Abstract
Benthic macroinvertebrate communities from different lake zones are known to respond differently to environmental parameters and human pressures. The objectives of this study were to explore the spatial and temporal variability of benthic communities, investigate the effect of environmental factors on their assemblages and assess their response to eutrophication in the profundal and sublittoral zones of three Greek eutrophic lakes (Volvi, Kastoria and Mikri Prespa). These lakes are subjected to different land uses in their catchment areas. Samplings were conducted bi-annually (spring and autumn). Sixteen taxa were collected in the sublittoral and eleven in the profundal zone of the studied lakes. Among them, some species were recorded for the first time in the studied lakes. The dominant species were Potamothrix hammoniensis (Michaelsen, 1901) (Oligochaeta), Chaoborus (Chaoborus) flavicans (Meigen, 1830) and Chironomus (Chironomus) gr. plumosus Linnaeus, 1758 (Diptera), reflecting the eutrophic status of these waterbodies. Benthic communities, their functional traits (microhabitat preferences and feeding types), diversity and rarity species differed among lakes. Especially Lake Prespa, as a potentially ancient lake, hosts an endemic oligochaete community. Altitude and eutrophication (expressed as P–PO4 and BOD5 concentrations) were the main environmental factors explaining spatial and temporal variability in the assemblages. Based on the Greek Lake Benthic invertebrate Index, the ecological quality of the studied lakes was estimated as good to moderate. Benthic macroinvertebrates from both lake zones are associated with eutrophication, which is related to anthropogenic activities. Therefore, these lake zones should be included in assessment methods linking benthic invertebrate assemblages to eutrophication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater ecosystems are one of the most diverse habitats on the Earth and reflect the effects of activities occurring in their basins (Magnuson et al. 2006). Multiple and varied threats, such as changing climate, invasions, harmful algal blooms, microplastic pollution, impact freshwater ecosystems (Reid et al. 2019). Their biodiversity decreases even faster than in terrestrial or marine systems, despite the efforts to prevent this loss (Collen et al. 2014). Therefore, long-term conservation and efficient management often request a multidisciplinary concept to determine how aquatic biodiversity, water and habitat quality dynamics relate across different water bodies (Hill et al. 2016).
Benthic macroinvertebrate communities are usually used in biomonitoring due to their high sensitivity and response to pressures (Bonada et al. 2006; Birk et al. 2012). They are present on organically rich substances in the upper layer of the sediment (Matisoff and Wang 1998). Benthic assemblages act as “ecosystem engineers” and make a crucial contribution to aquatic ecosystem services as sediment oxygenation via bioturbation, internal nutrient loading and other biogeochemical processes (Covich et al. 1999). Moreover, they play a fundamental role as key species in food chains and food webs (Covich et al. 1999). Potential food sources are phytoplankton, bacteria and organic debris (Biswas et al. 2009). In turn, they serve as prey for higher-class organisms such as fish (Zimmer et al. 2001) and they can act as biological indicators to track water quality conditions (e.g., Rossaro et al. 2007; Miler et al. 2013; Lazaridou et al. 2018).
In lakes, macroinvertebrate assemblages differ among the littoral, sublittoral and profundal lake zones, which are considered as subsystems, responding differently to natural variability and human-induced pressures (Pilotto et al. 2012, 2015). Within the profundal zone, shifts in species composition are related mostly to eutrophication (Bazzanti et al. 2012; Jyväsjärvi et al. 2012; Pilotto et al. 2012), whereas in sublittoral and littoral zones to morphological alterations of lakeshores (Miler et al. 2013; McGoff et al. 2013), acidification (Schartau et al. 2008) and wave action (Johnson et al. 2004; Stendera and Johnson 2008). Littoral zone microhabitats tend to exhibit high macroinvertebrate species richness, due to their heterogeneity and productivity, compared to other lake strata (Pilotto et al. 2015). In small lakes, sublittoral macroinvertebrate assemblages demonstrate lower variation in density and species number among years than those occupying the profundal lake area because of alternating oxygen conditions (Hämäläinen et al. 2003). In contrast to littoral and sublittoral benthic communities, profundal assemblages are often low in species richness and are primarily dominated by oligochaetes (Burlakova et al. 2018). However, community composition varies according to the studied region and relevant stressors (e.g., Sterling et al. 2016; Tolkkinen et al. 2016; Jonsson et al. 2017).
Until now, several international legislations, directives and guidelines have been established with the aim of promoting the assessment and conservation of biodiversity (i.e., the European Biodiversity Strategy to 2020; EC 2011) and the good ecological quality of water bodies (i.e., Water Framework Directive, WFD; EC 2000). The latter necessitates all European Member States to protect and improve the quality of their aquatic ecosystems concerning pressure-specific stressors. An essential step in the ecological quality assessment of water bodies is the quantification of single and combined effects of multiple stressors on biota (Solimini et al. 2009), recognizing that biogeographic, morphometric and geological factors, as well as water chemistry, may influence species composition (Heling et al. 2018). Additionally, the utility of different biological elements should be reliably proved before they can be applied in the assessments of the ecosystems (Tolonen et al. 2020).
However, a plethora of studies have yet been published on the use of benthic macroinvertebrates for river ecological quality assessments (e.g., Hering et al. 2010; Birk et al. 2012). On the contrary, substantially fewer surveys have addressed the efficiency of benthic macroinvertebrate assemblages for monitoring water quality in lakes (e.g., Johnson et al. 2004; Brauns et al. 2007) and even less have relied on distinct taxa assemblages in the sublittoral/profundal zones (e.g., Jyväsjärvi et al. 2014; Lau et al. 2017; Ntislidou et al. 2018). This is mainly due to sampling and identification difficulties as well as to the biogeographical and spatial variation of environmental lakes’ characteristics (Poikane et al. 2016). However, it is crucial to assess the impact of pressures because profundal benthic assemblages respond to eutrophication, whereas the littoral ones, which have the highest richness and diversity, mainly to morphological alterations of lakeshore (Miler et al. 2013). Among Mediterranean countries, studies focusing on benthic assemblages in Greek lakes are also scarce (Petridis 1993; Petridis and Sinis 1993, 1995, 1997; Kagalou et al. 2006; Bobori et al. 2018).
The aim of the present study contribute to fulfill these knowledge gaps and to: (a) investigate the diversity and structure of benthic macroinvertebrate communities in the profundal and sublittoral zones of three Greek lakes (Volvi, Kastoria and Mikri Prespa), (b) examine the environmental effects on benthic macroinvertebrate assemblages, and (c) explore benthic macroinvertebrate response to eutrophication. We also provide a dataset that contains benthic macroinvertebrate records of these lakes concerning their rarity and endemicity. Understanding the drivers that structure benthic macroinvertebrate distribution in lakes is of key interest to manage the effects of environmental changes. The present study will contribute to fill this gap, especially for the Mediterranean lakes.
Material and methods
Study area
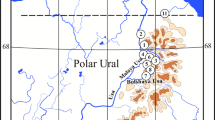
Three lakes, located in Northern Greece, were selected as case studies due to different land uses in their catchment areas (Fig. 1, Table 1). Lake Volvi (40° 37′ N and 23° 21′ E) is a relatively deep, warm monomictic lake (Vardaka et al. 2005) and constitutes part of the National Wetland Park of Lakes Koronia, Volvi and Macedonian Temp (Table 1, Fig. 1). Despite the minor industrial and domestic development in the catchment, agricultural activities are intensive, dominated by maize, alfalfa and cereal crops (Vogiatzis et al. 2008). Lake Kastoria (40° 30′ N, 21° 18′ E) is an urban, shallow, polymictic lake (Table 1, Fig. 1). Until 1995, it was the acceptor of sewage effluents, affecting thus its water quality and causing algal blooms (Moustaka et al. 2006). Lake Mikri Prespa (40° 44′ N, 21° 04′ E) is a shallow, polymictic lake (Vardaka et al. 2005) (Table 1, Fig. 1). Its catchment area is affected by minor anthropogenic pressures (cultivated plots and livestock; Tziritis 2014). The three lakes are under a strict protection status since they have been designated as areas of community interest (Birds and Habitats Directives; EC 2009/147, EEC 1992/43), while the two of them (Lakes Volvi and Mikri Prespa) are RAMSAR sites (RAMSAR 1974). Some of their limnological characteristics and land uses in their catchment areas, according to Corine Land Cover 2012 (EU 2018), are provided in Table 1.
Sampling and laboratory procedures
A total of 19 stations in the profundal and sublittoral zones of the studied lakes were sampled in autumn 2011 and early spring 2012, using an Ekman-Birge grab (three replicates, 225 cm2 sampling area) (Fig. 1). Sediment samples were sieved with a 200-μm mesh (Rosenberg and Resh 1993) and fixed in 4% neutralized formaldehyde. Macroinvertebrates were sorted and identified to species or genus level. Their abundance was converted to density as individual per m2 (ind. m−2). Chironomids and oligochaetes were slide-mounted prior to identification using the proper keys (Wiederholm 1983, 1986; Timm 2009). Immature stages of oligochaetes were identified based on the particular characters of the setae.
Water samples were collected at each station, 1 m above the sediment, using a Niskin-type sampler. Water temperature (°C), conductivity (mS cm−1), pH and dissolved oxygen (DO, mg L−1) were measured in situ using portable probes. Concentrations of nitrite (NO2–N, mg L−1), nitrate (NO3–N, mg L−1) and ammonium (NH4–N, mg L−1) nitrogen, phosphorus orthophosphate (PO4–P, mg L−1), total suspended solids (TSS, mg L−1) and biological oxygen demand (BOD5, mg L−1) were determined according to APHA (2005). Sampling depth (m) and Secchi disk depth (cm) were also recorded in situ, with the latter been used as a proxy of water transparency.
Diversity indices and functional traits
Benthic macroinvertebrate assemblages at each lake were described using the following diversity indices: Shannon–Wiener (H′, Shannon and Weaver 1949), Weighted Diversity Index (Hw′, Rossaro et al. 2011), Margalef (d′, Margalef 1958) and Pielou's evenness index (J′, Magurran 1988). The functional approach associates subgroups constituted of individuals of the same type as to microhabitat preferences and feeding types. We selected these traits due to their ability to indicate physical and ecological changes and their use in developing a trait-based bioassessment approach (Menezes et al. 2010). The Asterics software was used to estimate the functional traits for microhabitat preferences and feeding types (Table 2) (version 4.0.4; Wageningen Software Labs 2005).
Ecological quality assessment
To evaluate the ecological quality of the studied lakes, the Greek Lake Benthic invertebrate Index (GLBiI; Ntislidou et al. 2018) was applied. The index is based on three metrics, the number of taxa (Taxa_Tot) and the Simpson’s diversity index (Simpson_Tot) in the profundal and sublittoral zones and the relative contribution of Chironomidae in the profundal zone (%Chironomidae_Prof). The GLBiI is a multimetric index with a clear response to eutrophication, while the three metrics constituting the index are related to factors corresponding to lake hydromorphology and chemistry (Ntislidou et al. 2018).
Data analyses
The nonparametric Kruskal–Wallis and Mann–Whitney U tests were applied to extract significant spatial and temporal differences in environmental parameters and taxa distribution among stations and between sampling periods (autumn and spring), respectively. Pearson’s correlation analysis extracted relationships between environmental variables and benthic macroinvertebrate abundances. The above statistical analyses were performed using the statistical package SPSS (version 21.0).
The relative importance of environmental parameters in explaining the composition of benthic communities in the profundal and sublittoral zones was explored by redundancy analysis (RDA). Prior to this analysis, a detrended correspondence analysis (DCA) was applied, revealing that the total gradient length was less than three times the standard deviation, thus indicating a linear relationship between the environmental parameters and the benthic macroinvertebrate density data. To retain the significant environmental parameters in RDA, the Monte Carlo permutation test (499 permutations, p < 0.05) and the inflation factor (< 20) were used. Densities and environmental parameters were log(x + 1) transformed, except temperature and pH, which were standardized. All ordinations were performed with the statistical program CANOCO version 4.5.1 (ter Braak and Šmilauer 1998).
The similarity analysis routines ANalysis Of SIMilarity (ANOSIM) was applied to identify differences between profundal and sublittoral zones for benthic macroinvertebrate communities and functional trait categories. Additionally, the SIMilarity PERcentage Analysis (SIMPER) was performed to determine the taxa and functional trait categories that contributed the most to the observed similarities and differences between groups (profundal and sublittoral zones) with a cut-off of 90% (Clarke et al. 2014). Both analyses were based on the Bray–Curtis similarity index (Clarke et al. 2014) and were conducted using Primer v6 software (Clarke and Gorley 2006).
Results
Environmental parameters
The environmental parameters measured in the studied lakes are given in Table 3. The highest concentrations of PO4–P (0.034 mg L−1), NO3–N (0.770 mg L−1) and NO2–N (0.150 mg L−1) were recorded in Lake Volvi, whereas the highest NH4–N concentrations (0.514 mg L−1) in Lake Mikri Prespa. Within each lake, none of the environmental parameters exhibited significant differences among stations (Kruskal–Wallis test, p > 0.05), whereas a significant seasonal pattern was evident in all lakes (Mann–Whitney test p < 0.05). Higher eutrophication was noticed in Lake Volvi than in the other two lakes. Temporal significant (p < 0.05) differences among the majority of the environmental parameters were observed in Lake Volvi, apart from nitrogen nutrients (NO3–N, NO2–N, NH4–N). In lakes Kastoria and Mikri Prespa, five (DO, pH, conductivity, BOD5, transparency) and three (DO, pH, transparency) out of the 11 environmental parameters studied differed significantly (p < 0.05) between the sampling periods, respectively.
Benthic community composition and structure
A total of 53,466 specimens, belonging to 16 macroinvertebrate taxa, were identified with the most abundant belonging to Potamothrix hammoniensis (Oligochaeta) and Chaoborus (Chaoborus) flavicans (Diptera) (Table 4). Empty shells of Valvata sp. Müller, 1773 (in lakes Volvi and Kastoria), Unio sp. Retzius, 1788 (in Lake Kastoria) and Dreissena presbensis Kobelt, 1915 (in lakes Volvi and Mikri Prespa) were detected. The profundal zone was characterized by the highest densities and the lowest diversity (11 taxa, Table 4), with P. hammoniensis (67.5%) and C. (Chaoborus) flavicans (23.5%) dominated benthic assemblages (Fig. 2). The same taxa were also the most abundant in the sublittoral zone (69.3% and 18.6%, respectively) of the lakes, where 16 taxa were identified (Table 4).
In lakes Volvi and Kastoria, most benthic macroinvertebrates belonged to oligochaetes (> 50%) and Diptera (> 20%), followed by Nematoda and Hirudinea. Oligochaeta comprised more than 45% of the whole community (> 65% and 46%, respectively), with P. hammoniensis being the dominant species. However, in Lake Kastoria (stations S3, S5 in autumn and S1 in spring), the most abundant species was C. (Chaoborus) flavicans (> 39%). Diptera dominated (> 53%) the benthic macroinvertebrate assemblages of Lake Mikri Prespa, with C. (Chaoborus) flavicans being the dominant species, except stations S1 (autumn) and S2 (autumn and spring), where P. hammoniensis was more abundant (> 52%).
No spatial differentiation in benthic densities was observed among stations (Kruskal–Wallis test, p > 0.05) and between sampling periods (Mann–Whitney test p > 0.05) within each lake. The structure of benthic assemblages was also similar in both lake zones studied (ANOSIM: Lake Volvi R = 0.312, p = 0.07; Lake Kastoria R = 0.362, p = 0.178; Lake Mikri Prespa R = 0.131, p = 0.210). This similarity was owned mainly to the presence of P. hammoniensis and C. (Chaoborus) flavicans in both lake strata (Table 5).
Diversity indices and functional traits
All diversity indices exhibited their highest values in Lake Mikri Prespa (Fig. 3). Shannon–Wiener (H′), Weighted Diversity (Hw′) and Pielou's evenness (J') indices were highly correlated with depth (r = 0.647, r = 0.629 and r = 0.477, p < 0.05, respectively) and conductivity (r = 0.531, r = 0.639 and r = 0.559, p < 0.05, respectively) and Margalef index (d′) with depth and NO2–N (r = 0.354 and r = 0.354, p < 0.05, respectively) (Table 6).
Box-and-Whisker plots of benthic macroinvertebrate diversity indices a Shannon–Wiener (H′), b Weighted (Hw′), c Margalef (d′) and d Pielou's evenness (J′) estimated in lakes Volvi, Kastoria and Mikri Prespa. Solid lines at the center of boxes indicate median values, boxes represent inter-quartile ranges (25–75%), outer lines the highest and lowest values, and symbol * the outlier values
Benthic macroinvertebrate communities showed a preference for particulate organic matter (Pom; 28–78%) and mud (Pel; 16–37%) in all lakes in both zones (Fig. 4a). Considering the different feeding types, collectors/gatherers (fga) were dominant in both zones of lakes Volvi (80–83%) and Kastoria (49–65%), followed by predators (fpr) (32–46%) (Fig. 4b). In Lake Mikri Prespa, predators mostly contributed to the benthic macroinvertebrate composition of the profundal and sublittoral zone (Fig. 4b). However, no clear spatial pattern was evident between the two lake zones when feeding and habitat traits were compared (ANOSIM, Microhabitat preferences: R = 0.006, p = 0.341; Feeding type: R = − 0.026, p = 0.630). The SIMPER analysis, though, extracted the preference for particulate organic matter as the main trait differentiating the two lake zones (Table 7). The same analysis revealed that collectors/gatherers (fga; Profundal zone: 59.0% and sublittoral zone: 60.4%) contributed more than the other feeding groups to benthic assemblage structure in both profundal and sublittoral zones (Table 7). Generally, the low dissimilarity of benthic assemblages between the two zones, ranging from 19.6 to 21.2% (Table 7), indicates homogenous lake systems.
Ecological quality
The ecological quality of the studied lakes was assessed using the GLBiI. The lakes Volvi and Mikri Prespa were classified as water bodies of good quality (0.68 and 0.63, respectively), whereas the ecological quality of Lake Kastoria was classified as moderate (0.53). Apparently, not all the metrics included in the GLBiI assessed the quality of each lake with the same class. Only in the case of Lake Kastoria, the three metrics had the same quality class (“Taxa_Tol” = 0.53, “Simpson_Tot” = 0.50, “%Chironomidae_Prof” = 0.55). In the case of Lake Volvi, the metric “%Chironomidae_Prof” equaled 1.02, assessing thus the lake with high quality, whereas the rest metrics with moderate (“Taxa_Tot” = 0.44, “Simpson_Tot” = 0.60). Lake Mikri Prespa was classified as having good quality by two metrics (“Simpson_Tot” = 0.68, “%Chironomidae_Prof” = 0.62) and moderate by “Taxa_Tot” (0.59).
Relationships between environmental parameters and benthic macroinvertebrate communities
The RDA analysis applied on the profundal samples showed that two (altitude and depth) out of the 13 environmental parameters were the best predictors (Monte Carlo test, p < 0.05) in explaining benthic macroinvertebrate community composition. The first two ordination axes explained 49.6% of the total species variance and 85.4% of the species—environmental parameter relation. Axis I (eigenvalue 0.411) was related to altitude (intra-set correlation 0.618) and Axis II (eigenvalue 0.085) to P–PO4 and transparency (intra-set correlations 0.739 and − 0.663, respectively). Both axes separated stations of Lake Mikri Prespa from stations of the other lakes. Specifically, stations of Mikri Prespa were positively correlated with altitude (r = 0.618) and negatively with P–PO4 (r = − 0.246) (Fig. 5a). P. hammoniensis and C. (Chaoborus) flavicans were correlated with P–PO4 (Fig. 5b). Moreover, Peipsidrilus pusillus found in Mikri Prespa were negatively correlated with sampling depth (Fig. 5b).
Ordination of a sampling stations and b benthic macroinvertebrate densities in relation to environmental parameters with respect to the first and second axes of the redundancy analysis. Data referred to the profundal zone of lakes Volvi (V), Kastoria (K) and Mikri Prespa (P) in autumn (A) 2011 and spring (S) 2012. Alt: Altitude, DO: Dissolved Oxygen, S_Depth: Sampling Depth, Trans: transparency, TSS: total suspended solids, WT: water temperature. C_def: C. (Cryptochironomus) gr. defectus, C_flav: C. (Chaoborus) flavicans, C_plum: C. (Chironomus) gr. plumosus, Cerat: Ceratopogonidae, M_tener: M. tener, Nem: Nematoda, P_choreus: P. choreus, P_hamm: P. hammoniensis, P_pus: P. pusillus, Psam_sp.: Psammoryctides sp., T_tub: T. tubifex
Regarding the sublittoral zone, RDA revealed altitude and BOD5 as the most statistically significant (Monte Carlo test, p < 0.05) parameters affecting benthic macroinvertebrate assemblages. Axis I (eigenvalue 0.580) explained 72% of benthic taxa and environmental parameters variability and was positively correlated to altitude (intra-set correlation 0.833, Fig. 6a). Axis II (eigenvalue 0.098) was positively correlated with BOD5 (intra-set correlation 0.548, Fig. 6a). The cumulative percentage variance between benthic macroinvertebrates and environmental parameters explained by Axis II was 84.2%. Stations of Lake Mikri Prespa were related to altitude and characterized by M. tener and E. tendens (Fig. 6b). Stations from Lake Kastoria were associated with BOD5 and were defined by Psectrocladius (Psectrocladius) psilopterus and Ceratopogonidae.
Ordination of a sampling stations and b benthic macroinvertebrate densities in relation to environmental parameters with respect to the first and second axes of the redundancy analysis. Data referred to the sublittoral zone of lakes Volvi (V), Kastoria (K) and Mikri Prespa (P) in autumn (A) 2011 and spring (S) 2012. Alt: altitude, DO: dissolved oxygen, WT: water temperature. C_def: C. (Cryptochironomus) gr. defectus, C_flav: C. (Chaoborus) flavicans, C_plum: C. (Chironomus) gr. plumosus, C_virid: C. viridulum, Cerat: Ceratopogonidae, E_tend: E. tendens, Erb: Erpobdellidae, M_tener: M. tener, Nem: Nematoda, P_choreus: P. choreus, P_hamm: P. hammoniensis, P_pus: P. pusillus, Psam_sp.: Psammoryctides sp., P_psil: P. (Psectrocladius) psilopterus, Tany_sp.: Tanytarsus sp., T_tub: T. tubifex
Discussion
Understanding the structure of benthic macroinvertebrate communities in the different lake zones is essential in biomonitoring programs, as they are constrained by multiple drivers of changes and respond unevenly to distinct human disturbances (Pilotto et al. 2012). This study enhances our knowledge about the composition of benthic macroinvertebrates of the studied lakes. In sublittoral and profundal zones we found similar benthic assemblages composed of species known to be sensitive to eutrophication (Wiederholm 1980; Jónasson 2004). Our observations supported that benthic macroinvertebrates of these lake zones were also functionally homogenous. Moreover, our data revealed that the driving factor affecting benthic macroinvertebrate assemblages is eutrophication in the profundal and sublittoral zones of the studied lakes. Thus, our results suggest that communities belonging to these lake strata could be treated together regarding benthic macroinvertebrate responses to disturbance.
Our knowledge on benthic macroinvertebrate communities for the studied three lakes is dated before 25 years (Economidis 1991; Koussouris et al. 1987, 1989, 1991; Petridis and Sinis 1995, 1997) with a more recent exception (Ntislidou 2019). The total number of taxa referred in the previous studies was higher than in the present study [i.e., Volvi: 12 (Economidis 1991) and Mikri Prespa: 15 (Petridis and Sinis 1995)]. Among the macroinvertebrate taxa documented by Economidis (1991) and Petridis and Sinis (1995) are the littoral species Dicrotendipes nervosus (Staeger 1839) and Einfeldia sp., that were not found in the current study, due probably to the restriction of our sampling campaigns only in the profundal and sublittoral zones of the lakes, excluding the littoral zone, which is generally more diverse in Mediterranean lakes (Pilotto et al. 2015).
However, our surveys revealed the occurrence of some species, which have not been previously reported in the studied lakes. The chironomid species E. tendens, Cladopelma viridulum and P. (Psectrocladius) psilopterus are recorded for the first time in the Greek freshwaters (Ntislidou et al. 2019). Cryptochironomus (Cryptochironomus) gr. defectus was known in Greece, but it was recorded for the first time in Lake Volvi (Ntislidou et al. 2019). Among Oligochaeta, the rare species P. pusillus was until now only known from Northern Europe [i.e., Germany (Haybach and Timm 2013), Slovakia, Serbia and Austria (Šporka et al. 2008; Atanacković et al. 2011)]. Here, this species is recorded for the first from Lake Mikri Prespa. This potentially ancient lake hosts an endemic oligochaete community, isolated within narrow zoogeographical boundaries defined by the lakes Megali Prespa, Ohrid, Skadar and Dojran (Albrecht et al. 2012).
In the mud substrate of lakes, oligochaete is generally one of the prevailing components of the benthos, as is chironomid larvae (Wiederholm 1980). Accordingly, Tubificidae and Chironomidae were the dominant taxa in the three lakes studied. P. hammoniensis was dominant among the tubificids and C. (Chironomus) gr. plumosus mainly prevailed from chironomid species. P. hammoniensis is distributed worldwide, generally inhabiting silt and clay bottoms (Risnoveanu and Vadineanu 2002) and it is tolerant to pollution (van Haaren and Soors 2013). Chironomus (Chironomus) gr. plumosus can tolerate the hypoxic conditions that often prevail in organic-rich fine sediments (Heling et al. 2018). C. (Chaoborus) flavicans was also a dominant species in Lake Mikri Prespa, as in previous studies (Petridis and Sinis 1995, 1997), indicating the eutrophic state of the lake.
In the studied lakes, a typical benthic fauna of the Mediterranean profundal and sublittoral lake zones was recorded belonging to oligochaetes and chironomids (Bazzanti et al. 2012), which are well known to be sensitive to eutrophication (Wiederholm 1980; Lang 1990). The profundal zone was colonized by few taxa, very resistant to low water oxygenation. The structure of benthic assemblages in this zone is less complex than in other lake zones, as it is mostly affected by changes in lake productivity due to oxygen depletion and organic food material concentration (Bazzanti et al. 2017). In addition, our data indicated that sublittoral and profundal zones were dominated by the same species [P. hammoniensis and C. (Chaoborus) flavicans]. Generally, eutrophication reduces benthic macroinvertebrate diversity and abundance, as well as increases faunal similarity among depth zones (Bazzanti and Seminara 1987; Bazzanti et al. 2012).
Knowledge of community functional trait composition is crucial in studying community–environment relationships and human-induced ecosystems degradation (Heino 2008). The benthic macroinvertebrate functional traits have been extensively studied for river ecosystems (e.g., Bady et al. 2005; Bonada et al. 2007; Feld et al. 2014), dam-removal (e.g., Renöfält et al. 2013; Tullos et al. 2014; Sullivan and Manning 2017) and reservoirs (e.g., Fanny et al. 2013; Beghelli et al. 2020). Moreover, during the last years, Chironomidae, as one of the most diverse and widely distributed dominant group within aquatic macroinvertebrates, is claimed to be suitable for functional diversity across various environmental gradients (e.g., Serra et al. 2016; 2017; Milošević et al. 2018; Jiang et al. 2019; Antczak-Orlewska et al. 2020). However, few studies have been conducted for lake benthic communities (e.g., Bazzanti et al. 2017; Tolonen et al. 2018). The functional structure of macroinvertebrate assemblages in both zones of the studied lakes did not differ. The profundal and sublittoral habitats were characterized by the high presence of collectors/gatherers, alongside with predators. Especially in Lake Mikri Prespa, predators dominated, although the lake is populated by invertivorous fish (Petriki 2015). Collectors/gatherers are considered common benthic feeding traits in the profundal lake zone, favored by the sinking of organic matter, which constitutes the main source of nutrients in this lake stratum (Hamerlík and Brodersen 2010; Oliveira and Nessimian 2010; Frainer et al. 2016). Undoubtedly, shredders, grazers and miners lack from profundal and sublittoral zones due to the absence of light (Bazzanti et al. 2017). Regarding the functional trait microhabitat preference, macroinvertebrates showed a preference for particulate organic matter and mud. Both lake zones are characterized by homogenous habitats, fine sediments and no vegetation (Jónasson 2004). Still, much work has to be conducted to figure out the efficacy of using functional traits of lake macroinvertebrate communities (Heino 2008; Frainer et al. 2016; Tolonen et al. 2018).
The ecological quality of lakes assessed by the application of the GLBiI was characterized as “good” for lakes Volvi and Mikri Prespa and “moderate” for Lake Kastoria. Regarding each metric from GLBiI, the lowest values were estimated for the species richness (“Taxa_Tol”). Eutrophication decreases diversity and enhances homogeneity in the sublittoral and profundal zones (Bazzanti et al. 2017). The latest estimation of their ecological quality based on benthic macroinvertebrates was moderate (Ntislidou et al. 2018), due to human activities leading to their degradation (Latinopoulos et al. 2016; Petriki et al. 2017). Recovering these lakes necessitates more severe mitigation measures, which are included in the national River Basin Management Plans (RBMP, http://wfdver.ypeka.gr/en/home-en/) to fulfill the goals set by WFD.
The geographical factor (altitude) and eutrophication (P–PO4, BOD5) were the main environmental factors structuring benthic macroinvertebrate communities in the studied lakes. The inclusion of altitude in the RDA is likely a surrogate for climate effect and accounts for differences among lakes (Donohue et al. 2009). Shifts in climatic and geographic factors are reflected by other environmental properties, such as water and air temperature, precipitation, catchment area and vegetation cover characteristics (Hamerlík et al. 2010). Studies covering a wide range of altitude found a decrease of species richness with increasing altitude (e.g., Rahbek 1995). Our findings showed that benthic communities from Lake Prespa and especially P. pusillus, Tanytarsus sp. and Ceratopogonidae are related to altitude. Moreover, the positive relationships found between nutrients and sublittoral and profundal macroinvertebrate densities pose evidence that these benthic assemblages could provide useful information for the classification of lakes’ eutrophication status. Several studies have previously identified the importance of phosphorus as an essential factor in shaping macroinvertebrate communities (e.g., Fried‐Petersen et al. 2020; Heino and Tolonen 2017; Bazzanti et al. 2012). According to Heino and Tolonen (2017), total phosphorus sets limits to the distribution of certain species preferring high-primary-productivity lake habitats. In our study, P. hammoniensis, C. (Chaoborus) flavicans and M. tener are affected mainly by phosphorus concentration.
Lakes are unique systems, and they are especially vulnerable to nutrient enrichment caused by human activities. Thus, it is crucial to identify these impacts for lake ecosystems. Regarding lakes Volvi, Kastoria and Mikri Prespa, we showed that environmental parameters related to eutrophication influence the structure of sublittoral and profundal benthic communities of these lakes. The effects of these shifts are a reduction in the diversity of benthic macroinvertebrates and an increase in the number of taxa with a high tolerance for pollution. Since these lake ecosystems are located in protected areas, the present results could contribute to decision makers develop and implement management plans for maintaining ecosystems’ biodiversity and improving ecosystem services. Such programs of measures could include actions for reducing diffuse pollution, improving the coverage of the sanitation network, increasing the efficiency of the existing Waste Water Treatment Plants and restoring the riparian zones to improve their ecological status. Although the studied lakes are under the surveillance of the institutional protection management (e.g., Management Bodies), the existing protection should be enhanced more to ensure their integrity. Restoration programs that integrate hydromorphological, physicochemical and biological elements of the studied lake catchment areas are of high priority for future management strategies. Consequently, the understanding of the biological and ecological requirements of benthic assemblages, according to our outcomes, will contribute to this field.
Availability of data and material
Data are available on request from the authors.
References
Albrecht C, Hauffe T, Schreiber K, Wilke T (2012) Mollusc biodiversity in a European ancient lake system: lakes Prespa and Mikri Prespa in the Balkans. Hydrobiologia 682:47–59. https://doi.org/10.1007/s10750-011-0830-1
Antczak-Orlewska O, Płóciennik M, Sobczyk R, Okupny D, Stachowicz-Rybka R, Rzodkiewicz M, Siciński J, Mroczkowska A, Krąpiec M, Słowiński M, Kittel P (2020) Chironomidae morphological types and functional feeding groups as a habitat complexity vestige. Front Ecol Evol 8:480. https://doi.org/10.3389/fevo.2020.583831
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Atanacković A, Todorović DJ, Simić V, Tubić B, Vasiljević B, Gačić Z, Paunović M (2011) Oligochaeta community of the main Serbian waterways. Water Res Manag 1:47–54
Bady P, Dolédec S, Fesl C, Gayraud S, Bacchi M, Schöll F (2005) Use of invertebrate traits for the biomonitoring of European large rivers: the effects of sampling effort on genus richness and functional diversity. Freshw Biol 50:159–173. https://doi.org/10.1111/j.1365-2427.2004.01287.x
Bazzanti M, Seminara M (1987) Profundal macrobenthos in a polluted lake. Depth distribution and its relationship with biological indices for water quality assessment. Oecol Appl 8:15–26
Bazzanti M, Mastrantuono L, Solimini AG (2012) Selecting macroinvertebrate taxa and metrics to assess eutrophication in different depth zones of Mediterranean lakes. Fundam Appl Limnol 180:133–143. https://doi.org/10.1127/1863-9135/2012/0200
Bazzanti M, Mastrantuono L, Pilotto F (2017) Depth-related response of macroinvertebrates to the reversal of eutrophication in a Mediterranean lake: implications for ecological assessment. Sci Total Environ 579:456–465. https://doi.org/10.1016/j.scitotenv.2016.11.073
Beghelli FG, Cetra M, Marchese M, López-Dovál JC, Rosa AH, Pompêo ML, Moschini-Carlos V (2020) Taxonomic and non-taxonomic responses of benthic macroinvertebrates to metal toxicity in tropical reservoirs: the case of Cantareira Complex, São Paulo, Brazil. An Acad Bras Ciênc. https://doi.org/10.1590/0001-3765202020180962
Biswas JK, Rana S, Bhakta JN, Jana BB (2009) Bioturbation potential of chironomid larvae for the sediment–water phosphorus exchange in simulated pond systems of varied nutrient enrichment. Ecol Eng 35:1444–1453. https://doi.org/10.1016/j.ecoleng.2009.06.004
Birk S, Bonne W, Borja A, Brucet S, Courrat A, Poikane S, Solimini A, van de Bund W, Zampoukas N, Hering D (2012) Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecol Indic 18:31–41. https://doi.org/10.1016/j.ecolind.2011.10.009
Bobori DC, Ntislidou C, Petriki O, Chronis I, Kagalou I, Lazaridou M (2018) Macroinvertebrate and fish communities in the watershed of a re-constructed Mediterranean water body: link to the ecological potential. Environ Monit Assess 190:106. https://doi.org/10.1007/s10661-018-6484-y
Bonada N, Prat N, Resh VH, Statzner B (2006) Developments in aquatic insect biomonitoring: a comparative analysis of recent approaches. Annu Rev Entomol 51:495–523. https://doi.org/10.1146/annurev.ento.51.110104.151124
Bonada N, Rieradevall M, Prat N (2007) Macroinvertebrate community structure and biological traits related to flow permanence in a Mediterranean river network. Hydrobiologia 589:91–106. https://doi.org/10.1007/s10750-007-0723-5
Brauns M, Garcia XF, Walz N, Pusch MT (2007) Effects of human shoreline development on littoral macroinvertebrates in lowland lakes. J Appl Ecol 44:1138–1144. https://doi.org/10.1111/j.1365-2664.2007.01376.x
Burlakova LE, Kovalenko KE, Schmude KL, Barbiero RP, Karatayev AY, Lesht BM (2018) Development of new indices of Great Lakes water quality based on profundal benthic communities. J Great Lakes Res 44:618–628. https://doi.org/10.1016/j.jglr.2017.11.004
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E Ltd, Plymouth
Clarke KR, Gorley RN, Somerfield PJ, Warwick RM (2014) Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E Ltd, Plymouth
Collen B, Whitton F, Dyer EE, Baillie JE, Cumberlidge N, Darwall WR, Pollock C, Richman NI, Soulsby AM, Böhm M (2014) Global patterns of freshwater species diversity, threat and endemism. Glob Ecol Biogeogr 23:40–51. https://doi.org/10.1111/geb.12096
Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems: zoobenthic species influence energy flows and nutrient cycling. Bioscience 49:119–127. https://doi.org/10.2307/1313537
Donohue I, Donohue LA, Ainín BN, Irvine K (2009) Assessment of eutrophication pressure on lakes using littoral invertebrates. Hydrobiologia 633:105–122. https://doi.org/10.1007/s10750-009-9868-8
Economidis G (1991) Bionomic study of the benthic fauna of Lake Volvi. Dissertation, Aristotle University of Thessaloniki
EC (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23rd October 2000 establishing a framework for community action in the field of water policy. Official Journal of the European Communities, L327. Brussels, Belgium, European Commission
EC (2009) Directive 2009/147/EC of the European Parliament and of the Council of 30 November 2009 on the conservation of wild birds. Official Journal of the European Communities, L20. Brussels, Belgium, European Commission
EC (2011) Our life insurance, our natural capital: an EU bio- diversity strategy to 2020. Belgium, European Commission, Brussels
EEC (1992) Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Official Journal of the European Communities, L206. Brussels, Belgium, European Commission
EU (2018) Copernicus Land Monitoring Service 2018, European Environment Agency (EEA). https://land.copernicus.eu/. Accessed 28 Nov 2020
Fanny C, Virginie A, Jean-François F, Jonathan B, Marie-Claude R, Simon D (2013) Benthic indicators of sediment quality associated with run-of-river reservoirs. Hydrobiologia 703:149–164. https://doi.org/10.1007/s10750-012-1355-y
Feld CK, Bello F, Dolédec S (2014) Biodiversity of traits and species both show weak responses to hydromorphological alteration in lowland river macroinvertebrates. Freshw Biol 59:233–248. https://doi.org/10.1111/fwb.12260
Frainer A, Johansen KS, Siwertsson A, Mousavi SK, Brittain JE, Klemetsen A, Knudsen R, Amundsen PA (2016) Variation in functional trait composition of benthic invertebrates across depths and seasons in a subarctic lake. Fundam Appl Limnol 188:103–112. https://doi.org/10.1127/fal/2016/0839
Fried-Petersen HB, Araya-Ajoy YG, Futter MN, Angeler DG (2020) Drivers of long-term invertebrate community stability in changing Swedish lakes. Glob Chang Biol 26:1259. https://doi.org/10.1111/gcb.14952
Hämäläinen H, Luotonen H, Koskenniemi E, Liljaniemi P (2003) Inter-annual variation in macroinvertebrate communities in a shallow forest lake in eastern Finland during 1990–2001. Hydrobiologia 506:389–397. https://doi.org/10.1023/B:HYDR.0000008581.86095.0b
Hamerlík L, Brodersen KP (2010) Non-biting midges (Diptera: Chironomidae) from fountains of two European cities: micro-scale island biogeography. Aquat Insects 32:67–79. https://doi.org/10.1080/01650420903397645
Haybach A, Timm T (2013) First records of Peipsidrilus pusillus Timm, 1977 and Isochaetides michaelseni (Lastočkin, 1937) (Oligochaeta, Tubificidae) in the Upper Rhine (Germany). Lauterbornia 76:131–133
Heino J (2008) Patterns of functional biodiversity and function-environment relationships in lake littoral macroinvertebrates. Limnol Oceanogr 53:1446–1455. https://doi.org/10.4319/lo.2008.53.4.1446
Heino J, Tolonen KT (2017) Ecological drivers of multiple facets of beta diversity in a lentic macroinvertebrate metacommunity. Limnol Oceanogr 62:2431–2444. https://doi.org/10.1002/lno.10577
Heling CL, Stelzer RS, Drecktrah HG, Koenigs RP (2018) Spatial variation of benthic invertebrates at the whole-ecosystem scale in a large eutrophic lake. Freshw Sci 37:605–617. https://doi.org/10.1086/699386
Hering D, Borja A, Carstensen J, Carvalho L, Elliott M, Feld CK, Heiskanen AS, Johnson RK, Moe J, Pont D, Solheim AL, van de Bund W (2010) The European Water Framework Directive at the age of 10: a critical review of the achievements with recommendations for the future. Sci Total Environ 408:4007–4019. https://doi.org/10.1016/j.scitotenv.2010.05.031
Hill MJ, Ryves DB, White JC, Wood PJ (2016) Macroinvertebrate diversity in urban and rural ponds: Implications for freshwater biodiversity conservation. Biol Conserv 201:50–59. https://doi.org/10.1016/j.biocon.2016.06.027
Jiang X, Pan B, Song Z, Xie Z (2019) Do functional traits of chironomid assemblages respond more readily to eutrophication than taxonomic composition in Chinese floodplain lakes? Ecol Indic 103:355–362. https://doi.org/10.1016/j.ecolind.2019.04.029
Johnson RK, Goedkoop W, Sandin L (2004) Spatial scale and ecological relationships between the macroinvertebrate communities of stony habitats of streams and lakes. Freshw Biol 49:1179–1194. https://doi.org/10.1111/j.1365-2427.2004.01262.x
Jónasson PM (2004) Benthic invertebrates. In: Sullivan PE, Reynolds CS (eds) The lakes handbook: limnology and limnetic ecology, vol 1. Wiley-Blackwell, Oxford, pp 341–416
Jonsson M, Burrows RM, Lidman J, Fältström E, Laudon H, Sponseller RA (2017) Land use influences macroinvertebrate community composition in boreal headwaters through altered stream conditions. Ambio 46:311–323. https://doi.org/10.1007/s13280-016-0837-y
Jyväsjärvi J, Aroviita J, Hämäläinen H (2012) Performance of profundal macroinvertebrate assessment in boreal lakes depends on lake depth. Fundam Appl Limnol 180:91–100. https://doi.org/10.1127/1863-9135/2012/0205
Jyväsjärvi J, Aroviita J, Hämäläinen H (2014) An extended Benthic Quality Index for assessment of lake profundal macroinvertebrates: addition of indicator taxa by multivariate ordination and weighted averaging. Freshw Sci 33:995–1007. https://doi.org/10.1086/676914
Kagalou I, Economidis G, Leonardos Ι (2006) Assessment of a Mediterranean shallow lentic ecosystem (Lake Pamvotis, Greece) using benthic community diversity: response to environmental parameters. Limnologica 36:269–278. https://doi.org/10.1016/j.limno.2006.08.002
Koussouris Th, Diapoulis A, Balopoulos E (1987) Limnological situations in two shallow Greek Lakes (Kastoria and Mikri Prespa Lakes). GeoJournal 14:377–379. https://doi.org/10.1007/BF00208214
Koussouris Th, Diapoulis A, Balopoulos E (1989) Assessing the trophic status of Lake Mikri Prespa, Greece. Anns Limnol 25:17–24. https://doi.org/10.1051/limn/1989001
Koussouris Th, Diapoulis A, Bertahas I (1991) Evaluating trophic status and restoration procedures of a polluted lake, Lake Kastoria, Greece. GeoJournal 23:153–161. https://doi.org/10.1007/BF00241400
Lang C (1990) Quantitative relationships between oligochaete communities and phosphorus concentrations in lakes. Freshw Biol 24:327–334. https://doi.org/10.1111/j.1365-2427.1990.tb00713.x
Latinopoulos D, Ntislidou C, Kagalou I (2016) Multipurpose plans for the sustainability of the Greek lakes: emphasis on multiple stressors. Environ Process 3:589–602. https://doi.org/10.1007/s40710-016-0152-4
Lazaridou M, Ntislidou C, Karaouzas I, Skoulikidis N (2018) Harmonisation of a new assessment method for estimating the ecological quality status of Greek running waters. Ecol Indic 84:683–694. https://doi.org/10.1016/j.ecolind.2017.09.032
Lau DC, Vrede T, Goedkoop W (2017) Lake responses to long-term disturbances and management practices. Freshw Biol 62:792–806. https://doi.org/10.1111/fwb.12902
Magnuson JJ, Kratz TK, Benson BJ (2006) Long-term dynamics of lakes in the landscape: long-term ecological research on north temperate lakes. Oxford University Press, Oxford
Magurran AE (1988) Ecological diversity and its measurement. Groom helm, London
Margalef R (1958) Information theory in ecology. Gen Syst 3:36–71
Matisoff G, Wang X (1998) Solute transport in sediments by freshwater infaunal bioirrigators. Limnol Oceanogr 43:1487–1499. https://doi.org/10.4319/lo.1998.43.7.1487
McGoff E, Aroviita J, Pilotto F, Miler O, Solimini AG, Porst G, Jurca T, Donohue L, Sandin L (2013) Assessing the relationship between the Lake Habitat Survey and littoral macroinvertebrate communities in European lakes. Ecol Indic 25:205–214. https://doi.org/10.1016/j.ecolind.2012.09.018
Menezes S, Baird DJ, Soares AM (2010) Beyond taxonomy: a review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. J Appl Ecol 47:711–719. https://doi.org/10.1111/j.1365-2664.2010.01819.x
Miler O, Porst G, McGoff E, Pilotto F, Donohue L, Jurca T, Solimini AG, Sandin L, Irvine K, Aroviita J, Clark R, Pusch MT (2013) Morphological alterations of lake shores in Europe: a multimetric ecological assessment approach using benthic macroinvertebrates. Ecol Indic 34:398–410. https://doi.org/10.1016/j.ecolind.2013.06.002
Milošević D, Stojanović K, Djurdjević A, Marković Z, Stojković Piperac M, Živić M, Živić I (2018) The response of chironomid taxonomy- and functional trait-based metrics to fish farm effluent pollution in lotic systems. Environ Pollut 242:1058–1066. https://doi.org/10.1016/j.envpol.2018.07.100
Moustaka-Gouni M, Vardaka E, Michaloudi E, Kormas KA, Tryfon E, Mihalatou H, Gkelis S, Lanaras T (2006) Plankton food web structure in a eutrophic polymictic lake with a history in toxic cyanobacterial blooms. Limnol Oceanogr 51:715–727. https://doi.org/10.4319/lo.2006.51.1_part_2.0715
Ntislidou C, Lazaridou M, Tsiaoussi V, Bobori DC (2018) A new multimetric macroinvertebrate index for the ecological assessment of Mediterranean lakes. Ecol Indic 93:1020–1033. https://doi.org/10.1016/j.ecolind.2018.05.071
Ntislidou C, Bobori DC, Lazaridou M, Rossaro B (2019) First records of Chironomidae (Insecta: Diptera) species from Greek lakes. Acta Zoologica Bulgarica 71:25–28
Ntislidou C (2019) Assessment of the ecological quality of lake ecosystems using benthic macroinvertebrates. Dissertation, Aristotle University of Thessaloniki
Oliveira ALH, Nessimian JL (2010) Spatial distribution and functional feeding groups of aquatic insect communities in Serra da Bocaina streams, southeastern Brazil. Acta Limnol Bras 22:424–441. https://doi.org/10.4322/actalb.2011.007
Petridis D (1993) Macroinvertebrate distribution along an organic pollution gradient in Lake Lysimachia (Western Greece). Arch Hydrobiol 128:367–384
Petridis D, Sinis A (1993) Benthic macrofauna of Tavropos reservoir (central Greece). Hydrobiologia 262:1–12. https://doi.org/10.1007/BF00010985
Petridis D, Sinis A (1995) Benthos of Lake Mikri Prespa (North Greece). Hydrobiologia 304:185–196. https://doi.org/10.1007/BF02329313
Petridis D, Sinis A (1997) The benthic fauna of Lake Mikri Prespa. Hydrobiologia 351:95–105. https://doi.org/10.1007/BF02329313
Petriki O (2015) Development of a fish-based multi-metric index for the assessment of the ecological quality of Greek lakes. Dissertation, Aristotle University of Thessaloniki
Petriki O, Lazaridou M, Bobori DC (2017) A fish-based index for the assessment of the ecological quality of temperate lakes. Ecol Indic 78:556–565. https://doi.org/10.1016/j.ecolind.2017.03.029
Pilotto F, Free G, Cardoso AC, Wolfram G, Solimini AG (2012) Spatial variance of profundal and sublittoral invertebrate benthic communities in response to eutrophication and morphological pressures. Fundam Appl Limnol 180:101–110. https://doi.org/10.1127/1863-9135/2012/0206
Pilotto F, Bazzanti M, Di Vito V, Frosali D, Livretti F, Mastrantuono L, Pusch MT, Sena F, Solimini AG (2015) Relative impacts of morphological alteration to shorelines and eutrophication on littoral macroinvertebrates in Mediterranean lakes. Freshwat Sci 34:410–422. https://doi.org/10.1086/680523
Poikane S, Johnson RK, Sandin L, Schartau AK, Solimini AG, Urbanič G, Arbačiauskas K, Aroviita J, Gabriels W, Miler O, Pusch MT, Timm H, Böhmer J (2016) Benthic macroinvertebrates in lake ecological assessment: a review of methods, intercalibration and practical recommendations. Sci Total Environ 543:123–134. https://doi.org/10.1016/j.scitotenv.2015.11.021
Rahbek C (1995) The elevational gradient of species richness: A uniform pattern? Ecog 18:200–205
RAMSAR (1974) The RAMSAR convention on wetlands. www.ramsar.org. Accessed 28 Nov 2020
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PT, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873. https://doi.org/10.1111/brv.12480
Renöfält BM, Lejon AG, Jonsson M, Nilsson C (2013) Long-term taxon-specific responses of macroinvertebrates to dam removal in a mid-sized Swedish stream. River Res Appl 29:1082–1089. https://doi.org/10.1002/rra.2592
Risnoveanu G, Vadineanu A (2002) Observations on the population dynamics of Potamothrix hammoniensis (Michaelsen, 1901) (Tubificidae, Oligochaeta) in Lake Isacova in the Danube Delta. Hydrobiologia 479:23–30. https://doi.org/10.1023/A:1021012111295
Rosenberg D, Resh V (1993) Freshwater biomonitoring and benthic macroinvertebrates. Chapman Hall, New York
Rossaro B, Marziali L, Cardoso AC, Solimini A, Free G, Giacchini R (2007) A biotic index using benthic macroinvertebrates for Italian lakes. Ecol Indic 7:412–429. https://doi.org/10.1016/j.ecolind.2006.04.002
Rossaro B, Boggero A, Lods-Crozet B, Free G, Lencioni V, Marziali L (2011) A comparison of different biotic indices based on benthic macro-invertebrates in Italian lakes. J Limnol 70:109–122. https://doi.org/10.4081/jlimnol.2011.109
Schartau AK, Moe SJ, Sandin L, McFarland B, Raddum GG (2008) Macroinvertebrate indicators of lake acidification: analysis of monitoring data from UK, Norway and Sweden. Aquatic Ecol 42:293–305. https://doi.org/10.1007/s10452-008-9186-7
Schmedtje U, Colling M (1996) Ökologische Typisierung der aquatischen Makrofauna. Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft, München
Schweder H (1992) Neue Indices für die Bewertung des ökologischen Zustandes von Fließgewässern, abgeleitet aus der Makroinvertebraten-Ernährungstypologie. Limnol Aktuell 3:353–377
Shannon CE, Weaver W (1949) The Mathematical theory of communication. University of Illinois Press, Urbana
Serra SR, Cobo F, Graca MA, Doledec S, Feio MJ (2016) Synthesising the trait information of European Chironomidae (Insecta: Diptera): towards a new database. Ecol Indic 61:282–292. https://doi.org/10.1016/j.ecolind.2015.09.028
Serra SR, Graça MA, Dolédec S, Feio MJ (2017) Discriminating permanent from temporary rivers with traits of chironomid genera. Ann Limnol-Int J Lim 53:161–174. https://doi.org/10.1051/limn/2017004
Solimini AG, Ptacnik R, Cardoso AC (2009) Towards holistic assessment of the functioning of ecosystems under the Water Framework Directive. Trends Analyt Chem 28:143–149. https://doi.org/10.1016/j.trac.2008.10.015
Šporka F, Ofenböck T, Graf W (2008) Bratislavia palmeni (Munsterhjelm, 1905) (Naididae) and Peipsidrilus pusillus Timm, 1977 (Tubificidae) two rare Oligochaeta species new to the Austrian fauna. Lauterbornia 63:15–22
Stendera S, Johnson RK (2008) Habitat-specific stability and persistence of benthic invertebrate communities in boreal lakes. Fundam Appl Limnol 171:311–322. https://doi.org/10.1127/1863-9135/2008/0171-0311
Sterling JL, Rosemond AD, Wenger SJ (2016) Watershed urbanization affects macroinvertebrate community structure and reduces biomass through similar pathways in Piedmont streams, Georgia, USA. Freshw Sci 35:676–688. https://doi.org/10.1086/686614
Sullivan SMP, Manning DW (2017) Seasonally distinct taxonomic and functional shifts in macroinvertebrate communities following dam removal. PeerJ 5:e3189. https://doi.org/10.7717/peerj.3189
ter Braak CJF, Šmilauer P (1998) CANOCO Reference manual and User’s Guide to CANOCO for Windows. Software for Canonical Community Ordination (version 4). Centre for Biometry, Wageningen
Timm T (2009) A guide to the freshwater Oligochaeta and Polychaeta of Northern and Central Europe. Lauterbornia 66:1–235
Tolkkinen MJ, Mykrä H, Virtanen R, Tolkkinen M, Kauppila T, Paasivirta L, Muotka T (2016) Land use impacts on stream community composition and concordance along a natural stress gradient. Ecol Indic 62:14–21. https://doi.org/10.1016/j.ecolind.2015.11.015
Tolonen KT, Cai Y, Vilmi A, Karjalainen SM, Sutela T, Heino J (2018) Environmental filtering and spatial effects on metacommunity organisation differ among littoral macroinvertebrate groups deconstructed by biological traits. Aquat Ecol 52:119–131. https://doi.org/10.1007/s10452-018-9649-4
Tolonen KT, Karjalainen J, Hämäläinen H, Nyholm K, Rahkola-Sorsa M, Cai Y, Heino J (2020) Do the ecological drivers of lake littoral communities match and lead to congruence between organism groups? Aquat Ecol 54:839–854. https://doi.org/10.1007/s10452-020-09781-x
Tziritis EP (2014) Environmental monitoring of Micro Prespa Lake basin (Western Macedonia, Greece): hydrogeochemical characteristics of water resources and quality trends. Environ Monit Assess 186:4553–4568. https://doi.org/10.1007/s10661-014-3719-4
Tullos DD, Finn DS, Walter C (2014) Geomorphic and ecological disturbance and recovery from two small dams and their removal. PLoS ONE 9:e108091. https://doi.org/10.1371/journal.pone.0108091
van Haaren T, Soors J (2013) Aquatic Oligochaeta of the Netherlands and Belgium Identification Key to the Oligochaetes. KNNV publishers, Utrecht
Vardaka E, Moustaka-Gouni M, Cook KM, Lanaras T (2005) Cyanobacterial blooms and water quality in Greek Waterbodies. J Appl Phycol 17:391–401. https://doi.org/10.1007/s10811-005-8700-8
Vogiatzis M, Karydas C, Alexandridis T, Zalidis G, Silleos N (2008) An object-based approach for wetland habitats inventory and assessment using ALOS AVNIR-2 and field data. In: Lacoste H, Ouwehand L (ed) Proceedings of the ALOS Principal Investigators Symposium, Rhodes, Greece
Wiederholm T (1980) Use of benthos in lake monitoring. Water Pollut Control Fed 52:537–547
Wiederholm T (1983) Chironomidae of the Holarctic region. Keys and diagnoses: Part I: Larvae. Entomologica Scandinavica, Stockholm
Wiederholm T (1986) Chironomidae of the Holarctic region. Keys and diagnoses. Part I. Pupae. Entomologica Scandinavica, Stockholm
Zimmer KD, Hanson MA, Butler MG, Duffy WG (2001) Size distribution of aquatic invertebrates in two prairie wetlands, with and without fish, with implications for community production. Freshw Biol 46:1373–1386. https://doi.org/10.1046/j.1365-2427.2001.00759.x
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Télesphore Sime-Ngando.
Rights and permissions
About this article
Cite this article
Ntitslidou, C., Rossaro, B., Lazaridou, M. et al. What drives benthic macroinvertebrate dispersal in different lake substrata? The case of three Mediterranean lakes. Aquat Ecol 55, 1033–1050 (2021). https://doi.org/10.1007/s10452-021-09880-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-021-09880-3