Abstract
The littoral area of Lake Alchichica is heterogeneous. The presence of a discontinuous ring of stromatolites and diminishing water level in the lake has formed isolated areas and zones connected with the rest of the lake, which has favored the development of a varied and different littoral community along the perimeter of the lake. Several different biological groups are present. This chapter summarizes the studies developed over almost 30 years and includes data about benthic macroinvertebrates (BMI), heterotrophic protists, and littoral diatoms. One of the most notable assemblages is that of the aquatic BMI. Various studies have been carried out since 1993 with different results. The number of taxa observed has varied between 15 and 44 BMI taxa; the factors that had the most influence on species richness and density were salinity and macrophyte cover. In the most recent (2016) and intensive research, only 21 taxa were found. Dominant species were the tubificid worm Limnodrilus hoffmeisteri, (with presence in all the sample stations), followed by the amphipod Hyalella azteca (90% of presence), the leeches Helobdella stagnalis, Glossiphoniidae, and the midge Micropsectra (80%), the water boatmen Krizousacorixa tolteca, and the midges Cricotopus (Isocladius) triannulatus and Chironomus stigmaterus (now Chironomus alchichica) (60%). Regarding abundance, the oligochaete Limnodrilus hoffmeisteri (43%) and the amphipod Hyalella azteca (39%) contributed with the largest percentage (82%). In this last study, sediment texture played a crucial role in explaining BMI distribution. The BMI distribution along the littoral zone was heterogeneous. Total BMI biomass was 88.5 ± 309.5 mg C/m2. Temporarily, July displayed the lowest average biomass (37.52 ± 138.71 mg C/m2), while May the highest average biomass (129.75 ± 450.06 mg C/m2). Spatially, the biomass among sampling stations ranged from 25.72 ± 104.86 mg C/m2 to 151.48 ± 484.52 mg C/m2. The littoral ecosystem’s community structure simplifies as littoral-pelagic isolation increases, although it was not supported statistically. Detritivores and herbivorous BMI dominated numerically over carnivorous. There are 6 Functional Feeding Groups -FFG- in the littoral BMI assemblages: most of the BMI were collector-gatherers (40.5 ± 25.9%), shredders (35.2 ± 28.0%), and predators-engulfer (14.2 ± 15.7). Plant coverage and sedimentary organic matter content were the most critical factors in explaining the MIB biomass and distribution. Additionally, three new species of crustacean associated with the lake’s littoral zone have been described: an asellid isopod species, Caecidotea williamsi, cryptically inhabiting in tufa crevices, that constitutes the first report of an epigean asellid isopod inhabiting inland saline waters in America. C. williamsi shares this habitat with two new species of ostracods, Limnocytherina axalapasco and Candona alchichica.
MBI can consume heterotrophic protists. The composition and trophic function of the littoral protists of Lake Alchichica were studied by the colonization method of artificial polyurethane foam substrates (PFU). Throughout the 38 days that the study lasted, 39 species of heterotrophic protists belonging to 6 supergroups were identified within the taxonomic classification of eukaryotes by Adl et al. (2019). The Alveolata supergroup, which includes the ciliated protists, was the one with the highest number of species (15 spp.), followed by the Discoba supergroup (11 spp.), which includes several heterotrophic nanoflagellates (HNF) species and several small euglenoids. The maximum number of species (23 spp.) was observed at 28 days of exposure of the substrates, while the lowest number (17 spp.) appeared at 21 days. Species of HNF, although made up of small organisms, presented the highest relative abundances: Bodo caudatus (6159 ± 4494 ind/mL), Bodo saltans (1532 ± 982 ind/mL), and Spumella termo (1428 ± 1168 ind/mL).
On the other hand, the ciliates presented a broader range in their sizes but with lower abundances: Cyclidium glaucoma (53 ± 51 ind/mL), Uronema sp. (29 ± 31 ind/mL), and Stylonychia notophora (26 ± 49 ind/L). Amoebae and related species were at a medium level of abundance. Most of the identified species feed mainly on bacteria and other organisms such as cyanobacteria, eukaryotic microalgae, other flagellates, and other ciliates. In turn, these protists are consumed by different groups of larger organisms such as rotifers, crustaceans, oligochaetes, chironomids, and others. The conclusion is that littoral heterotrophic protists are essential as a trophic link between bacteria and other larger microorganisms.
Diatoms are fundamental primary producers in freshwater, marine, and aquatic ecosystems. Its characteristic structure, the frustule, is made of silicon dioxide, which is why these organisms actively participate in the silicon cycle on our planet. In the littoral zone of Lake Alchichica, a high species richness (40 spp.) was found in 8 sites studied over three periods and in four different substrates: epiphytic (on Ruppia maritima and Chara canescens), epilithic, and in the plocon. Pennate diatoms dominated, and the most frequently observed species were Navicymbula pusilla, Rophalodia gibberula, Gomphoneis olivaceum, Mastogloia smithii, Halamphora veneta, Mastogloia elliptica, Cocconeis placentula, Surirella striata, Anomoeneis sphavicula, and Anomoneis sp. Most of the species (23 spp.) occurred in the three samplings, and eight species were found in a single sample. Regarding their spatial distribution, only G. olivaceum and N. pusilla appeared in the eight sampling sites in October 2006 and March 2007. Six species more were found in all sites in March 2007. In September 2007, three species were present in all sites. Most species did not show a preference for some substrate since they grew on the four substrates collected. Cluster analysis showed that temporal variation had the most significant influence because samples were mainly grouped by the sampling date. Some of the plocon samples became the ones with the most remarkable difference in species composition. The Lake Alchichica diatoms seem to be of great importance as components of the littoral food web, as they are indeed a very significant food for most of the different groups of macroinvertebrates that inhabit it. Together with the planktonic diatom species, they indeed have a very relevant function within the lake’s silicon cycle.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 The Littoral Zoobenthos
The littoral zone of the lakes is an ecotone that forms an independent compartment comprising all trophic levels (primary producers, consumers, decomposers), a large diversity of ecological niches and food webs (herbivory and detrital), and a great diversity (Roldán and Ramírez 2008). The lake’s littoral area presents a high environmental heterogeneity, which leads to a diversity of habitats susceptible to being occupied by a rich and diverse benthic fauna Esteves (1988).
Lake Alchichica embraces a discontinuous stromatolites ring that runs parallel and close to the littoral. This carbonated structure jointly with the gradual decrease of the lake’s water level has resulted in different isolation degrees of separation between the littoral and pelagic zones; in this way, there are littoral regions that are in direct contact and interchange with the pelagic zone, while others, in the opposite end, are isolated (see Chap. 8).
The littoral area of Lake Alchichica characterizes an ample range of environmental and biological characteristics resulting in a wide variety of habitats exposed to high temporal variability associated to fluctuations in the weather conditions, water level, wave energy, and biological activity.
10.1.1 Background Studies
The first study of the littoral benthos of Lake Alchichica revealed 10 chironomid species ranging between four and seven in different littoral areas (Alcocer et al. 1993a). The most important chironomid numerically and in biomass contribution was Tanypus (Apelopia) sp. Alcocer et al. (1993b) reported 15 taxonomic groups of benthic macroinvertebrates (BMI) in the littoral of Lake Alchichica. Tubificids, hirudinean, amphipods, and chironomids were the most frequent and abundant groups. Macrophytes coverage and salinity played an essential role in explaining the taxonomic richness of BMI in Lake Alchichica.
Subsequently, Alcocer et al. (1998) reported 44 taxa of littoral BMI in Lake Alchichica. Four taxa composed up to 99% of the organisms present: oligochaetes, amphipods, midges, and leeches; the most important species were Limnodrilus hoffmeisteri, Hyalella azteca, Tanypus (Apelopia), and Stictochironomus sp. Once again, aquatic vegetation is the critical variable explaining taxonomic richness. Some studies provide additional information on the biology/ecology of specific taxa.
Hyalella azteca displayed a high density (13,496 ± 20,740 ind/m2) that remained constant throughout the annual cycle with no significant difference among the littoral zones (Alcocer et al. 2002). The vegetation type and coverage play a significant role in the amphipod dynamics. The population structure of H. azteca showed juveniles were numerically dominant (55–64%), followed by females (13–20%), males (10–17%), and ovigerous females (8–14%). The male to female ratio was 1:1.75. Fecundity was positively correlated to female size; the number of eggs carried by the ovigerous females was 4.5 ± 2.8 eggs/female.
Oligochaete species richness of Lake Alchichica (i.e., Limnodrilus hoffmeisteri and Tubifex tubifex) is among the lowest worldwide. High oligochaete densities and biomasses values (>45,000 ind/m2 and >2700 mg AFDW/m2) in the littoral zone of Lake Alchichica associated with surface sediments with high organic matter content. Density and biomass values of oligochaetes did not show significant seasonal differences (Peralta et al. 2002). Limnocytherina axalapasco and Candona alchichica in the littoral zone reached densities up to 3612 ± 4148 ind/m2 and 22,812 ± 16,045 ind/m2, respectively, and biomasses of 9.83 ± 11.6 mg C/m2 and 366.2 ± 281 mg C/m2, respectively (Hernández et al. 2010). Finally, Cletocamptus gomezi in the littoral zone reached a density of 4106 ± 10,962 ind/m2 with biomass of 3.66 ± 9.75 mg C/m2, with no statistical temporal differences in density or biomass (Alcocer et al. 2015).
10.1.2 Structure: Composition and Richness
A total of 53 taxa of benthic organisms (Table 10.1) has been mentioned to inhabit the littoral zone of Lake Alchichica (Acosta et al. 2017; Alcocer et al. 1993a, b, 1998, 2002; Cohuo-Durán et al. 2014; Escobar-Briones and Alcocer 2002; Peralta et al. 2002; Suárez-Morales et al. 2013).
However, a more recent study by Alcocer et al. (2016), considering 11 sampling stations equally distributed along the littoral zone of Lake Alchichica found only 21 macroinvertebrates. The taxonomic richness ranges from 2 to 13 with an average of 9 ± 3 taxa (Fig. 10.1).
The only species present in all localities (100%) is the tubificid worm Limnodrilus hoffmeisteri, followed by the amphipod Hyalella azteca (90%), the leeches Helobdella stagnalis, Glossiphoniidae, and the midge Micropsectra (80%), and the water boatmen Krizousacorixa tolteca, and the midges Cricotopus (Isocladius) triannulatus and Chironomus stigmaterus (now Chironomus alchichica) (60%).
10.1.3 Distribution and Seasonal Variations
The BMI distribution along the littoral zone was heterogeneous, which was not surprising considering the littoral area is environmentally diverse (see Chap. 8). Regarding abundance, the oligochaete Limnodrilus hoffmeisteri (43%) and the amphipod Hyalella azteca (39%) contributed with the largest percentage (82%) well above the other taxa (Fig. 10.2). Limnodrilus hoffmeisteri (0–80%) dominated in 60% of the sampling stations, while Hyalella azteca (18–100%) dominated in the other 40%. At located littoral areas, Micropsectra and Tanypus (Apelopia) (≈5%) reached higher abundances (≈10% and 5%, respectively).
The average density of the MIBs ranged from 7437 ± 5724 ind/m2 to 70,252 ± 14,407 ind/m2, with a global mean of 33,536 ± 20,463 ind/m2. The BMI abundance peak was associated with the diatom and cyanobacteria blooms, most likely providing abundant and fresh food (Pérez-Rodríguez et al. 2013). Sediment texture played a key role in explaining BMI distribution. The diversity of Shannon ranged from 0.002 to 1.69 with an average of 1.26 ± 0.54. Shannon’s maximum diversity fluctuated in a range of 0.33 to 3.06 with an average of 2.49 ± 0.84. Finally, Shannon’s evenness ranged from 0.002 to 0.73 with an average of 0.46 ± 0.20 (Alcocer et al. 2016).
Total BMI biomass was 88.5 ± 309.5 mg C/m2. Temporarily, July displayed the lowest average biomass (37.52 ± 138.71 mg C/m2), while May the highest average biomass (129.75 ± 450.06 mg C/m2). Spatially, the biomass among sampling stations ranged from 25.72 ± 104.86 mg C/m2 to 151.48 ± 484.52 mg C/m2 (Alcocer et al. 2016; Pérez-Rodríguez et al. 2013).
10.1.4 Function and Trophic Complexity
The community structure of the littoral ecosystem simplifies as littoral-pelagic isolation increases. Although simplification is graphically evident in a decrease in taxonomic richness, diversity, maximum diversity, and equity (but not in density), it is not supported statistically (Alcocer et al. 2016).
Detritivores (43.3 ± 23.1%) and herbivorous (40.1 ± 25.7%) BMI dominated numerically over carnivorous (16.6 ± 16.0%) in the littoral zone of Lake Alchichica (Fig. 10.3). Detritivores and herbivores were dominant in 40% of each of the littoral stations, in 10% were co-dominant, while 10% dominated the carnivorous.
According to Pérez-Rodríguez et al. (2013), there are four trophic guilds in the littoral BMI of Lake Alchichica. Herbivorous composed the highest percentage (50%, 155 ± 471.9 mg C/m2) of the total biomass, while filter feeders constituted the lowest percentage (3%, 39.4 ± 72.1 mg C/m2); detritivores accounted for 28% of the total biomass (85.8 ± 274.6 mg C/m2), while predators contributed 19% of the total biomass (47.1 ± 152.9 mg C/m2). Throughout the year, the biomass of detritivores and predators BMI remained similar, while herbivorous peaked in May, followed by a minimum in July. Filter feeders peak in January and diminished in July. Spatially, all littoral stations were different in their percentual composition of trophic guilds. In general, the stations with the highest vegetation cover presented the highest BMI biomass composed mainly of herbivores. Plant coverage and sedimentary organic matter content were the most critical factors in explaining the MIB biomass and distribution.
There are six Functional Feeding Groups -FFG- in the littoral BMI assemblages of Lake Alchichica (Fig. 10.4). Most of the BMI are collector-gatherers (40.5 ± 25.9%), shredders (35.2 ± 28.0%), and predators-engulfer (14.2 ± 15.7%), while piercers-predators, piercers-herbivorous and scrapers were scarce (<1%).
10.1.5 Cryptic Fauna
The shape of the lake’s basin approximates that of a cylinder (see Chap. 6). Nowadays, the lake has a reduced littoral area and a very abrupt slope with calcareous deposits called “tufa” (stromatolites). Tufa forms when carbonate-rich groundwater emerges in an alkaline-sodium lake, precipitating the carbonates in the form of calcite and, to a lesser extent, aragonite; microbial activity promotes carbonate precipitation (see Chap. 22). Tufa deposits that come in the form of a ring on the lake’s periphery resemble, by their appearance, coral reefs. More critical, tufa deposits constitute an available habitat for cryptic species.
Stromatolites are solid mounds or cylinders of carbonate deposition that hardly retain sediments preventing benthic organisms from settling. Nonetheless, Escobar-Briones and Alcocer (2002) found and described a new asellid isopod species, Caecidotea williamsi, cryptically inhabiting in tufa crevices. C. williamsi constitutes the first report of an epigean asellid isopod inhabiting inland saline waters in America. Hernández et al. (2010) found C. williamsi shares this habitat with the amphipod Hyalella azteca, and two ostracods, Limnocythere inopinata (now Limnocytherina axalapasco) and Candona sp. (now Candona alchichica).
C. williamsi is a small isopod (1–8 mm) with pigmented eyes. The body is mottled pale and light brown or gray. Many individuals inhabit empty trichopteran cases cemented within the tufa crevices. Its crevicular habitat explains its absence from other benthic habitats in the lake (Alcocer and Escobar-Briones 2007).
10.2 Littoral Heterotrophic Protists
10.2.1 Introduction
As previously mentioned, the presence of stromatolites in the littoral zone of Lake Alchichica favors the formation of numerous microenvironments, with a wide range of biological and environmental conditions and a great diversity of habitats. In this work, heterotrophic protists diversity associated with the littoral zone was studied in a lake site where the stromatolites’ littoral conditions combine with an area more related to open waters. To carry out the study, we use the colonization method of artificial polyurethane foam units (PFU) substrates proposed by Cairns et al. (1969). Cairns et al. (1979) found that the equilibrium in the number of protist species colonizing the PFU is reached around 21 days. In the present study, the substrates stayed in place until 38 days. During this period, the presence of 39 species of heterotrophic protists was observed in Alchichicica.
Protists perform numerous important functions in water bodies: autotrophic flagellates contribute to primary production through their ability to photosynthesize. Heterotrophic nanoflagellates (HNFs) are the primary consumers of bacteria, as are ciliates and small amoebae. Larger ciliates can consume, in addition to bacteria, flagellates, phytoplankton, diatoms, and other ciliates; large amoebae also consume algae, flagellates, ciliates, diatoms, etc. (Fenchel 1987). Protists are usually abundant in the littoral zone of water bodies since there are abundant and different types of food; in addition to diverse ecological niches, such as parts of aquatic plants, biofilms on surfaces (on the rocks and sediment), and filamentous algae. In turn, they are part of the food of larger organisms as rotifers, microcrustaceans (copepods, cladocerans), and macrocrustaceans such as ostracods or Hyalella (Monakov 2003).
In 1990, the study of protists from the littoral zone was carried out in Lake Alchichica using the method of polyurethane foam units (PFU) colonization (Cairns et al. 1969). It is a simple method that allows obtaining comparable samples at various sampling sites and at different times, reducing the difficulty in studying the various taxonomic groups and simplifying information on the community’s structure (Bamforth 1982; Pratt et al. 1986). Polyurethane foam constitutes a habitat used in different ways by protists: as a refuge, as a fixation site, and as a place for feeding.
The PFUs (three replicates for each sampling date) were placed in the littoral zone, in the outer part of the barrier formed by the stromatolites. The environmental conditions of the littoral are fully described in the Chap. 8. According to Plafkin et al. (1980) the PFU colonization process behaves like a colonization of islands and follows the McArthur-Wilson model.
Since the study was carried out with the water extracted from the PFU, it was not possible to quantify the species’ absolute density. The densities are in ind/mL referred to as one milliliter of water volume squeezed from the PFUs. In the present study, the substrates were in place until 38 days.
All the species observed are considered free-living (Table 10.2). In Fig. 10.5, it is evident that Alveolata (Ciliata) and Discoba groups had the highest number of species throughout the colonization process. After them, Stramenopiles and Amebozoa were the groups with the most species. All ciliates’ species are found within Alveolata, while species included in the trophic group of HNF (heterotrophic nanoflagellates) belong to the supergroups Discoba, Stramenopiles, and Holozoa.
Number of protists species by taxonomic supergroup and sampling day according to Adl et al. (2019)
Bodo caudatus (6159 ± 4494 ind/mL), Bodo saltans (1532 ± 982 ind/mL), and Spumella termo (1428 ± 1168 ind/mL) were the most abundant species in HNF. Actinophrys sol (480 ± 163 ind/mL), Trichamoeba osseossacus (471 ± 790 ind/mL) and Mayorella microeruca (377 ± 241 ind/mL) dominated the amoeba and related groups. In ciliates, Cyclidium glaucoma (53 ± 51 ind/mL), Uronema sp. (29 ± 31 ind/mL), Stylonychia notophora (26 ± 49 ind/mL) and Trochilia minuta (21 ± 31 ind/mL).
Theoretically, species number along the colonization process of the PFU must have a positive trend. It did not happen in Lake Alchichica because, at 8 and 14 days, 19 species were observed, but at 21 days, there was a decrease to 17 species. On day 28, an increase to 23 species occurred, and a slight decrease to 20 species on day 38.
10.2.2 Trophic Groups
Figure 10.6 shows the number of species according to their type of feeding (trophic group) throughout the sampling period. Pratt and Cairns (1985) criteria were used for flagellates and amebae, and the ciliates followed the one of Adl et al. (2019). Four trophic groups were found: bacterivores, predators, omnivores, and omnivores-cytrophic. Bacterivores consume bacteria by filtering them from the water; omnivores consume bacteria and other smaller protists, while cytrophic omnivores need to consume other protists to grow, but by consuming them, they also ingest bacteria.
Predators attack their prey, usually other protists or microalgae. From day 8 to 28, bacterivores had the largest number of species, followed by omnivores. However, on day 38, the omnivores equalized the number of bacterivorous.
The composition and dominance of heterotrophic protists in the littoral zone of Lake Alchichica provide valuable information about the biological conditions. The abundance of bacterivorous species indicates the presence of significant amounts of bacteria, which is common in littoral environments due to the fact that there is usually abundant decomposing organic matter (Arndt et al. 2000). This food favors the presence and growth of bacterivorous and detritivorous species of different sizes, among them, the bacterivorous flagellates which are the smallest. In the present study, several genus Bodo species, and Spumella, small bacterivorous, reached the highest abundances. Other important bacterivores were some small amoebae species such as Vexillifera sp. or F. nolandi.
The next largest trophic group was the omnivores. This group includes larger amoebae and the vast majority of ciliates. Omnivorous feed on bacteria, but they also consume other microorganisms such as small cyanobacteria, autotrophic flagellates, HNF, some diatoms, and various other microalgae types. A variant of this group is that of the omnivores-cytrophic, which in order to grow, they must consume protists, commonly flagellated, although they also feed on bacteria (Adl et al. 2019).
Although not very numerous, the predators’ group included species from the supergroup Stramenopila (Heliozoa), such as A. sol and A. eichornii, which are passive predators. In the same case, the ciliated suctor S. soliformis is also a passive predator of other ciliates and flagellates. Finally, the ciliate L. fasciola is an active predator that detects, attacks, and consumes other ciliate and flagellate species.
The environmental factor that seems to exert the most significant influence on the species richness of the littoral heterotrophic protists of Lake Alchichica is salinity. The lake water has a salinity of 8.5 ± 0.2 g/kg (Vilaclara et al. 1993). At the site where the PFUs were placed, the salinity was 7 ± 0.1 g/kg. This salinity seemed to harm the species richness of protists since in other nearby lakes, with freshwater or considerably less saline, up to 50% more species were observed than in Lake Alchichica (Lugo 1993). Several dominant species in the study, as the HNF B. saltans and R. nasuta, and the ciliate C. glaucoma can occur under both marine and freshwater conditions, but other species have a narrow range of tolerance and salinity may be a limit for their distribution (Arndt et al. 2000; Lynn 2008).
In conclusion, the Lake Alchichica littoral zone’s heterotrophic protists have a relevant ecological function as consumers of bacteria and other protists. In turn, they can be consumed by different groups of littoral macroinvertebrates, either directly or indirectly, as components of the organic detritus that constitute several of these invertebrates’ food. From the list of littoral fauna groups observed in Lake Alchichica, crustaceans (copepods, ostracods and Hyalella), oligochaetes, and nematodes, chironomids could be the protist’s predators (Monakov 2003). In this way, littoral heterotrophic protists constitute an energy transfer link from bacteria to a wide variety of macroscopic organisms, which in turn can be the food of fish (Alchichica’s silverside), amphibians (Alchichica’s axolotl), or even of aquatic birds that live temporarily or permanently in the lake.
10.3 Littoral Diatoms
10.3.1 Introduction
In Lake Alchichica, as in other environments, both freshwater and marine and saline, diatoms are essential biological components. In the plankton and the littoral zone, there are numerous species of this group. Diatoms are unicellular or filamentous autotrophic organisms, characterized by presenting a cell wall or frustule made of silicon dioxide. The frustule is formed by two valves (epi and hipovalve) that fit together and joined by a series of bands that form the belt or cingulum. The frustule presents perforations of various forms (striae, pores) or spine-like extensions, which, for a long time, were the basis of the group’s taxonomy.
Diatoms are of great importance in the silicon cycle in water bodies and soil. They constitute a fundamental food source for numerous groups of organisms, from protists (ciliates and amoebae) to larger organisms, such as cladocerans and copepods. In the littoral zone of water bodies, they can be the principal food of numerous groups of animals, among them those that make up the meiofauna of sediments, mollusks that scrape them from substrates, or even fish, which consume them together with debris or as epiphytes of plants and algae (Monakov 2003).
Benthic littoral diatoms can form mucilaginous layers on the bottom that help stabilize the substrate and reduce erosion (Grant et al. 1986). In littoral environments, diatoms contribute an essential fraction of primary production, which has been underestimated (Cahoon and Safir 2002), and they constitute the majority of the living organic fraction of sediments. Therefore, they can be considered to constitute most detritivores’ food (Admiraal et al. 1984).
As described in detail in Chap. 8 (The Littoral environment), in the littoral area of Lake Alchichica, it is possible to find a wide variety of microenvironments. In the deep (12 m) and rocky area of the littoral, there is a significant growth of the filamentous Chlorophyte Cladophora sp. Numerous species of epiphytic cyanoprokaryotes and also some diatoms colonized these filaments (Tavera and Komárek 1996).
In the shallow (<1 m) portion of the litoral zone, the submerged aquatic macrophyte Ruppia maritima and the Charophyte Chara canescens grow in several places, being substrates where benthic -epiphytic- diatoms grow.
10.3.2 Species Richness
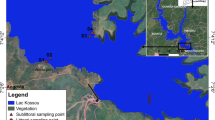
The samples were obtained at eight sites along the lake’s perimeter (Fig. 10.7) and three different dates: October 2006, March 2007, and September 2007. The substrates sampled were the submerged aquatic plant R. maritima and the charophyte C. canescens. Also, where possible, rock scraping or plocon samples were collected. Table 10.3 shows the substrates sampled by season and site. In total, the number of samples analyzed was 32.
In each sampling site and date, water temperature, water conductivity, pH, and dissolved oxygen concentration were measured.
Forty diatom species were found in the eight points sampled in the littoral zone. Table 10.4 shows the list of species. The species number increased in each sampling: 27 species in October 2006, 30 in March 2007, and 36 in September 2007.
Ten species were observed with a frequency ≥75% in the samples obtained (n = 32) during the study (Fig. 10.8): Navicymbula pusilla, Rophalodia gibberula, Gomphoneis olivaceum, Mastogloia smithii, Halamphora veneta, Mastogloia elliptica, Cocconeis placentula, Surirella striata, Anomoeoneis sphaerophora, and Navicula cryptocephala.
The species with a frequency percentage value <10% were Cocconeis placentula var. euglypta, Anomoeoneis costata (both 9.4%) and Caloneis westii (3.1%). Most of the species (23 spp.) occurred in the three samplings. In contrast, eight species were found in a single sample: Hyppodonta sp. (28.1%) and Gyrosigma acuminatum (15.6%) in October 2006. Anomoeoneis costata (9.4%) in March 2007. Cyclotella menenghiniana (31.2%), Nitzchia gracilis (18.8%), Epithemia sorex (15.6%), Cocconeis placentula var. euglypta (9.4%) and Caloneis westii (3.1%) in September 2007.
Regarding their spatial distribution, only G. olivaceum and N. pusilla appeared in the eight sampling sites in October 2006. In March 2007, more species were observed in all the sites: G. olivaceum and N. pusilla, also M. elliptica, E. alata, N. cryptocephala, C. placentula var. lineata, Nitzchia frustulum and Epithemia argus. In September 2007, only three species were present in all sites: E. argus, Rhopalodia gibberula and M. smithii.
Most of the species recorded in the study can live in wide ranges of salinity values, from freshwater environments to marine water. A. lybica, A. sphaerophora, C. clypeus, E. alata, M. smithii, and N. communis have only been found in saline environments. H. veneta, G. olivaceum, Hippodonta sp., D. pseudovalis and R. gibberula were species with high values of percentage of frequency (Frequency >75%). These are species that develop preferentially in saline environments, being rare in strictly freshwater environments (Hartley 1996; Krammer 2003; Novelo et al. 2007).
The majority of the species found belong to the group of pennate diatoms. Cyclotella menenghiniana, a central diatom, was observed in various littoral sites (Frequency 31.2%) only in September 2007. On several occasions, two other species of central diatoms were found in the littoral samples: Cyclotella alchichicana and C. choctawhatcheeana, which were not included in this study, since they are organisms that arrived from the pelagic zone, where they are common components of phytoplankton (see the chapter on phytoplankton), and their habitat is not the littoral zone.
Most species did not show a preference for some substrate since they grew on R. maritima, C. canescens, as epilithic, or as part of the plocon. Some exceptions were C.westii, only observed as epilithic, or G. acuminatum, which was only epiphytic; A. costata, E. sorex and P. brevissonii were not observed in the plocon samples.
The environmental conditions measured correspond to those generally observed in the littoral zone and are described fully in Chap. 8. The temperature varied between 16 and 25 °C, the dissolved oxygen between 5 and 13 mg/L, and the pH was basic to strongly basic (8–12 units). During the study, the conductivity range was between 8 (E5 October 2006) and 13 mS/cm (E1, E2, E6, and E7 March 2007). The highest values were associated with decreased water level and salts’ concentration in several sampled ponds.
Lake water’s electrical conductivity ranges between 12 and 13 mS/cm (Vilaclara et al. 1993). However, due to groundwater entry at various points in the littoral zone, the conductivity may be lower.
10.3.3 Temporal, Spatial, and by Substrate Variation
In Fig. 10.9, the analyzed samples’ grouping is observed by time of year, site, and sample type, using the Jaccard index as a similarity value and the UPGMA grouping method. Two groups were evident. The first of them (red box) includes the majority (9) of the sites and substrates from the October 2006 sampling (sampling 1), indicating that the composition of sites 1, 2, 3, 4, and 5 were similar, as well as the epilithic samples from sites 6 and 8 and the epiphytic from site 7. At site 4, which is penultimate to join the group, only a plocon sample was taken (similarity slightly less than 0.5).
Cluster analysis of Lake Alchichica’s diatom species (presence-absence data). Key: First number: sampling number (1–3); second number: sampling site (1–8). Substrates: Ep epiphytic, el epilithic, Pl plocon. Most of the epiphytic samples were from R. maritima. Only the epiphytic samples from site 2 are from C. canescens (12Ef and 32Ef)
In the second group (blue box), the samples from all the sites from the March 2007 sample joined first (sample 2) and then a group of 8 sites from sample 3 (September 2007). Plocon samples from sites 3 and 4 of the third sample are very similar (>0.85) and joined first with the epiphytic sample from station 7, and then with the epilithic sample from site 6, they are then joined with those of the group formed by the pair of plocon samples from sample 2.
Less similar samples, which later joined the previous group, were epilithic from sites 3 and 8 and epiphytic from sites 2, 8, and 5 of the third sample. Most different samples were from sampling date 1: the epilithic and the plocon from site 7, and the plocon from site 6, the latter being the least similar to all the rest. The analysis showed that the most remarkable similarities were temporary. The samples were grouped mainly according to the sampling dates, without the type of substrate from which they were obtained associating them. In some cases, the different substrates of the same site were similar, but in others, they were very different; for example, the epiphytic sample from site 7 of sample 1 had low similarity to the epiphytic and plocon samples from the same site.
The species richness of littoral diatoms of Lake Alchichica, considered a hyposaline lake, was greater than that of the hypersaline Mono lake (Nevada, USA), which only had 30 species (Herbst and Blinn 1998), but less than other hypersaline water bodies such as La Amarga lagoon in Argentina (53 spp.), or the Central Park lake, Turkey, with 126 spp. When Lake Alchichica is compared with freshwater bodies, the number of Lake Alchichica diatom species is considerably lower (e.g., Lake Nagpur, India, 92 spp.; Marjal Oliva-Pego, Spain. 51 spp.) (Maidana and Romero 1995; Kaoru et al. 1997; Sarode and Kamat 1980; Cantoral-Uriza and Sanjurjo 2008).
The species richness of littoral diatoms of Lake Alchichica was moderate, with a predominance of species capable of resisting a wide salinity range. However, species with preferentially saline or marine conditions were also present. Except for exceptional cases, no specificity of diatoms was observed for any substrate since most of them were observed indistinctly in several of them. Temporal variation had the most significant influence since the critical factor for grouping the samples was the sampling date. Some of the plocon samples became the ones with the most remarkable difference in species composition. The diatoms of Lake Alchichica seem to be of great importance from the littoral food webs’ point of view. They are indeed a very significant food for most of the different groups of macroinvertebrates that inhabit it. Together with the planktonic diatom species, they indeed have a very relevant function within the lake’s silicon cycle.
References
References for Sect. 10.1
Acosta R, Prat N, Ribera C, Michailova P, Hernández-Fonseca MC, Alcocer J (2017) Chironomus alchichica n. sp. (Diptera: Chironomidae) from Lake Alchichicha, Mexico. Zootaxa 4365(1):053–070. https://doi.org/10.11646/zootaxa.4365.1.3
Alcocer J, Lugo A, Estrada S, Ubeda M, Escobar E (1993a) Littoral chironomids of a Mexican plateau athalassohaline lake. Verhandlungen Internationale Vereinigung für theoretische und angewandte Limnologie 25:444–447
Alcocer J, Lugo A, Estrada S, Ubeda M, Escobar E (1993b) La macrofauna bentónica de los axalapazcos mexicanos. Actas del VI Congreso Español de Limnología 33:409–415
Alcocer J, Escobar E, Lugo A, Peralta L (1998) Littoral benthos of the saline crater-lakes of the basin of oriental, Mexico. Int J Salt Lake Res 7(2):87–108
Alcocer J, Escobar E, Peralta L, Álvarez F (2002) Population structure of the macrobenthic amphipod Hyalella azteca Saussure (Crustacea: Peracarida) on the littoral zone of six crater lakes. In: Escobar-Briones E, Álvarez F (eds) Modern approaches to the study of crustacea. Kluwer, Amsterdam, pp 111–115, 355 pp
Alcocer J, Escobar-Briones E (2007) On the ecology of Caecidotea williamsi Escobar-Briones & Alcocer (Crustacea: Isopoda: Asellidae) from Alchichica saline lake, Central Mexico. Hydrobiologia 576(1):103–109
Alcocer J, Hernández MC, Oseguera LA, Escobar E (2015) On the ecology of Cletocamptus gomezi Suárez-Morales, Barrera-Moreno & Ciros-Pérez 2013 (Crustacea, Copepoda, Harpacticoida) micro-endemic to Lake Alchichica, Central Mexico. J Limnol 74(2):302–309
Alcocer J, Escobar E, Řezníčková P, Oseguera LA (2016) La comunidad de macroinvertebrados bentónicos litorales como un reflejo de la heterogeneidad ambiental. Hidrobiológica 26(3):403–418
Cohuo-Durán S, Pérez L, Karanovic I (2014) On Limnocytherina axalapasco, a new freshwater ostracod (Podocopida: Limnocytheridae) from Mexican crater lakes. Rev Biol Trop 62(1):15–32
Escobar-Briones E, Alcocer J (2002) Caecidotea williamsi (Crustacea: Isopoda: Asellidae), a new species from a saline crater-lake in the eastern Mexican plateau. Hydrobiologia 477(1–3):93–105
Esteves FA (1988) Fundamentos de limnologia. Interciencia/FINEP, Rio de Janeiro, 575 pp
Hernández MC, Escobar E, Alcocer J (2010) Ensamble de crustáceos bentónicos en un lago salino tropical. Rev Mex de Biodiver 81:133–140
Peralta L, Alcocer J, Escobar E, Lugo A (2002) Oligochaetes from six tropical crater lakes in Central Mexico: community composition, species density and biomass. Hydrobiologia 467(1–3):109–116
Roldán Pérez G, Ramírez Restrepo JJ (2008) Fundamentos de limnología neotropical. Universidad de Antioquia, Colombia, 442 pp
Suárez-Morales E, Barrera-Moreno O, Ciros-Pérez (2013) A new species of Cletocamptus Schmankewitsch, 1875 (Crustacea, Copepoda, Harpacticoida) from a high altitude saline lake in Central Mexico. J Limnol 72:313–325
References for Sect. 10.2
Adl SM, Bass D, Lane CE, Lukes J, Schoch CL, Smirnov A, Agata S, Berney C, Brown MW, Burki F, Cárdenas P, Čepiča I, Chistyakova L, del Campo J, Dunthorn M, Edvardsen B, Eglit Y, Guillou L, Hampl V, Heiss AA, Hoppenrath M, James TY, Kankowska A, Karpov S, Kim E, Kolisko M, Kudryavtsev A, Lahr DJG, Lara E, Le Gall L, Lynn DH, Mann DG, Massana R, Mitchell EAD, Morrow C, Park JS, Pawlowski JW, Powell MJ, Richter DJ, Rueckert S, Shadwick L, Shimano S, Spiegel FW, Torruella G, Youssef N, Zlatogursky V, Zhang Q (2019) Revisions to the classification, nomenclature and diversity of eukaryotes. J Eukaryot Microbiol 66:4–119. https://doi.org/10.1111/jeu.12691
Arndt H, Dietrich D, Auer B, Cleven E-J, Gräfenhan T, Weitere M, Mylnikov AP (2000) Functional diversity of heterotrophic flagellates in aquatic ecosystems. In: Leadbeater BSC, Green JC (eds) The flagellates, Unity, diversity and evolution. Taylor & Francis, London, pp 240–268
Bamforth SS (1982) The variety of artificial substrates used for microfauna. In: Cairns J Jr (ed) Artificial Substrates. Ann Arbor Science Publishers, Michigan, pp 115–130
Cairns J Jr, Dahlberg ML, Dickson KL, Smith NR, Waller WT (1969) The relationship of fresh-water protozoan communities to the Mac-Arthur-Wilson equilibrium model. Am Nat 103:439–454
Cairns J Jr, Kuhn DL, Plafkin, JL (1979) Protozoan colonization of artificial substrates. pp. 34–57. In: Weitzel RL (ed.). Methods and measurements of periphyton communities: a review. ASTM STP 690. American Society for Testing and Materials. Philadelphia 9
Fenchel T (1987) Ecology of protozoa. The biology of free-living phagotrophic protists. Science Tech Publishers/Springer, Berlin
Lugo A (1993). Estudio de las comunidades litorales de protozoarios en seis lagos cráter del estado de Puebla, mediante el método de colonización de sustratos artificiales. Master Sciences Dissertation. Universidad Nacional Autónoma de México
Lynn DH (2008) The ciliated protozoa. Characterization, classification and guide to the literature. Springer, New York
Monakov AV (2003) Feeding of freshwater invertebrates. Kenobi Productions, Ghent
Pérez-Rodríguez Vania J, Alcocer-Durand J, Oseguera-Pérez LA, Escobar-Briones EG (2013) 2.4. Dinámica del carbono en sedimentos y biomasa expresada como carbono de los MIB de la zona litoral de Alchichica, Puebla. pp. 307–314. In: Paz Pellat F, Wong González J, Bazan M, Saynes V (eds.). Estado Actual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis a 2013. Programa Mexicano del Carbono, Colegio de Posgraduados, Universidad Autónoma de Chapingo e Instituto Tecnológico y de Estudios Superiores de Monterrey. México. 696 pp
Plafkin JL, Kuhn DL, Cairns J Jr, Yonge WH Jr (1980) Protozoan species accrual on artificial islands in differing lentic and wetland systems. Hydrobiologia 75:161–178
Pratt JR, Cairns J Jr (1985) Functional groups in the protozoa: roles in differing ecosystems. J Protozool 32:415–423
Pratt JR, Lang BZ, Kaesler RL, Cairns J Jr (1986) Effect of seasonal changes on protozoans inhabiting artificial substrates in a small pond. Arch Protistenkd 131:45–57
Vilaclara G, Lugo A, Chávez M, González H, Gaytán M (1993) Comparative description of crater-lakes basic chemistry in Puebla state, Mexico. Vehr Internat Verein Limnol 25:435–440
References for Sect. 10.3
Admiraal W, Peletier H, Brouwer T (1984) The seasonal succession patterns of diatom species on an intertidal mudflat: an experimental analysis. Oikos 42:30–40
Cahoon LB, Safir KA (2002) Distribution and biomass of benthic microalgae in Manukau Harbor, New Zealand. N Z J Mar Freshw Res 36:257–266
Cantoral-Uriza EA, Sanjurjo MA (2008) Diatomeas (Bacillarophyceae) del marjal Oliva-Pego (Comunidad Valencia España). Anales del Jardin Botánico de Madrid 65:111–128
Grant J, Bothmann UU, Mills EL (1986) The interaction between benthic diatom films and sediment transport. Estuar Coast Shelf Sci 23:225–238
Hartley B. Edited by Sims AP (1996) An atlas of British diatoms Lid. Great Britain, 601 pp
Herbst BD, Blinn DW (1998) Experimental mesocosms studies of salinity effects on the benthic algal community of a saline lake. J Phycol 34:772–778
Kaoru KM, Hisashi M, Kusucuoglu C, Karabiyikoglu M (1997) Diatom assemblages from inland saline lakes in the central part of Turkey – their application from quantitative reconstructions of paleosalinity changes during the Late Quaternary. Jpn Rev 8:235–249
Krammer K (2003) Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. In: Lange-Bertalot H (ed) Diatoms of Europe. Diatoms of European Inland Waters and comparable habitats 4, pp 1–530
Maidana NI, Romero OE (1995) Diatoms from the hypersaline “La Amarga” lake (La Pampa, Argentina). Diatom Res 16:163–188
Monakov AV (2003). Feeding of freshwater invertebrates. Kenobi Productions, Ghent
Novelo ME, Tavera SRL, Ibarra C (2007) Bacillariophyceae from karstic wetlands in Mexico J Cramer, Stuttgart, Germany
Sarode T, Kamat DN (1980) The diatomflora of Nagpur. India Nova Hedwigia 32:797–837
Tavera RL, Komárek J (1996) Cyanoprokaryotes in the volcanic lake of Alchichica, Puebla state, Mexico. Algol Stud 83:511–538
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alcocer, J. et al. (2022). The Littoral Community. In: Alcocer, J. (eds) Lake Alchichica Limnology. Springer, Cham. https://doi.org/10.1007/978-3-030-79096-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-79096-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79095-0
Online ISBN: 978-3-030-79096-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)