. Buse (2012) studied relict flightless saproxylic weevils and found that the frequency of these beetles was correlated with historical, but not with current, woodland size. Dubois et al. (2009) found that the current occurrence of the globally red-listed scarab Osmoderma eremita in France reflected 50 years old patterns in their dead wood substrate (Dubois et al. 2009). Both studies used historical information in the form of old maps to assess previous dead wood amounts.
A set-up of comparing sites with different temporal isolation and a control was used by Komonen et al. (2000), which is the only study considering a food-chain. The study focuses on three trophic levels: an old-growth red-listed specialist saproxylic fungus (Fomitopsis rosea), a moth living in the basidiomes of the fungus, and a parasite of the moth. They conclude that the surroundings outside the stand had a clear temporal effect on the abundance of the species (Komonen et al. 2000). A time-lag in the effect of isolation was clearly verified: isolation a long time ago had led to truncated food chains.
Differential effects on generalists and certain specialized species
Several studies detect different responses between species richness of generalist species and richness of species often used as old-growth indicator species (e.g. Nitare 2000) and/or red-listed species.
Berglund and Jonsson (2008) compared site occupancy of 29 saproxylic fungus species in Woodland Key Habitats (sensu Timonen et al. 2010) with different times since the logging took place in the surrounding forest. The temporal scale spanned from sites that had been isolated for the past 80–100 years, to sites where surrounding old growth had been removed less than 15 years ago. They also used natural mosaic landscapes that had been only marginally influenced by humans for >2,000 years as control. They found few overall effects, but some specialized red-listed species showed decreased occupancy in old isolates compared to recent, indicating a temporal effect of large-scale dead wood amounts.
In a study of saproxylic beetles on two polypore fungi, Jonsell and Nordlander (2002) compared three types of forests that differed in historical continuity and availability of breeding substrate. Most of the studied beetle species (25 spp. on Fomitopsis pinicola and 27 on F. rosea) were not affected by the continuity. Notably, however, quite a few red-listed species were more common on long-continuity sites, indicating that historical effects do affect the current occurrence. However, this pattern was not consistent among all the red-listed species (data on them were scarce, though).
Studies by Penttilä et al. (2006) and Laaksonen et al. (2008) applied a method where a specific isolation index of each patch was calculated, based on ca. 50 years history of forest transformation. This index was then used to test and reveal if and how the temporal history affects current, empirically measured assemblages. Both studies found clear impacts of timing of isolation but—again—there were obvious differences between different species. Penttilä et al. (2006) could verify that time-lagged effects (related to isolation) have impacts mostly on rare fungi species. Laaksonen et al. (2008) studied beetles and also found that rare species are affected but also some common species. The results of these studies were further supported and expanded by Berglund et al. (2011) and Nordén et al. (2013) who analysed the occurrence of fungi along a gradient of forest management intensity and duration across southern Finland. A clear landscape effect was observed so that a suitable dead wood substrate was most likely to be occupied in a region where management had its shortest history.
Kouki et al. (2012) also analysed occurrence patterns of beetles along a management gradient in southern Finland. Their study was experimental where similar dead wood substrates were created by burning comparable forests in different landscape context. There was a very clear landscape-level effect so that areas that had the longest management history attracted the least amounts of rare, red-listed and pyrophilous species. The results suggest a strong landscape-scale effect on the saproxylic beetle fauna.
Time-lagged landscape effects were also found by Paltto et al. (2006), who considered the proportion of broadleaved forest in the surrounding landscape 130 years ago and today (as a proxy for broadleaved dead wood) at several spatial scales, and its effect on saproxylic fungi. They found a clear time-lagged effect of historical habitat within 1 km radius on present species density, indicating a possible landscape-scale extinction debt. Similarly, in a study of species occurrence of 21 red-listed species (12 lichens and 9 fungi) on old oaks, Ranius et al. (2008) considered the effect of density of oaks 170 years ago and today, and found a time-lagged effect of habitat density for 11 of the species.
Sverdrup-Thygeson and Lindenmayer (2003) studied the effects of historical patterns in forest age classes and absence of logging versus present-day structures, at both the forest stand scale and the surrounding landscape (80 ha) scale, for the two red-listed fungi Phellinus nigrolimitatus and Cystostereum murrayi. They found that historical landscape structure was the best predictor for the current occurrence and abundance of P. nigrolimitatus, while no clear patterns emerged for C. murrayi. Contrary to Paltto et al. (2006), Sverdrup-Thygeson and Lindenmayer (2003) also found a positive stand-level effect of previous amounts of dead wood, while local effects were not measured in Ranius et al. (2008), who had parish area as the smallest scale level.
Overview and conclusions
We found the studies of the effects of spatial and temporal scale of dead wood on saproxylic species to be rather heterogeneous in relation to their focus, methods and scales studied. Consequently, general conclusions are difficult to draw. Still, certain trends arise from the published studies, in particular concerning the importance of a larger and longer scale perspective.
Small scale vs. large scale
In our compilation, five independent studies with specific focus on spatial scales identify large scale levels as most important for one or all subgroups studied (Table 3). Three studies identify small scale levels as most important (Table 3); two of these focus on single species. In the rest of the studies, both small and large scale effects were found to be important. Judging from this result, one might suggest that there is a slight tendency that more studies distinguish large scale effects as more significant, especially for red-listed species, but mostly this result illustrates the diverse responses of different species and different systems.
A possible landscape effect has also been hypothesized in previous reviews into this matter. A meta-analysis on dead wood volume and saproxylic biodiversity found the magnitude of correlation between the two to be relatively moderate, and suggest that one reason for this might be the stand-level focus of the studies included (Lassauce et al. 2011). Also Paillet et al. (2010), comparing managed and unmanaged forest in Europe, point to the possible overriding effect of adjacent landscape structures.
Time-lagged effects
The 14 studies addressing the temporal scale perspective also include a large-scale spatial perspective. Ecologically, this makes sense, as local (stand) continuity might be less relevant in practice. Most microhabitats have rather short life-times and the majority of species therefore have to respond to the dynamics on a larger scale (Hanski 2005).
Only one of the studies addressing temporal effects did not find a time-lagged effect, namely Andersson et al. (2012). This study also has a special set-up; the temporal effect is only measured locally (cut stumps removed or not 25 years ago) and it uses only general species richness as a response variable. The remaining studies on time-lagged effects analyse species belonging to different sub-groups, like red-listed species, separately. The strongest effects of previous landscape characteristics were found for rare or red-listed species, but the relative importance of different scales depended on the setting. As response to spatial scale depends so strongly on the dispersal limitations of different species, a differentiated response could be expected between common generalist species with high dispersal power and species that are specialized to stable, long-lived microhabitats (Southwood 1977). Habitat specialists may today live in fragments too isolated for successful exchange of genes and individuals (e.g. Henle et al. 2004), resulting in declining populations and a listing as threatened in national Red Lists.
Limitations
Considering the scale issues, caveats and limitations in comparing and generalizing the reviewed studies are obvious. One limitation is that most of the current data and studies originate from those European landscapes that have been under intensive forest management for prolonged periods. The accumulated effects of land-use may have influenced the species pool of these landscapes, especially those species that are most demanding in terms of forest naturalness. Thus, we might miss certain scale effects, in particular those that are time-scale related.
Another challenge inherent in the included studies is that the way the dead wood resource is measured, changes with scale. While detailed dead wood surveys can be conducted on a stand scale (Fig. 1a, b), this clearly becomes very difficult as scale increases to a landscape level (Fig. 1c, d). For the larger scales, proxies are instead used and the most commonly used proxy is the amount of old forest (e.g. Paltto et al. 2006; Økland et al. 1996). This means that the precision decreases with increasing scale, which can blur results.
Any scale-related analysis is challenging when only a few scales are included. In many studies covered here, a common comparison was between “small” and “large” scale or “stand” and “landscape” scales. This approach may prevent finding the real pattern of scale-dependency. One way to overcome this challenge is to use a continuous variable and compare effects over a large range of scales, which has been done in some of the analysed studies (e.g. Bergman et al. 2012; Franc and Götmark 2008). New data sources from remote sensing, like satellite imagery or data from airborne laser scanning, might radically change the accessibility of such wall-to-wall digital map input for analyses.
A more basic study design issue is also worth mentioning, namely that the inter-dependencies of several scale effects can change with the variability or span of dead wood conditions covered in a study. This was exemplified by the studies of Franc et al. (2007) and Götmark et al. (2011). By including more sites and thereby expanding the range on the axes of the local dead wood predictors from the study of Franc et al. (2007), Götmark et al. (2011) showed that while the significance of the large scale was still present, local species richness was now predicted by a combination of local, landscape and regional factors.
These limitations call for prudence when it comes to formulating management guidelines, as supported by other reviews on dead wood and saproxylic species that conclude the evidence presently available is insufficient for the formulation of convincing management recommendations (Davies et al. 2008; Ranius and Fahrig 2006). Still, other reviews suggest that thresholds of local dead wood amounts can be discerned (Müller and Bütler 2010) and even serve as guidelines for forest management (Junninen and Komonen 2011).
Conclusion
We conclude that the present state of knowledge on the importance of dead wood on different spatial and temporal scales for saproxylic biodiversity is still limited; we only found about 30 studies that addressed this topic in a way that made it possible to include them in a review. This means that the research field is still too immature to allow firm, quantitative management recommendations, or to try to differentiate between the response of different taxa. Still, some patterns emerge. Further research, on more species groups and in different systems (especially other geographical areas with other land-use histories), will indicate whether the tentative patterns suggested here are consistent across the saproxylic community.
Firstly, even with the considerable variation in response to dead wood patterns between different taxa, and sub-groups of saproxylic species, several of the studies reviewed here indicate that time-lagged effects deserve more attention, especially on a landscape scale (Fig. 1d). Secondly, several studies indicate that species specialized to microhabitats or conditions restricted to old forest habitats or to natural forest dynamics (like many red-listed species), respond differently to large-scale and long-time forestry than species with a broader ecological niche.
For future studies, we strongly recommend extending research to areas of forest with natural CWD levels, and to use them as reference areas or to establish long term monitoring sites on them. Such areas are still available in many parts of Russia, Canada and Alaska. We also advise more studies to simultaneously compare different taxa or different ecological or functional groups within the same landscape system. Comparisons between well and poorly dispersing species, generalists and specialists, or common and rare species could bring much to our knowledge on scale-related effects. Future studies that combine landscape-level forest planning with the response of functional groups in landscapes with different remaining species pools are especially urgent. Also, some saproxylic species groups like bryophytes, lichens, Hymenoptera and Diptera are poorly represented in the current research and need more focus in future studies. In addition, we suggest that more rigorous protocols should be applied in future studies so that, for example, measurements of dead wood amounts at different scales are comparable.
Even though exact scales and the quantitative magnitudes of their effects remain unresolved so far, there is plenty of evidence that any actions and measures that aim to promote the diversity of saproxylic species in managed forests require a “broad-scale approach”, i.e. that local management measures should be considered in the context of current landscape-scale and historical landscapes, to a larger extent than today.
References
Abrahamsson M, Jonsell M, Niklasson M et al (2009) Saproxylic beetle assemblages in artificially created high-stumps of spruce (Picea abies) and birch (Betula pendula/pubescens): does the surrounding landscape matter? Insect Conserv Diver 2:284–294
Alexander KNA (2008) Tree biology and saproxylic coleoptera: issues of definitions and conservation language. Rev Ecol-Terre Vie 9–13
Andersson J, Hjältén J, Dynesius M (2012) Long-term effects of stump harvesting and landscape composition on beetle assemblages in the hemiboreal forest of Sweden. For Ecol Manag 271:75–80. doi:10.1016/j.foreco.2012.01.030
Berglund H, Jonsson BG (2008) Assessing the extinction vulnerability of wood-inhabiting fungal species in fragmented northern Swedish boreal forests. Biol Conserv 141(12):3029–3039. doi:10.1016/j.biocon.2008.09.007
Berglund H, Hottola J, Penttilä R, Siitonen J (2011) Linking substrate and habitat requirements of wood-inhabiting fungi to their regional extinction vulnerability. Ecography 34(5):864–875. doi:10.1111/j.1600-0587.2010.06141.x
Bergman KO, Jansson N, Claesson K, Palmer MW, Milberg P (2012) How much and at what scale? Multiscale analyses as decision support for conservation of saproxylic oak beetles. For Ecol Manag 265:133–141. doi:10.1016/j.foreco.2011.10.030
Boulanger Y, Sirois L, Hebert C (2010) Distribution of saproxylic beetles in a recently burnt landscape of the northern boreal forest of Quebec. For Ecol Manag 260(7):1114–1123. doi:10.1016/j.foreco.2010.06.027
Brunet J, Isacsson G (2009) Restoration of beech forest for saproxylic beetles-effects of habitat fragmentation and substrate density on species diversity and distribution. Biodiver Conserv 18(9):2387–2404
Bunnell FL, Houde I (2010) Down wood and biodiversity: implications to forest practices. Environ Rev 18:397–421. doi:10.1139/a10-019
Buse J (2012) “Ghosts of the past”: flightless saproxylic weevils (Coleoptera: Curculionidae) are relict species in ancient woodlands. J Insect Conserv 16(1):93–102. doi:10.1007/s10841-011-9396-5
Buse J, Schröder B, Assmann T (2007) Modelling habitat and spatial distribution of an endangered longhorn beetle: a case study for saproxylic insect conservation. Biol Conserv 137(3):372–381
Calabrese JM, Fagan WF (2004) A comparison-shopper’s guide to connectivity metrics. Front Ecol Environ 2(10):529–536.
Cousins SAO, Ohlson H, Eriksson O (2007) Effects of historical and present fragmentation on plant species diversity in semi-natural grasslands in Swedish rural landscapes. Landsc Ecol 22(5):723–730. doi:10.1007/s10980-006-9067-1
Davies ZG, Tyler C, Stewart GB, Pullin AS (2008) Are current management recommendations for saproxylic invertebrates effective? A systematic review. Biodiver Conserv 17(1):209–234
Dubois GF, Vignon V, Delettre YR, Rantier Y, Vernon P, Burel F (2009) Factors affecting the occurrence of the endangered saproxylic beetle Osmoderma eremita (Scopoli, 1763) (Coleoptera: Cetoniidae) in an agricultural landscape. Landsc Urban Plan 91(3):152–159. doi:10.1016/j.landurbplan.2008.12.009
Franc N, Götmark F (2008) Openness in management: Hands-off vs partial cutting in conservation forests, and the response of beetles. Biol Conserv 141(9):2310–2321. doi:10.1016/j.biocon.2008.06.023
Franc N, Götmark F, Økland B, Nordén B, Paltto H (2007) Factors and scales potentially important for saproxylic beetles in temperate mixed oak forest. Biol Conserv 135(1):86–98
Gärdenfors U (ed) (2010) Rödlistade arter i Sverige: The 2010 Red List of Swedish Species. ArtDatabanken, Sveriges lantbruksuniversitet SLU, Uppsala. In Swedish and English
Gibb H, Hjälten J, Ball JP, Atlegrim O, Pettersson RB, Hilszczanski J, Johansson T, Danell K (2006) Effects of landscape composition and substrate availability on saproxylic beetles in boreal forests: a study using experimental logs for monitoring assemblages. Ecography 29(2):191–204
Gibb H, Hilszczanski J, Hjälten J, Danell K, Ball JP, Pettersson RB, Alinvi O (2008) Responses of parasitoids to saproxylic hosts and habitat: a multi-scale study using experimental logs. Oecologia 155(1):63–74
Götmark F, Asegard E, Franc N (2011) How we improved a landscape study of species richness of beetles in woodland key habitats, and how model output can be improved. For Ecol Manag 262(12):2297–2305. doi:10.1016/j.foreco.2011.08.024
Grove SJ (2001) Extent and composition of dead wood in Australian lowland tropical rainforest with different management histories. For Ecol Manag 154(1–2):35–53
Grove SJ (2002) Saproxylic insect ecology and the sustainable management of forests. Annu Rev Ecol Syst 33:1–23
Hanski I (2005) The shrinking world: ecological consequences of habitat loss. International Ecology Institute, Oldendorf/Luhe
Hanski I, Ovaskainen O (2002) Extinction debt at extinction threshold. Conserv Biol 16(3):666–673. doi:10.1046/j.1523-1739.2002.00342.x
Henle K, Davies KF, Kleyer M, Margules C, Settele J (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13(1):207–251
Jackson HB, Baum KA, Cronin JT (2012) From logs to landscapes: determining the scale of ecological processes affecting the incidence of a saproxylic beetle. Ecol Entomol 37(3):233–243. doi:10.1111/j.1365-2311.2012.01355.x
Janssen P, Fortin D, Hebert C (2009) Beetle diversity in a matrix of old-growth boreal forest: influence of habitat heterogeneity at multiple scales. Ecography 32(3):423–432. doi:10.1111/j.1600-0587.2008.05671.x
Johansson V, Snäll T, Ranius T (2013) Estimates of connectivity reveal non-equilibrium epiphyte occurrence patterns almost 180 years after habitat decline. Oecologia 172(2):607–615. doi:10.1007/s00442-012-2509-3
Jonsell M, Nordlander G (2002) Insects in polypore fungi as indicator species: a comparison between forest sites differing in amounts and continuity of dead wood. For Ecol Manag 157(1–3):101–118. doi:10.1016/s0378-1127(00)00662-9
Jonsson BG, Kruys N, Ranius T (2005) Ecology of species living on dead wood: lessons for dead wood management. Silva Fennica 39(2):289–309
Junninen K, Komonen A (2011) Conservation ecology of boreal polypores: a review. Biol Conserv 144(1):11–20. doi:10.1016/j.biocon.2010.07.010
Kålås JA, Viken Å, Bakken T (2006) Norwegian Red List. Norwegian Biodiversity Information Centre (in Norwegian and English), Trondheim
Kehler D, Bondrup-Nielsen S (1999) Effects of isolation on the occurrence of a fungivorous forest beetle, Bolitotherus cornutus, at different spatial scales in fragmented and continuous forests. Oikos 84(1):35–43
Komonen A, Penttilä R, Lindgren M, Hanski I (2000) Forest fragmentation truncates a food chain based on an old-growth forest bracket fungus. Oikos 90(1):119–126
Kouki J, Hyvarinen E, Lappalainen H, Martikainen P, Similä M (2012) Landscape context affects the success of habitat restoration: large-scale colonization patterns of saproxylic and fire-associated species in boreal forests. Divers Distrib 18(4):348–355. doi:10.1111/j.1472-4642.2011.00839.x
Kuussaari M, Bommarco R, Heikkinen RK, Helm A, Krauss J, Lindborg R, Öckinger E, Pärtel M, Pino J, Roda F, Stefanescu C, Teder T, Zobel M, Steffan-Dewenter I (2009) Extinction debt: a challenge for biodiversity conservation. Trends Ecol Evol 24(10):564–571
Laaksonen M, Peuhu E, Varkonyi G, Siitonen J (2008) Effects of habitat quality and landscape structure on saproxylic species dwelling in boreal spruce-swamp forests. Oikos 117(7):1098–1110
Lassauce A, Paillet Y, Jactel H, Bouget C (2011) Deadwood as a surrogate for forest biodiversity: meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms. Ecol Indic 11(5):1027–1039. doi:10.1016/j.ecolind.2011.02.004
Lindbladh M, Abrahamsson M, Seedre M, Jonsell M (2007) Saproxylic beetles in artificially created high-stumps of spruce and birch within and outside hotspot areas. Biodiver Conserv 16(11):3213–3226
Lindenmayer D, Hobbs RJ, Montague-Drake R, Alexandra J, Bennett A, Burgman M, Cale P, Calhoun A, Cramer V, Cullen P, Driscoll D, Fahrig L, Fischer J, Franklin J, Haila Y, Hunter M, Gibbons P, Lake S, Luck G, MacGregor C, McIntyre S, Mac Nally R, Manning A, Miller J, Mooney H, Noss R, Possingham H, Saunders D, Schmiegelow F, Scott M, Simberloff D, Sisk T, Tabor G, Walker B, Wiens J, Woinarski J, Zavaleta E (2008) A checklist for ecological management of landscapes for conservation. Ecol Lett 11(1):78–91
Moretti M, Barbalat S (2004) The effects of wildfires on wood-eating beetles in deciduous forests on the southern slope of the Swiss Alps. For Ecol Manag 187(1):85–103
Müller J, Bütler R (2010) A review of habitat thresholds for dead wood: a baseline for management recommendations in European forests. Eur J For Res 129(6):981–992. doi:10.1007/s10342-010-0400-5
Münzbergova Z, Cousins SAO, Herben T, Plackova I, Milden M, Ehrlen J (2013) Historical habitat connectivity affects current genetic structure in a grassland species. Plant Biol 15(1):195–202. doi:10.1111/j.1438-8677.2012.00601.x
Nitare J (ed) (2000) Signalarter. Indikatorer på skyddsvärd skog: flora över kryptogamer. Skogsstyrelsens förlag, Jönköping
Nordén B, Appelqvist T (2001) Conceptual problems of ecological continuity and its bioindicators. Biodivers Conserv 10:779–791
Nordén J, Penttilä R, Siitonen J, Tomppo E, Ovaskainen O (2013) Specialist species of wood-inhabiting fungi struggle while generalists thrive in fragmented boreal forests. J Ecol 101(3):701–712. doi:10.1111/1365-2745.12085
Økland B, Bakke A, Hagvar S, Kvamme T (1996) What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway. Biodiver Conserv 5(1):75–100
Paillet Y, Berges L, Hjälten J, Odor P, Avon C, Bernhardt-Römermann M, Bijlsma RJ, De Bruyn L, Fuhr M, Grandin U, Kanka R, Lundin L, Luque S, Magura T, Matesanz S, Meszaros I, Sebastia MT, Schmidt W, Standovar T, Tothmeresz B, Uotila A, Valladares F, Vellak K, Virtanen R (2010) Biodiversity differences between managed and unmanaged forests: meta-analysis of species richness in Europe. Conserv Biol 24(1):101–112. doi:10.1111/j.1523-1739.2009.01399.x
Paltto H, Nordén B, Götmark F, Franc N (2006) At which spatial and temporal scales does landscape context affect local density of red data book and indicator species? Biol Conserv 133(4):442–454
Penttilä R, Lindgren M, Miettinen O, Rita H, Hanski I (2006) Consequences of forest fragmentation for polyporous fungi at two spatial scales. Oikos 114(2):225–240
Ranius T, Fahrig L (2006) Targets for maintenance of dead wood for biodiversity conservation based on extinction thresholds. Scand J For Res 21(3):201–208
Ranius T, Eliasson P, Johansson P (2008) Large-scale occurrence patterns of red-listed lichens and fungi on old oaks are influenced both by current and historical habitat density. Biodiver Conserv 17(10):2371–2381. doi:10.1007/s10531-008-9387-3
Ranius T, Johansson V, Fahrig L (2011a) Predicting spatial occurrence of beetles and pseudoscorpions in hollow oaks in southeastern Sweden. Biodiver Conserv 20(9):2027–2040. doi:10.1007/s10531-011-0072-6
Ranius T, Martikainen P, Kouki J (2011b) Colonisation of ephemeral forest habitats by specialised species: beetles and bugs associated with recently dead aspen wood. Biodiver Conserv 20(13):2903–2915. doi:10.1007/s10531-011-0124-y
Rassi P, Alanen A, Kanerva T, Mannerkoski I (eds) (2001) The 2000 Red List of Finnish species. Ministry of the Environment and Finnish Environment Institute, Helsinki (In Finnish with English summary)
Rukke BA, Midtgaard F (1998) The importance of scale and spatial variables for the fungivorous beetle Bolitophagus reticulatus (Coleoptera, Tenebrionidae) in a fragmented forest landscape. Ecography 21(6):561–572
Sahlin E, Schroeder LM (2010) Importance of habitat patch size for occupancy and density of aspen-associated saproxylic beetles. Biodiver Conserv 19(5):1325–1339. doi:10.1007/s10531-009-9764-6
Saint-Germain M, Drapeau P (2011) Response of saprophagous wood-boring beetles (Coleoptera: Cerambycidae) to severe habitat loss due to logging in an aspen-dominated boreal landscape. Landsc Ecol 26(4):573–586. doi:10.1007/s10980-011-9587-1
Schiegg K (2000a) Are there saproxylic beetle species characteristic of high dead wood connectivity? Ecography 23(5):579–587
Schiegg K (2000b) Effects of dead wood volume and connectivity on saproxylic insect species diversity. Ecoscience 7(3):290–298
Siitonen J (1994) Decaying wood and saproxylic Coleoptera in two old spruce forests: a comparison based on two sampling methods. Ann Zool Fennici 31:89–95
Siitonen J (2001) Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forest as an example. Ecol Bull 49:11–41
Sirami C, Jay-Robert P, Brustel H, Valladares L, Le Guilloux S, Martin JL (2008) Saproxylic beetle assemblages of old holm-oak trees in the mediterranean region: role of a keystone structure in a changing heterogeneous landscape. Rev Ecol-Terre Vie 101–114
Southwood TRE (1977) Habitat, the templet for ecological strategies? J Anim Ecol 46:337–365
Stokland JN, Siitonen J, Jonsson BG (2012) Biodiversity in dead wood. Cambridge University Press, Cambridge
Sverdrup-Thygeson A, Lindenmayer DB (2003) Ecological continuity and assumed indicator fungi in boreal forest: the importance of the landscape matrix. For Ecol Manag 174(1–3):353–363
Sverdrup-Thygeson A, Skarpaas O, Odegaard F (2010) Hollow oaks and beetle conservation: the significance of the surroundings. Biodiver Conserv 19(3):837–852. doi:10.1007/s10531-009-9739-7
Swift TL, Hannon SJ (2010) Critical thresholds associated with habitat loss: a review of the concepts, evidence, and applications. Biol Rev 85(1):35–53. doi:10.1111/j.1469-185X.2009.00093.x
Thomas CD, Kunin WE (1999) The spatial structure of populations. J Anim Ecol 68:647–657
Timonen J, Siitonen J, Gustafsson L, Kotiaho JS, Stokland JN, Sverdrup-Thygeson A, Monkkonen M (2010) Woodland key habitats in northern Europe: concepts, inventory and protection. Scand J For Res 25(4):309–324. doi:10.1080/02827581.2010.497160
Acknowledgments
Thanks to colleagues at INA, Univ. of Life Sciences, and at NINA for discussions and comments, to two anonymous reviewers for constructive and relevant suggestions, to Leonie Gough for checking the English language and to Tuva Sverdrup-Thygeson for assistance with illustrations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jörg Brunet.
Rights and permissions
About this article
Cite this article
Sverdrup-Thygeson, A., Gustafsson, L. & Kouki, J. Spatial and temporal scales relevant for conservation of dead-wood associated species: current status and perspectives. Biodivers Conserv 23, 513–535 (2014). https://doi.org/10.1007/s10531-014-0628-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0628-3