Abstract
Context
Although saproxylic beetles use deadwood in industrial exotic forest plantations, deadwood and historical land use patterns may interact among each other making difficult the implementation sustainable management intended to conserve saproxylic beetle diversity.
Objectives
We assessed the additive and interactive effects of deadwood and landscape-scale variables on alpha (α) and gamma (γ) diversity of saproxylic beetles.
Methods
We installed 1034 traps in 80 stands of pine/eucalyptus plantations, clear-cuts and native forest distributed in 29 1-km radius landscape units. Deadwood amount/diversity and composition (native vs. exotic) were estimated for each habitat. A 14-year image time series was used to estimate the cover of native forest and the temporal coefficient of variation of clear-cut cover, CV(CC), an indicator of how extensive clear-cut areas have been in each landscape.
Results
The amount/diversity of deadwood affected positively the α-diversity of all species, but its effect turned negative in clear cut stands. Exotic deadwood had an overall negative effect on α diversity of fungivores and was more marked as the cover of native forest increased within landscapes. The γ diversity of all species and predators responded negatively to CV(CC), while fungivores responded negatively to the current native forest cover. Deadwood and landscape-scale management had nonlinear effects on γ diversity, with the deadwood composition effect being dependent on clear-cut cover. All species and predators were less diverse as the proportion of exotic deadwood increased, but this effect turned positive within landscapes with high CV(CC).
Conclusions
Landscape-scale forest management has long- and short-term effects on saproxylic beetles that are modulated by deadwood and propagate through species functional dimensions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding how wildlife responds to land use change involves considering the extent to which populations, communities and ecosystems are resilient to novel human-caused environmental conditions (e.g., Haila 2002; Fahrig et al. 2011). Wild animals are particularly vulnerable to short- and long-term decrease in the amount and quality of resources available in the matrix (Kupfer et al. 2006; Tscharntke et al. 2012). Such a dynamic matrix is inherent to landscapes dominated by industrial exotic forest plantations managed under the clear-cutting system (Keenan and Kimmins 1993). Stands of exotic trees, such as Pinus spp. or Eucalyptus spp., may accumulate large amounts of logging waste over time and support a dense understory, thus increasingly turning into a suitable habitat for wildlife (Lindenmayer and Hobbs 2004; Brockerhoff et al. 2008). However, not all species may benefit from the resources and conditions available in exotic forest plantations. Depending on their ecological traits (e.g., micro-habitat specialization, trophic guild position, or dispersal ability), animal species can be more, or less sensitive to anthropogenic changes in habitat suitability (Ewers and Didham 2006; Gámez-Virués et al. 2015). In this sense, saproxylic (deadwood dependent) organisms may be negatively affected by forestry management that reduces drastically the quantity and quality of deadwood, as well as the level of habitat naturalness (Franc et al. 2007; Müller and Bütler 2010; Gossner et al. 2013).
Short harvesting cycles (e.g. 10–20 year rotations) cause a break down in the continuous supply and a growing scarcity of deadwood microhabitats (Jonsson and Siitonen 2012) through their mechanical destruction, burning or early removal for biofuel (Rudolphi and Gustafsson 2005; Lassauce et al. 2012). Forestry management also affects diversity and physical–chemical properties of deadwood. Compared to unmanaged native forests (e.g., deciduous forest of Nothofagus spp.), exotic deadwood substrates in forest plantations (e.g., stands of Pinus radiata) tend to be smaller and less diverse, but also more resinous and fibrous, with the latter properties slowing down the decaying process (Fierro et al. 2017; Ulyshen et al. 2018 and herein). Although the activity of the wood-decaying community would tend to increase with the age of plantations, deadwood quality may also decrease as management simplifies understory or canopy cover (Gossner et al. 2016; Johansson et al. 2017), as well as after the application of insecticides, herbicides and fertilizers (Miller and Miller 2004).
Saproxylic species usually exhibit threshold (nonlinear) responses to deadwood available at different spatial scales (e.g., Müller and Bütler 2010). However, preferences or requirements for deadwood also vary between functional groups (e.g., predators, fungivores and detritivores; Vanderwel et al. 2006; Andersson et al. 2012), but also change with habitat degradation, fragmentation and loss (e.g., Komonen et al. 2000; Sverdrup-Thygeson et al. 2014). In landscapes dominated by industrial exotic forest plantations managed under the clear-cutting system, the amount and suitability of deadwood can vary substantially depending on the disturbance regime caused by forest management (Müller and Bütler 2010; Jonsson and Siitonen 2012; Ulyshen et al. 2018). Such a marked heterogeneity in deadwood quality and quantity may make the effects of deadwood on saproxylic beetles unpredictable in landscapes dominated by exotic plantations, thus preventing the implementation of sustainable forest management (Jonsson 2012; Sverdrup-Thygeson et al. 2014). Therefore, the conservation of saproxylic beetles in landscapes dominated by industrial exotic forest plantations requires that forestry management integrates deadwood and land use dynamics across multiple scales (Ranius and Kindvall 2006; Rubene et al. 2017). Three potential drivers of saproxylic beetle diversity can be particularly important to consider for sustainable forestry management:
Current land cover composition
The relative amounts of native forest and exotic forest plantations to landscape scale are thought to be important for wildlife conservation (Swart et al. 2018). Large remnants of native forest may function as reservoirs of saproxylic beetle diversity by providing large, stable and rich deadwood microhabitats while promoting the recolonization of small remnants and exotic forest plantations (Vandekerkhove et al. 2013; Bouget and Parmain 2015). Conversely, clear-cut stands can be a hostile habitat for many organisms (Niemelä et al. 1993; Janssen et al. 2017), acting as potential dispersal barriers for saproxylic species specialized on decomposed wood (Hjältén et al. 2010; Jonsell and Schroeder 2014).
Historic land management
The effects of forestry on saproxylic fauna can accumulate on successive harvest rotations (Ranius and Kindvall 2006; Sverdrup-Thygeson et al. 2014). Steady deforestation usually causes rapid decline of biodiversity while increasing the extinction risk of several species, as found in tropical forests (Leidner et al. 2010). However, landscapes dominated by exotic forest plantations usually sustain a characteristic biodiversity, dominated by generalist species that use new resources, as the case of mammals (Moreira-Arce et al. 2015), birds (Vergara and Simonetti 2004), epigeic insects (Grez et al. 2003), and saproxylic beetles (Fierro et al. 2017). Since forest plantations may act as temporal reservoirs (e.g., sink habitats) of old‐forest specialized species (Pawson et al. 2010), landscapes with long-term retention of native forests should exhibit greater beetle diversity (e.g., Sverdrup-Thygeson et al. 2014). Conversely, an extensive clear-cut regime leading to landscapes dominated by even-aged plantation stands should exhibit declining diversity and those effects should be more evident over long periods.
Deadwood interactions
The fine-grained quantity and quality of deadwood have effects that interact with environmental variables at coarser spatial scales, ultimately shaping the response of saproxylic beetle metapopulations to habitat amount and connectivity (Schiegg 2000; Buse et al. 2010). Exotic tree plantations offer large amounts of deadwood, but of low quality in terms of palatability, diversity, size, decay, and lifespan (Ulyshen et al. 2018). Some studies suggest that regardless of deadwood quality, saproxylic beetles respond differently to deadwood depending on habitat type (e.g., native vs. plantation), with deadwood-habitat interactions being more frequent in specialized species, such as predators and fungivores (Fierro et al. 2017). Novel resources available in exotic forest plantations, as well as stand differences in terms of the age and composition of trees (Micó et al. 2013), deadwood dynamics (Jonsson 2012) and the number of colonizing adults (Schroeder et al. 2007) influence the patterns of deadwood use by saproxylic beetles, with these interactive deadwood effects being more evident at the landscape scale (Vergara et al. 2017).
In this study we assess habitat- and landscape-scale diversity (alpha and gamma diversity, respectively) of saproxylic beetles in landscapes units comprising native forest remnants surrounded by industrial exotic forest plantations of Monterrey pine (Pinus radiata) and Tasmanian blue gum (Eucalyptus globulus) in the Coastal Range of central Chile. Based on the above theoretical and empirical knowledge, the objective of this study was to answer the following questions:
-
i.
Is historic land management more important than the current land use pattern in accounting for saproxylic beetle diversity at different spatial scales?
-
ii.
Do deadwood effects on saproxylic beetle diversity depend on historic land management and current land use pattern?
Materials and methods
Study landscapes
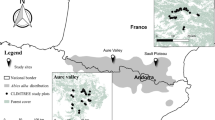
The study covered a total area of ca. 700 km2 located in the coastal range of Central Chile (35° 36′ 10″ S, 72° 20′ 60″ W to 36° 00′ 36″ S, 72° 20′ 60″ W; Fig. 1). In this landscapes land-cover are composed of, in order of dominance, industrial plantations of the exotic Monterrey pine and Tasmanian blue gum (hereafter referred to as pine and eucalyptus, respectively), clear-cut stands and remnants of native forest, locally known as “Maulino forest” (Donoso 1993). Native forest is dominated by the deciduous Nothofagus glauca, accompanied by a mix of sclerophyll and evergreen shrub and tree species.
We used an updated land-cover map (Fig. 1) to select 29 1-km radius (314.2 ha) landscape units, identifying within these four types of forest cover (hereinafter referred as “habitat”) adding a total of 80 stands: (1) pine plantation stands; (2) eucalyptus plantation stands; (3) clear-cut stands (i.e., 0 to 3-year plantations); and (4) native forest remnants. Pine and eucalyptus plantation stands were > 14 ha and > 10 years old on average, while clear-cut stands were > 24 ha. Native forest occurred mostly as small or medium remnants (usually ranging between 5 and 100 ha), but also a few large forest fragments (> 500 ha) were present within the study area (Fig. 1). Landscapes were selected, first, based on the current amount of native forest by including landscapes with low (6% to 20%) and high (20% to 50%) native forest cover (Table 1; Table S1). We also included landscape units with different levels of clear-cut cover (0% to 60%; Table 1). All the selected landscapes were distanced by more than 350 m (Fig. 1). In order to ensure spatial and statistical independence between landscape-scale data, we checked for spatial autocorrelation in the residuals of the best‐ranked models (see the Statistical analysis section) based on the Moran’s I test statistic available in the ape package of R (Paradis and Schliep 2018). We did not find evidence against the assumption of spatial independence among the errors of the models fitted to α and γ diversity data (Table S2 and Table S3, respectively).
Landscape variables
Habitat variables were measured between 1998 and 2012 (14 year; Fig. 2). For the current landscape unit characterization, we used SPOT 5 and Digital globe satellite images downloaded from Google Earth and georeferenced in QGIS 3.0.0 (QGIS Development Team 2018). Percentage of each habitat was estimated in relation to the landscape unit area (Table 1). For the historical landscape unit characterization, bi-annual time series of habitats from 1998 to 2012 were generated using Google Earth Engine (Fig. 2; Gorelick et al. 2017). We used Landsat with 30 m of pixel resolution, creating ca. 90 training polygons per image (a total of 732 polygons), considering the three habitats. Multi spectral classification method based on a RandomForest model was trained using the created polygons (Shelestov et al. 2017; see accuracy of classification in Table S4). From the 14-year classification of land use were estimated two predictor variables: (1) The temporal coefficient of variation of clear-cut cover, CV(CC), within the landscape unit (Table 1; Table S1), which was considered to represent the temporal synchrony in clear-cut harvesting. Larger CV(CC) values indicated a regime of extensive clear-cut areas, while small values result in patterns of uneven-aged stands in the landscape units. Within the studied landscape units, clear cut was highly variable in time, with a mean CV(CC) of ca. 120% and a maximum of 233% (Table 1). (2) The average cover of native forest was considered to be proportional to the long-term retention of native forest within each landscape unit (Table 1; Table S1).
Time-series of land cover maps from 1998 to 2006 of the study area shown in Fig. 1. Forest cover (%) estimated for each year is shown at the top left. Circles represent the 29 studied landscape units
Deadwood data
We collected information about 69 habitat variables associated with the availability and quality of deadwood by setting six 0.25 ha (deadwood plots) in each of the habitats sampled within landscape units (total n = 480 deadwood plots) (Table S5). Habitat variables included vegetation attributes (e.g., understory cover), but most of them were direct measures of deadwood, such as the number, volume and diversity of deadwood pieces, making the distinction among decay stages (early, intermediate and late), substrates (logs or stumps) and tree species (exotic trees or native trees; Table S5). To avoid the edges influence on deadwood availability and quality and vegetation attributes, the deadwood plots were located ca. 100 m from the edge (e.g., Aragón et al. 2015). Native deadwood was available in exotic plantations and clear-cuts, such as stumps and large logs that had remained after the native forest replacement by forest plantations. Quantification of vegetation and deadwood variables was made following the method described in detail in Fierro et al. (2017) and Fierro and Vergara (2019).
In order to reduce the total number of deadwood variables, an exploratory Canonical Partial Least Square Regression (PLS-CA) was developed using the R package plsdepot (Sanchez 2012; Lê et al. 2008; R Development Core Team 2018). PLS-CA functions deal with statistical collinearity and reduced sampling size (Indahl et al. 2009; Sanchez 2012). Through PLS-CA we selected deadwood variables based on their explained variance, retaining a subset of 24 variables with an R2 > 0.5 (Table S6). These selected variables were then grouped using a Principal Component Analysis (PCA), whose two first components (PC1 and PC2) accounted for 72.8% of the total variance (Fig S1). The scores of the two first components were included as predictor variables in a later regression analysis (see Statistical analysis section). The components were interpreted as based on factor loadings (Table S7). Variables with loading 0.70, or greater, were considered to be significantly correlated with a component. We distinguished two groups of deadwood variables highly correlated with PC1 and PC2. The first component (hereafter referred to as “DWI”) was positively correlated with the number and volume of logs and stumps of exotic tree species, but also negatively correlated with the number and volume of logs and stumps of native tree species of different size and decay stages (see details in Table S7 and Fig S1). The second component (hereafter referred to as “DWII”) was positively correlated with the diversity and amount of deadwood (i.e., the term “amount” referring to the total number and volume of all deadwood pieces, logs and stumps; Tables S4 and Fig S1). Thus, significant effects of DWI on beetle diversity were interpreted as beetles responding to tree species composition of deadwood (native vs. exotic), while significant effects of DWII were considered to reflect the beetle’s response to the diversity and amounts of deadwood.
Beetle data
Saproxylic beetles were sampled by randomly installing window-pitfall-traps (hereafter WPT) distributed across the habitats within 29 landscape units (Fig. 2). WPT are pitfall traps modified to incorporate transparent intercept panels, which makes them appropriate to capture beetles flying close to the ground in addition to individuals moving on the ground, as typically exhibited by saproxylic beetles searching for fallen logs and low stumps (Fierro et al. 2017). WPT contained about 200 ml of a mixture of 2 parts 20% neat glycerol and 80% water, with a few drops of an odourless detergent and 30 g salt (NaCl). A total of 13 WPT 100 m apart and > 100 m from the nearest habitat edge (as possible), were set in each habitat type covering > 16 ha within landscape units. Since the four habitat types were not completely replicated across the landscape units, the total number of traps was 1,034 traps (i.e., 13 traps per stand, installed in 80 stands, discounting 6 traps lost by foxes and understory birds). Captures were carried out during 25 days, from November to December (late austral spring) during two consecutive years (2012 and 2013), with WPT being removed at the end of the period. Beetles collected were placed in 96% alcohol to later identify and classify at the lowest taxonomic level, consulting taxonomic keys and the insect collection of the Chilean Natural History Museum. Based on the trophic ecology of their larvae (e.g., Elgueta and Arriagada 1989; Beutel and Leschen 2005; Leschen et al. 2010), beetles species were classified as saproxylic, as well as in four functional groups constituted by seven trophic guilds (e.g., Bouget et al. 2005; Micó et al. 2015; Fierro et al. 2017): fungivores (mycophages and xylomycophages), detritivores (saproxylophages, xylophages and saprophages), predators (zoophages) and omnivores (polyphages). Pooling species in those functional groups facilitated the later analysis of diversity and ecological interpretation of these results.
Diversity estimates
Alpha (α) diversity of beetles (i.e., diversity at the local, habitat level) was estimated by using the Hill numbers (Hill 1973; Jost 2006) qD of order q = 0, q = 1 and q = 2 using the iNext package in R environment specifying input data as sampling-unit-based incidence frequencies (Hsieh et al. 2016). Sample-size-based rarefaction and extrapolation sampling curves (Chao et al. 2014) were fitted to beetles data of the 13 WPT’s installed in each habitat sampled within landscape units. From the adjustment of these curves we calculated 0D, 1D and 2D, corresponding respectively to species richness (SR), exponential of the Shannon diversity Index (SHDI), and the inverse of Simpson diversity Index (SDI). The α-diversity was estimated for all species and the three main functional groups of saproxylic beetles (Fungivores, Detritivores and Predators), while Omnivores were excluded from diversity analysis because only nine species were recorded. Inventory completeness of sampling was evaluated with sample coverage suggested by Chao and Jost (2012), which is a less biased estimator of sample completeness. Sample coverage has values from 0 (minimal completeness) to 100 (maximal completeness). Gamma (γ) diversity of beetles (i.e., diversity at the landscape unit level) was estimated using the adipart function of the vegan package (Oksanen et al. 2011) in R environment. We used a partitioning procedure in which γ-diversity is calculated from the additive contribution of α diversity averaged across all habitats within a landscape unit and the sum of beta (β) diversities between these habitats (Crist and Veech 2006).
Statistical analysis
Generalized Linear Mixed Models (GLMM) were used to analyze α and γ diversity estimates of all species and functional groups of saproxylic beetles. Gaussian and Poisson distributions were assumed for α and γ diversity, respectively, and implemented with the glmer (R package nlme, Pinheiro et al. 2019) and lme (R package lme4, Bates et al. 2015) functions. Although α and γ diversity were analyzed separately, GLMMs included the same predictor variables: (1) deadwood factors derived from PCA (DWI and DWII; see above Deadwood data section); (2) metrics of the current landscape units (percentage of native forest and clearcut cover); and (3) metrics of the historical landscape units (CV of clear-cutting harvesting and temporal mean of native forest cover). Since deadwood was quantified at the habitat level, we obtained estimates of deadwood in each landscape units by the averaging weigh of DWI and DWII, using the relative area of each habitat within landscape units as a weighting factor. The interactions between deadwood and landscape variables were also included in the GLMM of α and γ diversities. Habitat type was specified as a four-level factor (with native forest as a reference level) in α diversity models, and its interactions with deadwood and landscape variables were estimated. Model correlation structure was specified by including the year of sampling and the habitat within landscape units as random factors.
We used the dredge function of the MuMIn package in R (Barton 2014) to generate models with all possible combinations of predictors and rank them by the Akaike’s information criterion corrected for small sample size (AICc). Candidate models with ∆AICc < 2 were considered the most parsimonious and retained. The model.avg function was used to estimate model averaged parameters from the set of best‐ranked models. We checked for collinearity using variance inflation factor (VIF) and Spearman correlation (rs) analysis. Predictors with VIF > 3 and rs > 0.7 were considered to be collinear and candidate models containing collinear predictors were excluded from the analysis (Fox and Monette 1992; see also Bouget et al. 2013; Table S8). Values of α diversity were log transformed and those with sampling coverage lower than 70% were discarded from analysis. To check for the presence of overdispersion in the distribution of γ diversity we used the dispersion_glmer function in the blmeco package of R (Korner-Nievergelt et al. 2015). From this analysis, we determined that the scale (θ) parameter ranged between 0.67 and 1.00, giving evidence of the validity of the Poisson assumption (with θ > 1.40 being indicative of overdispersion).
Results
A total of 38,873 individuals of 303 species of saproxylic beetle species belonging to 41 families were recorded within the 29 landscape units (n = 1034 traps). Predators were the most diverse group (n = 128 species 18,501 individuals), followed by fungivores (n = 99 species 15,721 individuals), detritivores (n = 67 species, 4108 individuals) and omnivores (n = 9 species, 543 individuals). At the habitat level, α diversity metrics (q = 0, q = 1, q = 2) varied among species groups (Table 2) and study landscapes (Table S9). Fungivores were, on average, the most diverse group at the habitat level, followed by predators and detritivores, while sample coverage averaged across all landscape units was ca. 92% for all species, fungivores and predators (see α diversity metrics in Table 2). At the landscape unit level, γ diversity values ranged between 37.2 ± 1.70 (mean ± SE) and 17.0 ± 1.38 species per landscape unit for fungivores and detritivores, respectively (Table 2; Table S10).
Response of α diversity to deadwood, habitat and landscape variables
In general, the diversity of saproxylic beetles at the habitat level (α diversity) was affected by habitat type and deadwood attributes, as shown by the best supported GLMM (Table 3, see details of models and coefficients in Tables S11 and S12). The same patterns were exhibited for the interaction between habitat type and deadwood attributes in fungivores and all species (Table 3; Tables S11 and S12). Particularly, fungivores responded to native forest cover and its interaction with deadwood (Table 3; Tables S11 and S12). Model-averaged coefficients of the best supported models showed that Shannon (and partially, Simpson) diversity of all species, fungivores and predators decreased in non-native habitats compared to native forest, while all diversity metrics of predators decreased in non-native habitats (Tables 3 and S12). Richness of all species decreased as the proportion of exotic deadwood increased at the habitat-level, as shown by a negative effect of DWI (Table 3; Fig. 3). The amount and diversity of deadwood (DWII) affected positively the richness of all species and detritivores as well as the Shannon diversity of all species and fungivores (Table 3; Fig. 3). Deadwood effects also changed with habitat type and current landscape composition, as shown by interaction terms (Table 3). The Simpson diversity and richness of fungivores increased with both the amount/diversity of deadwood (DWII) and proportion of exotic deadwood (DWI) only in eucalyptus, but this effect was not supported for the other habitats (Table 3; Fig. 4). The interaction between native forest cover and proportion of exotic deadwood (DWI) implied a decline in fungivores richness that became more pronounced as the proportion of native forest increased in the landscape (Table 3; Fig. 4). The interaction between clear cut habitat and amount/diversity of deadwood (DWII) implied that the positive effect of DWII on the Shannon diversity of all species turned negative in clear cut stands (Table 3; Fig. 4).
Predicted response of α diversity of saproxylic beetles to deadwood composition (Deadwood I) and amount/diversity of deadwood (Deadwood II). Deadwood I and Deadwood II are the standardized scores of the first two Principal Components (PC1 and PC2), based on deadwood related variables (see “Materials and Methods” section). The lines correspond to predictions from the best supported GLMMs (Table 3; Table S12), while the shaded areas represent the 2.5th–97.5th percentiles. The points are observed data values
Predicted response of α diversity of saproxylic beetles to the interactions between deadwood composition (Deadwood I) and amount/diversity (Deadwood II) with either the habitat type (eucalyptus and clear cut) or native forest cover. Predictions are based on best supported GLMM (Table 3; Table S12). Deadwood I × forest cover interaction is shown for landscape units with ≤ 10% and ≥ 40% of native forest cover. Lines, shadow areas, and points are as explained in Fig. 3
Response of γ diversity to deadwood and landscape variables
The diversity of saproxylic beetles at the landscape unit level (γ diversity) responded to deadwood attributes, current and historic landscape unit variables, as well as the interaction between the latter variables with deadwood (Table 4, see also Tables S13 and S14). However, these effects were mainly found for all species and predators (Table 4). The composition (DWI) and amount/diversity (DWII) of deadwood quantified at the landscape unit level affected negatively the γ diversity of all species and predators (Table 4; Table S14). All species and predators also responded negatively to CV(CC), i.e., the coefficient of variation (%) in clear-cut cover (Table 4; Fig. 5), while fungivores responded negatively to the current cover of native forest (Table 4; Fig. 5). Deadwood and landscape unit variables also interacted, resulting in nonlinear response of γ diversity. The interaction between CV(CC) and deadwood composition (DWI) implied a reduction in γ diversity of all species and predators as the proportion of exotic deadwood increased in landscapes with relatively low values of CV(CC) (Table 4; Fig. 6; note that the positive coefficient for these interaction terms resulted from the product between the negative individual effects for DWI and CV(CC)). However, the negative effect of DWI turned positive in landscape units with a great variability in clear-cut cover, especially in landscape units with CV larger than the mean CV value (Fig. 6). We also found support for a negative effect of the interaction between the current clear-cut cover (CC) and deadwood composition (DWI) on γ diversity of all species and detritivores, which involved different effects of deadwood composition (DWI) between landscape units with low and high clear-cut cover. In landscape units with CC < 40%, the γ diversity of all species increased as the proportion of exotic deadwood became higher, while in landscape units with CC > 40% DWI had a negative effect on the γ diversity of all species (Table 4; Fig. 6). For the case of detritivores, the significant DWI × CC effect resulted in γ diversity increasing with CC when the proportion of native deadwood was relatively high, but the effect of CC turned negative in landscape units with a high proportion of exotic deadwood (Fig. 6). Finally, detritivores responded to the interaction between clear-cut cover (CC) and deadwood diversity/amount (DWII) (Table 4). In landscape units with a low amount/diversity of native deadwood, γ diversity of detritivores increased as CC became larger, but the effect of CC turned negative in landscape units with high amount/diversity of deadwood (Fig. 6).
Contour plots showing predicted values of γ diversity of saproxylic beetles for different combinations of deadwood attributes (deadwood I and deadwood II) and clear-cut cover estimates, including the coefficient of variation of clear-cut cover, CV(CC), and current clear-cut cover. The predictions are based on GLMM containing significant interaction terms (Table 4; Table S14). The degree of darkness is proportional to diversity values
Discussion
The results of this study are consistent with the responses of saproxylic beetle diversity predicted under landscape-scale management scenarios. Landscape-scale forest management is expected to have effects that propagate through the spatial, temporal and functional dimensions, with these effects being modulated by the deadwood available at different spatial scales. From these results we can state the following four factors relevant for deadwood based-forest management:
Native forest is a primary habitat for saproxylic beetles
We demonstrated a reduction of local (α) diversity from native to exotic forest plantations. These negative effects were more noticeably on the Shannon than Simpson diversity metric, indicating that such an impoverishment of diversity results from changes in rare and common species, rather than the dominant ones. The lack of response of detritivores to exotic forest plantations contrasted with the decline of higher trophic predators and fungivores, as described for pine plantations (Fierro et al. 2017) and forest management (Komonen et al. 2000; García-López et al. 2016). Larvae of Staphylinidae and Carabidae species, the most abundant and diverse predators, usually have narrow ecological requirements, preying on fungivore larvae specialized in habitat conditions inherent to native forest (Rainio and Niemelä 2003; Vanderwel et al. 2006; Klimaszewski et al. 2017). Indeed, extreme temperature and humidity conditions in exotic forest plantations would cause the density of certain wood-decaying fungi to decline, which in turn would cascade to higher trophic levels (Brin and Bouget 2018).
Deadwood is a major, but not a consistent, predictor of saproxylic beetle diversity
At the habitat scale, saproxylic beetles tended to be more diverse as the amount and diversity of deadwood increased, but this positive effect was reversed at the landscape scale for all species and predators. Thus, although the quantity of deadwood is a stronger diversity indicator at the habitat scale (e.g., Müller and Bütler 2010), it should be interpreted with caution when it is scaled up to the landscape level, especially in dynamic landscapes dominated by forest plantations (Ranius and Kindvall 2006). A decreased γ diversity of predators in landscape units with high deadwood amounts suggests that forest management promotes deadwood microhabitats unsuitable for predators (Fierro et al. 2017). The composition of deadwood available at the habitat and landscape scales also influenced the α and γ diversity of all species and predators, with exotic deadwood being an indicator of low diversity. Predators can be highly vulnerable to exotic deadwood effects, which propagate up the food web (e.g., low fungi abundance causes fungivorous diversity to be lower, thus reducing prey availability) and become more intense at the landscape scale (e.g., Holt et al. 1999).
Landscape-scale forest management has short- and long-term effects
Land cover variables estimated over different temporal ranges were important drivers of γ diversity, but these effects also varied between species functional groups. Conversely, α diversity of saproxylic beetles was weakly influenced by landscape-scale variables.
Contrary to expectations, we did not find a positive effect of native forest amount on saproxylic beetle diversity, but the cover of native forest affected negatively the α and γ diversity of fungivores. The lack of a positive effect of native forest cover suggests that the amount of forest in the study landscape units (ranging between 6.0 and 49.3%) is not a limiting factor for beetle metapopulations. A reduction of native forest cover favors the prevalence of edges in the landscape, making edge dwelling species more diverse (Fahrig 2001). Exotic forest plantations promote the creation of soft edges between native forest and exotic plantations, thus favoring the abundance of understory plants, vertebrates and invertebrates (Vergara and Simonetti 2006). Thus, environmental conditions prevailing in forest boundaries may increase fungal development on deadwood, in turn improving habitat quality for fungivores (Vodka and Cizek 2013).
Clear-cut cover was an important landscape-scale factor for saproxylic beetle diversity, but our results make an important distinction in terms of the temporal scale at which clear cut cover affects diversity and interacts with deadwood. Although we did not find additive effects of the current clear-cut cover, γ diversity of all species and predators decreased when long-term management led to extensive clear-cut areas, i.e., landscape units with large CV(CC) values. The synchronic cut of forest stands not only homogenizes exotic habitats in the landscape, but also causes extreme environmental conditions to occur in extensive areas. Populations of species sensitive to clear-cut may decline over time as the size of clear-cut stands increases (e.g., Hakkarainen et al. 1996). After massive cutting of stands, small populations have few opportunities to recovery due to the Allee effect and reduced rescue effect from clearcut acting as movement barriers (Thomas and Hanski 1997; Jonsson and Siitonen 2012). In order to persist, predators usually require more habitat area than that required by their prey (Ryall and Fahrig 2005), thus predator–prey interactions can be very sensitive to the extensive cutting of exotic plantations functioning as a secondary habitat for saproxylic beetles (Fierro and Vergara 2019).
Deadwood and land management have synergistic nonlinear effects
Deadwood available at different spatial scales had effects on beetle diversity that were mediated by forest management. The response of α diversity to deadwood was mostly influenced by the habitat type, while γ diversity responded differently to deadwood depending on current and historic landscape-scale management. The Shannon diversity index was lower in clear-cut stands containing high levels of logging waste, indicating that fresh exotic deadwood is of low quality for most saproxylic beetles (Ulyshen et al. 2018). Eucalyptus plantations also interacted positively with deadwood amount, suggesting that the quality of eucalyptus plantations can be improved by leaving logging waste on the ground.
The composition of deadwood had nonlinear effects on γ beetle diversity through its interaction with clear-cut cover at different temporal scales. Deadwood composition affected all species, predators and detritivores, but also these effects were different depending on how clear-cut harvesting was applied, in terms of synchrony and cover amounts. However, the clear-cut cover × DWI and CV(CC) × DWI effects were contrasting, since the former was negative while the latter was positive. Such interactions may indicate that relative quality of native deadwood increases in landscapes dominated by clear-cut stands, while exotic deadwood micro-habitats become particularly suitable for saproxylic beetles in landscapes with a low clear-cut cover. Additionally, exotic deadwood is more used by beetles when forest plantation stands are synchronically cut, while native deadwood supports more beetle species when landscapes are composed of uneven-aged stands. These interactions between clear-cut cover and deadwood composition pose insights into the landscape-scale forest management. In this regard, exotic and native deadwood should promote beetle diversity at different development stages of plantations. Native deadwood would be more important for beetle diversity just after forest stands are cut, while the importance of exotic deadwood would become greater in landscapes dominated by adult plantations (Fierro et al. 2017; Seibold and Thorn 2018; Ulyshen et al. 2018). Therefore, clear-cut harvesting resulting in even-aged forest stands should consider the retention of logging waste by avoiding its burning or removal for biofuel (Hjältén et al. 2010). Forest stands varying in quality and quantity of logging waste may contribute to increase the diversity of saproxylic beetles through increasing the compositional landscape heterogeneity (Fahrig et al. 2011). Moreover, the spatial distribution of deadwood may interact with landscape structure influencing the functional landscape connectivity of dispersal-limited species. Thus, the ecosystem services (e.g., nutrient cycling) provided by saproxylic beetles could become stressed as forest stands become functionally unconnected. As a conclusion, we recommend that asynchrony in stand cutting and long-term retention of logging waste should be incorporated into landscape-scale planning of forestry plantations in order to transform landscapes into mosaics of native forest and uneven-aged stands with high amounts and diversity of deadwood.
References
Andersson J, Hjältén J, Dynesius M (2012) Long-term effects of stump harvesting and landscape composition on beetle assemblages in the hemiboreal forest of Sweden. Forest Ecol Manag 271:75–80
Aragón G, Abuja L, Belichón R, Martínez I (2015) Edge type determines the intensity of forest edge effect on epiphytic communities. Eur J Forest Res 134:443–451
Barton K (2014) MuMIn: multi-model inference. R package version 1.10.5
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Beutel RG, Leschen RAB (2005) Handbook of zoology. Volume 4. Arthropoda, insecta. Coleoptera beetles. Volume 1. Morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). Walter de Gruyter, Berlin
Bouget C, Brustel H, Nageleisen LM (2005) Nomenclature des groupes écologiques d’insectes liés au bois, synthèse et mise au point sémantique. C R Soc Bio 328:936–948
Bouget C, Larrieu L, Nusillard B, Parmain G (2013) In search of the best local habitat drivers for saproxylic beetle diversity in temperate deciduous forests. Biodivers Conserv 22:2111–2130
Bouget C, Parmain G (2015) Effects of landscape design of forest reserves on Saproxylic beetle diversity. Conserv Biol 30:92–102
Brin A, Bouget C (2018) Biotic interactions between saproxylic insect species. In: Ulyshen MD (ed) Saproxylic insects: diversity, ecology and conservation zoological monograph 1. Springer, New York, pp 471–513
Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J (2008) Plantation forests and biodiversity, oxymoron or opportunity? Biodivers Conserv 17:925–951
Buse J, Levanony T, Timmc A, Dayan T, Assmann T (2010) Saproxylic beetle assemblages in the Mediterranean region, impact of forest management on richness and structure. Forest Ecol Manag 259:1376–1384
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers, a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547
Crist TO, Veech JA (2006) Additive partitioning of rarefaction curves and species–area relationships: unifying α, β and γ diversity with sample size and habitat area. Ecol Lett 9:923–932
Donoso C (1993) Estructura y dinámica de los bosques dominados por las especies de Nothofagus. In: Donoso C (ed) Bosques Templados de Chile y Argentina. Editorial Universitaria, Santiago, Chile, pp 303–370
Elgueta M, Arriagada G (1989) Estado actual del conocimiento de los coleópteros de Chile (Insecta, Coleoptera). Rev Chil Entomol 17:5–60
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:117–142
Fahrig L (2001) How much habitat is enough? Biol Conserv 100:65–74
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
Fierro A, Grez AA, Vergara P, Ramírez-Hernández A, Micó E (2017) How does the replacement of native forest by exotic forest plantations affect the diversity, abundance and trophic structure of saproxylic beetle assemblages? Forest Ecol Manag 405:246–256
Fierro A, Vergara P (2019) A native long horned beetle promotes the saproxylic diversity in exotic plantations of Monterrey pine. Ecol Indic 96:532–539
Fox J, Monette G (1992) Generalized collinearity diagnostics. J Am Stat Assoc 87:178–183
Franc N, Götmark F, Økland B, Norden B, Paltto H (2007) Factors and scales potentially important for saproxylic beetles in temperate mixed oak forest. Biol Conserv 135:86–98
Gámez-Virués S, Perović DJ, Gossner MM, Börschig C, Blüthgen N, de Jong HD, Simons NK, Klein AM, Krauss J, Maier G, Scherber C, Steckel J, Rothenwohrër C, Steffan-Dewenter I, Weiner CN, Weisser W, Werner M, Tscharntke T, Westphal K (2015) Landscape simplification filters species traits and drives biotic homogenization. Nat Commun 6:8568
García-López A, Martínez-Falcón AP, Micó E, Estrada P, Grez AA (2016) Diversity distribution of saproxylic beetles in Chilean Mediterranean forests: influence of spatiotemporal heterogeneity and perturbation. J Insect Conserv 20:723–736
Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R (2017) Google Earth Engine: planetary-scale geospatial analysis for everyone. Remote Sens Environ 202:18–27
Gossner MM, Lachat T, Brunet J, Isacsson G, Bouget C, Brustel H, Brandl R, Weisser WW, Müller J (2013) Current near-to-nature forest management effects on functional trait composition of saproxylic beetles in beech forests. Conserv Biol 27:605–614
Gossner MM, Wende B, Levick S, Schall P, Floren A, Linsenmair KE, Steffan-Dewenter I, Shulze ED, Weisser WW (2016) Deadwood enrichment in European forests—which tree species should be used to promote saproxylic beetle diversity? Biol Conserv 201:92–102
Grez AA, Moreno P, Elgueta M (2003) Coleópteros (Insecta: coleoptera) epígeos asociados al bosque Maulino y plantaciones de pino aledañas. Rev Chil Entomol 29:9–18
Haila Y (2002) A conceptual genealogy of fragmentation research: from island biogeography to Landscape Ecology. Ecol Appl 12:321–334
Hakkarainen H, Koivunen V, Korpimäki E, Kurki S (1996) Clear-cut areas and breeding success of Tengmalm’s owls Aegolius funereus. Wildlife Biol 2:253–259
Hill MO (1973) Diversity and eveness—unifying notations and its consequences. Ecology 54:427–432
Hjältén J, Stenbacka F, Andersson J (2010) Saproxylic beetle assemblages on low-stumps, high-stumps and logs, implications for environmental effects of stump harvest. Forest Ecol Manag 260:1149–1155
Holt RD, Lawton JH, Polis GA, Martinez ND (1999) Trophic rank and the species-area relationship. Ecology 80:1495–1504
Hsieh TC, Ma KH, Chao A, McInerny G (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456
Indahl UG, Liland KH, Næs T (2009) Canonical partial least squares-a unified PLS approach to classification and regression problems. J Chemometr 23:495–504
Janssen P, Fuhr M, Cateau E, Nusillard B, Bouget C (2017) Forest continuity acts congruently with stand maturity in structuring the functional composition of saproxylic beetles. Biol Conserv 205:1–10
Johansson T, Gibb H, Hjältén J, Dynesius M (2017) Soil humidity, potential solar radiation and altitude affect boreal beetle assemblages in deadwood. Biol Conserv 209:107–118
Jonsell M, Schroeder M (2014) Proportions of saproxylic beetle populations that utilize clear-cut stumps in a boreal landscape—biodiversity implications for stump harvest. Forest Ecol Manag 334:313–320
Jonsson BG (2012) Population dynamics and evolutionary strategies. In: Stokland J, Siitonen J, Jonsson BG (eds) Biodiversity in deadwood. Cambridge University Press, Cambridge, pp 338–355
Jonsson BG, Siitonen J (2012) Deadwood and sustainable forest management. In: Stokland J, Siitonen J, Jonsson BG (eds) Biodiversity in deadwood. Cambridge University Press, Cambridge, pp 302–337
Jost L (2006) Entropy and diversity. Oikos 113:363–375
Keenan RJ, Kimmins JP (1993) The ecological effects of clear-cutting. Environ Rev 1(2):121–144
Klimaszewski J, Brunke T, Work L Venier (2017) Rove beetles (Coleoptera, Staphylinidae) as bioindicators of change in boreal forests and their biological control services in agroecosystems: Canadian case studies. In: Betz O, Irmler U, Klimaszewski J (eds) Biology of rove beetles (Staphylinidae)—life history, evolution, ecology and distribution. Springer, New York, pp 161–181
Komonen A, Penttilä R, Lindgren M, Hanski I (2000) Forest truncates a food chain based on an old growth forest bracket fungus. Oikos 90:119–126
Korner-Nievergelt F, Roth T, von Felten S, Guelat J, Almasi B, Korner-Nievergelt P (2015) Bayesian data analysis in ecology using linear models with R, BUGS and Stan. Elsevier, New York
Kupfer JA, Malanson GP, Franklin SB (2006) Not seeing the ocean for the islands: the mediating influence of matrix-based processes on forest fragmentation effects. Global Ecol Biogeogr 15:8–20
Lassauce A, Lieutier F, Bouget C (2012) Woodfuel harvesting and biodiversity conservation in temperate forests: effects of logging residue characteristics on saproxylic beetle assemblages. Biol Conserv 147:204–212
Lê S, Josse J, Husson F (2008) FactoMineR: an R Package for Multivariate Analysis. J Stat Softwt 25:1–18
Leidner AK, Haddad NM, Lovejoy TE (2010) Does tropical forest fragmentation increase long-term variability of butterfly communities? PLoS ONE 5:e9534
Leschen RAB, Beutel RG, Lawrence JF (2010) Handbook of zoology. Arthropoda, insecta. Part 38. Coleoptera, beetles. Volume 2. Morphology and systematics (Elateroidea, Bostrichiformia, Cucujiformia partim). Walter de Gruyter, Berlin
Lindenmayer DB, Hobbs RJ (2004) Fauna conservation in Australian plantation forests—a review. Biol Conserv 119(2):151–168
Micó E, García-López A, Brustel H, Padilla A, Galante E (2013) Explaining the saproxylic beetle diversity of a protected Mediterranean area. Biodivers Conserv 22:889–904
Micó E, García-López A, Sánchez A, Juárez M, Galante E (2015) What can physical, biotic and chemical features of a tree hollow tell us about their associated diversity? J Insect Conserv 19:141–153
Miller KV, Miller JH (2004) Forestry herbicide influences on biodiversity and wildlife habitat in southern forests. Wildl Soc B 32:1049–1060
Moreira-Arce D, Vergara PM, Boutin S, Simonetti JA, Briceño C, Acosta-Jamett G (2015) Native forest replacement by exotic plantations triggers changes in prey selection of mesocarnivores. Biol Conserv 192:258–267
Müller J, Bütler R (2010) A review of habitat thresholds for deadwood, a baseline for management recommendations in European forests. Eur J Forest Res 129:981–992
Niemelä J, Langor D, Spence JR (1993) Effects of clear-cut harvesting on boreal ground-beetle assemblages (Coleoptera: Carabidae) in western Canada. Conserv Biol 7:551–561
Oksanen J, Blanchet FG, Kindt R, Legendre P, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H (2011) Vegan: community ecology package. R package version 1:17-11
Paradis E, Schliep K (2018) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528
Pawson SM, Ecroyd CE, Seaton R, Shaw WB, Brockerhoff EG (2010) New Zealand’s exotic plantation forests as habitats for threatened indigenous species. New Zeal J Ecol 34:342–355
Pinheiro J, Bates D, DebRoy S, Sarkar, D., R Core Team (2019). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3:1-141
QGIS Development Team (2018) QGIS development team QGIS geographic information system. Open source geospatial foundation project
R Development Core Team (2018) R, a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rainio J, Niemelä J (2003) Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers Conserv 12:487–506
Ranius T, Kindvall O (2006) Modelling the amount of coarse woody debris produced by the new biodiversity-oriented silvicultural practices in Sweden. Biol Conserv 119:51–59
Rubene D, Schroeder M, Ranius T (2017) Effectiveness of local conservation management is affected by landscape properties: species richness and composition of saproxylic beetles in boreal forest clearcuts. Forest Ecol Manag 399:54–63
Rudolphi J, Gustafsson L (2005) Effects of forest-fuel harvesting on the amount of deadwood on clear-cuts. Scan J Forest Res 20:235–242
Ryall KL, Fahrig L (2005) Habitat loss decreases predator–prey ratios in a pine-bark beetle system. Oikos 110:265–270
Sanchez G (2012) Plsdepot: partial least squares (PLS) data analysis methods
Schiegg K (2000) Effects of deadwood volume and connectivity on saproxylic insect species diversity. Écoscience 7:290–298
Schroeder LM, Ranius T, Ekbom B, Larsson S (2007) Spatial occurrence of a habitat-tracking saproxylic beetle inhabiting a managed forest landscape. Ecol Appl 17:900–909
Seibold S, Thorn S (2018) The importance of dead-wood amount for saproxylic insects and how it interacts with dead-wood diversity and other habitat factors. In: Ulyshen MD (ed) Saproxylic insects: diversity, ecology and conservation. Zoological monograph 1. Springer, New York, pp 607–637
Shelestov A, Lavreniuk M, Kussul N, Novikov A, Skakun S (2017) Exploring google earth engine platform for big data processing: classification of multi-temporal satellite imagery for crop mapping. Front Earth Sci 5:17
Sverdrup-Thygeson A, Gustafsson L, Kouki J (2014) Spatial and temporal scales relevant for conservation of dead-wood associated species: current status and perspectives. Biodivers Conserv 23:513–535
Swart RC, Pryke JS, Roets F (2018) Arthropod assemblages deep in natural forests show different responses to surrounding land use. Biodivers Conserv 27:583–606
Thomas CD, Hanski I (1997) Butterfly metapopulations. In: Hanski I, Gilpin ME (eds) Metapopulation biology: ecology, genetics, and evolution. Academic Press, New York, pp 359–386
Tscharntke T, Tylianaskis J, Rand T, Didham R, Fahrig L, Batáry P, Bengtsson J, Clough Y, Crist TO, Dormann CF, Ewers RM, Fründ J, Holt RD, Holzschuh A, Klein AM, Kleijn D, Kremen C, Landis DA, Laurance W, Lindenmayer D, Scherber C, Sodhi N, Steffan-Dewenter I, Thies C, van der Putten WH, Westphal C (2012) Landscape modulation of biodiversity patterns and processes—eight hypotheses. Biol Rev 87:661–685
Ulyshen MD, Pawson SM, Branco M, Horn S, Hoebeke ER, Gossner MM (2018) Utilization of non-native wood by saproxylic insects. In: Ulyshen MD (ed) Saproxylic insects: diversity, ecology and conservation. Zoological monograph 1. Springer, New York, pp 797–834
Vandekerkhove K, Thomaes A, Jonsson BG (2013) Connectivity and fragmentation: island biogeography and metapopulation applied to old-growth elements. In: Kraus D, Krumm F (eds) Integrative approaches as an opportunity for the conservation of forest biodiversity. European Forest Institute, Joensuu, pp 103–115
Vanderwel MC, Malcolm JR, Smith SM, Islam N (2006) Insect community composition and trophic guild structure in decaying logs from eastern Canadian pine–dominated forests. Forest Ecol Manag 225:190–199
Vergara PM, Meneses LO, Grez AA, Quiroz MS, Soto GE, Pérez-Hernández CG, Díaz PA, Hahn HI, Fierro A (2017) Occupancy pattern of a long-horned beetle in a variegated forest landscape: linkages between tree quality and forest cover across spatial scales. Landsc Ecol 32:279–293
Vergara PM, Simonetti JA (2004) Avian responses to fragmentation of the Maulino Forest in central Chile. Oryx 38:383–388
Vergara PM, Simonetti JA (2006) Abundance and movement of understory birds in a Maulino forest fragmented by pine plantations. Biodivers Conserv 15:3937–3947
Vodka S, Cizek L (2013) The effects of edge-interior and understorey-canopy gradients on the distribution of saproxylic beetles in a temperate lowland forest. Forest Ecol Manag 304:33–41
Acknowledgements
Dr. Andrés Fierro gratefully thanks J. Simonetti, as well as ARAUCO SA. We thank FONDECYT 1095046, 1180978, and 3180112, and DICYT 021875VE-POSTDOC (USACH). Dagoberto Aravena and Nicolas Aravena for the logistic helping, and Mario Elgueta (National Museum of Natural History) for it comments about specimen identification.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fierro, A., Vergara, P.M., Grez, A.A. et al. Landscape-scale management of exotic forest plantations: synergy between deadwood and clear-cutting synchrony modulates saproxylic beetle diversity. Landscape Ecol 35, 621–638 (2020). https://doi.org/10.1007/s10980-019-00966-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-019-00966-w