Abstract
Current occurrence patterns of species associated with ancient trees may reflect higher historical habitat densities, because the dynamics of the habitat and the colonisation-extinction processes for many inhabiting species are expected to be slow. We tested this hypothesis in southeast Sweden by analysing species occurrence per parish for twelve redlisted lichen species and nine redlisted fungus species in relation with current density of big oaks, the density of oaks in the 1830s and connectivity with parishes with the species present. For most species, the occurrence was positively related with current density of habitat (for 18 species out of 21) and parish area (for 16 species). Historical habitat density was positively related with occurrence for 11 species, while connectivity with current occurrences in the surroundings was positive for the occurrence of 12 species and negative for the occurrence of two. For lichen species the connectivity measure that best explained the variation was at a larger spatial scale as compared to fungus species. Even if the density of old oaks remains in the future, inhabiting species will most likely decline because their distribution patterns are not in equilibrium with the current habitat density. Therefore, to allow long-term persistence of inhabiting species the number of old oaks should be increased. Areas where such an increase is most urgent could be identified based on species occurrence data and current habitat density, but because species data will always be incomplete data on the historical habitat distribution is valuable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss is one of the main factors causing global and regional extinctions, and consequently threatened species are often associated with declining habitats. For such species, analyses of current occurrence patterns may give a too positive view of their persistence probabilities, if populations remain in landscapes where the habitat recently has become too scarce to allow long-term persistence (e.g. Hanski et al. 1996). One way to analyse to what extent occurrence patterns are in equilibrium with the current habitat is to correlate the current occurrence pattern with both historical and current habitat distribution (e.g. Petit and Burel 1998). A positive relationship between species richness and historical habitat amount, in combination with previous habitat loss, indicate that the species richness is not in equilibrium with the current habitat amount, and will decline in the future even if the current habitat amount and distribution will remain (Lindborg and Eriksson 2004). It is however relatively rare that such relationships are documented, probably because data on historical habitat amount and distribution is scarce. For the current occurrence of vascular plants, a strong effect of local management history up to 300 years ago has been revealed (e.g. Cousins and Eriksson 2002; Gustavsson et al. 2007) and also of historical (50–100 years ago) connectivity (Lindborg and Eriksson 2004). In a study of epiphytic lichens on aspen, species richness was found to be better explained by historic (110–140 years ago) woodland structure than by modern woodland structure (Ellis and Coppins 2007). However, in that study they did not control for the possible effect of current stand size and quality. For a beetle, its distribution has been found to be significantly better explained by the 1952 landscape structure than by the current one (Petit and Burel 1998). When no relationship has been obtained between current distribution patterns and the historical habitat distribution at certain points of time, a relationship with landscape history has in some cases been revealed by metapopulation modelling. For three saproxylic fungi (Gu et al. 2002) and one saproxylic beetle (Schroeder et al. 2007), the outcome from a model assuming a dynamic landscape fitted better with field data than when assuming a constant landscape.
In natural forests, the density of big trees is often high (Nilsson et al. 2002). In contrast, such trees are typically absent from managed forests, because trees are harvested at less than half their maximum life-time (Nilsson 1997). In Europe, big trees have also been frequent in agricultural landscapes, but they have become scarcer because they have been cut down, or not replaced by new trees when they have died (e.g. Petit and Burel 1998). For that reason, many organisms associated with old or big trees are threatened in the present-day landscape (e.g. in Sweden: Berg et al. 1994). Generally, long-lived species exploiting long lasting resources respond slower to environmental changes as compared to species associated with more ephemeral resources (Hanski and Ovaskainen 2002). Because old trees are a long lasting habitat, it is likely to find an effect of historical habitat availability on the current distribution pattern of inhabiting species. Rose (1976) suggested that some lichens associated with old trees are present only in forest stands with a long continuity. However, he did not make any systematic analyses to support that hypothesis. Paltto et al. (2006) tested the effect of habitat history, and found a positive correlation between the number of wood-inhabiting fungi species (indicator species + red-listed species) and the proportion of broadleaved forest 130 years ago, but failed to detect any such relationship for lichens. Such a positive pattern could arise either because the species richness had been higher in landscapes with a higher habitat density historically and the extinction rate after that have been slow, or because the current habitat quality, e.g. the density of large living and dead trees, is higher in landscapes with a high historical density of broadleaved trees.
In this study, we examined the current occurrence patterns of lichens and fungi associated with old oaks (Quercus robur and Q. petraea) in relation to current and historical habitat density in southeast Sweden. We used records of red-listed species, which have been surveyed especially during the last 15 years, e.g. as indicator species of woodland key-habitats (Nitare 2000). Historical oak data were from about 1830. Especially during 1830–1850, the amount of oaks decreased rapidly. This was mainly because until the 1830s, oaks on land owned by farmers belonged to the Swedish state. After that, farmers were allowed to remove oaks, which they did to a high extent (Eliasson 2002); according to reports from Kalmar county to the Forest committee in 1855, oak was almost extinct there and in 1862 the state forester on Gotland reported that oak remained almost only on church land (Eliasson and Nilsson 1999). We analysed species richness, as well as the occurrence of individual species, per parish in relation to current and historical habitat density, area of the parish, and connectivity to other parishes with the species present. By using connectivity measures based on different spatial scales, we analysed at which spatial scale the species’ occurrences are correlated with dispersal sources in the surrounding. For one of our five counties of study we could access data on the current size and sun exposure of the oaks. We used these data to examine the correlation between current habitat quality and historical habitat density, because if such a correlation exists, it may confound the causes of the species’ occurrence patterns.
Methods
Study region
Our study included five counties (Blekinge, Kalmar, Östergötland, Södermanland, and Gotland) in southeast Sweden. In the study area, there were totally 410 parishes, which had a median size of 51.9 km2 (min = 1.6 km2, max = 414.8 km2). By restricting the study to southeast Sweden, we avoided the much wetter western part of Sweden. Thus, the study region was homogenous in terms of climatic conditions; at the meteorological stations within our study counties, the annual mean temperature varies between 6.0 and 7.3°C, and annual mean precipitation between 400 and 631 mm (Anonymous 2006).
Study species
We searched for study species that are red-listed, associated with large oaks (Thor and Arvidsson 1999), and present in at least ten of the study parishes. We obtained 12 lichens and 9 fungi that fulfilled these criteria, and all occurred in at least three of the five study counties. For every parish we compiled presence/absence data of each study species. Species records were obtained from a data base from 24th October, 2006, provided by the Swedish Species Information Centre. 84% of the records were from 1992 to 2003. During that period, the records were mainly originating from systematic surveys of potential forest key-habitats. Even though the nation-wide survey of woodland key habitats is far from including every suitable tree in Sweden, the sampling effort had been high and distributed all over the study region (Berg et al. 2002). The records in the database confirmed that living oak trees are the major habitat for all study species. However, for most of the species there were some records also from other broad-leaved trees (especially lime tree (Tilia cordata), ash (Fraxinus excelsior), and elm (Ulmus glabra)), and for several fungi there were also some records from downed trees.
Current habitat density
Current habitat density was obtained from two surveys (Fig. 1a). Firstly, The County Administration Board of Östergötland has surveyed all big (>1 m in breast height diameter) and hollow (independent on size) trees in all habitat types over the entire county of Östergötland. In total, 29,820 oak trees were surveyed, including information on position, species, and diameter. Secondly, we obtained data from a nation-wide survey of semi-natural pastures and meadows carried out by The Swedish Board of Agriculture. The number of big trees had been estimated, and it had been documented whether there were oaks among these trees or not. In the county of Östergötland, 4264 big trees were documented from sites with oaks in this survey, implying that about 14 % of the total number of big or hollow oaks was included. Due to a strong correlation between the estimates from the two surveys (Table 1), we concluded that the data from the pasture and meadow survey was an acceptable proxy for the current habitat density. Thus, for Östergötland, we used data from the total survey, while for the other counties (Blekinge, Kalmar, Södermanland, and Gotland) we used the estimate from the meadow and pasture survey multiplied by 7.0 (i.e. 29,820/4264).
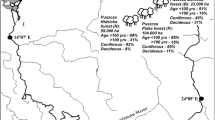
Current and historical density of oaks per parish in southeast Sweden. (a) Current density of oaks (number of big oaks/km²) based on the nation-wide survey of pastures and meadows and the survey of old and hollow oaks in the county of Östergötland. (b) Density of oaks around 1830 (total number of oaks/km²)
The current and historical density of oaks was estimated as the number of trees per square kilometre land area. The centres of the parishes are churches that remain over time. When parishes had been split or fused between the time points for the historical and current surveys, we used data for the total area of the parishes. Borders between the parishes may be changed over time. We minimised the effect of this by estimating the historical density of oaks by dividing the number of oaks with an area estimate from 1876 (Anonymous 1876), while the current density of oaks was obtained by dividing with the current area.
Historical habitat density
The historical habitat density was obtained from oak surveys carried out around 1830. We yielded such data for 367 out of 410 parishes (Fig. 1b). In those surveys, the number of oaks was counted in a standardized way at all land owned by the peasants (tax land) and in some counties also at the land belonging to the state (crown land). The land owned by the nobility (noble land) was excluded from the surveys. In the calculations of oak density per parish, we assumed that the distribution of land into the three different categories (tax, crown and noble) was proportional to the distribution of land tax (in Swedish: “mantal”). By peasants at that time, the distribution of land tax was used to share common resources and burdens, and it is therefore likely that the land tax distribution was rather close to the land area distribution. Secondly, it was assumed that the density of oaks was the same on land owned by the nobility as on other land (Eliasson 2002). This is supported by the state forester Forssbeck who in 1813 noted that oaks in the eastern part of Östergötland were as common on noble land as on tax and crown land, but in other parts less common on noble land. On the other hand, the navy officer af Borneman in his grand survey in Östergötland 1822 noted that there were more oaks on noble land than on other kinds of land, but of the same bad quality for the navy (Eliasson and Nilsson 1999).
Statistics
We analysed presence/absence of each species in relation to parish area, current habitat density, historical habitat density, and current connectivity by multiple logistic regression. Species richness was analysed in relation to the same independent variables with multiple linear regression. Connectivity was calculated as a value specific for each species and parish, according to the following function (Hanski 1999):
in which S j is the connectivity of parish j, α is a constant that scales the connectivity kernel, d ij is the distance between the midpoints of the parishes i and j, p is either 1 (= species presence) or 0 (= species absence) and Q is the amount of habitat (in this case the current number of big or hollow oaks). For analyses of species richness, we estimated a connectivity measure by replacing p by the number of species present.
In the estimate of connectivity, we took into account occurrences of the study species per parish in all Swedish counties with significant occurrences of oak (Skåne, Halland, Blekinge, Kronoberg, Kalmar, Jönköping, Västra Götaland, Östergötland, Södermanland, Stockholm, Örebro, Uppsala, Västmanland, and Gotland). Thus, Denmark was the closest land area not taken into account in our connectivity measure, situated 110 km from the southwestern border of our study area. We calculated six connectivity measures for each species, using different connectivity kernels (1/α = 2, 4, 8, 16, 32, and 64 km) and thus reflecting connectivity at different spatial scales.
By forward selection, we included statistically significant (P < 0.05) variables to the regression models. We included either none or one connectivity measure in each model. If any connectivity measure was significant, we compared models with only one connectivity measure in each, and selected that with the highest Nagelkerke R 2 value.
For the county of Östergötland, we calculated Pearson correlation coefficients between two different measures of current habitat density, historical habitat density, number of species present, and characteristics that may reflect current habitat quality (mean tree diameter and openness).
Results
Species richness of both lichens and fungi was positively related with parish area, current density of habitat, connectivity and historical density of habitat (Table 2, Fig. 1 and 2). For lichens, the strongest relationship was found with a connectivity measure at a larger spatial scale (1/α = 64 km) than for fungi (1/α = 4 km; Fig. 3). For most species, the occurrence was positively related with current density of habitat (for 18 out of 21) and parish area (for 16). Historical habitat density was positively related with occurrence for 11 species, while connectivity was positive for the occurrence of 12 species and negative for the occurrence of 2 (Table 3). There were no differences between lichens and fungi in the proportion of significant relationships (P > 0.05, chi-square) for any of these four variables. There was a significant (P = 0.030, Mann-Whitney U-test) difference for one variable: for lichen species richness the connectivity measure that generated the best fit was at a larger scale in comparison to fungus species richness (Fig. 3). There were no significant differences in the proportion of significant relationships between threatened species (red-list categories CR, EN, VU), and those classified as near threatened (NT).
R² values for multiple linear regression models with the connectivity variable based on different values of 1/α. n = 367. Dependent variables: number of fungus species, and number of lichen species, respectively. Independent variables: parish area, current density of big oaks, historical density of oaks, and connectivity
There was a relatively strong relationship between density of oaks estimated from the total number of big and hollow oaks, and the density estimated from the number of big oaks in the meadow and pasture survey (Table 1). Species richness values for lichens and fungi were positively related with the density of big oaks independent of the measure that was used, but there was a stronger relationship with data from the total survey in comparison to the meadow and pasture survey. Neither lichen nor fungus species richness values were significantly related with openness or tree diameter (Table 1).
Discussion
Effects of historical habitat density
As expected, increasing current habitat density and parish area increased the number of fungus and lichen species, as well as the frequency of occurrence for most species (Table 2 and 3). For species richness, and for the occurrence of about a half of the study species, there was a positive relationship also with the historical habitat density. Such a pattern may arise either because the current habitat quality is higher in landscapes with a high historical density of oaks, or because the species richness had been higher in landscapes with a higher oak density historically, and the extinction rate after that have been slow. However, in our case the first explanation is unlikely, because we failed to find significant relationships between species richness and habitat quality (tree diameter and openness; Table 1). Thus, the observed correlation with historical habitat density is most likely reflecting that historical colonisations and extinctions affect the current occurrence patterns. Consequently, species richness of our study species are probably not in equilibrium with the current habitat density, but the number of species is currently higher due to higher historical habitat density. An extinction debt has been reported for lichens and fungi in boreal forest that has been fragmented during the last few decades (Berglund and Jonsson 2005), and also in a study of epiphytic lichens on aspen, the historical (110–140 years ago) woodland structure was more important for the species richness than the current woodland structure (Ellis and Coppins 2007). The present study deals with a longer period of time than previous studies; in our study the main habitat decline occurred more than 150 years ago. This long time lag may be explained by the fact that old oaks are a long-lasting habitat. In oak pastures in southeast Sweden, several of the study lichen species are observed on oaks that are 200–450 years, but trees older than that are rare (Ranius et al. 2008, in press). This suggests that the study species may occur on the same tree over a period of at least 100–200 years.
Colonisation rates and ranges of the focal species
Our results indicate that today many of the study species have a limited colonisation ability; if the species had the capacity to colonise suitable trees immediately wherever they occurred, presence/absence of the species would only correlate with current habitat density and quality, but not with historical habitat density. Also at a smaller spatial scale, observed spatial structure of epiphytic lichens suggests a limited colonisation rate (Löbel et al. 2006). Furthermore, colonisation rate of saproxylic fungi has been found to be strongly affected by the abundance of fruit bodies at a local scale (Edman et al. 2004). Thus, the limited colonisation ability suggested by the present large-scale study is in line with results from previous studies, which only have been done at a smaller spatial scale.
Lichens were on average related with connectivity at a larger spatial scale in comparison to fungi (Table 2 and 3). If everything else is equal, a wider dispersal range generates a relationship with connectivity at a larger scale. Therefore, based on our results we hypothesize that epiphytic lichen species on old oaks disperse, on average, further away than wood-inhabiting fungi on the same substrate. However, spatial autocorrelation might reflect also other processes and patterns than just dispersal distances, and therefore other types of studies are necessary to test this hypothesis.
Implications for conservation
To allow long-term persistence of the biota that today constitutes the extinction debt requires an increase in the number of old oaks. Areas where such an increase is most urgent could be identified based on species occurrences and the current habitat density. However, because most of the biota associated with old trees are currently not known in detail, and extensive surveys of species-rich groups, such as insects and cryptogams, are unlikely over large spatial scales even in the future, historical data are also valuable. The biggest extinction debt is expected in landscapes which have gone through the largest decline of oaks most recently. Consequently, historical data add important information when determining where conservation efforts are most needed.
References
Anonymous (1876) Sveriges areal beräknad af Generalstabens topografiska afdelning. Kungl. Statistiska centralbyrån, Stockholm
Anonymous (2006) Väder och Vatten nr 13. SMHI, Norrköping (In Swedish)
Berg Å, Ehnström B, Gustafsson L, Hallingbäck T, Jonsell M, Weslien J (1994) Threatened plant, animal, and fungus species in Swedish forests: distribution and habitat associations. Conserv Biol 8:718–731
Berg Å, Gärdenfors U, Hallingbäck T, Norén M (2002) Habitat preferences of red-listed fungi and bryophytes in woodland key habitats in southern Sweden—analyses of data from a national survey. Biodivers Conserv 11:1479–1503
Berglund H, Jonsson BG (2005) Verifying an extinction debt among lichens and fungi in Northern Swedish boreal forests. Conserv Biol 19:338–348
Cousins SAO, Eriksson O (2002) The influence of management history and habitat on plant species richness in a rural hemi-boreal landscape, Sweden. Landscape Ecol 17:517–529
Edman M, Kruys N, Jonsson BG (2004) Local dispersal sources strongly affect colonization patterns of wood-decaying fungi on spruce logs. Ecol Appl 14:893–901
Eliasson P (2002) Skog, makt och människor. En miljöhistoria om svensk skog 1800–1875. Kungliga Skogs-och Lantbruksakademien, Stockholm
Eliasson P, Nilsson SG (1999) Rättat efter Skogarnes aftagande – en miljöhistorisk undersökning av den svenska eken under 1700- och 1800-talen. Bebyggelsehistorisk tidskrift 37:33–64
Ellis CJ, Coppins BJ (2007) 19th century woodland structure controls stand-scale epiphyte diversity in present-day Scotland. Diversity Distrib 13:84–91
Gu W, Heikkilä R, Hanski I (2002) Estimating the consequences of habitat fragmentation on extinction risk in dynamic landscapes. Landscape Ecol 17:699–710
Gärdenfors U (2005) The 2005 red list of Swedish species. ArtDatabanken, Sveriges lantbruksuniversitet, Uppsala
Gustavsson E, Lennartsson T, Emanuelsson M (2007) Land use more than 200 years ago explains current grassland plant diversity in a Swedish agricultural landscape. Biol Conserv 138:47–59
Hanski I (1999) Metapopulation ecology. Oxford University Press, New York
Hanski I, Ovaskainen O (2002) Extinction debt at extinction thresholds. Conserv Biol 16:666–673
Hanski I, Moilanen A, Gyllenberg M (1996) Minimum viable metapopulation size. Am Nat 147:527–541
Lindborg R, Eriksson O (2004) Historical landscape connectivity affects the present plant species diversity. Ecology 85:1840–1845
Löbel S, Snäll T, Rydin H (2006) Species richness patterns and metapopulation processes—evidence from epiphyte communities in boreo-nemoral forests. Ecography 29:169–182
Nilsson SG (1997) Forests in the temperate-boreal transition: natural and man-made features. Ecol Bull 46:61–71
Nilsson SG, Niklasson M, Hedin J, Aronsson G, Gutowski JM, Linder P, Ljungberg H, Mikusiński G, Ranius T (2002) Densities of large living and dead trees in old-growth temperate and boreal forests. For Ecol Manag 161:189–204
Nitare J (2000) Signalarter—indikatorer på skyddsvärd skog. Skogsstyrelsen, Jönköping [Indicator species for assessing the nature conservation value of woodland sites: a flora of selected cryptogams. In Swedish, with an English summary]
Paltto H, Nordén B, Götmark F, Franc N (2006) At which spatial and temporal scales does landscape context affect local density of Red Data Book and indicator species? Biol Conserv 133:442–454
Petit S, Burel F (1998) Effects of landscape dynamics on the metapopulation of a ground beetle (Coleoptera, Carabidae) in a hedgerow network. Agric Ecosyst Environ 69:243–252
Ranius T, Johansson P, Berg N, Niklasson M (2008) The influence of tree age and microhabitat quality on the occurrence of crustose lichens associated with old oaks. J Veg Sci. doi:10.3170/2008-8-18433.
Rose F (1976) Lichenological indicators of age and environmental continuity in woodlands. In: Brown DH, Hawksworth DL, Bailey RH (eds) Lichenology: progress and problems. Academic Press, London, United Kingdom, pp 279–307
Schroeder LM, Ranius T, Ekbom B, Larsson S (2007) Spatial occurrence of a habitat-tracking saproxylic beetle inhabiting a managed forest landscape. Ecol Appl 17:900–909
Thor L, Arvidsson G (eds) (1999) Rödlistade lavar i Sverige. Artfakta. Artdatabanken, SLU, Uppsala [Swedish Red Data book of lichens]
Acknowledgements
Erik Öckinger has provided valuable comments to the manuscript. This study is based on data provided by The County Administration Board of Östergötland, The Swedish Board of Agriculture, and Swedish Species Information Centre. It has been done within Ranius’ project “Predicting extinction risks for threatened wood-living insects in dynamic landscapes” financed by The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas), and has also been funded through a grant from the Swedish Board of Agriculture for further developing environmental monitoring and assessment at SLU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ranius, T., Eliasson, P. & Johansson, P. Large-scale occurrence patterns of red-listed lichens and fungi on old oaks are influenced both by current and historical habitat density. Biodivers Conserv 17, 2371–2381 (2008). https://doi.org/10.1007/s10531-008-9387-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-008-9387-3