Abstract
Karst freshwater ecosystems are considered biodiversity hotspots, highlighting their sensitivity and vulnerability to environmental change. However, our understanding of the distribution and ecology of macroinvertebrates in lotic karst habitats is still incomplete. Therefore, to fill the knowledge gaps, the current study was conducted in the Dinaric and Carpathian–Balkan Mountains in Serbia, Southeastern Europe. We studied aquatic macroinvertebrates and their relationship with environmental parameters at 25 sampling sites in three habitat types (springs, the upper reaches, and tufa barriers) in 12 rivers and streams in Serbia, seasonally, between 2019 and 2022. We recorded 85,072 individuals within 206 taxa. Most environmental variables were comparable among the three habitat types, which most probably resulted in comparable abundance and diversity of benthic macroinvertebrates. However, taxa richness was lower in springs compared to upper reaches and tufa barriers. Environmental parameters had a greater impact than spatial effects on shaping the macroinvertebrate community. Moreover, IndVal analysis revealed a list of 30 indicator taxa associated with specific habitat types. We emphasized that highly specialized species support vulnerable functions in high-diversity karst freshwater ecosystems. Therefore, this study establishes a scientific foundation for implementing effective management strategies for these unique aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Karst terrain is known for its unique hydrology and landforms, which result from the combination of highly soluble rock, mainly limestone or dolomite, and well-developed secondary (fracture) porosity (Ford & Williams, 2007). Covering approximately 12% of the Earth's dry ice-free surface, karst regions and their aquifers are an indispensable resource for agriculture, industry, and the public drinking water supply in many areas (Hartmann et al., 2014; Dražina et al., 2017). Tufa barriers with various morphological forms are unique hydrogeological structures associated with karst freshwaters and usually form barrage lakes on longitudinal profiles of karstic rivers (Rodriguez et al., 2020). Tufa is a hollow, porous rock created from the deposition of dissolved calcium carbonate in cold freshwater that commonly contains biological remains of different organisms (Ford & Pedley, 1996). The formation process of the tufa barriers is caused by very complex, clearly defined geomorphological, physical, chemical, and biological parameters which together indicate “healthy” and preserved natural environments (Chen et al., 2004; Golubić et al., 2008; Špoljar et al., 2011; Batoćanin et al., 2023). Among biological components, bryophytes (i.e., aquatic moss) are the most abundant vegetation in lotic karst ecosystems and the major component of intense tufa formation, creating porous deposits and diverse microhabitats for micro- and macrofauna (Miliša et al., 2006; Šemnički et al., 2012). In addition to aquatic mosses, the other important organisms involved in the formation of tufa are cyanobacteria and eukaryotic algae, as well as aquatic macroinvertebrates (Carthew et al., 2003; Matoničkin Kepčija et al., 2006; Arp et al., 2010).
Karst habitats have long been recognized as biodiversity hotspots with many endemic, rare, and threatened species (Clements et al., 2006; Sertić Perić et al., 2011; Vilenica et al., 2018a, b). However, due to their high biodiversity and exposure to various anthropogenic pressures, karst freshwater habitats are extremely fragile and vulnerable (Kazakis et al., 2018; Ridl et al., 2018). Droughts predicted for the future are expected to greatly influence the hydrology of karst regions, hence various models have been developed to study the influence of climate change on these freshwater habitats (Hao et al., 2006; Guo et al., 2020; Nerantzaki & Nikolaidis, 2020). For these reasons, karst ecosystems have been consistently recognized as globally significant, earning their place in national conservation management plans in various regions worldwide (Clements et al., 2006).
The number of studies on the diversity, distribution, and ecology of macroinvertebrates in Southern European karst lotic ecosystems has increased in recent decades, particularly in the Dinaric Western Balkan ecoregion in Croatia and Bosnia and Herzegovina (Hrovat et al., 2009; Savić et al., 2017; Pozojević et al., 2021). However, there is limited data available for other regions of Southern Europe, with Serbia standing out in this context. Karst freshwater habitats can be found in the Carpathian–Balkan Mountains in the east and the Dinaric area in the west of Serbia, generally at altitudes exceeding 500 m.a.s.l. (Batoćanin et al., 2023). The bibliography of tufa accumulations in Serbia is insufficient, and information about the biota in these areas is completely missing. In recent years, attention has been given to individual smaller deposits in the context of environmental protection and geological heritage (Miljković et al., 2020; Batoćanin et al., 2023).
This study aims to fill knowledge gaps about the distribution and ecology of macroinvertebrates in the Dinaric and Carpathian–Balkan Mountains in Serbia, Southeastern Europe. We assume that aquatic macroinvertebrates show varying diversity patterns in longitudinal distribution across different types of karst freshwater habitats (Vannote et al., 1980; Vilenica et al., 2017). Additionally, we expect to discover rare, specialized, and new taxa highlighting the vulnerability of freshwater karst ecosystems and supporting their status as biodiversity hotspots. To achieve our objectives, we (a) identified the qualitative and quantitative specificities in the macroinvertebrate community composition across 25 sampling sites and three habitat types (springs, upper reaches, and tufa barriers) comprising 12 karst rivers and streams in Serbia; (b) explored the influence of different environmental variables on the macroinvertebrate communities to recognize the most influential ones shaping the composition and diversity, and (c) assessed the influence of seasonal and spatial variation on macroinvertebrate communities within the investigated karst lotic habitats.
Materials and methods
Study area
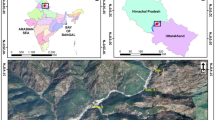
The main data source for this study consisted of hydrobiological surveys conducted in 12 Serbian karst rivers and streams, distributed across two ecoregions (according to Paunović et al., 2012): the Dinaric Western Balkans (Ecoregion 5—the Sopotnica, Panjica, Gostiljska, Raška Rivers, and the Banjski Stream) and the Eastern Balkan/Carpathian–Balkan Mountains (Ecoregion 7—the Grza, Lisine, Mirovštica, Miliva, Malo Vrelo, Gradašnička Rivers, and the Bigar Stream) (Fig. 1). These rivers were selected due to their diverse habitat types typical of karst systems, including springs, streams, lakes, and notably underwater tufa barriers, waterfalls, and cascades (Miljković et al. 2020; Batoćanin et al. 2023). The study encompassed 25 sites within 3 habitat types: springs (7 sites), the upper reaches of rivers and streams (6 sites), and tufa barriers (12 sites) (Fig. 2). A detailed description of the sites, including geographical coordinates, altitudes, types of substrates, and riparian vegetation, is provided in Table 1.
Sample collection and processing
Macrozoobenthos samples were collected once during four seasons (spring, summer, autumn, and winter) between 2019 and 2022 at each of the 25 sampling sites. At each sampling site, six subsamples of macrozoobenthos were collected from the most dominant substrate types with a 0.0625-m2 Surber sampler of 250-mm mesh, according to EN ISO 27828:1994 and EN ISO 10870:2012. The organisms attached to stone surfaces or other substrates were collected manually by tweezers and, if necessary, scraped with a fine brush. The six subsamples were merged into a single composite sample. Hence, the total number of macrozoobenthos samples in our study was 99, comprising 28 from springs, 24 from upper reaches, and 47 from tufa barriers. The samples were immediately preserved with 96% ethanol solution, transported, and stored at the Institute of Biology and Ecology, Faculty of Science, University of Kragujevac, Republic of Serbia. Collected materials were analyzed under a stereomicroscope (NIKON SMZ800, Nikon Corporation, Tokyo, Japan) and a microscope (Nikon Eclipse E100, Nikon Corporation, Tokyo, Japan). The material was identified to the lowest possible taxonomic level using available identification literature and the authors’ comparative collections (Conci & Nielsen, 1956; Aubert, 1959; Olmi, 1976; Rozkošný, 1980; Nilson, 1997; Zwick, 2004; Eiseler, 2005; Timm, 2009; Waringer & Graf, 2011; van Haaren & Soors, 2013). Early instar insect larvae and some taxonomically demanding groups such as Hirudinea and some Diptera (e.g., family Chironomidae, genus Simulium), could not be identified at the species level. Abundances are expressed as the number of individuals/m2.
Concurrently with collecting benthic macroinvertebrate samples in the field, the environmental variables were measured using standard methods (EN 10870, 2012). The water temperature (WT), hydrogen ion concentration (pH), conductivity (EC), and water hardness (H) were measured in situ using combined digital HANNA instruments (Hanna Instruments Ltd., Leighton Buzzard, UK), and the concentrations of dissolved oxygen (DO) and saturation (OSAT) were measured with an oximeter [Mettler Toledo SevenGo (Duo) Schwerzenbach, Switzerland]. For complete laboratory analysis, 1 l of water was collected at each site during each sampling period, packed in polyethylene bottles, and transported to the laboratory on the same day. In the laboratory, the ammonium-nitrogen (NH4-N), ammonia-nitrogen (NH3-N), nitrate-nitrogen (NO3-N), total nitrogen (N), orthophosphate (PO4-P), phosphorus pentoxide (P2O5), and total phosphorus (P) concentrations were determined using a photometer (Aqualytic AL400, Nhat Anh Company, Vietnam) with appropriate reagents following APHA (2012) procedures. Substrate composition (stones, gravel, sand, clay, anoxic mud, detritus, and tufa), vegetation cover (algae and macrophytes), and canopy coverage were determined by visual estimation.
Data analysis
To estimate differences in the community composition and diversity of macroinvertebrates among the three habitat types (i.e. springs, upper reaches, tufa barriers), the taxonomic community metrics (the number of individuals, taxa richness, and Shannon, 1948 and Simpson, 1949 diversity indices) were calculated for each sampling site and the date and results were expressed as means and standard deviations for each site (i.e., combining dates). Accordingly, the Kruskal–Wallis H test was used to determine significant differences in the total habitat score, environmental variables, and diversity indices among the three groups (i.e., habitat types; springs, upper reaches, and tufa barriers). In addition, for post hoc pairwise comparisons, the Mann‒Whitney U test was used. Performing a pairwise permutational multivariate analysis of variance (pairwise PERMANOVA) allowed us to test significant differences between macroinvertebrate communities of springs, upper reaches, and tufa barriers (Van Den Wollenberg, 1977). The correlation between environmental variables and taxonomic community metrics was tested using Spearman's rank correlation.
Furthermore, the macroinvertebrate metacommunity targets were analyzed. Understanding variation in biodiversity typically requires the consideration of different factors operating at different spatial scales (Medeiros et al., 2020). The multiscale distance relationships among different habitats within each karst river and stream were modeled and described via spatial variables using distance-based Moran’s eigenvector maps (dbMEMs; Borcard and Legendre, 2002; Borcard et al., 2004; Dray et al., 2006). Moran's eigenvector maps (dbMEMs) and derived approaches have proven very helpful in studying the spatial and temporal distributions of aquatic macroinvertebrate communities (Brind’Amour et al., 2018). Moran’s eigenvector map variables describe the spatial variability along a spatial scale. High dbMEM values indicate broad-scale patterns of relationships between sample sites, while low dbMEM values represent fine-scale relationships (Gronroos et al., 2013). The dbMEMs are calculated using geographical coordinates, and as outputs, the orthogonal spatial variables are obtained. Only dbMEMs with positive autocorrelation were analyzed further for variance partitioning. Since spatial factors have a strong impact on macroinvertebrate communities, spatial metacommunity patterns may become blurred because some sites are too far from each other. To avoid this, we separately performed this analysis for each subset of investigated rivers and streams (group 1—Ecoregion 5, 5 watercourses and 8 sites; group 2—Ecoregion 7, 7 watercourses and 17 sites). The relative importance of spatial and local environmental variables for the composition of macroinvertebrate community was subsequently tested by variance partitioning analysis (VPA) for each karst river and stream (Oksanen et al., 2007). Before VPA, macroinvertebrate abundance data were Hellinger transformed (Legendre & Gallagher, 2001). To select environmental variables that were included in the VPA the forward selection with two criteria, exceeding the critical P-value (P = 0.05) and exceeding the adjusted R2 value of the global model (Blanchet et al., 2008) was applied, using the function ‘R2adj’ in the R package (Dray et al., 2009). The statistical design was set to [Spatial (S) + Environmental (E) parameters], pure (E|S and S|E) and shared (S and E) effects of explanatory variable groups on the response variables (i.e., the macroinvertebrate community). The significance of total and pure effects was calculated using the function ‘ANOVA’ (Oksanen et al., 2007). As a result, Venn diagrams were generated.
Finally, the Indicator value (IndVal) analysis (Dufrêne & Legendre, 1997) was used to identify potential bioindicators of benthic macroinvertebrates at each habitat type (springs, upper reaches, and tufa barriers). IndVal analysis has been advocated as a useful ecological method for assessing associations between species and the habitats on which they rely. In addition, indicator values can be estimated for any given level of clustering, which constitutes a useful property of the approach. This analysis is based on the specificity and fidelity measured for each taxon in an assemblage, with indicator values ranging from 0 to 100% and reaching a maximum when all individuals of a taxon are recorded in only one habitat type (high specificity) and when the taxon is present in all samples of that habitat type (high fidelity) (Dufrêne & Legendre, 1997; Podani & Csányi, 2010). These two terms are multiplied and then scaled to express the indicator value of a species relative to the cluster as a percentage. Only macroinvertebrate taxa exhibiting a significant (P < 0.05) maximal indicator value for a given habitat, were identified as indicators in each habitat, whereas indicator taxa with IndVals above the threshold of 25% were retained as the most significant. The significance of the indicator value for each species was tested using a Monte Carlo randomization test with 1,000 permutations. All the statistical analyses were performed using the statistical software “R” Core Team version 4.2.2 (Oksanen et al., 2007).
Results
Environmental variables
Water temperature varied from 4.9° C (Raš1) to 13.9° C (Gra3) (Table S1). Dissolved oxygen ranged from 8.86 to 12.62 mg/l, and oxygen saturation ranged from 84 to 131.7% (Table S1). Conductivity and hardness were uniform across sites (Table S1). Inorganic nutrient concentrations were low across sites (Table S1). Among all investigated environmental variables, only pH and altitude significantly differed between the three habitat types (Kruskal–Wallis H test, P < 0.05; Table 2). pH was circumneutral to slight basic across habitat types, being lower at springs and higher at tufa barriers (Table 2). Other environmental variables were comparable among the three habitat types (Table 2).
Macroinvertebrate community composition
During this study, we recorded 85,072 individuals and 206 taxa, of which 126 were identified at the species level, 66 at the genus level, and 13 at the family level (Table S2). They all belonged to 18 benthic systematic groups (Table S2). Overall, Trichoptera was the most diverse order (60 taxa), followed by Diptera (34 taxa), Ephemeroptera (24 taxa), and Plecoptera (22 taxa) (Table S2).
The highest taxonomic richness in all three habitat types was recorded among Trichoptera, followed by Diptera (Table 3). Additionally, Ephemeroptera exhibited higher taxonomic diversity on the tufa barriers and in the upper reaches, both with 18 taxa, while Plecoptera, with 14 taxa, were more abundant in the springs than Ephemeroptera (Table 3). The dominant systematic group with the highest abundance in all habitats was Amphipoda (Gammarus balcanicus Schäferna, 1923 exclusively) (Table 3). Gastropods, primarily the species Bythinella istoka Glöer & Pešić, 2014, and B. dispersa Radoman, 1976, appeared in large numbers at some sampling sites of the tufa barriers (Spo3, Panj3) (Table S2; Table 3). Both qualitatively and quantitatively, the groups Nematoda, Decapoda, Neuroptera, Isopoda, Odonata, and Bivalvia were the least represented (Table 3).
Macroinvertebrate community patterns
Taxa richness was the only taxonomic community metric that significantly differed among the three habitat types (Kruskal–Wallis H test, P < 0.05, Table 4). Taxa richness was significantly lower in springs than in upper reaches (Mann–Whitney U test, U = 159.5, Z = − 3.23, P < 0.05) and tufa barriers (Mann–Whitney U test, U = 391.5, Z = − 2.91, P < 0.05). However, no significant difference was detected between the upper reaches and tufa barriers (Mann–Whitney U test, U = 519.5; Z = 0.53, P > 0.05). Also, there were significant differences in macroinvertebrate communities among the three habitat types (PERMANOVA, F = 5.43, P < 0.05). No significant differences were found in the macroinvertebrate communities between seasons for each habitat type (PERMANOVA, F = 1.15, P > 0.05). Analysis of correlation coefficients showed that Simpson’s diversity index negatively correlated with pH values (Spearman’s rank correlation, R = − 0.29, P < 0.05), while the number of individuals positively correlated with altitude (R = − 0.30, P < 0.05, Table S3).
Statistically significant differences (Mann–Whitney U test, P < 0.05) between Ecoregions 5 and 7 were observed for taxa richness, Shannon, and Simpson diversity indices (Table 5), with all taxonomic metrics being higher for Ecoregion 5 (Table 5). The environmental variables that showed significant statistical differences between Ecoregions 5 and 7 were elevation, dissolved oxygen, oxygen saturation, conductivity, and hardness (Mann–Whitney U test, P < 0.05, Table S4). For Ecoregion 5, analysis of correlation coefficients showed that taxa richness positively correlated with pH (Spearman’s rank correlation, R = 0.54, P < 0.05) and oxygen saturation (R = 0.49, P < 0.05, Table S5). In Ecoregion 7, significant negative correlations were observed between pH and taxa richness (R = − 0.29, P < 0.05), as well as the Shannon (R = − 0.30, P < 0.05) and Simpson (R = − 0.38, P < 0.05) diversity indices (Table S5). As shown by the VPA results, both environmental and spatial factors played important roles in structuring macroinvertebrate communities in Ecoregions 5 and 7 (Fig. 3). Twenty-four percent of the variation in the overall metacommunity was accounted for by the two variable groups. According to the results, the pure effect of environmental factors (approximately 16% in two variable groups) was slightly more influential than spatial effects (6% in Ecoregion 5 and 8% in Ecoregion 7). The residuals (74% in Ecoregion 5 and 76% in Ecoregion 7) are the variations left unexplained by the canonical model for both ecoregions (Fig. 3).
Venn diagram illustrating the variation partitioning analysis. The contributions of physical and chemical properties (X1) and spatial distance (X2) to the community composition of macroinvertebrates in karst rivers and streams in Ecoregions 5 (a) and 7 (b). The fraction between two overlapping circles represents the variation explained between the components, while the residuals are the variation left unexplained by the canonical model
IndVal analysis revealed a list of 30 taxa (17 with IndVal > 0.25) that are associated with specific habitat types (Table 6). We found ten potential indicator taxa for the spring sites, four of which (Elmis sp., Thremma anomalum McLachlan 1876, Ancylus fluviatilis O. F. Müller, 1774, Elmis maugetii Latreille, 1802) had significant IndVals (> 0.25, Table 6). Mayflies [Baetis rhodani (Pictet, 1843) and Rhithrogena semicolorata (Curtis, 1834)] were found to be characteristic of upper reaches sites. The habitats of the tufa barriers were distinguished by a particularly high number (15) of potential indicator taxa, of which 11 had significant IndVals (family Chironomidae, Tinodes unicolor (Pictet, 1834), Ephemera danica Müller, 1764, Riolus subviolaceus (Müller, 1817), Eiseniella tetraedra (Savigny, 1826), family Lumbricidae, Dugesia gonocephala (Duges, 1830), Simulium sp., Haplotaxis gordioides (Hartmann, 1819), Sialis lutaria (Linnaeus, 1758) and Rhyacophila tristis Pictet, 1834) (Table 6).
During this research, we recorded new data on the distribution of several rare and endangered species of macroinvertebrates, such as Helicopsyche bacescui Orghidan & Botosaneanu 1953, Thremma anomalum McLachlan 1876, Drusus discolor (Rambur, 1842), Beraeodes minutes (Linnaeus, 1761), Protonemura praecox (Morton, 1894), Taeniopteryx hubaulti Aubert, 1946., Cordulegaster heros Theischinger, 1979, Austropotamobius torrentium (Schrank, 1803), Grossuana euxina serbica Radoman, 1973 and others (Table S2, species marked in bold). These species are protected by national regulations and are on the list of strictly protected and protected species according to the Rulebook on the Declaration and Protection of Strictly Protected and Protected Wild Species of Plants, Animals, and Fungi (Official Gazette of RS No. 5/2010, 47/2011, 32/2016, and 98/2016). In addition, we recorded three species for the first time in Serbia: Dixa puberula Löw, 1849, Dixa maculata Meigen, 1818, and Chaetopterygopsis maclachlani Stein, 1874.
Discussion
Most of the measured environmental variables were comparable among habitats. High water quality, reflected in the high concentration of dissolved oxygen and oxygen saturation, was recorded at all sites. These values correspond to those obtained in recent research from karst rivers in Croatia (Ridl et al., 2018), Slovenia (Hrovat et al., 2009), and Austria (Bednar et al., 2017). A pH gradient was observed from the springs to the upper reaches and finally to the tufa barriers, as expected (Vilenica et al., 2017), primarily due to the reduction in carbon dioxide dissolved in the water, which is one of the key factors for tufa deposition (Chen et al., 2004). The specific environmental conditions, such as thermal stability, high values of dissolved oxygen, and low amounts of inorganic nutrients described at the sampling sites, most likely resulted in a rather high diversity of macroinvertebrate communities, constituting nearly 20% of the macroinvertebrate taxa richness documented in Serbia (Petrović, 2014). Compared to the data from neighboring regions (e.g., Hrovat et al., 2009; Ridl et al., 2018; Vilenica et al., 2018a, b), the benthic macroinvertebrate fauna of the karst ecosystems in Serbia can be defined as quite rich and diverse at the regional scale. As expected, springs hosted lower taxa richness, as aquatic macroinvertebrate diversity typically increases downstream along the longitudinal profile of a stream (Erman & Erman, 1995). These findings are supported by research conducted in Croatian karst hydrosystems on various aquatic insects, including Trichoptera (Previšić et al., 2007; Pozojević et al., 2021), Ephemeroptera (Vilenica et al., 2017), and some Diptera such as Empididae (Ivković et al., 2012) and Simuliidae (Ivković et al., 2014). According to the River Continuum Concept (Vannote et al., 1980), macroinvertebrate communities in springs are composed of species that can function within a narrow temperature range on a restricted nutritional base. On the other hand, high community diversity and species richness usually reflect high habitat diversity (Miliša et al., 2006). Therefore, the high diversity and species richness at tufa barriers could be the consequence of the variety of microhabitats (i.e. presence of different aquatic mosses and algae) and the availability and diversity of food resources (Miliša et al., 2006; Ivković et al., 2014; Vilenica et al., 2017).
Taxa richness in the studied karst springs (i.e., 124 taxa) was lower compared to that in Bulgaria (i.e., 148 taxa in 20 systematic groups) (Vidinova et al., 2022), but higher compared to that in the Julian Alps (i.e., 76 taxa) (Mori, 2005). The dominance of insect taxa, primarily Ephemeroptera, Plecoptera and Trichoptera (collectively referred to as EPT), is expected and supported by previous studies (Maiolini et al., 2011; Cíbik et al., 2021). We recorded 64 EPT taxa (out of a total of 91 insect taxa), a substantially higher number compared to the 28 EPT taxa recorded for 50 karst springs in central Bosnia and Herzegovina (Savić et al., 2017). Trichoptera was the most speciose EPT order in springs, corroborating the results of previous studies (e.g. Maiolini et al., 2011; Savić et al., 2017). Ephemeroptera was less diverse than Plecoptera, which is consistent with the findings of Maiolini et al. (2011) and Cíbik et al. (2021) and could be related to the thermal preferences of mayflies, which are generally adapted to warmer conditions (Haidekker & Hering, 2007) and are more often abundant in the upper reaches (Vilenica et al., 2017). As isolated habitats, springs make immigration difficult for non-flying macroinvertebrates (e.g., Oligochaetes) (Dumnicka, 2006). Karst springs in the studied region had lower Oligochaeta taxa richness (i.e., 10 taxa) compared to karst springs in the Western Carpathians (i.e., 37 taxa) (Beracko et al., 2022). The lack of suitable microhabitats and food resources, primarily due to low concentrations of phosphorus and nitrate resulting in low periphyton productivity compared to lower sections, justifies the smaller number of Oligochaeta taxa (Beracko et al., 2022).
We found that springs have a higher proportion of cold-water stenotherm species and species with increased vulnerability to climate change, such as the endangered European species Arcynopterix dichroa (McLachlan, 1872) and T. hubaulti (Tierno de Figueroa et al., 2010; Fochetti, 2020; Simović et al., 2023), as well as C. maclachlani (Bálint et al., 2011). Moreover, the A. dichroa and C. maclachlani are European rare and circumpolar species that have survived glacial cycles through altitude shifts in isolated periglacial populations in the high mountains (Bálint et al., 2011; Theissinger et al., 2012). Also, highly specialized taxa such as T. anomalum, P. praecox, and P. meyeri were typically abundant in the Balkans (Ridl et al., 2018). The distribution of the genus Elmis in karst rivers in Serbia corresponds to its known habitat preferences (Mičetić Stanković et al., 2018, 2022).
The family Chironomidae had the highest association with tufa barriers because they play a significant role in the ecological processes of tufa environments, inhabiting crevices, pores, and surfaces covered in algae and macrophytes (Čmrlec et al., 2013; Dorić et al., 2024). Tufa barriers can create favourable conditions for the development of dense, rapidly growing populations of filter-feeding insect larvae, such as Simuliidae (Ivković et al., 2014) or Dixidae (Ivanković et al., 2019), which, in our study, were collected from many tufa barrier sites. Our study confirmed the presence of two Dixa species (D. puberula and D. maculata), while the karst regions of Croatia documented five species from the genus Dixa (Ivanković et al., 2019). Ivković & Pont (2016) reported certain Limnophora species at sites on tufa barriers in Croatia. However, in our case, their occurrence was rare, probably due to lower temperatures as a limiting factor for the development of these larvae (Ivković & Pont, 2016). The most suitable habitat types for the Ephemeroptera fauna in our study were the upper reaches of streams and rivers; tufa barriers were less favourable, while springs were the least favourable. These findings correspond with results from tufa-depositing habitats of the Dinaric Karst in Croatia (Vilenica et al., 2017). However, our results revealed a higher Ephemeroptera taxa richness in each of the three types of habitats. This result may be a consequence of the absence of fish predators at some sampling sites, as reported during fieldwork. Moreover, the local people does not recall fish inhabiting some rivers (e.g., Sop, Gos, Gra). Furthermore, several mayfly species, such as Paraleptophlebia werneri Ulmer, 1920 and Ephemera danica Müller, 1764, prefer habitats on tufa barriers and cascades, while B. rhodani and R. semicolorata are characteristic of fast-flowing upper reaches, which is in accordance with the literature (Vilenica et al., 2017, 2021).The strong inclination of Sialis lutaria, the only Megaloptera species documented in our investigation, toward tufa barriers, corresponds with the conclusion of Vilenica et al. (2018a). These habitats offer favorable living conditions for diverse aquatic insects, such as mayflies, which can serve as a crucial food source for Megaloptera (Vilenica et al., 2018a).
The diversity of Trichoptera observed at the tufa barriers in our research closely resembled that found in the Plitvice Lakes National Park in Croatia (Previšić et al., 2007; Šemnički et al., 2012), highlighting the dominance of Rhyacophilidae, Polycentropodidae, Psychomyiidae, and Limnephilidae. We reported the highest average richness of Trichoptera taxa on tufa barriers, followed by those in the upper reaches and springs, consistent with findings by Pozojević et al. (2021). Furthermore, Tinodes unicolor (Pictet, 1934) was predominantly found in tufa barrier habitats, consistent with the observations made by Previšić et al. (2007). Additionally, our results indicate that tufa barriers can also serve as habitats for species such as T. anomalum and H. bacescui, which have a disjunctive European distribution and inhabit small areas in mountainous regions (Petrović, 2014; Rimcheska et al., 2015). According to our knowledge, this is the first finding of these species on tufa barriers. The abundant presence of rare or endangered species from the Drusinae subfamily, such as Drusus annulatus (Stephens, 1837) (Sop3), D. discolor (Sop3), and Ecclisopteryx madida (McLachlan, 1867) (Lis1, Mvr2), validates their preference for tufa habitats featuring aquatic mosses exposed to strong water currents (Martini & Waringer, 2021). According to Waringer et al. (2011), 64% of Drusinae species are endemic to the Dinaric Western Balkan ecoregion. Furthermore, species of the genera Drusus and Ecclysopteryx have been suggested as good examples of cold stenothermic species, which are among the most threatened by climate warming (Pauls et al., 2006). The abundant populations of pollution-sensitive snails, such as B. istoka (stenoendemic) and B. dispersa recorded at some of our sampling sites (Big1, Panj2, Sop3) indicate favorable habitat conditions and low levels of anthropogenic pressure (Gojšina et al., 2022). This is consistent with studies that have identified large populations of Bythinella species in well-oxygenated and calcareous conditions (Dumnicka et al., 2007; Bednar et al., 2017).
The Odonata species richness in the study area could be considered low compared to the Odonata fauna of the karst rivers of neighboring countries, such as Croatia (Vilenica, 2017) and Montenegro (Pešić et al., 2017). The low water temperature, low productivity, and smaller amounts of water were most likely limiting factors for the occurrence of dragonflies. Additionally, the sampling methodology used could influence this list of Odonata species, as the sampling nets cover only a small area of a habitat/microhabitat (Vilenica, 2017). All investigated habitats were inhabited exclusively by lotic species, such as Calopteryx virgo (Linnaeus, 1758), Cordulegaster bidentata (Selys, 1843), and C. heros, as expected (Vilenica, 2017). The findings of C. heros, as valuable indicators of natural, unaltered water flows, are key to nature conservation and the formation of the NATURA2000 network of protected areas (Holuša & Holušová, 2022).
PERMANOVA did not reveal statistically significant differences in the composition of the macroinvertebrate communities between seasons, which is not consistent with the results of Sertić Perić et al. (2011) and Rađa & Šantić (2014). Such seasonal uniformity within macroinvertebrate communities results from the specificity of karst lotic ecosystems, which maintain stable environmental (physical and chemical) conditions throughout the year. Therefore, the stable habitat conditions shaped the macroinvertebrate communities, reflecting the autecology of the recorded taxa (Hrovat et al., 2009; Lewin et al., 2013; Moog et al., 2017; Pozojević et al., 2021).
Spatial processes played a lesser role in shaping macroinvertebrate communities within Ecoregion 5 (6%) and Ecoregion 7 (8%), while local environmental factors were more significant drivers. However, all models exhibited high levels of unexplained variation, a common issue in similar studies (Rezende et al., 2014; Medeiros et al., 2020) due to the lack of environmental factors available to differentiate between sites. In their natural habitat, macroinvertebrates face a variety of factors that operate simultaneously. Hence, to determine the effects of different environmental variables on macroinvertebrates, an analysis of multiple parameters, such as sediment type, habitat characteristics, and geomorphological and biogeographical factors (Verschut et al., 2015) is necessary. The relative importance of biotic factors (interspecific interactions) can also be significant (Peeters et al., 2004). Efforts to collect more site-specific data are crucial to validate these results.
Most investigated habitats are easily reachable and have multiple purposes. Moreover, without alternative solutions, karst springs are the only potential for current and future water supplies for some parts of the Balkan Peninsula (Pešić et al., 2020). Although some of the habitats studied are protected on the national level, to varying degrees (Ranđelović & Avramović, 2004; Telbisz et al., 2021; Marjanović et al., 2022), they are exposed to various anthropogenic influences. Slightly elevated amounts of nitrates, orthophosphates, and ammonium-nitrogen were detected at some sites (i.e., Gra3Au, Grz2Su, Lis3Au; Mil1Sp), which may be attributed to recreational activities in the form of restaurants located along the banks of the river as well as in picnic areas surrounding rivers. This should be considered when protecting these vulnerable karst river systems and their communities because the rapid expansion of tourism threatens karst landscapes featuring tufa accumulations throughout Europe (Escarpinati et al., 2011; Megerle, 2021), and carbonate deposition rates decline significantly as a result of phosphate pollution (Capezzuoli et al., 2014).
Conclusions
The study showed that tufa-depositing lotic habitats in the Dinaric and Carpathian–Balkan Karst regions of Serbia are crucial for maintaining the high biodiversity of Southern European freshwater ecosystems. Most environmental variables were comparable among the habitat types, as were most of the taxonomic community metrics, with only the springs having lower taxa richness compared to the upper reaches and tufa barriers. This study illustrates the importance of considering both environmental and spatial factors in shaping macroinvertebrate communities. Additionally, we documented new distribution and ecological trait data for several rare and protected species in Serbia. Finally, we emphasize that this study contributes to filling existing knowledge gaps regarding karst freshwater habitats and benthic communities in the Balkan Peninsula. This knowledge can be used in future conservation and protection management procedures for these unique aquatic ecosystems in this part of Europe. Future activities should include systematic ecological studies in a larger number of study sites and microhabitats, also encompassing lentic habitats.
Data availability
Enquiries about data availability should be directed to the authors.
References
American Public Health Association (APHA), 2012. Standard Methods for Examination of Water and Wastewater, 22nd ed. American Public Health Association Press, Washington, DC:
Arp, G., A. Bissett, N. Brinkmann, S. Cousin, D. De Beer, T. Friedl, K. I. Mohr, T. R. Neu, F. Shiraishi, E. Stackebrandt & B. Zippel, 2010. Tufa-forming biofilms of German karst water streams: microorganisms, exopolymers, hydrochemistry, and calcification. Geological Society London: Special Publications 336: 83–118. https://doi.org/10.1144/sp336.6.
Aubert, J., 1959. Plecoptera. In: Insecta Helvetica, Fauna, Vol. 1. Imprimerie La Concorde, Lausanne: pp. 91–136.
Bálint, M., S. Domischc, C. H. M. Engelhardt, P. Haase, S. Lehrian, J. Sauer, K. Theissinger, S. U. Paulus & C. Nowak, 2011. Cryptic biodiversity loss linked to global climate change. Nature Climate Change 1: 313–318. https://doi.org/10.1038/nclimate1191.
Batoćanin, N., W. Wróblewski, I. Carević, U. Durlević, V. Gajić & A. Valjarević, 2023. Facies and origin of tufa deposits from the Gostilje River Basin and the Sopotnica River Basin (SW Serbia). Applied Sciences 13: 3190. https://doi.org/10.3390/app1305319.
Bednar, J. P., M. Trobej, M. Schagerl & J. Waringer, 2017. Which factors shape macrozoobenthic communities in tufa springs? Results from Austrian meteogene travertine-depositing sites. Hydrobiologia 799: 293–307. https://doi.org/10.1007/s10750-017-3228-x.
Beracko, P., J. Cíbik, P. Macko & T. Lánczos, 2022. Three biodiversity facets and assembly mechanism of the oligochaete community in the karst spring environment. Hydrobiologia 849: 603–624. https://doi.org/10.1007/s10750-021-04728-1.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Forward selection of explanatory variables. Ecology 89: 2623–2632. https://doi.org/10.1890/07-0986.1.
Borcard, D. & P. Legendre, 2002. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecological Modelling 153: 51–68. https://doi.org/10.1016/S0304-3800(01)00501-4.
Borcard, D., P. Legendre, C. Avois-Jacquet & H. Tuomisto, 2004. Dissecting the spatial structure of ecological data at multiple scales. Ecology 85: 1826–1832. https://doi.org/10.1890/03-3111.
Brind’Amour, A., S. Mahévas, P. Legendre & L. Bellanger, 2018. Application of Moran Eigenvector Maps (MEM) to irregular sampling designs. Spatial Statistics 26: 56–68. https://doi.org/10.1016/j.spasta.2018.05.004.
Capezzuoli, E., A. Gandin & M. Pedly, 2014. Decoding tufa and travertine (freshwater carbonates) in the sedimentary record. The state of the art. Sedimentology 61: 1–21.
Carthew, K. D., R. N. Drysdale & M. P. Taylor, 2003. Tufa deposits and biological activity, Riversleigh, Northwestern Queensland. In Roach, I. C. (ed), Advances in Regolith CRC LEME, Glen Osmond: 55–59.
Chen, J., D. D. Zhang, S. Wang, T. Xiao & R. Huang, 2004. Factors controlling tufa deposition in natural waters at waterfall sites. Sedimentary Geology 166: 353–366. https://doi.org/10.1016/j.sedgeo.2004.02.003.
Cíbik, J., P. Beracko, I. Krno, T. Lánczos, T. Navara & T. Derka, 2021. The taxonomical and functional diversity of three groups of aquatic insects in Rheocrene karst springs are affected by different environmental factors. Limnologica 91: 125913. https://doi.org/10.1016/j.limno.2021.125913.
Clements, R., N. S. Sodhi, M. Schilthuizen & K. L. Peter, 2006. Limestone karsts of Southeast Asia: imperiled arks of biodiversity. BioScience 56: 733. https://doi.org/10.1641/0006-3568(2006)56[733:lkosai]2.0.co;2.
Čmrlec, K., M. Ivković, P. Šemnički & Z. Mihaljević, 2013. Emergence phenology and microhabitat distribution of aquatic Diptera community at the outlets of barrage lakes: effect of temperature, substrate and current velocity. Polish Journal of Ecology 61: 135–144.
Conci, C. & C. Nielsen, 1956. Odonata, Fauna d’Italia. Sotto gli dell' Accademia Nazionale Iteliana di Entomologia e dell'Unione Zoologica Italiana, Vol. I. Edizioni Calderini, Bologna: 200–277.
Dorić, V., I. Pozojević, V. Baranov, Z. Mihaljević & M. Ivković, 2024. Long-term chironomid emergence at a karst tufa barrier in Plitvice Lakes National Park, Croatia. InSects 15: 51. https://doi.org/10.3390/insects15010051.
Dray, S., P. Legendre & P. R. Peres-Neto, 2006. Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbor matrices (PCNM). Ecological Modelling 196: 483–493. https://doi.org/10.1016/j.ecolmodel.2006.02.015.
Dray, S., P. Legendre & G. Blanchet, 2009. Packfor: forward Selection with permutation (Canoco p. 46). R package version 0.0-8.
Dražina, T., M. Špoljar, B. Primc & I. Habdija, 2017. Distribution of rotifers and other meiofauna in the bryophytes and hyporheic zone of a karst hydrosystem – an example of a nested community. Marine and Freshwater Research 68: 43–52. https://doi.org/10.1071/MF14291.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
Dumnicka, E., 2006. Composition and abundance of Oligochaetes (Annelida: Oligochaeta) in spring of Kraków-Czestochowa Upland (Southern Poland): effect of spring encasing and environmental factors. Polish Journal of Ecology 54: 231–242.
Dumnicka, E., J. Galas & P. Koperski, 2007. Benthic invertebrates in karst springs: does substratum or location define communities? International Review of Hydrobiology 92: 452–464. https://doi.org/10.1002/iroh.200610991.
Eiseler, B., 2005. Identification key to the mayfly larvae of the German Highlands und Lowlands. Lauterbornia 53: 1–112.
Erman, N. & D. Erman, 1995. Spring permanence, Trichoptera species richness, and the role of drought. Journal of the Kansas Entomological Society 62: 50–64.
Escarpinati, S. C., R. Roque, P. Boroso Medina & J. Raizer, 2011. Macroinvertebrate community in recreational areas in a karst river (Bonito, Brazil): implication for biomonitoring of tourist activities. Tourism and Karst Areas 4: 121–130.
European Committee for Standardization (CEN), 1994. EN 27828, Water Quality – Methods for Biological Sampling – Guidance on Hand-Net Sampling of Benthic Macroinvertebrates, EN 27828:1994. European Committee for Standardization.
European Committee for Standardization (CEN), 2012. EN ISO 10870, Water Quality – Guidelines for the Selection of Sampling Methods and Devices for Benthic Macroinvertebrates in Freshwaters, EN 10870:2012. European Committee for Standardization.
Fochetti, R., 2020. Diversity, Threats, decline and conservation of European stoneflies (Plecoptera, Insecta). In Reference Module in Earth Systems and Environmental Sciences. Elsevier. https://doi.org/10.1016/B978-0-12-821139-7.00014-3.
Ford, T. D. & H. M. A. Pedley, 1996. Review of tufa and travertine deposits of the world. Earth-Science Reviews 41: 117–175. https://doi.org/10.1093/jis/14.1.54.
Ford, D. & P. Williams, 2007. Karst Hydrogeology and Geomorphology. Wiley, Chichester: 562. https://doi.org/10.1002/9781118684986.
Gojšina, V., M. Vujić & V. Marković, 2022. A new record of Bythinella istoka Glöer & Pešić, 2014 (Gastropoda: Bythinellidae) from Serbia. Acta Zoologica Bulgarica 74: 329–332.
Golubić, S., G. Violante, A. Plenković-Moraj & T. Grgasović, 2008. Travertines and calcareous tufa deposits: an insight into diagenesis. Geologica Croatica 61: 363–378. https://doi.org/10.4154/GC.2008.28.
Gronroos, M., J. Heino, T. Siqueira, V. L. Landeiro, J. Kotanen & L. M. Bini, 2013. Metacommunity structuring in stream networks: roles of dispersal mode, distance type, and regional environmental context. Ecology and Evolution 3: 4473–4487.
Guo, B., W. Zang & W. Lue, 2020. Spatial–temporal shifts of ecological vulnerability of Karst Mountain ecosystem – impacts of global change and anthropogenic interference. Science of the Total Environment 741: 140256. https://doi.org/10.1016/j.scitotenv.2020.140256.
Haidekker, A. & D. Hering, 2007. Relationship between benthic insects (Ephemeroptera, Plecoptera, Coleoptera, Trichoptera) and temperature in small and medium-sized streams in Germany: a multivariate study. Aquatic Ecology 42: 463–481. https://doi.org/10.1007/s10452-007-9097-z.
Hao, Y., T. C. J. Yeh, Z. Gao, Y. Wang & Y. Zhao, 2006. A gray system model for studying the response to climatic change: the Liulin karst springs, China. Journal of Hydrology 328: 668–676. https://doi.org/10.1016/j.jhydrol.2006.01.022.
Hartmann, A., N. Goldscheider, T. Wagener, J. Lange & M. Weiler, 2014. Karst water resources in a changing world: review of hydrological modeling approaches. Reviews of Geophysics 52: 218–242. https://doi.org/10.1002/2013RG000443.
Holuša, O. & K. Holušová, 2022. Population density and abundance of the northernmost population of Cordulegaster heros (Anisoptera: Cordulegastridae) in Europe (Czech Republic) with notes on its biogeographical range. Diversity 14: 854. https://doi.org/10.3390/d14100854.
Hrovat, M., G. Urbanič & I. Sivec, 2009. Community structure and distribution of Ephemeroptera and Plecoptera larvae in lowland karst rivers in Slovenia. Aquatic inSects 31: 343–357. https://doi.org/10.1080/01650420902811919.
Ivanković, L., M. Ivković & I. Stanković, 2019. Perennial phenology patterns and ecological traits of Dixidae (Insecta, Diptera) in lotic habitats of a barrage lake system. Limnologica 76: 11–18. https://doi.org/10.1016/j.limno.2019.03.001.
Ivković, M. & A. C. Pont, 2016. Long-time emergence patterns of Limnophora species (Diptera, Muscidae) in specific karst habitats: tufa barriers. Limnologica 61: 29–35. https://doi.org/10.1016/j.limno.2016.09.003.
Ivković, M., V. Mičetić Stanković & Z. Mihaljević, 2012. Emergence patterns and microhabitat preference of aquatic dance flies (Empididae; Clinocerinae and Hemerodromiinae) on a longitudinal gradient of barrage lake system. Limnologica 42: 43–49. https://doi.org/10.1016/j.limno.2011.07.003.
Ivković, M., M. Kesić, Z. Mihaljević & M. Kúdela, 2014. Emergence patterns and ecological associations of some haematophagous blackfly species along an oligotrophic hydrosystem. Medical and Veterinary Entomology 28: 94–102. https://doi.org/10.1111/mve.12019.
Kazakis, N., K. Chalikakis, N. Mazzilli, C. Ollivier, A. Manakos & K. Voudouris, 2018. Management and research strategies of karst aquifers in Greece: literature overview and exemplification based on hydrodynamic modelling and vulnerability assessment of a strategic karst aquifer. Science of the Total Environment 643: 592–609. https://doi.org/10.1016/j.scitotenv.2018.06.184.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280. https://doi.org/10.1007/s004420100716.
Lewin, I., I. Czerniawska-Kusza, K. Szoszkiewicz, A. E. Ławniczak & S. Jusik, 2013. Biological indices applied to benthic macroinvertebrates at reference conditions of mountain streams in two ecoregions (Poland, The Slovak Republic). Hydrobiologia 709: 183–200. https://doi.org/10.1007/s10750-013-1448-2.
Maiolini, B., M. Carolli & L. Silveri, 2011. Ephemeroptera, Plecoptera and Trichoptera in springs in Trentino (South-eastern Alps). Journal of Limnology 70: 122–133. https://doi.org/10.3274/JL11-70-S1-09.
Marjanović, M., J. Milenković, M. Lukić, N. Tomić, A. Antić, R. S. Marković, J. Atanasijević, D. Božić, S. Buhmiler, M. Radaković, A. R. Radivojević, A. Langović Milićević, M. B. Gavrilov & S. B. Marković, 2022. Geomorphological and hydrological heritage of Mt. Stara Planina in SE Serbia: from river protection initiative to potential geotouristic destination. Open Geosciences 14: 275–293. https://doi.org/10.1515/geo-2022-0340.
Martini, J. & J. Waringer, 2021. Dynamic microhabitat shifts in space and time of caddisfly larvae (Insecta: Trichoptera) in a first-order calcareous mountain stream. Biologia 76: 2527–2541. https://doi.org/10.1007/s11756-021-00741-w.
Matoničkin Kepčija, R., I. Habdija, B. Primc-Habdija & M. Miliša, 2006. Simulid silk pads enhance tufa deposition. Archiv Für Hydrobiologie 166: 387–409. https://doi.org/10.1127/0003-9136/2006/0166-0387.
Medeiros, A. S., D. Milošević, D. R. Francis, E. Maddison, S. Woodroffe, A. Long, I. R. Walker, L. Hamerlík, R. Quinlan, P. Langdon, K. P. Brodersen & Y. Axford, 2020. Arctic chironomids of the northwest North Atlantic reflect environmental and biogeographic gradients. Journal of Biogeography 48: 511–525. https://doi.org/10.1111/jbi.14015.
Megerle, H. E., 2021. Calcerous tufa as invaluable geotopes endangered by (over-)tourism: a case study in the UNESCO Global Geopark Swabian Alb, Germany. Geosciences 11: 198. https://doi.org/10.3390/geosci-ences1105019.
Mičetić Stanković, V., B. Bruvo Mađarić, M. A. Jäch & M. Kučinić, 2018. Elmis rietscheli Steffan, 1958 (Insecta: Coleoptera: Elmidae) in Croatia: first record and DNA barcoding. Natura Croatica 27: 185–194. https://doi.org/10.20302/nc.2018.27.9.
Mičetić Stanković, V., B. Bruvo Madarić & M. Kučinić, 2022. Ubiquitous but ignored? A case of water beetle in Southeastern Europe. Diversity 14: 26. https://doi.org/10.3390/d14010026.
Miliša, M., R. M. Kepčija, I. Radanović, A. Ostojić & I. Habdija, 2006. The impact of aquatic macrophyte (Salix sp. and Cladium mariscus (L.) Pohl.) removal on habitat conditions and macroinvertebrates of tufa barriers (Plitvice Lakes, Croatia). Hydrobiologia 573: 183–197. https://doi.org/10.1007/s10750-006-0271-4.
Miljković, Đ, L. Miljković & M. Jovanović, 2020. Conservation of geomorphological heritage in the Homolje Area (Eastern Serbia) – current state and perspective. Geoconservation Research 3: 49–61. https://doi.org/10.30486/gcr.2020.1903646.1024.
Moog, O., W. Graf, B. Janecek & T. Ofenböck, 2017. Sensitive taxa. In Moog, O. & A. Hartmann (eds), Fauna Aquatica Austriaca 3rd ed. BMLFUW, Wien.
Mori, N., 2005. Macroinvertebrate communities of karst springs in the Julian Alps in relation to environmental factors. Natura Sloveniae 5: 17.
Nerantzaki, S. D. & N. P. Nikolaidis, 2020. The response of three Mediterranean karst springs to drought and the impact of climate change. Journal of Hydrology 591: 125296. https://doi.org/10.1016/j.jhydrol.2020.125296.
Nilson, A., 1997. Aquatic insects of North Europe. In A Taxonomic Handbook. Odonata–Diptera, Vol. 2. Apollo Books, Stenstrup: 93–427.
Official Gazette of RS No. 5/2010, 47/2011, 32/2016, and 98/2016: Rulebook on the Declaration and Protection of Strictly Protected and Protected Wild Species of Plants, Animals, and Fungi. Convention on the Conservation of Migratory Species of Wild Animals.
Oksanen, J., R. Kindt, P. Legendre, B. O’Hara, M. H. H. Stevens & M. J. Oksanen, 2007. The Vegan Package-Community Ecology Package. R package version 2007, 2: 5-6.
Olmi, M., 1976. Coleoptera, Dryopidae, Elminthidae. In Fauna d’Italia. Sotto gli auspici dell' Accademia Nazionale Iteliana di Entomologia e dell'Unione Zoologica Italiana, Vol. XII. Edizioni Calderini, Bologna: 19–234.
Pauls, S. U., H. T. Lumbsch & P. Haase, 2006. Phylogeography of the montane caddisfly Drusus discolor: evidence for multiple refugia and periglacial survival. Molecular Ecology 15: 2153–2169. https://doi.org/10.1111/j.1365-294x.2006.02916.x.
Paunović, M., B. Tubić, M. Kračun, V. Marković, V. Simić, K. Zorić & A. Atanacković, 2012. Ecoregions delineation for the territory of Serbia. Water Research and Management 2: 65–74.
Peeters, E. T., R. Gylstra & J. H. Vos, 2004. Benthic macroinvertebrate community structure in relation to food and environmental variables. Hydrobiologia 519: 103–115.
Pešić, V., B. Gligorović, A. Savić & P. Buczyński, 2017. Ecological patterns of Odonata assemblages in karst springs in central Montenegro. Knowledge and Management of Aquatic Ecosystems 418: 3. https://doi.org/10.1051/kmae/2016035.
Pešić, M., S. Milić, M. Nujkić & M. Marić, 2020. Determination of heavy metal concentration and correlation analysis of turbidity: a case study of the Zlot Source (Bor, Serbia). Water, Air, and Soil Pollution. https://doi.org/10.1007/s11270-020-4453-x.
Petrović, A., 2014. Mogućnost korišćenja baze podataka u strategiji konzervacije biodiverziteta makrobeskičmenjaka kopnenih voda na nacionalnom nivou. PhD, Univerzitet u Kragujevcu, Prirodno-matematički fakultet, Kragujevac.
Podani, J. & B. Csányi, 2010. Detecting indicator species: some extensions of the IndVal measure. Ecological Indicators 10: 1119–1124. https://doi.org/10.1016/j.ecolind.2010.03.010.
Pozojević, I., M. Ivković, K. A. Cetinić & A. Previšić, 2021. Peeling the layers of caddisfly diversity on a longitudinal gradient in karst freshwater habitats reveals community dynamics and stability. InSects 12: 234. https://doi.org/10.3390/insects12030234.
Previšić, A., M. Kerovec & M. Kučinić, 2007. Emergence and composition of Trichoptera from karst habitats, Plitvice Lakes Region, Croatia. International Review of Hydrobiology 92: 61–83. https://doi.org/10.1002/iroh.200510921.
Rađa, B. & M. Šantić, 2014. Community structure of aquatic insects in the karstic Jadro River in Croatia. Journal of Insect Science. https://doi.org/10.1093/jis/14.1.54.
Ranđelović, N. & D. Avramović, 2004. Protected nature areas, flora and vegetation in vicinity of Soko Banja. Natura Montenegrina 3: 379–386.
Rezende, R. S., A. M. Santos, C. Henke-Oliveira & J. J. F. Gonçalves, 2014. Effects of spatial and environmental factors on benthic a macroinvertebrate community. Zoologia 31: 426–434. https://doi.org/10.1590/s1984-46702014005000001.
Ridl, A., M. Vilenica, M. Ivković, A. Popijač, I. Sivec, M. Miliša & Z. Mihaljević, 2018. Environmental drivers influencing stonefly assemblages along a longitudinal gradient in karst lotic habitats. Journal of Limnology 77: 412–427. https://doi.org/10.4081/jlimnol.2018.1816.
Rimcheska, B., V. Slavevska-Stamenković, H. Ibrahimi, S. Smiljkov, M. Ristovska & M. Paunović, 2015. First record of the genus Helicopsyche von Siebold, 1856 (Trichoptera: Helicopsychidae) from the Republic of Macedonia. Acta Zoologica Bulgarica 67: 443–446.
Rodriguez, P., N. Vučković & M. Kerovec, 2020. New species of aquatic oligochaetes (Annelida: Clitellata) from tufa barriers in Croatia. Zootaxa 4758: 442–460. https://doi.org/10.11646/zootaxa.4758.3.2.
Rozkošný, R., 1980. Klíč vodnich lareu hmyzu, Vydala academia nakladatelstvi Československé akademie véd, Praha:
Savić, A., D. Dmitrović & V. Pešić, 2017. Ephemeroptera, Plecoptera, and Trichoptera assemblages of karst springs in relation to some environmental factors: a case study in central Bosnia and Herzegovina. Turkish Journal of Zoology 41: 119–129. https://doi.org/10.3906/zoo-1512-31.
Šemnički, P., A. Previšić, M. Ivković, K. Čmrlec & Z. Mihaljević, 2012. Tufa barriers from a caddisfly’s point of view: streams or lake outlets? International Review of Hydrobiology 97: 465–484. https://doi.org/10.1002/iroh.201101500.
Sertić Perić, M., M. Miliša, R. M. Kepcija, B. Primc-Habdija & I. Habdija, 2011. Seasonal and fine-scale spatial drift patterns in a tufa depositing barrage hydrosystem. Fundamental and Applied Limnology 178: 131–145. https://doi.org/10.1127/1863-9135/2011/0178-0131.
Shannon, C. E., 1948. A mathematical theory of communication. The Bell System Technical Journal 27(379–423): 623–656.
Simović, P., V. Simić, D. Milošević & A. Petrović, 2023. New records of species Taeniopteryx hubaulti Aubert, 1946 and Taeniopteryx schoenemundi (Mertense, 1923) (Plecoptera: Taeniopterygidae) in Serbia. Journal of Entomological Research Society 25: 155–166. https://doi.org/10.51963/jers.v25i1.2274.
Simpson, E., 1949. Measurement of diversity. Nature. https://doi.org/10.1038/163688a0.
Špoljar, M., D. Štafa, A. Ostojić, T. Dražina, R. Matoničkin-Kepčija, K. Kralj Borojević & B. Princ, 2011. Tufa deposition in a karst stream as an indicator of water quality (Papuk Nature Park, Croatia). Ribararstvo, Croatian Journal of Fisheries 69: 137–151.
Telbisz, T., J. Ćalić, J. Kovačević-Majkić, R. Milanović, J. Brankov & J. Micić, 2021. Karst geoheritage of Tara National Park (Serbia) and its geotouristic potential. Geoheritage 13: 88. https://doi.org/10.1007/s12371-021-00612-5.
Theissinger, K., M. Bálint, K. A. Feldheim, P. Haase, J. Johannesen, I. Laube & S. U. Pauls, 2012. Glacial survival and post-glacial recolonization of an Arctic-Alpine freshwater insect (Arcynopteryx dichroa, Plecoptera, Perlodidae) in Europe. Journal of Biogeography 40: 236–248. https://doi.org/10.1111/j.1365-2699.2012.02793.x.
Tierno de Figueroa, J. M., M. J. López-Rodríguez, A. Lorenz, W. Graf, A. Schmidt-Kloiber & D. Hering, 2010. Vulnerable taxa of European Plecoptera (Insecta) in the context of climate change. Biodiversity and Conservation 19: 1269–1277. https://doi.org/10.1007/s10531-009-9753-9.
Timm, T., 2009. A guide to the freshwater Oligochaeta and Polychaeta of Northern and Central Europe. Lauterbornia 66: 1–235.
Van Haaren, T. & J. Soors, 2013. Aquatic Oligochaetes of The Netherlands and Belgium, KNNV Publishing, Zeist:
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137. https://doi.org/10.1139/F80-017.
Verschut, T. A., E. Meineri & A. Basset, 2015. Biotic interactions affect the colonization behavior of aquatic detritivorous macroinvertebrates in a heterogeneous environment. Estuarine, Coastal and Shelf Science 157: 120–128.
Vidinova, Y., V. Tyufekchieva, M. Ihtimanska, V. Evtimova, E. Varadinova, M. Todorov & G. Georgieva, 2022. Benthic macroinvertebrate assemblages in karst springs ecosystems in Bulgaria. Acta Zoologica Bulgarica 16: 15–19.
Vilenica, M., 2017. Ecological traits of dragonfly (Odonata) assemblages along an oligotrophic Dinaric karst hydrosystem. Annales De Limnologie 53: 377–389. https://doi.org/10.1051/limn/2017019.
Vilenica, M., V. Mičetić Stanković, M. Sartori, M. Kučinić & Z. Mihaljević, 2017. Environmental factors affecting mayfly assemblages in tufa-depositing habitats of the Dinaric Karst. Knowledge and Management of Aquatic Ecosystems 418: 14. https://doi.org/10.1051/kmae/2017005.
Vilenica, M., M. Bilić, V. Mičetić Stanković & M. Kučinić, 2018a. Mayfly ecological traits in a European karst spring: species, microhabitats, and life histories. Community Ecology 19: 248–258. https://doi.org/10.1556/168.2018.19.3.6.
Vilenica, M., V. Mičetić Stanković, Z. Mihaljević & M. Kučinić, 2018b. Alderfly assemblages (Megaloptera, Sialidae) along an oligotrophic karst hydrosystem: spatial patterns and species–environment relationship. Biologia 73: 493–503. https://doi.org/10.2478/s11756-018-0056-z.
Vilenica, M., M. Rumišek, F. Rebrina, R. Matoničkin Kepčija, K. Medak, V. Gulin & A. Brigić, 2021. Dinaric karst intermittent rivers harbour some rare mayfiles (Insecta, Ephemeroptera). Natura Croatica 30: 377–387. https://doi.org/10.20302/NC.2021.30.24.
Waringer, J. & W. Graf, 2011. Atlas der mitteleuropäischen Köcherfliegenlarven: Atlas of Central-European Trichoptera Larvae, Erik Mauch Verlag, Dinkelscherben:
Waringer, J., W. Graf, T. Pitsch, S. U. Pauls, A. Previšić & M. Kučinić, 2011. Description of the larval stage of Drusus mixtus (Pictet, 1834) (Trichoptera: Limnephilidae: Drusinae) with notes on its ecology and zoogeography. Limnologica 41: 249–255. https://doi.org/10.1016/j.limno.2010.10.006.
Zwick, P., 2004. Key to the West Palaearctic genera of stoneflies (Plecoptera) in the larval stage. Limnologica 34: 315–348. https://doi.org/10.1016/S0075-9511(04)80004-5.
Acknowledgements
The authors express their gratitude to Andrija Dumanović for his help with the figure's design and graphic image processing. The authors thank all anonymous reviewers for their comprehensive review, valuable and constructive comments, and suggestions, which helped improve the quality of this manuscript.
Funding
This work was supported by the Serbian Ministry of Science, Technological Development, and Innovation under grant No. 451-03-66/2024-03/200122 and 451-03-65/2024-03/200122.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Verónica Ferreira
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Simović, P., Milošević, D., Simić, V. et al. Benthic macroinvertebrates in a tufa-depositing environment: a case study of highly vulnerable karst lotic habitats in Southeast Europe. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05629-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05629-9