Abstract
Logging significantly reduces the proportion of late-seral stands in managed boreal landscapes. Availability of habitat elements typical of these stand types, such as standing dead wood, decreases, and dependant species may have their abundance reduced or become locally extirpated, potentially affecting the ecosystem processes/services in which they take part. We evaluated the impact of habitat loss on saprophagous wood-boring beetles (Coleoptera: Cerambycidae) in an aspen-dominated landscape intensively logged for the past 30 years. Sixty natural snags of middle decay class were chosen along a gradient of habitat loss and disturbance age, cut down and dissected for beetle larvae. We then assessed relationships between species occurrence and percentage of residual cover and age of disturbance at spatial scales ranging from 40 to 2000 m radii. The most common species, Anthophylax attenuatus, showed no response, being abundant regardless of the intensity of habitat loss. The second most common species, Bellamira scalaris, showed a negative response, especially in sites which had been fragmented for a longer time. A third species, Trachysida mutabilis, showed an inverse trend, having a higher probability of presence where habitat loss was more severe. Our study shows that some saprophagous wood-borers do react negatively to habitat loss, but that within a relatively homogenous group the response can vary significantly between species. Saprophagous wood-borers should be considered potentially sensitive to habitat loss, and their response to fragmentation remains to be evaluated on a longer time frame.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most ecosystem processes and services depend to some extent on biodiversity. The functional characteristics of plant and animal communities, together with abiotic factors, shape the properties of a given ecosystem. Species loss can alter these properties and thus affect the processes and services they provide (Hooper et al. 2005). Values associated with biodiversity have been much discussed in the last decades, and concerns about the potential impact of human activities on the biota are now more commonly expressed, if not taken into account (Millennium Ecosystem Assessment 2005).
Habitat loss is currently seen as the primordial factor in the decline of biodiversity (Pimm and Raven 2000; Fahrig 2003). Habitat loss and fragmentation commonly result in species extinction in regions of high endemism, as exemplified by studies of tropical forests (Turner 1996; Brooks et al. 2002, 2003). In less heterogeneous and diverse biomes such as the boreal forest, species extinction is not as much of an immediate threat, but species can become locally or regionally extinct and resulting community impoverishment can hinder or disrupt crucial ecosystem processes, such as pollination, trophic webs and nutrient cycling (Didham et al. 1996). Habitat loss can affect populations through a decrease and fragmentation of available habitat per se (e.g., species needing large territories), increased costs associated with foraging and dispersal (Desrochers and Hannon 1997; Bélisle et al. 2001; Gobeil and Villard 2002), elevated predation risk (Robinson et al. 1995; Donovan et al. 1997) and habitat alteration (e.g., edge effect) (Saunders et al. 1991; Ewers and Didham 2006). Because some of these mechanisms can interact strongly with many life history traits of a given species, the impact of habitat loss and fragmentation is likely to differ between species. Hence, trophic level, dispersal ability, body size and niche breadth are all likely to influence the response of the species to matrix contrast and fragment isolation, size and shape complexity (Tscharntke et al. 2002; Henle et al. 2004; Ewers and Didham 2006).
In the boreal forest, industrial logging has replaced natural disturbances such as fire, becoming the main disturbance in these ecosystems (Östlund et al. 1997; Angelstam 1998; Perera and Baldwin 2000; Yaroshenko et al. 2001; Schroeder and Perera 2002; Bergeron et al. 2004; Belleau et al. 2007; Boucher et al. 2009). Even-aged management with clearcutting as the dominant harvesting practice is used throughout the boreal forest. Cutblocks are generally spatiotemporally aggregated for logistic and economic purposes, and such an approach can result in some contexts in massive habitat loss at the landscape scale (Gauthier et al. 2009; Drapeau et al. 2009a). Logging does not result in land conversion in the boreal forest and, hence, some structure can be regained in early-seral stands after a few decades. However, specific habitat elements associated with late-seral stands, such as dead wood, reappear on a much longer time frame (Hély et al. 2000; Sénécal et al. 2004; Harper et al. 2006). Thus, species associated with such habitat elements are considered to be much more sensitive to forest management (Berg et al. 1994; Grove 2002; Drapeau et al. 2009b).
Saprophagous wood-boring beetles (e.g., Cerambycidae) are generally considered important actors of the wood decay process, as their tunnelling structurally weakens dead wood and helps decay fungi penetrate radially into the sapwood (Edmonds and Eglitis 1989). Such species already face important dispersal constraints because their host material is of variable and unpredictable availability, both temporally and spatially, as tree mortality is in part governed by stochastic factors (Sénécal et al. 2004). In landscapes having suffered important habitat loss, further rarefaction and an exacerbated spatial structuralization of host material could result in dispersal costs too high to maintain populations of some of these species on the long-term, at least in the more isolated habitat patches, and thus locally affect wood decay and nutrient cycling. Some work has been published on the response of saproxylic beetles in general to habitat connectivity and fragmentation (Schiegg 2000; Gibb et al. 2006; Webb et al. 2008; Brunet and Isacsson 2009), in most instances showing some degree of response to lack of habitat connectivity, especially in small, specialized fungivorous red-listed species. However, little such data has been collected for wood-boring species (i.e., in opposition to subcortical species) as capture rates of adult wood-boring species rarely reflect effective use of snags by wood-borers in habitat patches (Webb et al. 2008). Wood-borers’ adult phase strictly consists of dispersal and mating, and usually lasts only for a few weeks, whereas they may spend several years as larvae within the wood. A more direct approach such as wood dissection of snags which targets larval stages instead of adults is likely to yield more specimens and more informative data on the functional role of snags in remnant habitat patches for saprophagous wood-boring beetles (Saint-Germain et al. 2007a).
Saprophagous wood-borers make up an important element of the biodiversity of boreal forests and play an important functional role in wood decay (Edmonds and Eglitis 1989), nutrient cycling processes (Grove 2002) and in food webs (Murphy and Lenhausen 1998; Nappi et al. 2003; Nappi et al. 2010). Previous studies have shown that this group is especially sensitive to forest management (e.g., decreased dead wood availability, habitat fragmentation) (Berg et al. 1995; Grove 2002). The main objective of our study was to determine how aspen-feeding saprophagous wood-boring beetles responded to severe loss of mature forest cover in an aspen-dominated boreal mixed-wood managed landscape. Loss of forest cover should eventually translate to decreases in oviposition and feeding host availability for adults. We expected a positive relationship between the amount of residual forest cover and the probability of presence of wood-feeding larvae in sampled snags collected in remnant patches of clearcut areas (thus a negative impact of habitat loss). We also expected a stronger negative impact in areas that had been disturbed for a longer period of time, as response to habitat loss is often delayed in time, especially when the species under study has specific life history traits (e.g., longevity) or if the disturbance creates an initial burst in host availability (i.e., residual tree mortality induced by harvesting). Lastly, we also expected similar responses in all Cerambycidae species found in our study, although not necessarily at the same spatial scale, as they could be considered as constituting a rather homogenous group in terms of habitat requirements (Craighead 1923; Linsley and Chemsak 1976).
Methods
Study area
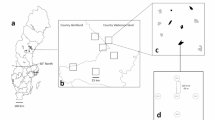
The study area was located on the boreal shield region in western Quebec, Canada. Centroid of all sampling plots was located at 48°25′ N, 79°26′ W. This area of the boreal mixed-wood forest is characterized by deep glaciolacustrine clay deposits. Annual mean temperature and precipitation are of 1°C and 800–900 mm respectively (Environment Canada 2009). Most of the stands on the landscape under study originated from a 1922 fire. These stands were dominated, in order of importance, by trembling aspen Populus tremuloides Michaux, black spruce Picea mariana (Miller), jack pine Pinus banksiana Lambert, white birch Betula papyrifera Marshall, balsam fir Abies balsamea (L.), and white spruce Picea glauca (Moench). Eighty-plus year-old aspen stands growing on such clay deposits can be considered as overmature, with a high density of large-diameter snags. First records of logging in this landscape date from 1976, and the largest surface areas were clearcutted in 1984–1986, 1989–1992, 1996–1998 and 2000–2002 (13.4, 10.6 and 6.1 km2 respectively, within Fig. 1 boundaries).
Maps showing study area with sampling sites and non-coniferous residual forest over 50 years old (NCRF50+) in light gray as of in 1976 (before first significant anthropogenic disturbances), in 1998 and 2008 (year of sampling), according to forest inventory maps. NCRF50+ totalled 69.1% in 1976, 36.9% in 1998 and 31.0% in 2008 of total area (including water)
Insect sampling
Most wood-borers associated with dead aspen usually colonize their host trees several years after their death, and larvae are far more abundant in snags of middle decay classes (Saint-Germain et al. 2007a). To assess if and how these species are affected by habitat loss, we elected to sample natural snags for larvae by snag dissection. Snags were chosen among residual patches selected according to their spatial context. Twelve patches were selected among three groups: (1) OC (“old cutblocks”), in sectors where most cutblocks were over 15 years old; (2) RC (“recent cutblocks”), in sectors where most cutblocks were between 5 and 10 years old; and (3) CTR (“controls”), with limited disturbance or very recent cutblocks (<2 years old). Figure 1 shows location of these patches. Information on logging history was taken from forest inventory maps and data forwarded by Tembec, Inc., the logging company active on the territory (Légaré, pers. com.). In each of the 12 patches, five aspen snags characterized as of “middle decay class” according to their visual appearance (criteria adapted from Maser et al. 1979) and of a minimum of 15 cm dbh (diameter at breast height) were localised and felled in September 2008, for a total of 60 snags. A 1 m section was taken from each between 0.5 and 1.5 m above ground and brought to the laboratory. One m sections were then cut into 20 cm pieces with a chainsaw and then dissected with wood chisels and hatchets. All larvae found within the wood were collected, boiled for a few seconds and then preserved in 70% ethanol. Larvae were identified to species whenever possible by M. Saint-Germain using keys from Craighead (1923) and Švácha and Danilevsky (1986, 1987, 1988). Among the main species identified, Anthophylax, Bellamira and Trachysida are all monospecific genera in boreal aspen, and are thoroughly described in Craighead (1923). Two species of Trigonarthris occurs in boreal aspen, and the larval stage as been described at generic level only. Collected larvae were thus identified as Trigonarthris sp., but could be either T. minnesotana (Casey) or T. proxima (Say), or both.
Snag characterization
Diameter at breast height was measured for each snag upon their selection. Following the felling of the tree, a hemispherical photograph was taken from the cut surface upwards to measure canopy openness, as some European studies have shown that some beetle species can favor either sun-exposed or shaded conditions (Sverdrup-Thygeson and Ims 2002). Photographs were analyzed with the software Gap Light Analyzer 2.0 (Frazer et al. 1999). Also at the time of felling, a wood disk was taken at 1.5 m above ground to measure wood density. Decay state is an important factor in host selection for dead-host wood-borers, and visual appearance does not necessarily give a good estimation of it (Saint-Germain et al. 2007a, b). Therefore, we decided to use measurements of wood density (calculated by dividing dry weight/volume of the whole disk) in the analyses.
Spatial context metrics
We decided to use residual habitat as an explanatory variable instead of habitat loss (i.e., clearcut area) because it is more biologically meaningful. We excluded habitats that do not produce suitable hosts such as open water, swamps, rocky outcrops and other unproductive terrain. We then determined what type(s) of stand as classified in Quebec Ministry of Natural Resources forest inventory maps should be considered as suitable habitat. Stands are classified in terms of tree species composition either by dominant/secondary species or by a more general appellation (coniferous, mixed, shade-tolerant or -intolerant broadleaf) especially for early-seral or highly mixed stands. Developmental stage is assessed by age class (10, 30, 50, 70, 90, >120). Most insect species targeted in this study use only aspen to breed among tree species common in the study area (Linsley and Chemsak 1976; Saint-Germain et al. 2007a). We thus had the options of using stands with a definite level of aspen component in our calculations of available habitat (e.g., dominant or merely present), or rely on more general classifications as total cover or total non-coniferous cover. Non-coniferous stands of age classes 50 years old and over were chosen following preliminary analyses involving different definitions of suitable habitat (see below).
Another important consideration when studying habitat loss and fragmentation is the spatial scale at which a response can be expected in the target organism. This spatial scale would be expected to be species-specific and also to vary among habitats showing different spatial structures. We used a technique and software package developed by Holland et al. (2004, 2005) to identify the spatial scales appropriate for our study species. The Focus program selects multiple sets of spatially independent sites (here snags) and conducts regressions between the habitat variable (here residual cover) and species abundance (here larval density). The process is repeated at different spatial scales and the mean regression coefficients obtained are used to identify the scale at which the species is most responsive. Responses of each of the four most abundant species were tested at the following spatial scales: 40, 250, 400, 600, 800, 1000, 1200, 1500, 1750 and 2000 m. The spatial scale identified as the most significant for a given species was used in all subsequent analyses.
In addition to the amount of habitat loss, time elapsed since the disturbance can be an important factor in the detection of habitat loss effects. Such effects may begin to appear only after a number of generations have passed (extinction debt; Tilman et al. 1994). This delay in the decline of species in fragmented habitat may be particularly expected in long-lived species. In our case, at least two of the species under study are believed to have a larval development spanning at least in some cases over 5 years or more. Thus, the species could very well be assessed as ‘present’ in a patch in which no further colonization had succeeded in the last 4–5 years. To include this temporal aspect in our analyses, we calculated for each snag, within a radius of 1200 m, how many years had elapsed since mature forest cover decreased under 50% using logging history data (Table 1). This 50% criterion may appear to be high when compared to traditionally accepted extinction thresholds. However, the intent of this index is to assess the global age of local disturbance, and not to assess the extent of habitat loss. In this sense, the 50% criterion appeared as more appropriate than 40 or 30% criteria considering the structure of our data. The 1200 m radius scale was chosen as being intermediate among all significant spatial scales identified using the Focus program (Holland et al. 2004, 2005).
No analyses involving other spatial metrics frequently used in habitat fragmentation studies (e.g., patch size, shape complexity) were conducted in this study. In our view, the way ‘habitat’ is considered in this context cannot easily be applied to holometabolous wood-feeing insects. In these, dead wood constitutes the habitat of the immature stages, while the adults, outside of their maturation feeding phase (if the species has one), acts strictly as dispersers searching for potential hosts to lay eggs, and thus have no definite ‘habitat’ that can be attributed to them. Hence, the mere distance between the point of origin of the adult and the future host is the critical element, much more than the structure of the habitat around it, although this structure can influence dispersal success (e.g., through differential energy expenditure, predation risk, etc.). However, there is no clear-cut distinction between the two, as in one is habitat and the other is not; e.g., open areas may require less energy to disperse but involve higher predation risk. Matrix contrast is thus expected to be low for these species. Suitable habitat does not equate to remnant patch but rather to individual snags within patches, and two snags closer to each other but located in separate patches could be considered as more connected than two snags located in the same patch but further apart. In this context, computing indices such as patch size and shape complexity would make little biological sense, and we opted to leave them out.
Statistical analyses
Responses of the four main taxa collected to snag characteristics (DBH, wood density, canopy openness) were first assessed using linear regression on square-root-transformed larval density (/m3) data for the two most abundant species (A. attenuatus and B. scalaris), and logistic regression on presence/absence data for all four frequent taxa. Percentages of surrounding non-coniferous forest cover were calculated in radii identified as appropriate using Focus for the taxa sampled. Effect of non-coniferous forest cover on the presence/absence of all four taxa and on squareroot-transformed larval density of A. attenuatus and B. scalaris were again assessed using logistic (for p/a data) and linear (for density) regressions respectively. When relationships with snag characteristics were significant, effect of surrounding forest cover was assessed using residuals of the relevant snag characteristic in a stepwise approach. To analyse the effect of the time elapsed since disturbance on larval density of A. attenuatus and B. scalaris, snags were classified in three groups according to the number of years elapsed since forest cover dropped under 50%: 0–6 years, 11–12 years and 15+ years. The effect of this index of age of disturbance on larval density was assessed using a Kruskal–Wallis non-parametric test. Logistic regressions were used to correlate presence/absence data to this same index.
Residuals of all regressions involving spatial context variables were tested to detect eventual spatial autocorrelation. No such effect was detected.
To validate our inclusion of all non-coniferous cover as suitable habitat in our spatial characterization, analyses of the effect of the proportion of forest cover on larval density of B. scalaris, and on presence/absence of B. scalaris and T. mutabilis were reconducted using other definitions of suitable habitat; calculated F-values and χ 2 obtained from simple linear and logistic regressions were then compared to assess the impact of using these different characterizations on the strength of relationships obtained. Hence in addition to non-coniferous cover (NC), definitions of suitable habitat used were: total forest cover (TC), forest cover with aspen component (dominant or secondary; AC), aspen-dominated stands (AD), and older, aspen-dominated stands (70+ y; OA).
Spatial computations were done using ArcView 3.2 (Environmental Systems Research Institute Inc., Redlands, California). Statistical analyses were performed on Systat 12 (Systat Software Inc., Chicago, Illinois) and JMP 8 (SAS Institute Inc., Cary, North Carolina).
Results
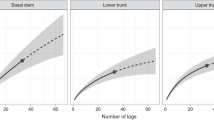
A total of 1042 cerambycid larvae were extracted from the 60 snags sampled. Of these, 81.6% were A. attenuatus, 11.4% B. scalaris, 2.3% Trigonarthris sp., 1.9% T. mutabilis, 0.4% Aegomorphus modestus (Gyllenhal). The remaining 2.4% were unidentifiable because of extreme small size or damaged/missing diagnostic anatomical parts. A. attenuatus was found in 70.0% of sampled snags, B. scalaris 40.0%, T. mutabilis 18.3% and Trigonarthris sp. 13.3%. Most relevant spatial scales identified using Focus were: 2000 m for A. attenuatus, 1200 m for B. scalaris, 2000 m for T. mutabilis and 800 m for Trigonarthris sp. (Fig. 2). The reader has to keep in mind that the 2000 m spatial scale was the largest for which analyses could be done, because of the spatial configuration of the study area and the need for spatially independent sites. Hence, species response to habitat characteristics may be even stronger at larger spatial scales. In the setting of our study, A. attenuatus and T. mutabilis were the most responsive to the 2000 m spatial scale. Snag characteristics and spatial metrics are summarized by sampling site on Table 1.
Quantification of species’ responses to habitat loss through a range of spatial scales (average Pearson correlation coefficient) produced using the Focus software (Holland et al. 2004). Averages are from 500 regressions using randomly selected spatially independent snags. Dotted lines show the spatial scales at which species response is stronger. Further analyses were based on these selected spatial scales
Effects of snag characteristics
No species responded to DBH or canopy openness, whether in terms of larval density or presence/absence. Two taxa responded to wood density, in terms of presence/absence, A. attenuatus, negatively (i.e., probability of presence higher in more decayed wood; χ 21 = 4.211; P = 0.0402), and Trigonarthris sp., positively (i.e., probability of presence higher in less decayed wood; χ 21 = 4.214; P = 0.0401) (Table 2), despite the limited range of wood density covered.
Effects of residual cover
No relationship was found between the proportion of non-coniferous forest cover and the occurrence of A. attenuatus or Trigonarthris sp., either in terms of larval density or presence/absence at the spatial scales identified using Focus (Table 2; Fig. 3). However, B. scalaris squareroot-transformed larval density significantly increased with the proportion of non-coniferous forest cover at the 1200 m (F 1,54 = 6.767; P = 0.0120) spatial scale. B. scalaris was thus less abundant in snags having less forest cover within these radii. The relationship with presence/absence for the same species was marginally significant (χ 21 = 3.707; P = 0.0542). In T. mutabilis, only presence/absence was analysed which showed an inverse trend, with probability of presence higher in snags having less suitable forest cover within a radius of 2000 m (χ 21 = 5.251; P = 0.0219) (Table 2; Fig. 3).
Response of the four main species to the proportion of residual non-coniferous forest cover in terms of density of larvae (A. attenuatus and B. scalaris) and presence-absence (all species). Spatial scales (radii) used to show response were identified using the Focus software (Holland et al. 2004)
Only B. scalaris showed a significant response to the disturbance age index (years elapsed since cover dropped below 50%). When considering presence/absence, the probability of presence decreased significantly in sites disturbed for a longer time (χ 21 = 4.383; P = 0.0363; Fig. 4). The effect was even stronger on B. scalaris larval density, which averaged 175 larvae/m3 in sites having reached the threshold in the last 0–6 years, while older sites (over 10 years) averaged close to 25 larvae/m3 (χ 22 = 6.802; P = 0.0333; Fig. 4).
Response of Bellamira scalaris to number of years elapsed since residual non-coniferous cover in a 1200 m radius dropped below 50%, in terms of presence absence (top) and density of larvae (bottom). Untransformed density of larvae was analysed with a non-parametric Kruskal–Wallis test, with standard error shown
Effect of classification of suitable habitat on strength of fit
Little variation in strength of fit was observed in the analyses between the proportion of suitable forest cover and larval density or presence/absence when using other classification approaches for defining suitable habitat. Suitable forest cover always had a significant effect on B. scalaris larval density regardless of the definition used, with the highest F-value obtained when using non-coniferous 50+ years old cover. Relationships were non-significant for B. scalaris presence/absence regardless of the definition used for suitable forest cover. For T. mutabilis, calculated χ 2 was highest when total cover was used (including coniferous stands), with significant relationships obtained when using total cover (TC), non-coniferous cover (NC), cover with aspen component (AC) and aspen-dominated stands (AD; Fig. 5).
Strenght of fit between density of larvae of B. scalaris (a), presence/absence of B. scalaris (b) and presence/absence of Trachysida mutabilis (c) and residual forest cover in a 1200 m radius calculated as total residual cover (TC), non-coniferous residual cover (NC), residual cover with aspen component (AC), aspen-dominated residual cover (AD) and older (70+ y) aspen-dominated residual cover (OA)
Discussion
Our results showed, among the four commonest species, three different types of responses to severe habitat loss having occurred on a fairly short time frame (~30 years). The most abundant species (A. attenuatus, present in 70% of snags with 81.6% of the total abundance) showed stable occurrence and abundance patterns regardless of the degree of habitat loss. The second most common species (B. scalaris, present in 40% of snags with 11.4% of the total abundance) showed a negative response to habitat loss both in terms of occurrence and abundance, especially where the landscape had been locally fragmented for a longer time. Finally, the rarer T. mutabilis (present in 18% of snags with 1.9% of total abundance) showed a weak but positive response to local habitat loss. Although we expected similar responses among all species of Cerambycidae, species-specific responses to habitat loss are not necessarily surprising because they can be strongly modulated by some life history traits.
Cerambycids live most of their lives as wood-boring larvae, necessarily within a single host. Upon completion of their larval development, emerging adults have a few weeks to find a mate, copulate and locate a suitable host for egg-laying. In the case of the species we studied, adults must locate a standing dead aspen of suitable diameter and decay stage within their adult life. The main constraint they face is thus one of dispersal. Background mortality is relatively high in aspen (Sénécal et al. 2004), at least in late-seral stands, and snags may remain within the species’ tolerance range in terms of decay stage for several years (Saint-Germain et al. 2007a, b). Host material could thus be expected to be plentiful in mature stands, with host-finding success being close to 100% in surviving adults. However, aspen snags are prone to breakage and their half-life have been calculated for eastern Canada to be somewhere between 9 (Vanderwel et al. 2006) and 15.1 years (Angers et al. 2010), which is amongst the shortest for boreal tree species. Given that aspen snags are generally colonized 5 years or more after their death (Saint-Germain et al. 2007b), the suitability period for cerambycids may be narrower than generally expected. Also, tree mortality tends to vary significantly between years (Sénécal et al. 2004; Angers et al. 2010); temporal gaps in mortality are thus likely to occasionally increase average distance between suitable hosts and thus increase dispersal costs and probability of failure to locate a suitable host. Habitat loss though timber harvesting removes a definite proportion of suitable hosts from the landscape and prevents further snag recruitment on the affected territory, no within-patch green tree retention being generally preserved in such operations in north-eastern North America. Habitat loss thus increases average distance between suitable hosts and may therefore constrain dispersal. For several organisms, high matrix contrast effects and sharp edges generated by clearcutting (Harper et al. 2004) can further complexify dispersal dynamics and increase probability of dispersal failure. However, this is probably not the case for boreal wood-boring species which are frequently observed or captured while dispersing in open habitat (Webb et al. 2008). Studies have even shown that some species prefer snags in open settings when looking for egg-laying hosts (Lindhe et al. 2005; Sahlin and Ranius 2009). In saprophagous wood-borers, a decrease of suitable host density in the landscape linked to habitat loss combined with temporal variability in snag recruitment could thus lead to occasional breakdown of population dynamics, and consequently to population decrease and local extinction. This could be the case for B. scalaris in our study area.
Some life history traits believed to affect species’ susceptibility to habitat loss do, however, vary among the studied species despite similar overall behaviour, e.g., body size, niche breadth (in terms of decay gradient suitable) and rarity. Theoretical and empirical work has provided general predictions on how specific traits should affect species susceptibility to habitat loss (Davies et al. 2000; Tscharntke et al. 2002; Ewers and Didham 2006). Despite some degree of correspondence, for example in rarity (abundant A. attenuatus shows no response while rarer B. scalaris drops in abundance), none of these predictions fit observed responses in all three cases. Other life history traits may also affect species response; however, gaps in our knowledge of these species’ life history preclude a definite understanding of why their response differed. Dispersal ability and fecundity are often linked with body size, but not always. Degree of specialization of species in terms of adult food source (all studied species are anthophilous as adults) also remains unknown. For example, a significant increase in the abundance of the pollen source of the anthophilous T. mutabilis adults in disturbed areas may have been responsible for the positive response of the species to habitat loss, despite a decrease in larval host material availability.
We can also ponder whether the time frame in which the study is set (average age of disturbance ~10–12 y) is sufficient to observe the true response of each species to severe habitat loss. An initial increase in mortality rates in trees along borders (see Sénécal et al. 2004; Mascarúa-López et al. 2006), and thus in suitable host density, may have led to a temporary population increase (or concentration) in the first few years following disturbance. Also, species’ longevity may result in some degree of population inertia, or delay in response even if the effects of habitat loss have taken effect (storage effect, but in the larval population; Warner and Chesson 1985). Emergence spanned over several years for a single generation of larvae could also have a bet-hedging type effect, with individuals emerging in years with good host recruitment ensuring the overall fitness of the cohort (Wikars 1994). Hence, given the time frame in which it is set, our study does not provide any certainty that either A. attenuatus or even T. mutabilis will not eventually also show a negative response to habitat loss.
Our results show that some saprophagous wood-borer populations do negatively react to a decrease in local forest cover. Observation of such negative effects of habitat loss prompts other questions. Does this decrease in abundance and/or loss of diversity in species linked to wood decay affect local rates of decay and nutrient cycling, which in their turn could affect on-site processes linked to soil fertility? Some researchers have advocated for a more functional approach to the study of habitat loss and fragmentation effects (Didham et al. 1996). A next step in the investigation of such questions could involve direct measurements of decay rates in fragments rather than assessing insect occurrence and abundance. Although B. scalaris did represent only 11.4% of larvae collected in our study, figures such as seen in Fig. 3b, were larval density drops from an average of 175 larvae/m3 to about 25 in sectors which have been disturbed for a longer time may suggest that indeed such rarefaction in wood-boring larvae (a 85% drop in larval activity for B. scalaris) may affect to some degree decay and falling rates of dead standing aspen.
The response of B. scalaris observed in our study indicates that management approaches like the one that was used in our study area may fail to sustain biological diversity, at least at the local scale. Species rarefaction indicates either that local snag recruitment is not sufficient, resulting in gaps in temporal connectivity (which would suggest the need for bigger remnant patches), or that remnant patches are too isolated from each other (which would suggest the need to increase spatial connectivity between remnant patches). Whereas B. scalaris responded negatively to habitat loss, the relationship we observed was linear. It is thus difficult at this stage to discuss any kind of threshold in disturbance level for managed landscapes that could allow appropriate continuation of ecosystem processes in which these species are involved. Future research should aim at pursuing a better understanding of species response to habitat loss, particularly in landscapes which have been under intensive management for longer periods of time, or maybe through alternate approaches, for example by looking at age structure of larval populations for long-lived species in different spatiotemporal contexts. The effective contribution of these species to food webs and the decay process should also be further assessed experimentally.
References
Angelstam PK (1998) Maintaining and restoring biodiversity in European boreal forests by developing natural disturbance regimes. J Veg Sci 9:593–602
Angers VA, Drapeau P, Bergeron Y (2010) Snag degradation pathways of four North American boreal tree species. For Ecol Manag 259:246–256
Bélisle M, Desrochers A, Fortin MJ (2001) Influence of forest cover on the movements of forest birds: a homing experiment. Ecology 82:1893–1904
Belleau A, Bergeron Y, Leduc A, Gauthier S, Fall A (2007) Using spatially explicit simulations to explore size distribution and spacing of regenerating areas produced by wildfires: recommendations for designing harvest agglomerations for the Canadian boreal forest. For Chron 83:72–83
Berg A, Ehnstrom B, Gustafsson L, Hallingback T, Jonsell M, Weslien J (1994) Threatened plant, animal, and fungus species in Swedish forests: distribution and habitat associations. Conserv Biol 8:718–731
Berg A, Ehnstrom B, Gustafsson L, Hallingback T, Jonsell M, Weslien H (1995) Threat levels and threats to red-listed species in Swedish forests. Conserv Biol 9:1629–1633
Bergeron Y, Flannigan M, Gauthier S, Leduc A, Lefort P (2004) Past, current and future fire frequency in the Canadian boreal forest: implications for sustainable forest management. Ambio 33:356–360
Boucher Y, Arseneault D, Sirois L (2009) Logging history (1820–2000) of a heavily exploited southern boreal forest landscape: insights from sunken logs and forestry maps. For Ecol Manag 258:1359–1368
Brook BW, Sodhl NS, Ng PKL (2003) Catastrophic extinctions follow deforestation in Singapore. Nature 424:420–423
Brooks TM, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Rylands AB, Konstant WR, Flick P, Pilgrim J, Oldfield S, Magin G, Hilton-Taylor C (2002) Habitat loss and extinction in the hotspots of biodiversity. Conserv Biol 16:909–923
Brunet J, Isacsson G (2009) Restoration of beech forest for saproxylic beetles—effects of habitat fragmentation and substrate density on species diversity and distribution. Biodivers Conserv 18:2387–2404
Craighead FC (1923) A classification and the biology of North American cerambycid larvae. Technical Bulletin no. 27, Canadian Department of Agriculture
Davies KF, Margules CR, Lawrence JF (2000) Which traits of species predict population declines in experimental forest fragments? Ecology 81:1450–1461
Desrochers A, Hannon SJ (1997) Gap crossing decisions by dispersing forest songbirds. Conserv Biol 11:1204–1210
Didham RK, Ghazoul J, Stork NE, Davis AJ (1996) Insects in fragmented forests: a functional approach. Trends Ecol Evol 11:255–260
Donovan TM, Jones PW, Annand EM, Thompson FR III (1997) Variation in local-scale edge effects: mechanisms and landscape context. Ecology 78:2064–2075
Drapeau P, Leduc A, Bergeron Y (2009a) Bridging ecosystem and multiple species approaches for setting conservation targets in managed boreal landscapes. Chapter 7. In: Villard M-A, Jonsson B-G (eds) Setting conservation targets in managed forest landscapes. Cambridge University Press, Cambridge, pp 129–160
Drapeau P, Nappi A, Imbeau L, Saint-Germain M (2009b) Standing deadwood for keystone bird species in the eastern boreal forest: managing for snag dynamics. For Chron 85:227–234
Edmonds RL, Eglitis A (1989) The role of the Douglas-fir beetle and wood borers in the decomposition of and nutrient release from Douglas-fir logs. Can J For Res 19:853–859
Environment Canada (2009) Canadian climate normals 1971–2000 [online]. Available from www.climate.weatheroffice.ec.gc.ca/climate_normals/index_e.html. Accessed 16 Sept 2009
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev Camb Philos Soc 81:117–142
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Frazer GW, Canham CD, Lertzman KP (1999) Gap Light Analyzer (GLA), Version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Simon Fraser University; The Institute of Ecosystem Studies, Burnaby, British Columbia; Millbrook, New York
Gauthier S, Vaillancourt MA, Morin H, Kneeshaw D, Leduc A, Drapeau P, Bergeron Y (2009) Ecosystem management in the boreal forest. Presses de l’Université du Québec, Québec, Canada, 539 pp
Gibb H, Hjaltén J, Ball J, Atlegrim O, Pettersson RB, Hilszczanski J, Johansson T, Danell K (2006) Effects of landscape composition and substrate availability on saproxylic beetles in boreal forests: a study using experimental logs for monitoring assemblages. Ecography 29:191–204
Gobeil JF, Villard MA (2002) Permeability of three boreal forest landscape types to bird movements as determined from experimental translocations. Oikos 98:447–458
Grove SJ (2002) Saproxylic insect ecology and the sustainable management of forests. Annu Rev Ecol Syst 33:1–23
Harper KA, Lesieur D, Drapeau P, Bergeron Y (2004) Forest structure and composition at young fire and cut edges in black spruce boreal forest. Can J For Res 34:289–302
Harper KA, Bergeron Y, Drapeau P, Gauthier S, De Grandpré L (2006) Changes in spatial pattern of trees and snags during structural development in Picea mariana boreal forests. J Veg Sci 17:625–636
Hély C, Bergeron Y, Flannigan MD (2000) Coarse woody debris in the southeastern Canadian boreal forest: composition and load variations in relation to stand replacement. Can J For Res 30:674–687
Henle K, Davies KF, Kleyer M, Margules C, Settele J (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13:207–251
Holland JD, Bert DG, Fahrig L (2004) Determining the spatial scale of species’ response to habitat. Bioscience 54:227–233
Holland JD, Fahrig L, Cappucino N (2005) Body size affects the spatial scale of habitat–beetle interactions. Oikos 110:101–108
Hooper DU, Chapin FS III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Lindhe A, Lindelow A, Asenblad N (2005) Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter. Biodivers Conserv 14:3033–3053
Linsley EG, Chemsak JA (1976) The Cerambycidae of North America. Part VI. No. 2: Taxonomy and classification of the subfamily Lepturinae. Univ Calif Publ Entomol 80:1–186
Mascarúa-López LE, Harper KA, Drapeau P (2006) Edge influence on forest structure in large forest remnants, cutblock separators, and riparian buffers in managed black spruce forests. Ecoscience 13:226–233
Maser C, Anderson RG, Cromack Jr K, Williams JT, Martin RE (1979) Dead and down woody material. In Thomas UV (ed). Wildlife habitats in managed forests: the Blue Mountains of Oregon and Washington. USDA Forest Service Agriculture Handbook no. 553
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: synthesis. Island Press, Washington
Murphy EC, Lehnhausen WA (1998) Density and foraging ecology of woodpeckers following a stand-replacement fire. J Wildl Manag 62:1359–1372
Nappi A, Drapeau P, Giroux JF, Savard JP (2003) Snag use by foraging Black-backed Woodpeckers in a recently burned eastern boreal forest. Auk 120:505–511
Nappi A, Drapeau P, Saint-Germain M, Angers V-A (2010) Effect of fire severity on long-term occupancy of saproxylic beetles and bark-foraging birds in burned boreal conifer forests. Int J Wildland Fire 19:500–511
Östlund L, Zackrisson O, Axelsson AL (1997) The history and transformation of a Scandinavian boreal forest landscape since the 19th century. Can J For Res 27:1198–1206
Perera AH, Baldwin DJB (2000) Spatial patterns in the managed forest landscape of Ontario. In: Perera AH, Euler DL, Thompson ID (eds) Ecology of a managed terrestrial landscape: patterns and processes of forest landscapes in Ontario. UBC Press, University of British Columbia, Vancouver, BC, pp 74–99
Pimm SL, Raven P (2000) Biodiversity: extinction by numbers. Nature 403:843–845
Robinson SK, Thompson FR, Donovan TM, Whitehead DR, Faaborg J (1995) Regional forest fragmentation and the nesting success of Migratory birds. Science 267:1987–1990
Sahlin E, Ranius T (2009) Habitat availability in forests and clearcuts for saproxylic beetles associated with aspen. Biodivers Conserv 18:621–638
Saint-Germain M, Drapeau P, Buddle CM (2007a) Host-use patterns of saproxylic phloeophagous and xylophagous Coleoptera adults and larvae along the decay gradient in standing black spruce and aspen. Ecography 30:737–748
Saint-Germain M, Drapeau P, Buddle CM (2007b) Occurrence patterns of aspen-feeding wood-borers (Coleoptera: Cerambycidae) along the wood decay gradient: active selection for specific host types or neutral mechanisms? Ecol Entomol 32:712–721
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Schiegg K (2000) Effects of dead wood volume and connectivity on saproxylic insect species diversity. Ecoscience 7:290–298
Schroeder D, Perera AH (2002) A comparison of large-scale spatial vegetation patterns following clearcuts and fires in Ontario’s boreal forests. For Ecol Manag 159:217–230
Sénécal D, Kneeshaw D, Messier C (2004) Temporal, spatial, and structural patterns of adult trembling aspen and white spruce mortality in Quebec’s boreal forest. Can J For Res 34:396–404
Švácha P, Danilevsky ML (1986) Cerambycoid larvae of Europe and Soviet Union (Coleoptera, Cerambycoidea) Part I. Acta Universitatis Carolinae – Biológica 30:1–176
Švácha P, Danilevsky ML (1987) Cerambycoid larvae of Europe and Soviet Union (Coleoptera, Cerambycoidea) Part II. Acta Universitatis Carolinae – Biológica 31:121–284
Švácha P, Danilevsky ML (1988) Cerambycoid larvae of Europe and Soviet Union (Coleoptera, Cerambycoidea) Part III. Acta Universitatis Carolinae – Biológica 32:1–205
Sverdrup-Thygeson A, Ims RA (2002) The effect of forest clearcutting in Norway on community of saproxylic beetles on aspen. Biol Conserv 106:347–357
Tilman D, May RM, Lehman C, Nowak M (1994) Habitat destruction and the extinction debt. Nature 371:65–66
Tscharntke T, Steffan-Dewenter I, Kruess A, Thies C (2002) Characteristics of insect populations on habitat fragments: a mini review. Ecol Res 17:229–239
Turner IM (1996) Species loss in fragments of tropical rain forests: a review of the evidence. J Appl Ecol 33:200–209
Vanderwel MC, Caspersen JP, Woods ME (2006) Snag dynamics in partially harvested and unmanaged northern hardwood forests. Can J For Res 36:2769–2779
Warner RR, Chesson PL (1985) Coexistence mediated by recruitment fluctuations: a field guide to the storage effect. Am Nat 125:344–366
Webb A, Buddle CM, Drapeau P, Saint-Germain M (2008) Use of remnant boreal forest habitats by saproxylic beetle assemblages in even-aged managed landscapes. Biol Conserv 141:815–826
Wikars LO (1994) Effects of fire and ecology of fire-adapted insects. Introductory research essay no. 12. Department of Zoology, Uppsala University, Sweden
Yaroshenko AY, Potapov PV, Turubanova SA (2001) The last intact forest landscapes of Northern European Russia. Greenpeace Russia, Moscow, 77 pp
Acknowledgments
Dave Gervais and Maryse Marchand provided help with fieldwork and wood dissection. Laboratories of the Lake Duparquet Research and Teaching Forest field station were used for dissection. TEMBEC Inc. gracefully provided logging history data for the study region. This project benefited from different funding sources including a Discovery grant to P. Drapeau from the Natural Science and Engineering Research Council of Canada (NSERC), a Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT)—Équipe grant (Drapeau and collaborators) and additional funding by the partners of the NSERC industrial chair in sustainable forest management of Univ. du Québec en Abitibi-Témiscamingue (UQAT) and Univ. du Québec à Montréal (UQAM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saint-Germain, M., Drapeau, P. Response of saprophagous wood-boring beetles (Coleoptera: Cerambycidae) to severe habitat loss due to logging in an aspen-dominated boreal landscape. Landscape Ecol 26, 573–586 (2011). https://doi.org/10.1007/s10980-011-9587-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-011-9587-1