Abstract
2,3-Butanediol (2,3-BD) has great potential for diverse industries, including chemical, cosmetics, agriculture, and pharmaceutical areas. However, its industrial production and usage are limited by the fairly high cost of its petro-based production. Several bio-based 2,3-BD production processes have been developed and their economic advantages over petro-based production process have been reported. In particular, many 2,3-BD-producing microorganisms including bacteria and yeast have been isolated and metabolically engineered for efficient production of 2,3-BD. In addition, several fermentation processes have been tested using feedstocks such as starch, sugar, glycerol, and even lignocellulose as raw materials. Since separation and purification of 2,3-BD from fermentation broth account for the majority of its production cost, cost-effective processes have been simultaneously developed. The construction of a demonstration plant that can annually produce around 300 tons of 2,3-BD is scheduled to be mechanically completed in Korea in 2019. In this paper, core technologies for bio-based 2,3-BD production are reviewed and their potentials for use in the commercial sector are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Concerns over climate change and finiteness of fossil reserves have driven the development of eco-friendly and sustainable bio-based chemicals. However, replacement of petro-based chemicals with bio-based ones is very limited primarily due to cost competitiveness issue. The first biological production of 2,3-BD was reported by Harden and Walpole who used Klebsiella pneumoniae as a host bacterium in 1906 [1]. Industrial production of bio-based 2,3-BD was tried during world war II for the production of 1,3-butadiene [2]. However, it was short lived as many countries rapidly developed less expensive petro-based 1,3-butadiene production processes using n-butene, butene, and ethanol [3, 4]. These facts suggest that bio-based 2,3-BD is not competitive as a raw material for the production of low-priced bulk and commodity chemicals.
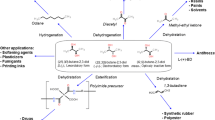
Up to date, 2,3-BD has been recognized as a chemical with low industrial value. Therefore, it is manufactured in very small quantities via a chemical process using butene, which needs to be pre-separated from cracked gas. Furthermore, upon production, 2,3-BD exists as three isomers: (2R,3R)-BD (also known as levo), (2R,3S)-BD (also known as meso), and (2S,3S)-BD (also known as dextro) [5, 6]. Since a chemical catalyst cannot recognize these isomers, fairly expensive separation and purification processes are required to obtain stereospecific 2,3-BD (Fig. 1).
Recently, many studies have elucidated the role of 2,3-BD and indicated that 2,3-BD isomers show quite different physicochemical characteristics and unique efficacies, especially in high value-added cosmetics, agriculture, and pharmaceutical industries [7,8,9,10,11,12]. Due to well-known advantages of biological processes in terms of production costs compared to chemical process, interest in biological production of 2,3-BD is increasing again [13].
In this paper, we reviewed current status of unit technologies including host strain development, feedstock utilization, fermentation, separation, and purification for bio-based 2,3-BD production. Potentials of diverse application areas for 2,3-BD depending on its isomers are also discussed.
Microorganisms
Metabolic pathways
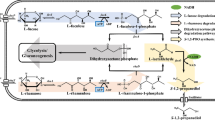
In biological system, the 2,3-BD formation pathway acts as a cellular carbon-and-energy storage system that regulates NADH/NAD+ ratio. This pathway may also be activated to prevent intracellular acidification via formation of neutral compound, 2,3-BD [13]. Most microorganisms that produce 2,3-BD have three consecutive metabolic conversion steps: pyruvate → α-acetolactate → acetoin and/or diacetyl → 2,3-BD (Fig. 2). First, pyruvate from glycolysis is converted to α-acetolactate via decarboxylation which is catalyzed by an α-acetolactate synthase. Next, α-acetolactate is anaerobically converted to R-acetoin via an α-acetolactate decarboxylase. It also produces diacetyl as a minor byproduct via a spontaneous reaction due to instability under aerobic conditions [14]. Diacetyl is further reduced to S-acetoin via a butanediol dehydrogenase or a diacetyl reductase with consumption of one NADH equivalent. Acetoin is an intermediate compound prior to the formation of 2,3-BD. Metabolic conversion of acetoin to 2,3-BD is reversible [12]. From these two types of acetoin (R-acetoin and S-acetoin), three types of 2,3-BD classified as (2R,3R), (2S,3S), and (2R,3S)-forms are determined by stereospecific termination enzymes such as a butanediol dehydrogenase and an acetoin reductase. Strains of Klebsiella and Enterobacter genera mainly form (2S,3S)-BD and (2R,3S)-BD, while members of Bacillus genus form (2R,3R)-BD and (2R,3S)-BD [15]. Although the metabolic significance of stereoisomer formation needs further study, mechanisms of stereoisomer formation have been investigated by many researchers. According to previous reports, a single strain may have either multiple enzymes for catalysis of the termination step or a single enzyme with bifunctional activities for 2,3-BD production (Fig. 2). For example, it has been reported that (2R,3S)-BD dehydrogenase derived from K. pneumoniae can catalyze the interconversion between acetoin and 2,3-BD [16]. In addition, it shows a strong diacetyl reductase activity. In B. licheniformis, there are two genes encoding (2R,3R)-BD and (2R,3S)-BD dehydrogenases to form (2R,3R)-BD and (2R,3S)-BD at 1:1 ratio [17]. Thus, 2,3-BD production pathway is highly complicated by enzyme reactions depending on strains. Theoretically, the maximum production yield of 2,3-BD via this pathway is 0.50 g/g·glucose (equivalent to 1 mol/mol) because two pyruvate molecules derived from glucose can be assembled into a single 2,3-BD molecule. In terms of redox balance, two moles of NADH are generated during glycolysis of 1 mol of glucose, resulting in a surplus of NADH via 2,3-BD synthetic pathway because only 1 mol NADH is required to reduce acetoin to 2,3-BD. Thus, several reduced byproducts such as lactate, succinate and ethanol are also generated to balance the redox status in cells under anaerobic condition [13, 18, 19]. However, it is possible that NADH/NAD+ ratio is regulated by respiration under aerobic condition via regeneration of NAD+. Based on the understanding of metabolic pathways, researchers have investigated and developed a number of strains to increase the production and stereoisomer selectivity of 2,3-BD. This will be described in the “Strain development”.
General biological routes for the production of three isomeric forms of 2,3-BD with mixed acid pathway. Dashed lines represent the route activated under aerobic conditions. Parenthesized number represents key enzymes required for 2,3-BD production. (1) α-acetolactate synthase; (2) α-acetolactate decarboxylase; (3) (2R,3S)-butanediol dehydrogenase (R-acetoin forming); (4) (2R,3S)-butanediol dehydrogenase (S-acetoin forming); (5) (2S,3S)-butanediol dehydrogenase (S-acetoin forming); (6) (2R,3R)-butanediol dehydrogenase; and (7) diacetyl reductase (S-acetoin forming)
Strain development
Microorganisms that can produce 2,3-BD are listed in Table 1. Native 2,3-BD producers can be classified into Gram-negative (mainly Enterobacteriaceae family) and Gram-positive (mainly Bacillus and Paenibacillus genera) bacteria. Several non-natural producers including Escherichia coli, Lactobacillus lactis, and Saccharomices cerevisiae have been engineered for the production of 2,3-BD. Most studies for the mass production of 2,3-BD have been carried out using bacteria in the family of Enterobacteriaceae, including those in Klebsiella, Enterobacter, and Serratia genera [15]. Although these aforementioned bacteria have been recognized as promising hosts, their pathogenicity (genera of risk group 2) has been pointed out as an obstacle for industrial uses. Exceptional among them is K. oxytoca, which is classified as a risk group 1 strain according to the US National Institutes of Health guidelines (April 2016). Interest in Bacillus strains producing 2,3-BD has been increasing recently because the genera of Bacillus and Paenibacillus are generally regarded as safe (GRAS) microorganisms. However, Bacillus strains have been reported to have rather low 2,3-BD production capabilities in terms of titer and productivity compared to Enterobacteriaceae strains. While most Bacillus species are difficult to cultivate and have high nutritional requirements, members belonging to Enterobacteriaceae could be used in a variety of substrates and are easy to cultivate in a simple medium [10, 15, 20]. In case of non-native producers, industrially relevant hosts such as E. coli, S. cerevisiae, and L. lactis have been studied for feasibility in large-scale production due to their well-characterized genetics and cultivation methods [6]. In addition, S. cerevisiae and L. lactis are known to exhibit a high degree of tolerance to alcohols and harsh industrial conditions [21, 22]. However, the application of strains carrying heterologous pathways may be restricted in some products such as food and agricultural industries. Therefore, during selection of an industrial host, the organism needs to exhibit high production efficiency as well as non-pathogenicity for cost effective and safe production of bio-based 2,3-BD. In addition, it is necessary to review living modified organism (LMO) regulations depending on the country and purpose of use. Toward this end, first, an appropriate high-performing strain should be selected and, if needed, metabolically engineered to regulate and balance its metabolism, thereby increasing metabolic flux until the desired end product is obtained (Table 1). It is also essential to design strains to control stereospecificity and obtain an optically pure 2,3-BD isomer. Notably, most 2,3-BD-producing microorganisms have a tendency to produce a mixture of stereoisomers [23]. Because physical property of each isomer is unique, it may be important to adjust the selectivity of isomers depending on the application.
Gram-negative bacteria
Klebsiella strains such as K. pneumoniae and K. oxytoca have been widely studied and engineered for 2,3-BD production because of their superior ability compared to others. To enhance 2,3-BD production, two basic strategies have been adopted. The first one is to reinforce the 2,3-BD formation route and the second is to block byproduct generation. For example, the budA and budB genes (encoding α-acetolactate decarboxylase and α-acetolactate synthase, respectively) involved in 2,3-BD synthesis were overexpressed in K. pneumonia resulting in the production of 101.53 g/L of 2,3-BD, with a productivity of 2.54 g/L/h [24]. As an example of the second strategy, inactivation of the ldhA (encoding lactate dehydrogenase) gene in a K. pneumoniae strain also increased 2,3-BD production with faster cell growth by reducing lactate formation, one of the major byproducts of the 2,3-BD formation pathway. Additional deletion of the adhE gene encoding alcohol dehydrogenase in the ldhA gene-deleted K. pneumoniae strain made it possible to produce 116 g/L of 2,3-BD with yield and productivity of 0.49 g/g·glucose and 2.21 g/L/h, respectively [25]. In addition to rational metabolic engineering, an in silico study has also been conducted for the prediction of strain improvement. Rathnasingh et al. have recently reported that in silico simulation-aided triple mutant K. pneumoniae strain by inactivating the ldhA, adhE, pta-ackA (encoding phosphotransacetylase and acetate kinase) genes could produce 91 g/L of 2,3-BD with a yield of 0.45 g/g·glucose and a productivity of 1.62 g/L/h in the mineral salt medium [26]. The approach was effective because metabolic characteristics of mutants were consistent with results of in silico simulation. Furthermore, specific target engineering is not always the best strategy to improve the performance because random approaches might be more effective for some strains. Random mutagenesis techniques have been adopted for the development of effective 2,3-BD-producing microorganisms. The highest 2,3-BD titer of 150 g/L after 38 h of fermentation was achieved using K. pneumoniae SDM strain which was generated by ion beam-based random mutation [27]. It resulted in the best production among reported wild-type strains. Several studies have been conducted to improve production and isomer selectivity of 2,3-BD. Klebsiella produces (2R,3S)-BD as a major isomer, although its optical purity is not satisfactory due to the presence of multiple butanediol dehydrogenases [6]. However, several reports have provided evidence that engineering could change isomer selectivity from (2R,3S)-BD to (2R,3R)-BD. To produce optically pure (2R,3R)-BD in the wabG gene-deleted non-pathogenic K. pneumoniae, the ldhA gene was deleted and two genes encoding glycerol dehydrogenase (encoded by dhaD and gldA) were overexpressed with concomitant deletion of the native 2,3-BD dehydrogenase (encoded by budC), which result in the production of (2R,3R)-BD at 61 g/L with (2R,3S)-BD at 1.4 g/L by fed-batch fermentation [28].
Similar strain development strategies used in K. pneumoniae have been replicated in K. oxytoca which is classified as a non-pathogen according to the US NIH guideline. Low pathogenesis and high 2,3-BD production might be key advantages of K. oxytoca for industrial uses. In one study, K. oxytoca mutant strain deficient in ethanol synthesis, a major byproduct, was successfully constructed by replacing the aldA gene (encoding aldehyde dehydrogenase) with a tetracycline resistance cassette. The mutant strain produced 130 g/L of 2,3-BD with yield and productivity of 0.48 g/g·glucose and 1.63 g/L/h, respectively, by fed-batch fermentation [29]. To reduce the synthesis of lactic acid, which is another major byproduct, deletion of the ldhA gene in a K. oxytoca strain was carried out and produced 115 g/L of 2,3-BD with yield and productivity of 0.41 g/g·glucose and 2.27 g/L/h, respectively [30]. In another study, the pflB gene (encoding pyruvate–formate lyase) was additionally deleted in the ldh deleted mutant based on in silico simulation to further enhance 2,3-BD production [31]. As a result, production yield was increased to 0.45 g/g·glucose with a similar titer of 113 g/L, suggesting that in silico simulation is a useful tool for accelerating strain development when target gene selection is difficult due to unknown metabolic pathways. Combining metabolic engineering-based deletion of adhE, pta-ackA, and ldhA genes and metabolic evolution can also improve strain performance. Adaptive evolution was carried out by transferring cultures serially more than 70 times in glucose-containing medium to screen for efficient glucose-utilizing strains since metabolically engineered strains exhibit lower growth rates. Adaptive evolution is an effective alternative to metabolic engineering which may be difficult to perform in some cases. The combination of metabolic engineering and adaptive evolution strategies made it possible to produce 117 g/L of 2,3-BD with yield and productivity of 0.49 g/g·glucose and 1.20 g/L/h by fed-batch fermentation [32]. To improve the production of optically pure 2,3-BD in K. oxytoca, Paenibacillus polymyxa-derived bdh gene (encoding 2R,3R-BD dehydrogenase) was overexpressed in budC (encoding 2R,3S-BD dehydrogenase), ldhA, and pflB-deleted K. oxytoca strain, resulting in 106.7 g/L of (2R,3R)-BD [(2R,3S)-BD, 9.3 g/L], with yield of 0.40 g/g and productivity of 3.1 g/L/h [28].
Enterobacter is also an opportunistic pathogen with a high 2,3-BD production potential [6]. Lactic acid is a major byproduct of fermentation by this species. In a previous study, deletion of the ldhA gene in E. aerogenes with optimization of both medium and aeration rate made it possible to produce 2,3-BD up to 118 g/L after 54 h of fed-batch fermentation [33]. To improve the optical purity, B. pumilus bdh gene was overexpressed with deletion of endogenous bdh in E. cloacae and pathways for the synthesis of major byproducts were blocked via deletion of ldh and frd (encoding fumarate reductase) genes. This engineered strain produced 152 g/L of optically pure (2R,3R)-BD [34]. This result may be the highest performance reported in studies that have focused on selectivity improvement. Selectivity is dependent on the strain employed as described above. Therefore, it is important to select a strain with a simple metabolic pathway, rather than multiple and/or non-specific dehydrogenases, to obtain optically pure 2,3-BD.
Lastly, Serratia is a representative microorganism belonging to family Enterobacteriaceae that can produce 2,3-BD. The metabolic pathway for the synthesis of 2,3-BD in S. marcescens has been recently characterized [35]. In previous reports, random mutagenesis of S. marcescens H30 which is not a metabolically engineered one has achieved 2,3-BD concentrations of 139 g/L and also exhibited the highest productivity at 3.49 g/L/h [36]. Although random mutation strain enhanced its performance, additional metabolic engineering further improved its performance. Because the 2,3-BD production pathway of S. marcescens is regulated by the swrR gene coding for serrawettin W1 synthase, deletion of the swrW gene in the same strain was performed to reduce the generation of foam and 152 g/L of 2,3-BD was produced with yield and productivity of 0.41 g/g·sucrose and 2.67 g/L/h [37].
These aforementioned studies have nicely demonstrated the value and potential of Klebsiella, Enterobacter, and Serratia as industrial strains. In summary, Gram-negative 2,3-BD-producing bacteria generally belonging to family Enterobacteriaceae can produce 2,3-BD effectively. In addition, their performance can be further improved via techniques such as metabolic engineering, in silico simulation, and evolutionary engineering.
Gram-positive bacteria
Besides Gram-negative bacteria belonging to family Enterobacteriaceae, Gram-positive bacteria belonging to Bacillus and Paenibacillus are also capable of producing 2,3-BD. They are recognized as promising hosts for industrial fermentation due to their safe characteristics. Recent reports have suggested that their production performances are comparable to those of Klebsiella species, although they face challenges such as process efficiency and requirement of rich-medium. Several strains including B. licheniformis, B. amlyloliquefaciens, and P. polymyxa have shown good performance. Thus, they are expected to be promising industrial producers. A newly isolated B. licheniformis strain 10-1-A could thermo-stably produce 115.7 g/L of 2,3-BD with high productivity and yield of 2.4 g/L/h and 0.47 g/g·glucose [38]. The main advantages of B. licheniformis are: (1) it is classified as a GRAS strain and (2) it can be grown at relatively high temperature of 50℃ which can reduce the risk of bacterial contamination. In another study, fed-batch fermentation of B. licheniformis DSM 8785 achieved maximum 2,3-BD concentration of 144.7 g/L with a productivity of 1.14 g/L/h by optimizing initial glucose concentration with stepwise feeding of nutrients [39]. Despite a relatively low productivity, the production titer appears to be similar to Gram-negative 2,3-BD producers.
Metabolic simplicity of 2,3-BD in Bacillus is another advantage because optically pure 2,3-BD can be produced by Bacillus strains via simple modifications. Cross-functional activities of enzymes for 2,3-BD production in Bacillus might not be complex as found in other bacteria. Most B. licheniformis and B. subtilis strains produce (2R,3R)-BD and (2R,3S)-BD at a ratio close to 1:1 [38, 40]. However, when the budC gene was deleted, optically pure (2R,3R)-BD isomer can be produced in B. licheniformis with a titer of 123.7 g/L. When the gdh gene was deleted, optically pure (2R,3S)-BD isomer can be produced with a titer of 90.1 g/L in B. licheniformis [17]. Also, B. subtilis can be engineered for production of (2R,3S)-BD stereoisomer. Systematic engineering strategies including reduction of byproducts, inactivation of (2R,3R)-BD dehydrogenase, incremental improvement of NADH availability, and overexpression of (2R,3S)-BD production enzymes made it possible to achieve 103.7 g/L of optically pure (2R,3S)-BD with a yield of 0.487 g/g·glucose [40]. These studies suggest that deletion of a single gene encoding 2,3-BD dehydrogenase in Bacillus is sufficient for the synthesis of 2,3-BD with a selectivity higher than 99%.
Paenibacillus polymyxa strain can naturally produce (2R,3R)-BD with high selectivity. It has been reported that 111 g/L of (2R,3R)-BD with a selectivity of 98% can be achieved in high nutritional medium (60 g/L of yeast extract) using wild-type strain of P. polymyxa [41]. Although high nutritional requirement is a disadvantage, stereospecific production of (2R,3R)-BD without genetic modification of P. polymyxa is a great advantage for industrial application.
Bacillus amyloliquefaciens is also regarded as a safe microorganism and a promising candidate for industrial production. Yang et al. [42] have newly isolated a GRAS strain of B. amyloliquefaciens designated as B10-127 that can produce 92.3 g/L of 2,3-BD with a productivity of 0.96 g/L/h. To further improve the strain’s performance, NADH availability and termination step of 2,3-BD synthesis were reinforced by overexpressing glyceraldehyde-3-phosphate dehydrogenase and 2,3-butanediol dehydrogenase, thereby increasing the production of 2,3-BD up to 132.9 g/L after 45 h of fermentation [43].
While the performance of Bacillus has been tremendously improved as described above, the need for high nutrient levels during cultivation and challenges associated with genetic engineering due to limited tools available should be resolved to facilitate the development of economically and industrially useful strains in the future.
Other microorganisms
Several efforts have also been made to produce 2,3-BD in various non-native producers [21, 22]. S. cerevisiae is a model eukaryote with a clear genetic background. It is known as an efficient producer of ethanol. Recently, it has attracted attention as a platform strain for the production of 2,3-BD. The production of 2,3-BD in S. cerevisiae can be improved stepwise by eliminating byproduct formation and redox rebalancing. In one study, pdc1 and pdc5 (encoding pyruvate decarboxylases) deletion mutants have been prepared to make glucose tolerant strain (MTH1 A81P point mutation). Then, 2,3-BD biosynthetic genes alsS and alsD genes (encoding α-acetolactate synthase and α-acetolactate decarboxylase, respectively) from B. subtilis, and endogenous bdh1 gene were overexpressed, resulting in the production of 96.2 g/L of (2R,3R)-BD with low yield and productivity of 0.28 g/g and 0.39 g/L/h [44]. The synthesis of optically pure (2R,3R)-BD has been facilitated by the characterization of endogenous bdh1 gene of S. cerevisiae [45]. Another study has found that redox rebalancing by overexpression of NADH oxidase (NoxE) following elimination of ethanol and glycerol biosynthetic pathways is effective for enhancing the productivity of 2,3-BD up to 1.43 g/L/h [46]. Thus, redox equilibrium mediated by NoxE overexpression is a key strategy to improve productivity by serving as an alternative to glycerol pathway that regulates NADH imbalance.
Another heterologous host L. lactis is an excellent strain for transforming dairy waste into value-added chemicals. In one study, L. lactis was engineered for the production of dual isomers (2R,3S)-BD and (2R,3R)-BD [22]. First all major byproduct pathways were eliminated by deleting genes encoding three lactate dehydrogenase (LDH) homologs, phosphotransacetylase (PTA), alcohol dehydrogenase (ADHE), and native butanediol dehydrogenases (BudAB). Subsequently, codon-optimized versions of butanediol dehydrogenase from E. cloace and alcohol dehydrogenase from A. xylosooxidans were introduced into the engineered strain to generate 51 g/L of (2R,3S)-BD and 32 g/L of (2R,3R)-BD from whey waste. This study demonstrates that optically pure synthesis of each isomer in L. lactis depends on the specific enzyme introduced.
Escherichia coli, a model strain for bacterial research, has also been utilized for 2,3-BD production by overexpressing a 2,3-BD gene cluster with its operon originating from E. cloacae subsp. dissolvens Another heterologous host SDM strain [47]. As a result, 73.8 g/L of optically pure (2R,3S)-BD was produced by fed-batch fermentation [47].
Based on these reports, it can be concluded that the production of 2,3-BD is possible through non-native and native producers. The low toxicity of 2,3-BD and the high activity of introduced enzymes in heterologous hosts can facilitate the production of 2,3-BD in various hosts. Because the effect of endogenous genes encoding 2,3-BD metabolism-related enzymes on non-native producers is minimized, synthesis of optically pure 2,3-BD with high selectivity may be an advantage.
Recently, cyanobacteria have also been selected as hosts for the production of 2,3-BD from abundant atmospheric carbon dioxide by way of photosynthesis. Nozzi et al. [48] have improved 2,3-BD production by cyanobacteria such as Synechococcus elongatus 7002 up to 1.6 g/L after 16 days of fermentation at a rate of 100 mg/L/day by systematic screening of different variables including: operon arrangement, copy number, light strength, inducer concentration, cell density at the time of induction, and nutrient concentration. Despite a low yield of 2,3-BD conversion, this study presents the possibility toward a sustainable process which does not rely on traditional carbon sources [49].
Available feedstocks
Diverse raw materials have been evaluated based on their cost and sustainability for industrial production of 2,3-BD (Fig. 3). Because raw material cost constitutes a large proportion of the total production cost, cheap and abundant biomasses have been investigated. Due to the resistance associated with the use of food resources in industrial production of biochemicals, an ideal solution would be utilizing waste products and excess biomass. Among non-cellulosic biomasses, relatively inexpensive and abundant substrates used include whey, sugarcane, cassava, Jerusalem artichoke, and crude glycerol [50,51,52,53]. Lignocellulosic biomass is also an attractive option for biorefinery because it is an abundant resource all around the world. In addition, it is available sustainably with a low cost.
Non-lignocellulosic biomass
Molasses, a byproduct of sugarcane industries, contains diverse nutrients including sucrose, minerals, organic compounds, and vitamins. Sugarcane molasses has been recognized as a suitable carbon source for fermentation due to its low price and rich sugar composition [54]. However, mixed sugars in molasses are used less efficiently than pure sucrose because substrate utilization pathways are subjected to carbon catabolite repression (CCR) [49]. Therefore, engineering of the regulatory system is needed to overcome this challenge. For example, high concentration of 2,3-BD exceeding 98 g/L has been obtained in a fed-batch fermentation using sugarcane molasses as a primary carbon source in sucrose regulator (ScrR) encoding gene-deleted E. aerogenes strain [55]. Sugarcane-derived raw sugar (sucrose) containing a low level of impurities has also been validated as a promising carbon source for efficient production of 2,3-BD in diverse microorganisms, although it is more expensive than molasses [56, 57].
Cassava is a relatively cheap and abundant starch crop widely cultivated in tropical and subtropical areas. It is available throughout the year due to easy planting and harvesting methods. In addition, it lacks competition in terms of price because cassava starch does not have substantial applications in food industry compared to corn starch [58]. It is known as one of the most efficient carbohydrate production crops since roots of cassava have high starch contents. It can be utilized in a number of diverse forms, including cassava starch, cassava powder, cassava baggasse, cassava chips, and fresh cassava roots. In one study, a newly isolated E. cloace strain was used for 2,3-BD production using cassava powder as carbon source [53]. Under optimal conditions, 93.9 g/L of 2,3-BD was produced after 47 h of simultaneous saccharification and fermentation (SSF) [53].
Jerusalem artichoke is an inexpensive and abundant non-grain raw material. Fresh Jerusalem artichoke tubers contain about 20% carbohydrates, of which inulin accounts for more than 70% [59]. Inulin is composed of multiple fructose units terminated by a glucose unit. It can be hydrolyzed by inulinase to fructose and glucose. Production of 2,3-BD by K. pneumoniae from Jerusalem artichoke tubers was successfully carried out, with a production of 91.6 g/L after 40 h of fed-batch SSF process [60]. Inulin hydrolysate has also been used for 2,3-BD production by thermophilic B. licheniformis strain, with a titer of 103 g/L and a high productivity of 3.4 g/L/h through a fed-batch SSF process [61]. To directly use Jerusalem artichoke tuber for 2,3-BD production without supplementation with inulinase, a P. polymyxa strain has been utilized as a host because this strain naturally secretes inulinase. By conducting one-step batch fermentation using raw inulin as a substrate, 36.92 g/L of (2R,3R)-BD with purity of 98% was produced [51].
Whey is a byproduct of the dairy industry. It contains about 5% lactose and 1% protein. Due to its ready availability in many countries, utilization of whey for 2,3-BD fermentation has been examined by many groups. However, concentrations of available sugars in whey are low. As a result, fermentation productivity using whey as a substrate is rather low compared to that with conventional substrates such as starch and sugar-based sources. Thus, cell immobilization technology has been developed to overcome such problem of low productivity [62]. However, it is still necessary to improve process efficiencies for industrial uses.
Glycerol is generated as a byproduct from ethanol fermentation, fat saponification, and biodiesel production. It is also a promising substrate for industrial production of 2,3-BD [63]. Of particular note, a growing surplus of glycerol in the world market is expected in the near future because the demand for biodiesel is growing and total weight of biodiesel-derived crude glycerol is equivalent to almost 10% of biodiesel production [64]. Biodiesel-derived crude glycerol has been used as the sole carbon source of the metabolically engineered K. oxytoca M1, where pduC (encoding glycerol dehydratase subunit) and ldhA genes are deleted to reduce the formation of byproducts such as 1,3-propanediol and lactate [65]. This double mutant strain could produce 131.5 g/L of 2,3-BD from crude glycerol with yield and productivity of 0.44 g/g·crude glycerol and 0.84 g/L/h, respectively, in fed-batch fermentation.
Lignocellulosic biomass
Lignocellulose is composed of carbohydrate polymers (cellulose and hemicellulose) containing different sugar monomers that are tightly bound to aromatic polymer (lignin). This compact structure makes it hard to directly use lignocellulosic biomass. Preprocessing under conditions of high temperatures with acid or ammonia solutions for hydrolysis of biomass to release free sugars is normally needed before using lignocellulosic biomass. Usually, acidic hydrolysis generates several toxic derivatives such as furfural and 5-hydroxymethylfurfural that can inhibit the growth of microorganisms and impede production performance [66]. Despite its low biosynthetic performance, lignocellulosic biomass is inexpensive and abundant without competing with the food industry, making it an attractive feedstock for biorefineries in the future. To produce 2,3-BD, various lignocellulosic biomasses from agricultural residues and wood such as corn cob, corn stover, and apple pomace have been utilized and it has been confirmed that industrial scale production of 2,3-BD using lignocellulosic biomasses is feasible [67, 68]. Because hydrolysis of lignocellulose yields mixtures of sugars containing predominantly glucose and xylose [69], strain engineering is normally required for efficient utilization and conversion of these sugars into 2,3-BD.
Corn stover hydrolysate has been investigated as a highly promising industrial substrate. Several demonstration studies have been carried out. In one study, carbon catabolite repression is eliminated by simultaneous deletion of glucose transporter-encoding gene ptsG and overexpression of a galactose permease encoding gene galP in E. cloace strain SDM to achieve simultaneous utilization of glucose and xylose. With additional engineering strategies for reducing byproduct formation and enhancing specific production of (2R,3R)-BD, 119.4 g/L of (2R,3R)-BD was produced using corn stover as a raw material after 51 h of fermentation, with a productivity of 2.3 g/L/h [67]. As another example, a newly isolated B. licheniformis X10 strain possessing high tolerance to fermentation inhibitors such as furfural, vanillin, formic acid, and acetic acid was used for fed-batch fermentation and 74 g/L of 2,3-BD was produced from corn stover hydrolysate with a productivity of 2.1 g/L/h [70].
Corncob molasses as a waste product derived from xylitol production is also one of the candidates for industrial production of 2,3-BD. For example, 78.9 g/L of 2,3-BD was produced by K. pneumoniae strain after 61 h of fed-batch fermentation [68]. Apple pomace is an abundant processing waste which remains after juice pressing. It has increasingly been used in biotechnology applications. Currently, apple pomace hydrolysate is used for microbial 2,3-BD production. For example, 113 g/L of 2,3-BD was produced using B. licheniformis NCIMB 8059 strain [67].
Other low cost and abundant biomasses such as empty palm fruit bunches and marine algal biomass have also been investigated as raw materials for 2,3-BD production [71, 72]. Available biomass could be enhanced depending on the production host employed by testing and evaluating various non-lignocellulosic types of biomass in the future.
Fermentation and recovery processes
Optimization of fermentation and recovery processes is essential for maximizing the performance of microorganisms and producing high purity products. Since enzymes catalyzing the 2,3-BD production pathway are significantly affected by oxygen, dissolved oxygen (DO) level that regulates fed-batch fermentation has been intensively studied for mass production of 2,3-BD. For recovery, various methods have been developed. Optimization studies are still underway to reduce production cost because it has been estimated that recovery from fermentation accounts for more than 50% of total cost [73]. As a pre-commercialization study, GS Caltex have developed whole fermentation and recovery processes for 2,3-BD production. Construction of a demonstration plant with an annual capacity of 300 tons is underway. Because it is designed to produce 2,3-BD for specialty chemical applications such as cosmetics, economic feasibility for commercialization of 2,3-BD is expected.
Fermentation
Fermentation efficiency of 2,3-BD production is affected by diverse parameters such as aeration, agitation, pH, temperature, amount of inoculum, and substrate concentration. Culture protocols classified as batch, fed-batch, continuous, and cell recycling with bioreactor configuration are also very important factors in fermentation operation [15].
It is generally agreed that control of oxygen supply is the most critical factor for efficient production of 2,3-BD. In earlier work, a trade-off relationship between biomass and 2,3-BD production depending on supplied oxygen level has been found [74]. Low oxygen levels are necessary to achieve high yield production of 2,3-BD. However, overall productivity of 2,3-BD is reduced because cell mass formation is decreased under low oxygen level [75]. Also, it is necessary to set an optimized oxygen level to enhance fermentation performance depending on strains employed because inactivation of α-acetolactate synthase occurs irreversibly under aerobic conditions [76]. To determine a proper oxygen supply regimen, parameters such as oxygen transfer rate (OTR), oxygen transfer coefficient (KLa), and respiratory quotient (RQ) have been utilized as determining factors to find optimal aerobic conditions in conventional studies [77,78,79]. However, these parameters are not easy to control under real fermentation conditions. Thus, finding more controllable parameters for large-scale fermentation is still needed. In this context, agitation speed-control based strategies have been investigated to optimize the fermentation condition for efficient production of 2,3-BD. In a two-stage agitation speed control strategy for K. oxytoca, agitation speed was set at 300 rpm to obtain high cell growth during the first 15 h of growth phase and agitation speed was subsequently reduced to 200 rpm to maximize 2,3-BD production [80]. In another study, the agitation speed was changed from 450 to 350 rpm when the remaining acetoin concentration was higher than 10 g/L to efficiently convert acetoin into 2,3-BD in K. oxytoca. These conditions made it possible to achieve about 113 g/L of 2,3-BD with yield and productivity of 0.45 g/g·glucose and 2.1 g/L/h, respectively [31]. Regarding final product accumulation, it was found that optical purity of 2,3-BD was also correlated with oxygen supply. When O2 availability is increased during fermentation, the ratio of (2R,3S)-BD was increased with concomitant reduction in optical purity of (2R,3R)-BD from 98 to 93% [81].
Control of culture pH has been extensively investigated as an important variable to enhance production of 2,3-BD during fermentation. Acidic conditions are generally feasible for the production of alcoholic compounds. From an evolutionary point of view, activation of 2,3-BD production might be triggered as a survival adaptation under acidic conditions due to the accumulation of organic acids because 2,3-BD is a neutral compound [82]. In this context, it has been found that acetic acid can function as an inducer for activation of the 2,3-BD production pathway and that 2,3-BD production by K. pneumoniae was increased more than 2-fold when acetic acid was supplemented at less than 1% [83]. Although low pH and supplementation of acetic acid to some extent were effective for 2,3-BD production, finding optimized pH conditions for each host strain is required because low pH is generally deleterious to cell growth. According to prior experimental results, optimum pH values for 2,3-BD production by K. oxytoca, K. pneumoniae, and E. aerogenes are in the range of 5–6 [13]. In contrast, Bacillus strains can produce much higher amounts of 2,3-BD at much higher pH than Enterobacteriaceae. A pH from 6.3 to 6.8 was optimal for P. polymyxa [84]. B. licheniformis could efficiently produce 2,3-BD at pH 6–7 [85]. At fixed pH level, additional enhancement of 2,3-BD production was achieved by applying a forced pH fluctuation method to trigger accumulation of 2,3-BD. By consecutively raising the pH levels at exact time intervals, more efficient conversion from glycerol to 2,3-BD was realized [86].
Temperature is also one important parameter for industrial fermentation in terms of cell growth, strain performance, and operational cost. Most 2,3-BD-producing bacteria have optimal growth temperatures from 30 to 37 °C. However, these moderate temperature conditions present some problems for industrial fermentation processes due to ease of contamination and increased cooling cost [87]. In this context, thermophilic 2,3-BD-producing bacteria B. licheniformis has great potential for industrial production because this strain is GRAS classified and optimally grows at 50 to 60 °C [38, 39].
Generally, the inoculum size used in 2,3-BD production affects the productivity, but not its yield, based on carbon source consumed [10, 88]. However, a few studies have reported that an increase in inoculum size has a positive effect on the yield [85]. Although inoculum size has an insignificant effect on overall performance, it is important to set optimal inoculum size because increased inoculum size may positively affect the productivity and yield of 2,3-BD [89]. To optimize fermentation conditions, substrate concentration may also be considered. Usually 5–10% of substrate is used for 2,3-BD fermentation, although relatively low concentration has also been used for industrial scale fermentation [90]. Basically, the production of 2,3-BD may be reduced at low substrate concentration during the growth phase, while production inhibition generally occurs at high substrate concentrations. Therefore, it is important to maintain adequate substrate concentrations below toxic level in the reactor. Also, operation mode and strain-dependent optimization of substrate concentration should always be implemented to enhance the production of 2,3-BD.
Besides operation parameters, fermentation modes including batch, fed-batch, continuous, cell recycle, and immobilized cell systems have also been tested to determine the most effective mode for industrial production of 2,3-BD. Batch mode is often favored due to the feasibility of recovery which requires no residual sugar in the fermentation broth. Although traditionally developed systems such as continuous culture, cell recycle, and immobilized cell systems have been demonstrated to have higher performance, especially for elevated productivity, they have limitations in terms of process stability, economic feasibility, and contamination problems [91,92,93]. Based on numerous studies, it is generally agreed that the most effective operation mode for 2,3-BD, which is less toxic than many other chemicals for industrial production, is fed-batch fermentation with intermittent feeding of substrate when residual nutrients are depleted. Industrial scale production of 2,3-BD has been successfully achieved via fed-batch fermentation using diverse microorganisms including those belonging to genera Klebsiella, Enterobacter, Paenibacillus, Serratia, and Bacillus (Table 1).
Separation and purification
Purification of 2,3-BD from fermentation broth containing dissolved and solid components is difficult due to its high boiling point and high affinity to water. For economic production of 2,3-BD, low cost and efficient separation and purification steps need to be set up. To date, diverse separation methods including unit processes such as steam stripping [94], solvent extraction [95], reverse osmosis [96], and pervaporation [97], and combined processes such as extraction and pervaporation [95], extraction and salting out [98], and alcohol precipitation and vacuum distillation [99], have been investigated to reduce energy consumption and increase product recovery. However, the scale-up of integrated process to produce high purity 2,3-BD has not been attempted due to economic challenges and the lack of 2,3-BD market. Steam stripping demands a large amount of energy. In addition, the high boiling point of 2,3-BD makes it hard to use conventional distillation for separation. Extraction and salting out mediated methods have limitations in terms of low yields and high demand for extractants or salts for separation. Large volumes of wastewater generated from unit processes also need to be considered because wastewater treatment costs are not negligible. Above all, a slow technology development is inevitable because 2,3-BD-based market is yet to be established. Therefore, improvements in separation and purification processes are required to increase production yields and purity based on the product-related market needs. Recently, GS Caltex, a Korean petrochemical company, has developed separation and purification processes for 2,3-BD from fermentation broth, achieving over 99.5% purity. Construction of a demo-scale plant is currently underway. The entire process can be divided into two stages. The first stage involves removal of insoluble particles from fermentation broth, using centrifugation and/or a filtration process to remove cells and small molecules such as proteins. The second stage is designed for the removal of soluble impurities using electrodialysis, ion exchange, evaporation, distillation process, and so on (Fig. 3). Electrodialysis is used to remove inorganic salts such as Na+, K+, Ca+, and Mg+. Filtrate depleted of inorganic salts is then passed through an ion exchange column to remove organic acids. The treated liquid devoid of impurities is subjected to evaporation and distillation to remove water and increase the purity of 2,3-BD [100]. In addition to these steps, decolorization and deodorization are added depending on the application that the product is destined for. Due to the nature of fermentation broth, the product has a slightly yellow-like color and fermentation odor after separation and purification. GS Caltex has also developed decolorization and deodorization processes to facilitate the production of colorless and odorless high-quality products via distillation after adding neutralizing agent and activated carbon to fully remove remained impurities [101]. The final product begins to be used in specialty products such as cosmetics currently. Although the cost of separation and purification still accounts for a large proportion of production cost, GS Caltex has continuously upgraded process efficiency and product quality. The technology developed by GS Caltex will be further validated before its implementation in commercial plants based on economics and marketability.
Potential applications
High price strategies are important to commercialize biochemicals. However, market entry has been difficult because conventionally produced biochemicals compete with petrochemicals for similar industrial purposes. In this regard, it is very important to identify the demand for high value-added areas that are differentiated from petrochemical products. In this regard, 2,3-BD is a promising industrial chemical with many potential applications (Fig. 4). It could be widely used in chemical, cosmetics, agricultural, pharmaceutical, and food industries. Conventionally, potential uses of 2,3-BD have mainly focused on chemical and fuel industries as a bulk production chemical. This scenario has delayed its commercialization using biological production processes, for economic reasons. Recently, however, emerging application areas for using this compound include cosmetics, agricultural, pharmaceutical, and food industries. Thus, 2,3-BD is attracting attentions again. These opportunities could contribute to a rise in value and market expansion of 2,3-BD.
Fuel, bulk chemical, and polymer industries
Industrial interest in 2,3-BD began during World war II because it could be converted to 1,3-butadiene via dehydration using chemical catalysis and then 1,3-butadiene could be used for the production of synthetic rubber [15]. While 1,3-butadiene is generated by 1,2-elimination of a water molecule, another dehydration product, methyl ethyl ketone (MEK), an effective fuel additive as well as an industrial solvent for resins and lacquers, is produced by pinacol rearrangement. After combination with MEK and hydrogenation reaction, high-quality aviation fuel octane can be produced from 2,3-BD [50]. (2R,3R)-BD can be used as antifreeze because its freezing point is lower than −30 °C. In addition, it can serve as a monomer to synthesize polymer [12]. 2,3-BD can also be used as a novel chain initiator and extender in the manufacture of polyol and polymeric isocyanate that are intermediates of polyurethanes [102].
Cosmetics and personal care industries
The cosmetics industry is now demanding cosmetic raw materials that are sustainable, eco-friendly, and natural. As a representative example, Loreal, the world’s top cosmetics company, announced its new sustainability commitment for 2020 “Sharing beauty with all” in 2013 (The Loreal Sustainability Commitment, 2013). It has created a framework with four clear commitments: innovating, producing, living, and developing sustainably. Consequently, 2,3-BD is one of the raw materials most suitable for mega-trend changes in the cosmetics industry. It has great potential as an ingredient of both cosmetics and personal care products.
Safety and efficacy are also important for use as a cosmetic raw material. 2,3-BD is expected to have minimal toxicity because it can be found easily in nature. Reliable toxicity test result can be found in Sigma-Aldrich’s safety data sheets for 2,3-BD and in safety assessment reports of alkane diols (Cosmetic ingredient review 2018 Safety Assessment of Alkane Diols as Used in Cosmetics), describing that there are no acute toxicity, skin or eye irritation, or skin sensitization, thus supporting the viability of 2,3-BD in cosmetics. Regarding safety issues, there are data suggesting that 2,3-BD could be utilized as antiseptic, humectant, emollient ingredients in cosmetics. According to a patent filed by global cosmetic company Amorepacific [8], compositions containing (2R,3S)-BD show antibacterial effects as a preservative to improve storage stability of the product compared to chemical preservatives such as parabens and phenoxyethanol which may potentially cause skin irritation as allergens. It has been noted that 2,3-BD could be contained in cream, lotion, powder, essence, pack, hand sanitizer, hand wash, body wash, cleansing cream, cleansing gel, cleansing foam, cleansing water, soap, and so on [8]. In addition, 2,3-BD has already been registered in INCI (International Nomenclature of Cosmetic Ingredients), IECIC (Inventory of Existing Cosmetic Ingredients in China), and others. Therefore, 2,3-BD can be used as a raw material for cosmetics. It is expected that it may be used as an alternative to 1,3-BD, a chemically derived material used in existing products.
Agricultural industries
2,3-BD is also expected to be used for various purposes in the agricultural industry. The most important factor to be considered before using 2,3-BD as an agricultural product is its safety to the environment and people. It is recognized to be a safe chemical because it is known to be a constituent of edible products such as fruits and wines [103]. Also, we found that 2,3-BD did not show any phytotoxicity effect on agricultural crops such as tomato, cucumber, or pepper (un-published data). Moreover, there will be little residual toxicity, a problem of chemical pesticides, because 2,3-BD is readily biodegradable. The efficacy of 2,3-BD as an active ingredient for growth and protection of plant species has also been validated. According to previous studies, (2R,3R)-BD can trigger substantial growth promotion and induced systematic resistance (ISR)-mediated protection effects against bacterial pathogen Erwinia carotovora in Arabidopsis [104, 105]. In addition, it has been confirmed that (2R,3R)-BD at a does range of 1 mg to 100 pg/plant in tobacco can induce its systematic resistance to Erwinia carotovora [106]. 2,3-BD has been reported to be effective against fungal diseases as well as bacterial diseases. Application of (2R,3R)-BD to the soil can reduce diseased leaf area of Agrostis stolonifera by 20–40% against fungal pathogens, Microdochium nivale, Rhizoctonia solani or Sclerotinia homoeocarpa compared to the water control. In addition, it can reduce the number of lesions per leaf area of Nicotiana benthamiana caused by Colletotrichum orbiculare by 77% [7, 11]. One of the most intriguing recent findings is that 2,3-BD is effective in controlling viral diseases. Currently, there is no effective anti-viral agent in the market. Recently, it has been reported that treatment with 2,3-BD can significantly reduce the incidence of naturally occurring viruses such as Cucumber mosaic virus and Tobacco mosaic virus compared to water control, thus increasing the yield of mature pepper fruits [107]. 2,3-BD has been reported to be effective for reducing diverse abiotic stresses. Induced drought tolerance of Arabidopsis mediated by leaf stomatal closure was detected when plants were treated with (2R,3R)-BD [9]. This phenomenon has been confirmed to be due to the induction of H2O2 and NO accumulation in guard cells [108], suggesting that 2,3-BD could be utilized as a resistant material to prevent or ameliorate drought disaster. Based on these multi-functional advantages of 2,3-BD, it is expected that agricultural products containing 2,3-BD as an active ingredient could be developed with a strong impact in the pesticide market soon.
Other applications
The pharmaceutical industry may have greater interest in this compound soon as it has been reported that 2,3-BD can trigger enhanced innate immunity and result in clearance of damaged liver cells by activating natural killer cell activity [109]. It has been shown that 2,3-BD has anti-inflammatory effects according to an earlier report. It can ameliorate endotoxin-induced acute lung injury in rats [110]. These findings imply that 2,3-BD could potentially be used for human therapeutics. Furthermore, it may have use as a food additive or a health supplement based on its efficacy as an immunity enhancer because 2,3-BD is natural substance that exists in naturally prepared or occurring products such as wine, beer, fermented foods, soils, and plants [104]. In addition, it is an eco-friendly substance that can be produced by microbial fermentation.
Conclusions
Bio-based chemical 2,3-BD is one of the compounds previously restricted to industrial production. Despite many benefits of 2,3-BD, its use has been limited due to low competitiveness of biological process for 2,3-BD production, high price of chemically synthesized 2,3-BD, and poor development of product applications. To facilitate technological development of biological processes, metabolic engineering studies have been extensively employed to overcome host limitations. A number of inexpensive and abundant carbon sources have been evaluated. In addition, scale-up has been realized based on the process development for fermentation, separation and purification. For example, GS Caltex, a Korean company, is planning to launch a demonstration plant with an annual capacity of 300 tons in 2019 based on a 10-year study of bio-based 2,3-BD production. In fact, chemically synthesized (2R,3S)-BD sold by Sigma-Aldrich is priced at more than $25,000/kg, a high price unsuitable for commercial use. Although chemically synthesized 2,3-BD is used only in trace amounts of reagents currently, we are trying to apply it in a wide range of products used in real life because biological processes can produce optically pure 2,3-BD at a low cost. It will soon be possible to identify 2,3-BD in various cosmetic products, further expanding its application in agriculture, pharmaceutical, and food industries in the near future. To this end, a continuous market analysis is needed because 2,3-BD is not currently available commercially. Despite many improvements to date, efforts to improve business and economic efficiency based on further technological and market development to address customer needs are continuously needed. Successful market entry of 2,3-BD as a biochemical product may represent a novel standard in biochemical product research.
References
Harden A, Walpole G (1906) 2,3-Butylene glycol fermentation by Aerobacter aerogenes. Proc R Soc Lond 77:399–405
Othmer D, Bergen W, Shlechter N, Bruins P (1945) Liquid–liquid extraction data. Ind Eng Chem Res 37:890–894
Jones MD (2014) Catalytic transformation of ethanol into 1,3-butadiene. Chem Cent J 8:53. https://doi.org/10.1186/s13065-014-0053-4
White WC (2007) Butadiene production process overview. Chem Biol Interact 166:10–14. https://doi.org/10.1016/j.cbi.2007.01.009
Gräfje H, Körnig W, Weitz H, Reiß W, Steffan G, Diehl H, Bosche H, Schneider K, Kieczka H (2012) Butanediols, butenediol, and butynediol. In: Ullmann’s encyclopedia of industrial chemical. https://doi.org/10.1002/14356007.a04_455
Yang Z, Zhang Z (2019) Recent advances on production of 2,3-butanediol using engineered microbes. Biotechnol Adv 37:569–578. https://doi.org/10.1016/j.biotechadv.2018.03.019
Cortes-Barco AM, Hsiang T, Goodwin PH (2010) Induced systemic resistance against three foliar diseases of Agrostis stolonifera by (2R,3R)-butanediol or an isoparaffin mixture. Ann Appl Biol 157:179–189. https://doi.org/10.1111/j.1744-7348.20
Baek HS, Woo BY, Yoo SJ, Joo YH, Shin SS, Oh MH, Lee JH, Kim SY (2016) Composition containing meso-2,3-butanediol. WO 2016064180 A1
Cho SM, Kang BR, Han SH, Anderson AJ, Park JY, Lee YH, Cho BH, Yang KY, Ryu CM, Kim YC (2008) 2R,3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol Plant Microbe Interact 21:1067–1075. https://doi.org/10.1094/MPMI-21-8-1067
Garg S, Jain A (1995) Fermentative production of 2,3-butnaediol: a review. Bioresour Technol 51:103–109. https://doi.org/10.1016/0960-8524(94)00136-O
Cortes-Barco AM, Goodwin PH, Hsiang T (2010) Comparison of induced resistance activated by benzothiadiazole, (2R,3R)-butanediol and an isoparaffin mixture against anthracnose of Nicotiana benthamiana. Plant Pathol 59:643–653
Syu MJ (2001) Biological production of 2,3-butanediol. Appl Microbiol Biotechnol 55:10–18. https://doi.org/10.1007/s002530000486
Celinska E, Grajek W (2009) Biotechnological production of 2,3-butanediol–current state and prospects. Biotechnol Adv 27:715–725. https://doi.org/10.1016/j.biotechadv.2009.05.002
Xiao Z, Xu P (2007) Acetoin metabolism in bacteria. Crit Rev Microbiol 33:127–140. https://doi.org/10.1080/10408410701364604
Ji XJ, Huang H, Ouyang PK (2011) Microbial 2,3-butanediol production: a state-of-the-art review. Biotechnol Adv 29:351–364. https://doi.org/10.1016/j.biotechadv.2011.01.007
Ui S, Mimura A, Ohkuma M, Kudo T (1999) Formation of a chiral acetoinic compound from diacetyl by Escherichia coli expressing meso-2,3-butanediol dehydrogenase. Lett Appl Microbiol 28:457–460. https://doi.org/10.1046/j.1365-2672.1999.00560.x
Ge Y, Li K, Li L, Gao C, Zhang L, Ma C, Xu P (2016) Contracted but effective: production of enantiopure 2,3-butanediol by thermophilic and GRAS Bacillus licheniformis. Green Chem 18:4693–4703. https://doi.org/10.1039/C6GC01023G
Magee R, Kosaric N (1987) The microbial production of 2,3-butanediol. Adv Appl Microbiol 32:89–161. https://doi.org/10.1016/S0065-2164(08)70079-0
Yang Z, Zhang Z (2018) Production of (2R, 3R)-2,3-butanediol using engineered Pichia pastoris: strain construction, characterization and fermentation. Biotechnol Biofuels 11:35. https://doi.org/10.1186/s13068-018-1031-1
Cho SM, Kang BR, Han SH, Anderson AJ, Park JY, Lee YH, Cho BH, Yang KY, Ryu CM, Kim YC (2008) 2R,3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol Plant Microbe Interact 21:1067–1075. https://doi.org/10.1094/MPMI-21-8-1067
Hong KK, Nielsen J (2012) Metabolic engineering of Saccharomyces cerevisiae: a key cell factory platform for future biorefineries. Cell Mol Life Sci 69:2671–2690. https://doi.org/10.1007/s00018-012-0945-1
Kandasamy V, Liu J, Dantoft SH, Solem C, Jensen PR (2016) Synthesis of (3R)-acetoin and 2,3-butanediol isomers by metabolically engineered Lactococcus lactis. Sci Rep. https://doi.org/10.1038/srep36769 (Article number: 36769)
Hohn-Bentz H, Radler F (1978) Bacterial 2,3-butanediol dehydrogenases. Arch Microbiol 116:197–203. https://doi.org/10.1007/BF00406037
Kim B, Lee S, Park J, Lu M, Oh M, Kim Y, Lee J (2012) Enhanced 2,3-butanediol production in recombinant Klebsiella pneumoniae via overexpression of synthesis-related genes. J Microbiol Biotechnol 22:1258–1263
Guo X, Cao C, Wang Y, Li C, Wu M, Chen Y, Zhang C, Pei H, Xiao D (2014) Effect of the inactivation of lactate dehydrogenase, ethanol dehydrogenase, and phosphotransacetylase on 2,3-butanediol production in Klebsiella pneumoniae strain. Biotechnol Biofuels 7:44. https://doi.org/10.1186/1754-6834-7-44
Rathnasingh C, Park JM, Kim DK, Song H, Chang YK (2016) Metabolic engineering of Klebsiella pneumoniae and in silico investigation for enhanced 2,3-butanediol production. Biotechnol Lett 38:975–982. https://doi.org/10.1007/s10529-016-2062-y
Ma C, Wang A, Qin J, Li L, Ai X, Jiang T, Tang H, Xu P (2009) Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl Microbiol Biotechnol 82:49–57. https://doi.org/10.1007/s00253-008-1732-7
Lee S, Kim B, Yang J, Jeong D, Park S, Lee J (2015) A non-pathogenic and optically high concentrated (R, R)-2,3-butanediol biosynthesizing Klebsiella strain. J Biotechnol 209:7–13. https://doi.org/10.1016/j.jbiotec.2015.06.385
Ji XJ, Huang H, Zhu JG, Ren LJ, Nie ZK, Du J, Li S (2010) Engineering Klebsiella oxytoca for efficient 2, 3-butanediol production through insertional inactivation of acetaldehyde dehydrogenase gene. Appl Microbiol Biotechnol 85:1751–1758. https://doi.org/10.1007/s00253-009-2222-2
Kim DK, Rathnasingh C, Song H, Lee HJ, Seung D, Chang YK (2013) Metabolic engineering of a novel Klebsiella oxytoca strain for enhanced 2,3-butanediol production. J Biosci Bioeng 116:186–192. https://doi.org/10.1016/j.jbiosc.2013.02.021
Park JM, Song H, Lee HJ, Seung D (2013) In silico aided metabolic engineering of Klebsiella oxytoca and fermentation optimization for enhanced 2,3-butanediol production. J Ind Microbiol Biotechnol 40:1057–1066. https://doi.org/10.1007/s10295-013-1298-y
Jantama K, Polyiam P, Khunnonkwao P, Chan S, Sangproo M, Khor K, Jantama SS, Kanchanatawee S (2015) Efficient reduction of the formation of by-products and improvement of production yield of 2,3-butanediol by a combined deletion of alcohol dehydrogenase, acetate kinase-phosphotransacetylase, and lactate dehydrogenase genes in metabolically engineered Klebsiella oxytoca in mineral salts medium. Metab Eng 30:16–26. https://doi.org/10.1016/j.ymben.2015.04.004
Jung MY, Ng CY, Song H, Lee J, Oh MK (2012) Deletion of lactate dehydrogenase in Enterobacter aerogenes to enhance 2,3-butanediol production. Appl Microbiol Biotechnol 95:461–469. https://doi.org/10.1007/s00253-012-3883-9
Li L, Li K, Wang Y, Chen C, Xu Y, Zhang L, Han B, Gao C, Tao F, Ma C, Xu P (2015) Metabolic engineering of Enterobacter cloacae for high-yield production of enantiopure (2R,3R)-2,3-butanediol from lignocellulose-derived sugars. Metab Eng 28:19–27. https://doi.org/10.1016/j.ymben.2014.11.010
Rao B, Zhang LY, Sun J, Su G, Wei D, Chu J, Zhu J, Shen Y (2012) Characterization and regulation of the 2,3-butanediol pathway in Serratia marcescens. Appl Microbiol Biotechnol 93:2147–2159. https://doi.org/10.1007/s00253-011-3608-5
Zhang L, Yang Y, Sun J, Shen Y, Wei D, Zhu J, Chu J (2010) Microbial production of 2,3-butanediol by a mutagenized strain of Serratia marcescens H30. Bioresour Technol 101:1961–1967. https://doi.org/10.1016/j.biortech.2009.10.052
Zhang L, Sun J, Hao Y, Zhu J, Chu J, Wei D, Shen Y (2010) Microbial production of 2,3-butanediol by a surfactant (serrawettin)-deficient mutant of Serratia marcescens H30. J Ind Microbiol Biotechnol 37:857–862. https://doi.org/10.1007/s10295-010-0733-6
Li L, Zhang L, Li K, Wang Y, Gao C, Han B, Ma C, Xu P (2013) A newly isolated Bacillus licheniformis strain thermophilically produces 2,3-butanediol, a platform and fuel bio-chemical. Biotechnol Biofuels 6:123. https://doi.org/10.1186/1754-6834-6-123
Jurchescu IM, Hamann J, Zhou X, Ortmann T, Kuenz A, Prusse U, Lang S (2013) Enhanced 2,3-butanediol production in fed-batch cultures of free and immobilized Bacillus licheniformis DSM 8785. Appl Microbiol Biotechnol 97:6715–6723. https://doi.org/10.1007/s00253-013-4981-z
Fu J, Huo G, Feng L, Mao Y, Wang Z, Ma H, Chen T, Zhao X (2016) Metabolic engineering of Bacillus subtilis for chiral pure meso-2,3-butanediol production. Biotechnol Biofuels 9:90. https://doi.org/10.1186/s13068-016-0502-5
Hassler T, Schieder D, Pfaller R, Faulstich M, Sieber V (2012) Enhanced fed-batch fermentation of 2,3-butanediol by Paenibacillus polymyxa DSM 365. Bioresour Technol 124:237–244. https://doi.org/10.1016/j.biortech.2012.08.047
Yang T, Rao Z, Zhang X, Lin Q, Xia H, Xu Z, Yang S (2011) Production of 2,3-butanediol from glucose by GRAS microorganism Bacillus amyloliquefaciens. J Basic Microbiol 51:650–658. https://doi.org/10.1002/jobm.201100033
Yang T, Rao Z, Zhang X, Xu M, Xu Z, Yang ST (2013) Improved production of 2,3-butanediol in Bacillus amyloliquefaciens by over-expression of glyceraldehyde-3-phosphate dehydrogenase and 2,3-butanediol dehydrogenase. PLoS One 8:e76149. https://doi.org/10.1371/journal.pone.0076149
Kim SJ, Seo SO, Jin YS, Seo JH (2013) Production of 2,3-butanediol by engineered Saccharomyces cerevisiae. Bioresour Technol 146:274–281. https://doi.org/10.1016/j.biortech.2013.07.081
Gonzalez E, Fernandez MR, Larroy C, Sola L, Pericas MA, Pares X, Biosca JA (2000) Characterization of a (2R,3R)-2,3-butanediol dehydrogenase as the Saccharomyces cerevisiae YAL060W gene product. Disruption and induction of the gene. J Biol Chem 275:35876–35885. https://doi.org/10.1074/jbc.M003035200
Kim S, Hahn JS (2015) Efficient production of 2,3-butanediol in Saccharomyces cerevisiae by eliminating ethanol and glycerol production and redox rebalancing. Metab Eng 31:94–101. https://doi.org/10.1016/j.ymben.2015.07.006
Xu Y, Chu H, Gao C, Tao F, Zhou Z, Li K, Li L, Ma C, Xu P (2014) Systematic metabolic engineering of Escherichia coli for high-yield production of fuel bio-chemical 2,3-butanediol. Metab Eng 23:22–33. https://doi.org/10.1016/j.ymben.2014.02.004
Nozzi NE, Case AE, Carroll AL, Atsumi S (2017) Systematic Approaches to Efficiently Produce 2,3-Butanediol in a Marine Cyanobacterium. ACS Synth Biol. https://doi.org/10.1021/acssynbio.7b00157
Yang T, Rao Z, Zhang X, Xu M, Xu Z, Yang ST (2017) Metabolic engineering strategies for acetoin and 2,3-butanediol production: advances and prospects. Crit Rev Biotechnol 37:990–1005. https://doi.org/10.1080/07388551.2017.1299680
Bialkowska AM (2016) Strategies for efficient and economical 2,3-butanediol production: new trends in this field. World J Microbiol Biotechnol 32:200. https://doi.org/10.1007/s11274-016-2161-x
Gao J, Xu H, Li QJ, Feng XH, Li S (2010) Optimization of medium for one-step fermentation of inulin extract from Jerusalem artichoke tubers using Paenibacillus polymyxa ZJ-9 to produce R, R-2,3-butanediol. Bioresour Technol 101:7087–7093. https://doi.org/10.1016/j.biortech.2010.03.143
Petrov K, Petrova P (2009) High production of 2,3-butanediol from glycerol by Klebsiella pneumoniae G31. Appl Microbiol Biotechnol 84:659–665. https://doi.org/10.1007/s00253-009-2004-x
Wang A, Xu Y, Ma C, Gao C, Li L, Wang Y, Tao F, Xu P (2012) Efficient 2,3-butanediol production from cassava powder by a crop-biomass-utilizer, Enterobacter cloacae subsp. dissolvens SDM. PLoS One 7:e40442. https://doi.org/10.1371/journal.pone.0040442
Akaraonye E, Moreno C, Knowles JC, Keshavarz T, Roy I (2012) Poly(3-hydroxybutyrate) production by Bacillus cereus SPV using sugarcane molasses as the main carbon source. Biotechnol J 7:293–303. https://doi.org/10.1002/biot.201100122
Jung MY, Park BS, Lee J, Oh MK (2013) Engineered Enterobacter aerogenes for efficient utilization of sugarcane molasses in 2,3-butanediol production. Bioresour Technol 139:21–27. https://doi.org/10.1016/j.biortech.2013.04.003
Xin Fengxue, Basu Anindya, Weng Michelle Cheung, Yang Kun-Lin, He J (2016) Production of 2,3-butanediol from sucrose by a klebsiella species. Bioenergy Res 9:15–22. https://doi.org/10.1007/s12155-015-9653-7
Song CW, Rathnasingh C, Park JM, Lee J, Song H (2018) Isolation and evaluation of Bacillus strains for industrial production of 2,3-butanediol. J Microbiol Biotechnol 28:409–417. https://doi.org/10.4014/jmb.1710.10038
Pervez S, Aman A, Iqbal S, Siddiqui NN, Ul Qader SA (2014) Saccharification and liquefaction of cassava starch: an alternative source for the production of bioethanol using amylolytic enzymes by double fermentation process. BMC Biotechnol 14:49. https://doi.org/10.1186/1472-6750-14-49
Szambelan K, Nowak J, Czarnecki Z (2004) Use of Zymomonas mobilis and Saccharomyces cerevisiae mixed with Kluyveromyces fragilis for improved ethanol production from Jerusalem artichoke tubers. Biotechnol Lett 26:845–848. https://doi.org/10.1023/B:BILE.0000025889.25364.4b
Sun LH, Wang XD, Dai JY, Xiu ZL (2009) Microbial production of 2,3-butanediol from Jerusalem artichoke tubers by Klebsiella pneumoniae. Appl Microbiol Biotechnol 82:847–852. https://doi.org/10.1007/s00253-008-1823-5
Li L, Chen C, Li K, Wang Y, Gao C, Ma C, Xu P (2014) Efficient simultaneous saccharification and fermentation of inulin to 2,3-butanediol by thermophilic Bacillus licheniformis ATCC 14580. Appl Environ Microbiol 80:6458–6464. https://doi.org/10.1128/AEM.01802-14
Champluvier B, Francart B, Rouxhet PG (1989) Co-immobilization by adhesion of beta-galactosidase in nonviable cells of Kluyveromyces lactis with Klebsiella oxytoca: conversion of lactose into 2,3-butanediol. Biotechnol Bioeng 34:844–853. https://doi.org/10.1002/bit.260340614
Ahn JH, Sang BI, Um Y (2011) Butanol production from thin stillage using Clostridium pasteurianum. Bioresour Technol 102:4934–4937. https://doi.org/10.1016/j.biortech.2011.01.046
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39. https://doi.org/10.1016/j.biotechadv.2008.07.006
Cho S, Kim T, Woo HM, Kim Y, Lee J, Um Y (2015) High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol Biofuels 8:146. https://doi.org/10.1186/s13068-015-0336-6
Thomsen MH, Thygesen A, Thomsen AB (2009) Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl Microbiol Biotechnol 83:447–455. https://doi.org/10.1007/s00253-009-1867-1
Bialkowska AM, Gromek E, Krysiak J, Sikora B, Kalinowska H, Jedrzejczak-Krzepkowska M, Kubik C, Lang S, Schutt F, Turkiewicz M (2015) Application of enzymatic apple pomace hydrolysate to production of 2,3-butanediol by alkaliphilic Bacillus licheniformis NCIMB 8059. J Ind Microbiol Biotechnol 42:1609–1621. https://doi.org/10.1007/s10295-015-1697-3
Wang A, Wang Y, Jiang T, Li L, Ma C, Xu P (2010) Production of 2,3-butanediol from corncob molasses, a waste by-product in xylitol production. Appl Microbiol Biotechnol 87:965–970. https://doi.org/10.1007/s00253-010-2557-8
Sheehan J, Himmel M (1999) Enzymes, energy, and the environment: a strategic perspective on the U.S. Department of Energy’s Research and Development Activities for bioethanol. Biotechnol Prog 15:817–827. https://doi.org/10.1021/bp990110d
Li L, Li K, Wang K, Chen C, Gao C, Ma C, Xu P (2014) Efficient production of 2,3-butanediol from corn stover hydrolysate by using a thermophilic Bacillus licheniformis strain. Bioresour Technol 170:256–261. https://doi.org/10.1016/j.biortech.2014.07.101
Kang IY, Park JM, Hong WK, Kim YS, Jung YR, Kim SB, Heo SY, Lee SM, Kang JY, Oh BR, Kim DH, Seo JW, Kim CH (2015) Enhanced production of 2,3-butanediol by a genetically engineered Bacillus sp. BRC1 using a hydrolysate of empty palm fruit bunches. Bioprocess Biosyst Eng 38:299–305. https://doi.org/10.1007/s00449-014-1268-4
Mazumdar S, Lee J, Oh MK (2013) Microbial production of 2,3 butanediol from seaweed hydrolysate using metabolically engineered Escherichia coli. Bioresour Technol 136:329–336. https://doi.org/10.1016/j.biortech.2013.03.013
Waldron KW (2010) Bioalcohol production: biochemical conversion of lignocellulosic biomass, 1st edn. Woodhead Pulishing Series in Energy, Sawston
Jansen NB, Flickinger MC, Tsao GT (1984) Production of 2,3-butanediol from d-xylose by Klebsiella oxytoca ATCC 8724. Biotechnol Bioeng 26:362–369. https://doi.org/10.1002/bit.260260411
Sablayrolles JM, Goma G (1984) Butanediol production by Aerobacter aerogenes NRRL B199: effects of initial substrate concentration and aeration agitation. Biotechnol Bioeng 26:148–155. https://doi.org/10.1002/bit.260260207
Kosaric N, Magee RJ, Blaszczyk R (1992) Redox potential measurement for monitoring glucose and xylose conversion by K. pneumoniae. Chem Biochem Eng Q 6:145–152
Beronio PB Jr, Tsao GT (1993) Optimization of 2,3-butanediol production by Klebsiella oxytoca through oxygen transfer rate control. Biotechnol Bioeng 42:1263–1269. https://doi.org/10.1002/bit.260421102
Fages J, Mulard D, Rouquet J, Wilhelm J (1986) 2,3-Butanediol production from Jerusalem artichoke, Helianfhus fuberosus, and by Bacillus polymyxa ATCC 12321. Optimization of kLa profile. Appl Microbiol Biotechnol 25:197–202
Zeng AP, Byun TG, Posten C, Deckwer WD (1994) Use of respiratory quotient as a control parameter for optimum oxygen supply and scale-up of 2,3-butanediol production under microaerobic conditions. Biotechnol Bioeng 44:1107–1114. https://doi.org/10.1002/bit.260440912
Ji XJ, Huang H, Du J, Zhu JG, Ren LJ, Hu N, Li S (2009) Enhanced 2,3-butanediol production by Klebsiella oxytoca using a two-stage agitation speed control strategy. Bioresour Technol 100:3410–3414. https://doi.org/10.1016/j.biortech.2009.02.031
Nakashimada Y, Kanai K, Nishio N (1998) Optimization of dilution rate, pH and oxygen supply on optical purity of 2, 3-butanediol produced by Paenibacillus polymyxa in chemostat culture. Biotechnol Lett 20:1133–1138. https://doi.org/10.1023/A:1005324403186
Van Houdt R, Aertsen A, Michiels CW (2007) Quorum-sensing-dependent switch to butanediol fermentation prevents lethal medium acidification in Aeromonas hydrophila AH-1N. Res Microbiol 158:379–385. https://doi.org/10.1016/j.resmic.2006.11.015
Yu EK, Saddler JN (1982) Enhanced production of 2,3-butanediol by Klebsiella pneumoniae grown on high sugar concentrations in the presence of acetic acid. Appl Environ Microbiol 44:777–784. https://doi.org/10.1155/2011/636170
Nakashimada Y, Marwoto B, Kashiwamura T, Kakizono T, Nishio N (2000) Enhanced 2,3-butanediol production by addition of acetic acid in Paenibacillus polymyxa. J Biosci Bioeng 90:661–664
Perego P, Converti A, Del Borghi M (2003) Effects of temperature, inoculum size and starch hydrolyzate concentration on butanediol production by Bacillus licheniformis. Bioresour Technol 89:125–131. https://doi.org/10.1016/S0960-8524(03)00063-4
Petrov K, Petrova P (2010) Enhanced production of 2,3-butanediol from glycerol by forced pH fluctuations. Appl Microbiol Biotechnol 87:943–949. https://doi.org/10.1007/s00253-010-2545-z
Fonseca GG, Heinzle E, Wittmann C, Gombert AK (2008) The yeast Kluyveromyces marxianus and its biotechnological potential. Appl Microbiol Biotechnol 79:339–354. https://doi.org/10.1007/s00253-008-1458-6
Grover BP, Garg SK, Verma J (1990) Production of 2,3-butanediol from wood hydrolysate by Klebsiella pneumoniae. World J Microbiol Biotechnol 6:328–332. https://doi.org/10.1007/BF01201306
Anvari M, Safari Motlagh MR (2011) Enhancement of 2,3-butanediol production by Klebsiella oxytoca PTCC 1402. J Biomed Biotechnol 2011:636170. https://doi.org/10.1155/2011/636170
Long SK, Patrick R (1963) The present status of the 2,3-butylene glycol fermentation. Adv Appl Microbiol 5:135–155. https://doi.org/10.1016/S0065-2164(08)70009-1
Dettwiler B, Dunn IJ, Heinzle E, Prenosil JE (1993) A simulation model for the continuous production of acetoin and butanediol using Bacillus subtilis with integrated pervaporation separation. Biotechnol Bioeng 41:791–800. https://doi.org/10.1002/bit.260410805
Dziewulski DM, Haughney HA, Das KP, Nauman EB (1986) Fed-batch with biomass recycle and batch production of 2,3-butanediol from glucose by Bacillus polymyxa. J Biotechnol 4:171–180. https://doi.org/10.1016/0168-1656(86)90044-1
Itoh N, Nakamura M, Inoue K, Makino Y (2007) Continuous production of chiral 1,3-butanediol using immobilized biocatalysts in a packed bed reactor: promising biocatalysis method with an asymmetric hydrogen-transfer bioreduction. Appl Microbiol Biotechnol 75:1249–1256. https://doi.org/10.1007/s00253-007-0957-1
Wheat J, Xleslie J, Tomkins R, Mitton H, Scott D, Ledingham G (1948) Production and properties of 2,3-butanediol. XXVIII. Pilot plant recovery of levo-2,3-butanediol from whole wheat mashes fermented by Aerobacillus polymyxa. Can J Res 26:469–496
Shao P, Kumar A (2009) Recovery of 2,3-butanediol from water by a solvent extraction and pervaporation separation scheme. J Membr Sci 329:160–168. https://doi.org/10.1016/j.memsci.2008.12.033
Sridhar S (1989) Zur Abtrennung von butandiol-2,3 aus Fermenter-Brühen mit Hilfe der Umkehrosmose. Chem Ing Tech 61:252–253. https://doi.org/10.1002/cite.330610316
Qureshi N, Meagher MM, Hutkins RW (2006) Recovery of 2,3-butanediol by vacuum membrane distillation. Sep Sci Technol 29:1733–1748. https://doi.org/10.1080/01496399408002168
Sun LH, Jiang B, Xiu ZL (2009) Aqueous two-phase extraction of 2,3-butanediol from fermentation broths by isopropanol/ammonium sulfate system. Biotechnol Lett 31:371–376. https://doi.org/10.1007/s10529-008-9874-3
Jeon S, Kim DK, Song H, Lee HJ, Park S, Seung D, Chang YK (2014) 2,3-Butanediol recovery from fermentation broth by alcohol precipitation and vacuum distillation. J Biosci Bioeng 117:464–470. https://doi.org/10.1016/j.jbiosc.2013.09.007
Jeon SJ, Nam HG (2019) Method of preparing diol. Korea Patent 1019751870000
Lee JJ, Jeon SJ, Nam HG (2019) Method of decolorization and deordorization of polyhydric alcohol. Korea Patent 1019695300000
Joanna P, Bogusław C (2006) New compounds for production of polyurethane foams. Appl Polym Sci 102:5918–5926. https://doi.org/10.1002/app.25093
Jiang B, Zhang Z (2010) Volatile compounds of young wines from cabernet sauvignon, cabernet gernischet and chardonnay varieties grown in the loess plateau region of china. Molecules 15:9184–9196. https://doi.org/10.3390/molecules15129184
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026. https://doi.org/10.1104/pp.103.026583
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci 100:4927–4932. https://doi.org/10.1073/pnas.0730845100
Han SH, Lee SJ, Moon JH, Park KH, Yang KY, Cho BH, Kim KY, Kim YW, Lee MC, Anderson AJ, Kim YC (2006) GacS-dependent production of 2R, 3R-butanediol by Pseudomonas chlororaphis O6 is a major determinant for eliciting systemic resistance against Erwinia carotovora but not against Pseudomonas syringae pv. tabaci in tobacco. Mol Plant Microbe Interact 19:924–930. https://doi.org/10.1094/MPMI-19-0924
Kong HG, Shin TS, Kim TH, Ryu CM (2018) Stereoisomers of the bacterial volatile compound 2,3-butanediol differently elicit systemic defense responses of pepper against multiple viruses in the field. Front Plant Sci 9:90. https://doi.org/10.3389/fpls.2018.00090
Wu L, Li X, Ma L, Borriss R, Wu Z, Gao X (2018) Acetoin and 2,3-butanediol from Bacillus amyloliquefaciens induce stomatal closure in Arabidopsis thaliana and Nicotiana benthamiana. J Exp Bot 69:5625–5635. https://doi.org/10.1093/jxb/ery326
Lai HC, Chang CJ, Yang CH, Hsu YJ, Chen CC, Lin CS, Tsai YH, Huang TT, Ojcius DM, Tsai YH, Lu CC (2012) Activation of NK cell cytotoxicity by the natural compound 2,3-butanediol. J Leukoc Biol 92:807–814. https://doi.org/10.1189/jlb.0112024
Hsieh SC, Lu CC, Horng YT, Soo PC, Chang YL, Tsai YH, Lin CS, Lai HC (2007) The bacterial metabolite 2,3-butanediol ameliorates endotoxin-induced acute lung injury in rats. Microbes Infect 9:1402–1409. https://doi.org/10.1016/j.micinf.2007.07.004
Fu J, Wang Z, Chen T, Liu W, Shi T, Wang G, Tang YJ, Zhao X (2014) NADH plays the vital role for chiral pure D-(-)-2,3-butanediol production in Bacillus subtilis under limited oxygen conditions. Biotechnol Bioeng 111:2126–2131. https://doi.org/10.1002/bit.25265
Acknowledgements
This work was supported by the Industrial Strategic Technology Development Program (No. 10050407) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, C.W., Park, J.M., Chung, S.C. et al. Microbial production of 2,3-butanediol for industrial applications. J Ind Microbiol Biotechnol 46, 1583–1601 (2019). https://doi.org/10.1007/s10295-019-02231-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-019-02231-0