Abstract
The role of benthic macroinvertebrate and fish communities for assessing the ecological quality of an artificial re-constructed, after 50 years of dryness, Mediterranean water body (Karla Reservoir, Greece) is presented. Moreover, we provide knowledge on the structure of the biological communities and their functioning role, for inspiring feature actions that will contribute to biodiversity protection and ecosystem services. Water (physicochemical parameters), benthic macroinvertebrates, and fish were monitored during a 2-year survey (2013–2015) in Karla and Kalamaki reservoirs and the inflowing ditches. A clear temporal pattern was evident for all sampling stations studied, differentiating the low- and high-flow period samples as to their physicochemical parameters. Redundancy analysis (RDA) revealed NO3-N, total nitrogen and total dissolved phosphorous as the most significant environmental parameters in explaining benthic invertebrate variance in ditches. Generally, tolerant to organic pollution macroinvertebrate taxa were abundant in ditches and reservoirs, while the fish fauna in Karla was composed almost exclusively of planktivorous and invertivorous species. Macroinvertebrate (GLBiI) and fish (GLFI) indices classified the ecological quality of Karla Reservoir as “poor” while ditches were classified as “bad” according to HESY-2. The anthropogenic pressures applied in the catchment and the benefits of improving water quality are discussed in the context of the implementation of Water Framework Directive 2000/60/EC for introducing sustainable management plans, taking into account some ecological restoration principles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The study of biological diversity is necessary for the conservation and sustainable management of natural resources, as was declared by the Convention on Biological Diversity in Rio de Janeiro (1992). Knowledge of taxonomic diversity and its dynamic is a fundamental requisite to conserve any ecosystem and to suggest management implications. Yet, the relationship among biological, chemical and physical components of the ecosystems is the basis of biological monitoring. For this reason, the Water Framework Directive 2000/60/EC (WFD; European Commission 2000) requires a solid evidence about the structure of the biological quality elements (BQEs) across European water bodies linking their variability to the ecological status. A diversity of biological indicators including phytoplankton, macrophytes, benthic invertebrates, fish and diatoms has been used to assess the water quality in European water bodies.
Benthic macroinvertebrates have been proved as suitable bioindicators of multiple stressors, based on their ability to respond to a variety of environmental variables (Hering et al. 2003; Brabec et al. 2004; Ofenböck et al. 2004; Bennett et al. 2011; Birk et al. 2012; Pardo et al. 2014). Benthic organisms provide important ecosystem services including mixing the sediment, nutrient cycling and channeling energy to higher trophic levels (Covich et al. 1999; Vander Zanden and Vadeboncoeur 2002; Karatayev et al. 2013). However, benthic invertebrate communities are controlled by many factors acting at multiple temporal and spatial scales such as catchment-scale variables (Townsend et al. 2003) and catchment geology (Allan and Johnson 1997), as well as microhabitat-scale variables (Brosse et al. 2003). Moreover, anthropogenic pressures may strongly affect macroinvertebrate communities classifying the hydromorphological alterations among the main stressors in water bodies (Moreno and Callisto 2006). The high availability of benthic fauna methods reflects the considerable tradition (Rosenberg and Resh 1993) of macroinvertebrate-based aquatic monitoring due to the limited mobility, variety of traits and adaptations of benthic animals.
On the other hand, fish are considered as potentially effective indicators of the conditions of aquatic ecosystems (Fausch et al. 1990; Hilty and Merenlender 2000; Joy and Death 2002; Whitfield and Elliott 2002), since they are sensitive to a wide array of stressors, relatively long-lived (reflecting long-term environmental degradation) and top predators in the aquatic ecosystems (integrating adverse effects on other components) and can be used to evaluate societal cost of degradation, due to their economic and aesthetic value (Karr 1981, Karr et al. 1986). Thus, fish communities can, in general, reflect watershed conditions and, consequently, have been so far used in biological monitoring to assess environmental health (Karr 1987; Oberdorff et al. 2002). Moreover, the biotic interactions associated with fish are important in determining the state of shallow lakes. High fish biomass is stabilizing the turbid state, while reduction in fish biomass leads to clear water state (Gulati and Van Donk 2002), enhancing thus fish manipulation as an efficient restoration tool in shallow lakes (Jeppesen et al. 1990). Moreover, it is well documented that water bodies affected by human activities and modification are more likely to be susceptible to invasive fish species. Thus, the structure and composition of the fish fauna can be used as indicators of conditions, which may need attention for remediation.
Reservoirs, like lakes, are geologically transitory systems and, on the scale of human life, are considered as permanent features on the landscape. The recently re-constructed Karla Reservoir (Thessaly, Greece) was formerly listed among the shallow natural lakes in Greece. In terms of biodiversity, the former Lake Karla endowed with a variety of habitats (pelagic, floating vegetation, shallow marshes with Juncus sp. and Typha sp., emergent vegetation and rocks) and had the ability to support a rich fish and bird fauna (Jerrentrup 1990). Thus, the re-colonization, the re-occupancy and consequently the variability of the benthic and fish communities constitute a great challenge regarding the re-established reservoir. In general, at regional scale, the mechanisms behind the re-colonization and the distribution patterns of both biocommunities are mainly related to the historic conditions, the abiotic factors of the habitat, the structure of the surrounding landscape and biological factors (Voshell and Simmons 1984; Pamplin et al. 2006). Multiple environmental stressors can also shape the spatial and temporal patterns of both communities; the range of physical, chemical and biological conditions experienced in a given site will support a characteristic set of macroinvertebrate and fish species. Any disturbances can disrupt ecosystem processes and alter community structure by affecting resource availability and physical and chemical conditions (Collier et al. 1998; Moreno and Callisto 2006).
Although this newly re-established water body is considered a vital aquatic ecosystem as it is listed in the network of Natura 2000 and has been characterized as a permanent wildlife refuge, 5 years after its re-filling, it is exposed to point and diffuse pollution sources leading already to a progressive eutrophication with frequent occurrence of algal blooms (Gkelis et al. 2017). In addition, scientific knowledge on the structure of the biological communities, hence on the functioning of the new reservoir, is still inadequate.
The main objective of this research was the assessment of benthic macroinvertebrate and fish assemblages in the re-constructed Karla Reservoir and the inflowing ditches. We also examined the following: (a) water physicochemical parameters and their possible influence in structuring benthic communities, (b) whether the biotic and abiotic environment in the ditches is linked to that in the reservoirs and (c) the use of benthic macroinvertebrate and fish as indicators of water ecological quality. Finally, we address management implications for improving the ecological potential of the re-constructed reservoir. The presented results constitute the first record after Karla’s re-filling.
Materials and methods
Study area
The former natural Lake Karla occupied the lowest part of its natural basin until 1962, when it completely dried for providing more agricultural land. The structure and function of Lake Karla was intimately linked with Pineios River, since a complex irrigation and drainage network connected the river with the lake. The river occasionally overflowed, and floodwaters rich in oxygen and nutrients drained into Karla. Moreover, surface runoff from the watershed supplied the lake with large quantities of freshwater through several streams. Because of its moderately sloping bed, the lake surface area fluctuated between 40 and 180 km2, depending on the water level fluctuation. Much of the surrounding farmland was inundated when floodwaters were held in the lake.
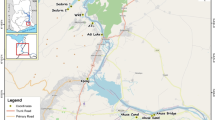
Nowadays, Karla undergoes restoration for establishing a “new” reservoir. The new constructed reservoir (Fig. 1) occupies a maximum area of about 38 km2, through the construction of two embankments, one in the eastern and one in the western part of the water body. The connection between Karla Reservoir and Pineios River is supported by a dense network of ditches (named 1T and 2T) (Fig. 1), which transfer the flood runoff from the river to Karla, as the reservoir is located in the lower part of the basin, simulating the pre-disturbance conditions. In addition, four collector channels (named S3, S4, S6, S7) (Fig. 1) drain the surface runoff from the higher-elevation zones of the watershed into the reservoir. The surface runoff of the lower elevation areas is pumped into the reservoir while the maximum allowable volume of reservoir is 180 hm3. However, ditches along with a secondary reservoir (Kalamaki Reservoir, Fig. 1), which serves for irrigation of the surrounding agricultural area, support floral and faunal elements that in turn might affect the biological processes of Karla. Nonetheless, the complex irrigation and drainage network may affect directly also the physicochemical properties in Karla Reservoir, due to the long-term water retention.
The climate in the area is typical continental, characterized by cold and wet winters and hot and dry summers. Mean annual precipitation and potential evapotranspiration in the watershed are about 560 and 775 mm respectively, while the mean annual temperature is 14.3 °C (Vasiliades and Loukas 2009). Geologically, the basin consists mainly of recent grains of various sizes originating from the former lake’s deposits. The plain comprises of aquifers, essentially sandy intercalations separated by layers of clay to silty-clay, and is bound by schist and karstic limestones or marbles. About 30.6% of the total area is covered by impermeable geological structures, while karstic and permeable structures cover a 14.5 and 54.9% of the plain.
Water sampling
Samples from the water column were collected using a Niskin-type sampler from three sampling stations in Karla Reservoir (KL1, KL2, KL3) and one in the adjacent Kalamaki Reservoir (KK) (Fig. 1). Water temperature (°C), pH, conductivity (μS/cm) and dissolved oxygen (DO, mg/l) were measured in situ using the Aqua Read AP-2000 probe (Kent, GB) and Secchi depth was also recorded. Concentrations of nitrate (NO3-N, mg/l), nitrite (NO2-N, mg/l) and ammonium (NH4-N, mg/l) nitrogen, total nitrogen (TN, mg/l), total phosphorus (TP, mg/l), total dissolved phosphorus (TDP, mg/l) and biological oxygen demand (BOD5, mg/l), in water samples were analyzed according to standard methods (APHA 2005). The same parameters (except Secchi depth) were also recorded in ditches 1T, 2T, 7T and 9T and channel S3 (Fig. 1). Water sampling was conducted bimonthly (the first year) and every 3 months (the second year) in reservoirs for two subsequently years (July 2013–July 2015), whereas biannually (September, March/April) in ditches, covering the same period.
Macroinvertebrate sampling
Benthic macroinvertebrates were sampled from the soft sediments at three stations (KL1, KL2, KL3) in Karla Reservoir (Fig. 1), using an Ekman-Birge grab (three replicates, 225 cm2 sampling area). Sampling was conducted in September (autumn) and March/April (spring) of the 2 years of survey, representing respectively the low- and high-flow conditions in the area. Samples were sieved with a 200-mm mesh and fixed in 10% v/v neutralised formaldehyde. After sorting, benthic macroinvertebrates were identified to the lowest possible taxon (Klink and Moller Pillot 2003; Brooks et al. 2007; Vallenduuk and Moller Pillot 2007; Timm 2009; Orendt and Spies 2014) and their abundance was converted to density (ind/m2).
Benthic macroinvertebrates were also sampled in the ditches (KR1, KR2, KR4, KR5, KR7) (Fig. 1). The KR3 station, belonging to collector channel S3 flowing into Karla Reservoir (Fig. 1), was drained during the survey and KR6 (Fig. 1) was not sampled, due to the cemented substrate. Sampling was conducted using a 250 mm × 230 mm, D-shaped pond net (0.9 mm mesh size, ISO 7828: 1985; EN 27828: 1994) according to the semi-quantitative 3-min kick/sweep method (Armitage and Hogger 1994) plus 1-min sweeping the bank vegetation, when existed (Wright 2000; Kemitzoglou 2004). All microhabitats were covered proportionally, based on a matrix of possible river habitats (Lazaridou et al. 2018; modified from Chatzinikolaou et al. (2006)). The same sampling procedure was also followed at the littoral zone of Kalamaki Reservoir (KK), due to its coarse substrate. Benthic samples were sieved with a 500-mm mesh and fixed in 10% v/v neutralised formaldehyde. Specimens from the ditches were identified mainly to family level (except of Ostracoda, Hydracarina, Araneae and Oligochaeta, apart from Tubificidae) using taxonomic keys (Tachet et al. 2010).
Fish sampling
Fish sampling in the reservoir was conducted once, in June 2014, due to the favorable hydrological conditions (i.e. the greatest quantity of water) prevailing at the time. A total of 12 benthic gillnets with multiple mesh sizes (Nordic type: 30 m × 1.5 m, length × height, 5–55 mm, from knot-to-knot) were used to meet the requirements of the European sampling protocol CEN (2005). Gill nets were set in the afternoon and lifted the next morning, ensuring a stable soak time of about 12 h. In addition, electrofishing (CEN 2003; FAME Consortium 2004) was conducted in the main ditches (stations KR2 and KR8) that inflow in Karla, biannually, in June and November 2014. All captured fish were identified to species level (Kottelat and Freyhof 2007), and their total length (TL, cm) and weight (W, g ± 0.1) were measured. The abundance of each species caught was expressed in terms of number (NPUE, individuals/m2 of gillnet or sampling area) and weight (BPUE, g/m2 of gillnet or sampling area).
Ecological quality assessment
Following the Guidance document, No 13 (European Commission 2005) established by the Water Framework Directive 2000/60/EC, biological indices in compliance with WFD were applied to evaluate the ecological quality of the ditches and the new Karla Reservoir. Specifically, the Greek Lake Benthic invertebrate Index (GLBiI; Ntislidou et al. 2016) and the Greek Lake Fish Index (GLFI; Petriki et al. 2016, 2017) were applied for assessing the ecological quality of Karla Reservoir. Moreover, the Hellenic Evaluation System 2 (HESY2) (Lazaridou et al. 2018), based on benthic macroinvertebrates, was used for estimating the water quality in ditches which belong to the R-M3 river type.
Data analyses
Cluster analysis with SIMPROF routine permutation test for assessing statistically significant groups (Clarke and Warwick 2001) was applied on the physicochemical and benthic macroinvertebrate data, to identify possible spatial or temporal patterns. Prior to the analysis, we assumed each biotic and abiotic parameter as means representing spring (March/April samples; high-flow conditions) and autumn (September samples; low-flow conditions). Consequently, data were log(x + 1) transformed with the exception of water temperature and pH, which were standardized. Euclidean distance and Bray-Curtis similarity indices were used for the extraction of the resemblances for the physicochemical and benthic macroinvertebrate data, respectively (Clarke and Warwick 2001). Then, the analysis of similarities (ANOSIM) was used, to test whether the differences between the groups, resulted from Cluster analysis, were statistically significant. In addition, the similarity percentages (SIMPER) analysis was performed to identify which parameters and taxa contributed most (cut-off contribution 90%) for similarities and dissimilarities within and between groups (Clarke et al. 2014). The above statistical analyses were applied using Primer v6 software (Clarke and Gorley 2006).
Detrended correspondence analysis (DCA) (indirect gradient analysis) (program CANOCO version 4.5.1; Ter Braak and Šmilauer 1998) was performed to the benthic macroinvertebrate data, in order to verify if they corresponded linearly to the environmental gradient or their abundances peaked around an environmental optimum (unimodal response) (Ter Braak and Šmilauer 1998). In this study, the length of the gradient of the first axis was three times less than the length of the within-sample standard deviation (Ter Braak and Šmilauer 1998). Thus, redundancy analysis (RDA) was further applied to link the environmental parameters to species. In order to decide which parameters will be kept in RDA, the Monte Carlo permutation test was performed to select the statistically significant (p < 0.05) environmental parameters while the inflation factor (> 20) was used for assessing multi-collinearity.
Results
Physicochemical parameters
Physicochemical parameters in Karla and Kalamaki reservoirs, as well as, the adjacent ditches are summarized in Table 1. The newly established Karla Reservoir is an alkalic, “warm” water body. Its water temperature reached 31 °C during the surveyed period, while the mean water temperature was about 20 °C (Table 1). Nitrate-N was the most important form in the dissolved inorganic nitrogen pool (NO3-N, NO2-N, NH4-N). TN and TP exhibited maximum values in Karla Reservoir during the low-flow season; mean ammonium-N value was lower in Karla (0.7 mg/l) compared to that in Kalamaki Reservoir (3.9 mg/l) and the ditches (3.2 mg/l), but always higher than the limit considering fish life (0.2 mg/l; Directive 2006/44/EC; European Commission 2006). TP reached 29.2 mg/l in Karla Reservoir, 25.6 mg/l in Kalamaki Reservoir and 3 mg/l in the ditches (Table 1). Water in Karla did not show any “clear-water phase” (Balvay et al. 1990; Talling 2003) during the monitoring period; the Secchi depth ranged between 5 and 30 cm, reflecting its turbid character. The organic load, expressed by BOD5, ranged from 2.8 to 9.6 mg/l in Karla, 3.1 to 9.1 mg/l in Kalamaki and 1.1 to 11.2 mg/l in the ditches.
Cluster analysis revealed a clear temporal pattern (Fig. 2) representing the high- (spring, group a) and low-flow (autumn, group b) periods (ANOSIM; R = 0.993, p = 0.001). SIMPER analysis extracted temperature (57.02% contribution), pH (20.67%), TDP (4.31%) and TP (4.19%) as the main parameters differentiating these groups. Indeed, the majority of the parameters monitored during the study exhibited higher mean values in autumn than in spring (Fig. 3). However, within each group, no clear spatial pattern was evident, as to the grouping of the sampling stations (Fig. 2). Nonetheless, stations KR1 and KR5 were differentiated from the rest of the ditches, grouped with the reservoirs in autumn (Fig. 2), mainly due to TDP (19.96% contribution) and pH (17.36%) according to SIMPER analysis.
Benthic macroinvertebrates
A total of 56,489 individuals belonging to 11 taxa (Oligochaeta: Potamothrix hammoniensis; Chironomidae: Chironomus plumosus gr, Procladius sp., Microchironomus tener, Tanytarsini, Cryptochironomus sp., Pollypedilum nubeculosum gr; Ceratopogonidae, Valvatidae, Lymnaeidae, Cordulegasteridae) were identified in Karla Reservoir. The most abundant species were Potamothrix hammoniensis (8840 ind/m2) and Microchironomus tener (5000 ind/m2) in station KL3 during the low-flow period in 2013 and 2014, respectively. The latter was found in all stations; however, its presence was not confirmed in station KL2 during the low-flow sampling in 2013. Spatially, the higher abundances were recorded in station KL3 (Fig. 4). In Kalamaki Reservoir, 9 families (174 individuals) were recorded: Hydrobiidae, Tubificidae, Erpobdellidae, Valvatidae, Physidae, Asellidae, Chironomidae, Curculionidae and Hydrophilidae. These families are tolerant to organic pollution (in accordance to HESY; Artemiadou and Lazaridou 2005), except of Hydrobiidae, which is tolerant to moderate levels of organic pollution.
A total of 7936 benthic macroinvertebrate specimens, belonging to 33 taxonomic groups, were sampled from the ditches. The seasonal and spatial variation of their abundances is presented in Fig. 5a. The highest abundance was recorded in station KR4 (1852 individuals) during autumn 2013, whereas the lowest (3 individuals) in station KR5 in autumn 2014. The most abundant families were Chironomidae (100%) and Hydrobiidae (91%) in stations KR5 and KR2, during the high-flow period in 2014 and 2015, respectively. Generally, tolerant to organic pollution taxa (Artemiadou and Lazaridou 2005) were abundant in almost all stations except KR2 in autumn 2015 (Fig. 5b).
Cluster analysis with SIMPROF test applied to benthic macroinvertebrate data in ditches revealed that almost all stations had similar assemblages. However, when applying the same analyses with reservoirs’ benthic data, Kalamaki and Karla reservoirs were differentiated (SIMPER; average dissimilarity 50.4), mainly due to the presence of Asellidae (contribution 26.08%) and Hydrobiidae (contribution 17.2%) only in Kalamaki Reservoir. When RDA analysis was applied for explaining benthic species variance in the ditches, three parameters, NO3-N, TN and TDP, were extracted as statistically significant (Monte Carlo test; p < 0.05) (Fig. 6), whereas TP and NH4-N were excluded, due to their high inflation factor (> 20). The first and all canonical axes of RDA analysis were statistically significant (p = 0.008 and p = 0.004, respectively), while the first two ordination axes of RDA explained 54% of the total species variance. Axis I (eigenvalue 0.313, p = 0.008) was related to NO3-N (intra-set correlation value 0.807) and TN (intra-set correlation value 0.762) while axis II (eigenvalue 0.227, p = 0.004) to TDP (intra-set correlation value − 0.401). Mainly, samples of stations KR1 and KR5 in both periods (spring and autumn 2014) and KR2 spring 2014 were ordered at the negative side of axis I, having the lowest NO3-N values while at the uppermost side of axis I, the KR4 autumn 2013 sample was placed. The rest of the samples were ordered around the 0 point of the two axes.
Fish
The fish fauna in the whole basin consisted of 10 species, the most (7) being recorded in Karla Reservoir (Table 2). Specifically, a total of 3492 specimens (total weight 82.7 kg) were caught by benthic gillnets. The most abundant species in terms of number (NPUE) and biomass (BPUE) was Lepomis gibbosus (77.06 and 64.44% respectively) (Fig. 7). Omnivorous species (Table 2) contributed 22.79% in the total biomass, while the introduced species 77.09% in the numerical abundance of the catch. Finally, the most abundant species in the ditches were Gambusia holbrooki (2.81 ind/m2) and Cyprinus carpio (5.41 g/m2), in terms of numbers and biomass respectively, while three species (Gobio feraeenis, Gambusia holbrooki and Knipowitchia thessala) were exclusively recorded there.
Ecological quality
Both indices GLFI and GLBiI classified the ecological quality of Karla Reservoir as “poor” (ecological quality ratio equal to 0.21 and 0.38, respectively) and therefore the ecological potential was estimated as “poor”. The HESY2 revealed a “bad” ecological quality in stations KR4 (autumn 2013 and spring 2015) and KR5 (spring 2014 and autumn 2014) and “poor” in the rest of the ditches (Table 3). Thus, the ecological potential of the ditches was classified as “poor”.
Discussion
The importance of restoring Karla water body and reversing the environmental conditions caused by anthropogenic activities was considered of high importance by the European Union (Natura 2000 network, 92/43/EC) and National Authorities (Laws 1650/86 and 3937/2011) for offering multi services, i.e. social, economic and ecological sustainable development, to the region and not just creating a new reservoir. Lake Karla, prior to the 1960s supported a diverse and very important ecosystem in the region, storing water for agricultural uses and fisheries and recharging the groundwater. However, the alteration of the watershed’s function resulted to the significant diminish of the groundwater aquifer. As a result, sea water intrusion and frequent flooding events were evident, affecting soil salinity and alkalinity increment. Moreover, nutrient enrichment through extended agrochemical application was recorded, leading to biodiversity loss and elimination of nature conservation values (Zalidis et al. 2004).
The water analysis through Karla’s water column, carried out in the 1950s (Ananiadis 1956) revealed high concentrations of dissolved nutrients and low of dissolved oxygen near the bottom, classifying the lake as eutrophic. According to the present nutrient profile, it becomes evident that Karla Reservoir is a eutrophic system, with apparent signals of hypertrophication during the warm period.
Although Kalamaki Reservoir is not characterized as a water body in the national inventory, results showed that both reservoirs had similar water quality, most possible due to their connection via a complex irrigation and drainage network. This knowledge could be useful in designing a future spatial sampling strategy in an optimal way, reducing the number of sampling stations and associated cost. The clear temporal differentiation in ditches between the low- (autumn) and high-flow (spring) periods revealed, also, the necessity of addressing management applications for agricultural activities in the study region. Additionally, nitrogen (NO3-N) concentrations (significantly correlated with RDA axis I) were low at KR1 and KR5 stations (corresponding to the Karla’s inflows), as well as in Karla Reservoir in spring. High NO3-N concentrations in ditch stations were observed during autumn. At the same time, the nutrients’ concentrations were also increased in Karla Reservoir, revealing that its water quality is clearly affected by water quality in ditches. Indeed, nitrogen loads, derive from point and non-point (agricultural) sources of pollution from the catchment (EGY 2013), enrich Karla Reservoir, as has also been observed in other Greek lakes (Latinopoulos et al. 2016). These loads lead to water degradation, favoring phytoplankton succession and dominance (Gkelis et al. 2017). On the other hand, the same was not evident for TDP, since high concentrations were observed in autumn in Karla, despite the low levels in ditches. It is feasible that the increase of phosphorus concentrations in Karla resulted by internal loading processes which are likely to occur, since the sediment has a long fertilization experience during the period of dryness (Chamoglou et al. 2014). Karla Reservoir is affected by both agricultural and industrial land uses in the surrounding area, leading to eutrophication and shifting algal community towards bloom-forming toxic cyanobacterial species (Gkelis et al. 2017). Having an extremely high water retention time, and no outflow makes the situation worse. The above support the assumption, referred to several other lakes (Lijklema 1994; Gulati and van Donk 2002), that Karla Reservoir acts as a “sink” for phosphorus. Moreover, since the two nutrients exhibit a N/P ratio < 10 (occasionally close to 0), the cyanobacteria dominance is favored, limiting nitrogen from the water column (Gkelis et al. 2017).
RDA and HESY2 applied to benthic macroinvertebrate data from the ditches revealed the worst quality in KR4 (“bad quality”). The rest of the ditches’ stations were also correlated with other physicochemical factors (e.g. TN, conductivity and BOD5) apart from the NO3-N and TDP, and they consequently had “poor” and “bad” quality. Additionally, KR1, which is located at the entrance of 1T ditch in Karla Reservoir, had the lowest NO3-Ν concentrations and the highest number of benthic macroinvertebrate taxa. Nonetheless, nitrate-nitrogen has been considered, among others, as an important stressor for driving benthic invertebrate richness (Camargo and Alonso 2006; Yuan 2010) through direct and indirect effects (Dodds and Welch 2000).
Although reservoirs share some common features with lakes, they have larger nutrient inputs and stronger water level fluctuations, leading to pronounced eutrophication and producing a shift in the biological structure. Their ageing process has been studied extensively (Straškraba et al. 1993; Joniak et al. 2003), highlighting that the first phase after the flooding is characterized by high nutrient concentrations while the next phase by the stabilization of the trophic status which is highly associated with the catchment’s influence. Yet, during this phase, biocommunities reach high densities and there is usually a dominance of certain phytoplankton species (Straškraba et al. 1993). Unfortunately, there are no data from the initial filling period of Karla Reservoir, thus the time lag of the first phase is quite undistinguishable. Regarding the nutrient profile assessed during the monitoring period along with the biocommunity abundances and the algal blooms during the last years (Gkelis et al. 2017; Sidiropoulos et al. 2017; personal observations), we assumed that the new reservoir is already in the very early of the second phase, keeping also a high trophic status. This is also supported by the fact that the zooplanktic community is dominated by small-size zooplankton species (Stabouli et al. 2012), which is frequently observed in shallow warm lakes (Beklioglu et al. 2007; Alexakis et al. 2013).
Five out of the 10 fish species (Alburnus thessalicus, Cobitis vardarensis, Gobio feraeensis, Knipowitchia thessala and Pachychilon macedonicum) that were recorded during the present study are endemics of Greece or near endemics (they are present in the adjacent countries), while Carassius gibelio, Lepomis gibbosus and Gambusia holbrooki are introduced species. Generally, the number of species recorded in the newly established Karla Reservoir and at the stations located in ditches was smaller than the one referred to the former natural lake and its basin. Specifically, 18 fish species are recorded in the basin (Ananiadis 1956; Economidis 1991; Economidis et al. 2003), among which is Cobitis stephanidisi. The species had a very restricted distribution, only around the former Lake Karla and in some springs of east Thessaly (Chasampali and Velestino springs which however are drained; Economidis et al. 2003). Unfortunately, it is nowadays considered as extinct (Legakis and Maragou 2009), a fact that is also supported by our results, although the limited sampling effort we applied in the ditches, due to the prevailing unfavorable conditions.
Nevertheless, the newly established fish community in the reservoir was dominated by cyprinids, which have good adaptability to highly eutrophic conditions (Jeppesen et al. 1997; Olin et al. 2002; Nurminen et al. 2010) as those prevailing in Karla Reservoir. However, it lacks the presence of any top predator, posing thus limitations to biomanipulation processes through top-down control. Lepomis gibbosus, which was recorded as the most abundant species in the reservoir, and feeds on small fish and invertebrates (Kottelat and Freyhof 2007), could pose direct top-down effects to control the entire fish community (Gliwicz 2002). The species dominance confirms its adaptation ability attributed to its remarkably great plasticity of its life history traits and phenotypes (Fox 1994; Copp and Fox 2007; Naspleda et al. 2012) and verifies its characterization as one of the most successful fish invaders in inland waters of Europe (Fox et al. 2007). Generally, the fish fauna of the new reservoir is composed almost exclusively of planktivorous and invertivorous species, thus the “top-down” effect (Blindow et al. 1993) is quite weak to control the eutrophic conditions. Since the new reservoir does not host any piscivorous species, their potential introduction might affect biodiversity via competition with the existing species for resources, alteration of habitats or even extinction of other species (Sidiropoulos et al. 2017). Moreover, the high recorded benthic macroinvertebrate abundances seem to favor the dominance of invertivorous species, both in lake and in ditches, affecting the continuous re-suspension of the sediment, which in turn involves on the turbidity and recycling of nutrients (Scheffer 2004). In addition, the role of fish as sources vs. sinks of nutrients (Vanni et al. 2013) has to be further examined.
The “poor” ecological quality of Karla Reservoir assessed by the application of the fish (GLFI) and the benthic invertebrate (GLBiI) indices agrees with the poor ecological potential, reflected in the physicochemical parameters, which in many cases exceeded the proposed limits for the welfare of fish (Directive 2006/44/EC; European Commission 2006). Moreover, this poor ecological quality triggers concern regarding the achievement of “good ecological potential” (GEP), set by the WFD 2000/60/EC, the biodiversity goals according to the Habitats Directive 92/43/EC (European Commission 1992) and the fulfillment of ecosystem services. Karla Reservoir has now been designated as “heavily modified water body”; thus, the goal of achieving a GEP is an open issue.
Finally, the present outcomes demonstrated the intense degradation of Karla’s basin. All technical works, however, designed in the frame of the implementation of the management plan developed in the area have not yet been completed (Sidiropoulos et al. 2017). The quality of water reservoir could perhaps be improved by completing the construction of a peripheral buffer zone, thus eliminating the nutrient’s input from the catchment. Moreover, the fully completion of the management plan will settle the hydraulic function among ditches and reservoirs, covering thus partially the irrigation demand of the area, which is estimated equal to 47 million m3. Besides, the operation of Karla Reservoir and the associated works will stop the over-pumping of underground aquifers for irrigation needs.
Permanent quality monitoring should be installed in the inflowing ditches and the reservoir, as the area is significantly affected by human activities, mainly agricultural, with strong water resource demands. Furthermore, re-establishment of specific habitats as vegetation patches and macrophytes beds is of great importance as refuges for fish and macroinvertebrates. The absence of any submerged vegetation is probably the result of the low-clarity conditions along with the irregular hydrological alterations. In that case, the absence of any habitat connectivity also enhances the internal loading (Gonzalez et al. 1988), leading in even high eutrophication.
Furthermore, we strongly suggest the implementation of a biomanipulation scheme, as a complementary management measure, capable to shift the top-down control. Since there is no piscivorous fish species, their possible introduction might be considered with caution for avoiding unknown impacts on endemics and natives. Because the new reservoir also falls under the EC Habitats Directive, there is a requirement for compatible restoration measures in order to achieve “a favorable conservation status”. As a preliminary step, the removal of some opportunistic and invader species, such as Carassius gibelio, and the regulation of Lepomis gibbosus through intensive fishing could be an effective mitigation measure.
References
Alexakis, D., Kagalou, I., & Tsakiris, G. (2013). Assessment of pressures and impacts on surface water bodies of the Mediterranean. Case study: Pamvotis Lake, Greece. Environmental Earth Sciences, 70(2), 687–698. https://doi.org/10.1007/s12665-012-2152-7
Allan, J., & Johnson, L. (1997). Catchment scale analysis of aquatic ecosystems. Freshwater Biology, 37(1), 107–111. https://doi.org/10.1046/j.1365-2427.1997.00155.x
Ananiadis, C. I. (1956). Limnological study of lake Karla. Bulletin Institute Oceanography, 1083, 1–19.
APHA, A. W. W. A. (2005). WEF, 2005. Standard methods for the examination of water and wastewater, 21st edn. Washington, DC, New York: American Public Health Association.
Armitage, P. D., & Hogger, J. (1994). Invertebrates Ecology and Methods of Survey. Bedfordshire: Sandy RSPB.
Artemiadou, V., & Lazaridou, M. (2005). Evaluation score and interpretation index for the ecological quality of running waters in Central and Northern Hellas. Environmental Monitoring and Assessment, 110(1–3), 1–40. https://doi.org/10.1007/s10661-005-6289-7
Balvay, G., Gawler, M., & Pelletier, J. P. (1990). Lake trophic status and the development of the clear-water phase in Lake Geneva. In M. M. Tilzer & C. Serruya (Eds.), Large lakes, Ecological Structure and Function (pp. 580–591). Berlin: Springer.
Beklioglu, M., Romo, S., Kagalou, I., Quintana, X., & Bécares, E. (2007). State of the art in the functioning of shallow Mediterranean lakes: workshop conclusions. Hydrobiologia, 584(1), 317–326. https://doi.org/10.1007/s10750-007-0577-x
Bennett, C., Owen, R., Birk, S., Buffagni, A., Erba, S., Mengin, N., Murray-Bligh, J., Ofenböck, G., Pardo, I., van de Bund, W., Wagner, F., & Wasson, J. G. (2011). Bringing European river quality into line: an exercise to intercalibrate macro-invertebrate classification methods. Hydrobiologia, 667(1), 31–48. https://doi.org/10.1007/s10750-011-0635-2
Birk, S., Bonne, W., Borja, A., Brucet, S., Courrat, A., Poikane, S., Solimini, A., van de Bund, W., Zampoukas, N., & Hering, D. (2012). Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecological Indicators, 18, 31–41. https://doi.org/10.1016/j.ecolind.2011.10.009
Blindow, I., Andersson, M., Hargeby, A., & Johansson, S. (1993). Long term pattern of alternative stable states in two shallow eutrophic lakes. Freshwater Biology, 30(1), 159–167. https://doi.org/10.1111/j.1365-2427.1993.tb00796.x
Brabec, K., Zahrádková, S., Pařil, P., Němejcová, D., Kokeš, J., & Jarkovský, J. (2004). Assessment of organic pollution effect considering differences between lotic and lentic stream habitats. In D. Hering, P. F. M. Verdonschot, O. Moog, & L. Sandin (Eds.), Integrated assessment of running waters in Europe (pp. 331–346). Netherlands: Springer. https://doi.org/10.1007/978-94-007-0993-5_20
Brooks, S. J., Langdon, P. G., & Heiri, O. (2007). The Identification and use of Palaearctic Chironomidae Larvae in Palaeoecology, QRA Technical Guide No. 10. London: Quaternary Research Association.
Brosse, S., Arbuckle, C. J., & Townsend, C. R. (2003). Habitat scale and biodiversity: influence of catchment, stream reach and bed form scales on local invertebrate diversity. Biodiversity and Conservation, 12(10), 2057–2075. https://doi.org/10.1023/A:1024107915183
Camargo, J. Α., & Alonso, A. (2006). Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environmental International, 32(6), 831–849. https://doi.org/10.1016/j.envint.2006.05.002
CEN. (2003). Water Quality – Sampling of Fish with Electricity. CEN/TC 230, Ref. No. EN 14011, 1–16.
CEN. (2005). Water quality—Sampling of Fish with Multimesh Gillnets. Ref. No. 14757, 1–29.
Chamoglou, M., Papadimitriou, T., & Kagalou, I. (2014). Keys-descriptors for the functioning of a Mediterranean reservoir: the case of a new Lake Karla-Greece. Environmental Processes, 1(2), 127–135. https://doi.org/10.1007/s40710-014-0011-0
Chatzinikolaou, Y., Dakos, V., & Lazaridou, M. (2006). Longitudinal impacts of anthropogenic pressures on benthic macroinvertebrate assemblages in a large transboundary Mediterranean river during the low flow period. Acta Hydrochimica et Hydrobiologica, 34(5), 453–463. https://doi.org/10.1002/aheh.200500644
Clarke, K. R., & Warwick, R. M. (2001). Change in marine communities: an approach to statistical analysis and interpretation. Plymouth: Primer-E, Ltd.
Clarke, K. R., & Gorley, R. N. (2006). PRIMER V6: User Manual-tutorial. Plymouth: PRIMER-E, Ltd.
Clarke, K. R., Gorley, R. N., Somerfield, P. J., & Warwick, R. M. (2014). Change in marine communities: an approach to statistical analysis and interpretation (3rd ed.). Plymouth: PRIMER-E Ltd.
Collier, K. J., Ilcock, R. J., & Meredith, A. S. (1998). Influence of substrate type and physic-chemical conditions on macroinvertebrate faunas and biotic indices of some lowland Waikato, New Zealand, streams. New Zealand Journal of Marine and Freshwater Research, 32(1), 1–19. https://doi.org/10.1080/00288330.1998.9516802
Copp, G. H., & Fox, M. G. (2007). Growth and life history traits of introduced pumpkinseed (Lepomis gibbosus) in Europe, and the relevance to its potential invasiveness. In F. Gherardi (Ed.), Biological invaders in inland waters: Profiles, distribution, and threats (pp. 289–306). Netherlands: Springer. https://doi.org/10.1007/978-1-4020-6029-8_15
Covich, A. P., Palmer, M. A., & Crowl, T. A. (1999). The role of benthic invertebrate species in freshwater ecosystems. Bioscience, 49(2), 119–127. https://doi.org/10.2307/1313537
Dodds, W. K., & Welch, Ε. B. (2000). Establishing nutrient criteria in streams. Journal of the North American Benthological Society, 19(1), 186–196. https://doi.org/10.2307/1468291
Economidis, P. S. (1991). Check list of freshwater fishes of Greece: recent status of threats and protection. Athens: Hellenic Society for the Protection of Nature.
Economidis, P. S., Bobori, D. C. & Pergantis, F. (2003). Ichthyologic Study in the Area of the Previous Lake Karla. Technical Report, Aristotle University of Thessaloniki, 1–157 (in Greek).
EGY. (2013). River Basin Management Plan of Thessaly. Report in Greek. Accessed on: 01/05/2017. Available from: http://wfd.ypeka.gr/pdf/GR08_Sxedio_diaxeirisis%20neron_ ypogegrammeno.pdf.
EN 27828 (1994). Water quality - Methods of biological sampling - Guidance on handnet sampling of aquatic benthic macro-invertebrates (ISO 7828 1985). European Committee for Standardization, 1–16.
European Commission. (1992). Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Official Journal of European Communities, 206, 7.
European Commission. (2000). Directive 2000/60/EC of the European Parliament and the Council of the 23 October 2000 establishing a framework for Community action in the field of water policy. Official Journal of European Communities, 327, 1–73.
European Commission. (2005). Guidance on the Intercalibration Process 2004–2006. Guidance document no. 14, 1-26.
European Commission. (2006). Directive 2006/44/EC of the European Parliament and of the Council of 6 September 2006 on the quality of fresh waters needing protection or improvement in order to support fish life. Official Journal of European Union, 264, 20.
FAME Consortium (2004). Manual for the application of the European Fish Index – EFI. A fish based method to assess the ecological status of European rivers in support of the water Framework Directive. Version 1.1, January 2005.
Fausch, K. D., Lyons, J. O. H. N., Karr, J. R., & Angermeier, P. L. (1990). Fish communities as indicators of environmental degradation. American Fisheries Society Symposium, 8, 123–144.
Fox, M. G. (1994). Growth, density, and interspecific influences on pumpkinseed sunfish life histories. Ecology, 75(4), 1157–1171. https://doi.org/10.2307/1939439
Fox, M. G., Vila-Gispert, A., & Copp, G. H. (2007). Life-history traits of introduced Iberian pumpkinseed Lepomis gibbosus relative to native populations. Can differences explain colonization success? Journal of Fish Biology, 71(sd), 56–69.
Gkelis, S., Panou, M., Chronis, I., Zervou, S.K., Christophoridis, C., Manolidi, K., Ntislidou, C., Triantis, M.T., Kaloudis, T., Hiskia, A., Kagalou, I., & Lazaridou, M. (2017). Monitoring a newly re-born patient: Water quality and cyanotoxin occurrence in a reconstructed shallow Mediterranean lake. Advances in Oceanography and Limnology, 8(1), 33–51. https://doi.org/10.4081/aiol.2017.6350.
Gliwicz, Z. M. (2002). On the different nature of top-down and bottom-up effects in pelagic food webs. Freshwater Biology, 47(12), 2296–2312. https://doi.org/10.1046/j.1365-2427.2002.00990.x
Gonzalez, S. M., Jeppensen, E., Goma, J., Sondergaard, M., Jensen, J., Lauridsen, T., Graneli, W., & Solander, D. (1988). Influence of aquatic macrophytes on phosphorus cycling in lakes. Hydrobiologia, 170, 245–266.
Gulati, R. D., & van Donk, E. (2002). Lakes in the Netherlands, their origin, eutrophication and restoration: state-of-the-art review. Hydrobiologia, 478(1/3), 73–106. https://doi.org/10.1023/A:1021092427559
Hering, D., Buffagni, A., Moog, O., Sandin, L., Sommerhäuser, M., Stubauer, I., Feld, C., Johnson, R., Pinto, P., Skoulikidis, N., Verdonschot, P., & Zahrádková, S. (2003). The development of a system to assess the ecological quality of streams based on macroinvertebrates—design of the sampling program within the AQEM project. International Review of Hydrobiology, 88(3–4), 345–361. https://doi.org/10.1002/iroh.200390030
Hilty, J., & Merenlender, A. (2000). Faunal indicator taxa selection for monitoring ecosystem health. Biological Conservation, 92(2), 185–197. https://doi.org/10.1016/S0006-3207(99)00052-X
ISO 7828 (1985). Water Quality – Methods of Biological Sampling – Guidance on Handnet Sampling of Aquatic Benthic Macro-Invertebrates. Ref. No. ISO 7828–1985. International Organization for Standardization, Ref. No. ISO 7828–1985, 1–12.
Jeppesen, E., Jensen, J. P., Kristensen, P., Søndergaard, M., Mortensen, E., Sortkjaer, O., & Olrik, K. (1990). Fish manipulation as a lake restoration tool in shallow, eutrophic, temperate lakes 2: threshold levels, long-term stability and conclusions. Hydrobiologia, 200(1), 219–227.
Jeppesen, E., Janse, I., Sondergaard, M., & Lauridsen, T. (1997). Top down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia, 342(343), 151–164.
Jerrentrup, H. (1990). The fauna of the former Lake Karla. In P. Gerakis (Ed.), Conservation and Management of the Greek Wetlands (pp. 527–535). Thessaloniki: WWF, IUCN and Aristotle University.
Joniak, T., Kowalczewska-Madura, K., & Kozak, A. (2003). Trophic state of a lowland reservoir during 10 years after restoration. Hydrobiologia, 506(1–3), 759–765.
Joy, M. K., & Death, R. G. (2002). Predictive modelling of freshwater fish as a biomonitoring tool in New Zealand. Freshwater Biology, 47(11), 2261–2275. https://doi.org/10.1046/j.1365-2427.2002.00954.x
Karatayev, A. Y., Burlakova, L. E., Vander Zanden, M. J., Lathrop, R. C., & Padilla, D. K. (2013). Change in a lake benthic community over a century: evidence for alternative community states. Hydrobiologia, 700(1), 287–300. https://doi.org/10.1007/s10750-012-1238-2
Karr, J. R. (1981). Assessment of biotic integrity using fish communities. Fisheries, 6(6), 21–27. https://doi.org/10.1577/1548-8446(1981)006<0021:AOBIUF>2.0.CO;2
Karr, J. R. (1987). Biological monitoring and environmental assessment: a conceptual framework. Environmental Management, 11(2), 249–256. https://doi.org/10.1007/BF01867203
Karr, J. R., Fausch, K. D., Angermeier, P. L., Yant, P. R., & Schlosser, I. J. (1986). Assessing biological integrity in running waters: a method and its rationale. Illinois Natural History Survey Special Publication, 5, 1–28.
Kemitzoglou, D. (2004). The effectiveness of the semi-quantitative sampling method using benthic macroinvertebrates. Master Thesis. Aristotle University of Thessaloniki, Greece.
Klink, A. G., & Moller Pillot, H. K. M. (2003). Chironomidae Larvae: key to the higher taxa and species of the lowlands of Northwestern Europe. Amsterdam: Biodiversity Center of ETI, CD-ROM.
Kottelat, M., & Freyhof, J. (2007). Handbook of European freshwater fishes Cornol, Switzerland: Publications Kottelat.
Latinopoulos, D., Ntislidou, C., & Kagalou, I. (2016). Multipurpose plans for the sustainability of the Greek lakes: emphasis on multiple stressors. Environmental Processes, 3(3), 589–602. https://doi.org/10.1007/s40710-016-0152-4
Lazaridou, M., Ntislidou, C., Karaouzas, I., & Skoulikidis, N. (2018). Harmonization of a new assessment method for estimating the ecological quality status of Greek running waters. Ecological Indicators, 84, 683–694. https://doi.org/10.1016/j.ecolind.2017.09.032
Legakis, A., & Maragou, P. (2009). The Red Data Book of threatened animal species of Greece. Athens: Hellenic Zoological Society.
Lijklema, L. (1994). Nutrient dynamics in shallow lakes: effects of changes in loading and role of sediment-water interactions. Hydrobiologia, 275(1), 335–348.
Moreno, P., & Callisto, M. (2006). Benthic macroinvertebrates in the watershed of an urban reservoir in southeastern Brazil. Hydrobiologia, 560(1), 311–321. https://doi.org/10.1007/s10750-005-0869-y
Naspleda, J., Vila-Gispert, A., Fox, M. G., Zamora, L., & Ruiz-Navarro, A. (2012). Morphological variation between non-native lake and stream dwelling pumpkinseed Lepomis gibbosus in the Iberian Peninsula. Journal of Fish Biology, 81(6), 1915–1935. https://doi.org/10.1111/j.1095-8649.2012.03416.x
Ntislidou, C., Lazaridou, M., Tsiaoussi, V., & Bobori D. (2016). Report on the development of the national assessment method for the ecological quality of natural lakes in Greece, using the Biological Quality Element “Benthic invertebrates” (GLBiI, Greek Lake Benthic invertebrate Index). Aristotle University of Thessaloniki, School of Biology, 1–25.
Nurminen, L., Pekcan-Hekim, Z., & Horppila, J. (2010). Feeding efficiency of planktivorous perch Perca fluviatilis and roach Rutilus rutilus in varying turbidity: an individual-based approach. Journal of Fish Biology, 76(7), 1848–1855. https://doi.org/10.1111/j.1095-8649.2010.02600.x
Oberdorff, T., Pont, D., Hugueny, B., & Porcher, J. P. (2002). Development and validation of a fish-based index for the assessment of ‘river health’ in France. Freshwater Biology, 47(9), 1720–1734. https://doi.org/10.1046/j.1365-2427.2002.00884.x
Ofenböck, T., Moog, O., Gerritsen, J., & Barbour, M. (2004). A stressor specific multimetric approach for monitoring running waters in Austria using benthic macro-invertebrates. In Integrated Assessment of Running Waters in Europe (pp. 251–268). Netherlands: Springer Netherlands.
Olin, M., Rask, M., Ruuhljärvi, J., Kurkilahti, M., Ala-Opas, P., & Ylönen, O. (2002). Fish community structure in mesotrophic and eutrophic lakes of southern Finland: the relative abundances of percids and cyprinids along a trophic gradient. Journal of Fish Biology, 60(3), 593–612. https://doi.org/10.1111/j.1095-8649.2002.tb01687.x
Orendt, C., & Spies, M. (2014). Chironomus (Meigen) (Diptera: Chironomidae). Key to the larvae of importance to biological water analysis in Germany and adjacent areas. Bilingual edition (German/English). Leipzig: 1–24.
Pamplin, P. A. Z., Almeida, T. C. M., & Rocha, O. (2006). Composition and distribution of benthic macroinvertebrates in Americana Reservoir (SP, Brazil). Acta Limnologica Brasiliensia, 18(2), 121–132.
Pardo, I., Gómez-Rodríguez, C., Abraín, R., García-Roselló, E., & Reynoldson, T. B. (2014). An invertebrate predictive model (NORTI) for streams and rivers: sensitivity of the model in detecting stress gradients. Ecological Indicators, 45, 51–62. https://doi.org/10.1016/j.ecolind.2014.03.019
Petriki, O., Lazaridou, M., & Bobori, D. C. (2016). Report on the development of the national assessment method for the ecological quality of natural lakes in Greece, using the Biological Quality Element “Fish” (GLFI, Greek Lake Fish Index), Aristotle University of Thessaloniki, School of Biology, 1–24.
Petriki, O., Lazaridou, M., & Bobori, D. C. (2017). A fish-based index for the assessment of the ecological quality of temperate lakes. Ecological Indicators, 78, 556–565. https://doi.org/10.1016/j.ecolind.2017.03.029
Rosenberg, D. M., & Resh, V. (1993). Freshwater biomonitoring and benthic macroinvertebrates. New York: Chapman & Hall.
Scheffer, M. (2004). Ecology of shallow lakes. Dordrecht/Boston/London: Kluver Academic Publishers.
Sidiropoulos, P., Chamoglou, M., & Kagalou, I. (2017). Combining conflicting, economic, and environmental pressures: evaluation of the restored Lake Karla (Thessaly, Greece). Ecohydrology & Hydrobiology, 17(3), 177–189. https://doi.org/10.1016/j.ecohyd.2017.04.002
Stabouli, Z., Papadimitriou, T., & Kagalou, I. (2012). Assessment of the ecological status of Lake Karla, using populations of zooplankton. Proceedings of the 34th Hellenic Society of Biological Sciences, Trikala, 17-19 June, 260-261.
Straškraba, M., Tundisi, J. G., & Duncan, A. (1993). State-of-the-art of reservoir limnology and water quality management. In M. Straškraba, J. G. Tundisi, & A. Duncan (Eds.), Comparative reservoir limnology and water quality management (pp. 213–288). Netherlands: Springer. https://doi.org/10.1007/978-94-017-1096-1_13
Tachet, H., Richoux, P., Bournaud, M., & Usseglio-Polatera, P. (2010). Invertébrés d’eau douce. Systématique, biologie, écologie. Paris: CNRS Editions.
Talling, J. F. (2003). Phytoplankton-zooplankton seasonal timing and the ‘clear-water phase’ in some English lakes. Freshwater Biology, 48(1), 39–52. https://doi.org/10.1046/j.1365-2427.2003.00968.x
Ter Braak, C. J. F., & Šmilauer, P. (1998). CANOCO Reference manual and User’s Guide to CANOCO for Windows. Software for Canonical Community Ordination (version 4). Wageningen: Centre for Biometry.
Timm, T. (2009). A guide to the freshwater Oligochaeta and Polychaeta of Northern and Central Europe. Lauterbornia, 66, 1-235.
Townsend, C. R., Doledec, S., Norris, R., Peacock, K., & Arbuckle, C. (2003). The influence of scale and geography on relationships between stream community composition and landscape variables: description and prediction. Freshwater Biology, 48(5), 768–785. https://doi.org/10.1046/j.1365-2427.2003.01043.x
Vallenduuk, H. J., & Moller Pillot, H. K. M. (2007). Chironomidae larvae of the Netherlands and adjacent lowlands. Zeist: KNNV Publishing.
Vander Zanden, M. J., & Vadeboncoeur, Y. (2002). Fishes as integrators of benthic and pelagic food webs in lakes. Ecology, 83(8), 2152–2161.
Vanni, M. J., Boros, G., & McIntyre, P. B. (2013). When are fish sources vs. sinks of nutrients in lake ecosystems? Ecology, 94(10), 2195–2206. https://doi.org/10.1890/12-1559.1
Vasiliades, L., & Loukas, A. (2009). Hydrological response to meteorological drought using the Palmer drought indices in Thessaly, Greece. Desalination, 237(1–3), 3–21. https://doi.org/10.1016/j.desal.2007.12.019
Voshell, J. R., & Simmons, G. M. (1984). Colonization and succession of benthic macroinvertebrates in a new reservoir. Hydrobiologia, 112(1), 27–39. https://doi.org/10.1007/BF00007663
Whitfield, A. K., & Elliott, M. (2002). Fishes as indicators of environmental and ecological changes within estuaries: a review of progress and some suggestions for the future. Journal of Fish Biology, 61(sA), 229–250. https://doi.org/10.1111/j.1095-8649.2002.tb01773.x
Wright, J. F. (2000). An introduction to RIVPACS. In J. F. Wright, D. W. Sutcliffe, & M. T. Furse (Eds.), Assessing the Biological Quality of Fresh Waters: RIVPACS and Other Techniques (pp. 1–24). Ambleside: Freshwater Biological Association.
Yuan, L. L. (2010). Estimating the effects of excess nutrients on stream invertebrates from observational data. Ecological Applications, 20(1), 110–125. https://doi.org/10.1890/08-1750.1
Zalidis, G. C., Takavakoglou, V., Panoras, A., Bilas, G., & Katsavouni, S. (2004). Re-establishing a sustainable wetland at former Lake Karla, Greece, using Ramsar restoration guidelines. Environmental Management, 34(6), 875–886. https://doi.org/10.1007/s00267-004-0022-0
Acknowledgements
The authors are grateful for research’s financial support by the Management Body of Ecodevelopment Area of Karla—Mavrovouni—Kefalovriso—Velestino, co-financed by the European Union (ERDF: European Regional Development Fund- ERDF) and Greek Nation in the framework of the Operational Program “Environment and Sustainable Development” 2007–2013 - Priority Axis “Protecting Nature and Biodiversity”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bobori, D.C., Ntislidou, C., Petriki, O. et al. Macroinvertebrate and fish communities in the watershed of a re-constructed Mediterranean water body: link to the ecological potential. Environ Monit Assess 190, 106 (2018). https://doi.org/10.1007/s10661-018-6484-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6484-y