Abstract
The 1,4-diacids including succinic, malic, and fumaric acids are naturally occurring C4 building blocks that are regarded as major metabolic intermediates in most eukaryotic or prokaryotic microorganisms. During the past decade, 1,4-diacids have established themselves as forerunners of the biorefinery platform chemicals. There is significant global interest in producing bulk chemicals from renewable resources using microorganisms. Compared with conventional production methods derived from fossil fuel feedstocks, the synthesis of 1,4-diacids from sugars or natural biomass using microorganism producers is highly efficient and has the potential to reduce world dependence on fossil fuels. This chapter reviews microorganism producers, cultivation, separation technologies, alternative substrates of lignocellulosic biomass, and integration strategies to provide analysis of the strategies and economics of 1,4-diacid commercial-scale production.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- C4 dicarboxylic acids
- Microbial production
- Biomass
- Cultivation strategies
- Downstream processing
- In situ product recovery
- Techno-economics challenges
1 Introduction
1.1 Platform Chemical Production Using a Biorefinery Concept
Platform chemicals act as the elementary materials for synthesizing many chemical intermediates and useful polymers . Presently, most marketable platform chemicals are synthesized by petrochemical processes that use crude oil , natural gas, or coal as the starting materials [1]. C4 platform chemicals mainly consist of carboxylic acids, dicarboxylic acids (such as succinic, malic, and fumaric acids ), alcohols, amino (di) carboxylic acids, diols, diamines, and dienes [2]. In the past 10 years, biorefinery of C4 platform chemical from biomass has been considered as a substitute for petrochemical-based approaches, which would lower the risk of energy security, limit use of fossil oils, and reduce environment problems associated with greenhouse gas emissions.
C4 platform chemical production from bio-resources includes efficient improvement of natural strains or engineered microorganisms for fermentation, optimization of separation and purification , integration of suitable unit operations . Price-competitive and sustainable resource-derived feedstocks are important for the entire cost evaluation. Succinic, together with fumaric and malic acids , have been well-known as the important building block chemicals that can be made from sugars or biomass substrates [3]. Figure 8.1 shows a bio-based strategy for the refinery of these three platform organic acids . Improvement of industrial strains capable of making the target products at high concentration, yields , and productivity is the first task. The optimization of downstream processes plays an important role in reducing the total production cost which is the major competing factor compared with existing petrochemical processes.
1.2 Current State and Perspectives of C4 Dicarboxylic Acids— Succinic, Malic, and Fumaric Acids
Existing commercial production of C4 dicarboxylic acids remains dependent on petrochemical production methods. The application extends to agricultural , pharmaceutical, food , adsorbents, and polymer industries [4]. C4 dicarboxylic acids, together with their derivatives, have a 15 × 109 US dollar market as bulk chemicals [5].
The U.S. Department of Energy has listed succinic acid as one of the top twelve building block chemicals for over 10 years [3]. U.S. Food and Drug Administration has approved succinic acid as a safe chemical. Succinic acid acts as an anticarcinogenic agent and as an insulinotropic agent in the pharmaceutical area.
In the chemical industry, succinic acid is a precursor for the production of numerous high value compounds such as 1,4-butanediol , N-methyl-2-pyrrolidone, γ-butyrolactone, tetrahydrofuran , and 2-pyrrolidone . Succinic acid is a precursor to many specialized polymers . For example, polybutylene succinic acid (PBS) is a promising biopolymer [3,4,5]. The production of succinic acid by microorganisms has been widely studied not only in traditional rumen bacteria, but also in many other host organisms. The major metabolic pathway uses the reductive branch of the tricarboxylic acid (TCA) cycle .
The growing succinic acid market is stimulating the commercialization of bio-based succinic acid . Many famous companies, such as Reverdia, Myriant, Succinity and BioAmber, have already touched the stage of industrialization.
Fumaric acid is generally used as beverage ingredient and food acidulant [6], or antibacterial agent in the feed industry. Fumaric acid can be polymerized to biodegradable polymers . Fumaric acid is regarded as a key intermediate in the production of L-malic acid and L-aspartic acid, which are used in sweeteners, beverages and other areas [7]. Bio-based fumaric acid was studied previously, but biorefinery of fumaric acid is being replaced by petrochemical synthesis methods in the market . Rhizopus species are well-known as native producers of fumaric acid via TCA pathway . Until now, few efforts have been made to improve the production processes of Rhizopus sp. through metabolic engineering , which suggests that this field is rich in potential opportunities. Many other microorganisms, including S. cerevisiae and genetic modified E. coli , have been engineered for fumaric acid production.
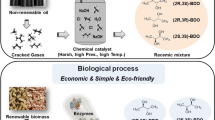
Malic acid has various applications in the food, beverage, metal cleaning, pharmaceuticals and plastics industries [3, 8]. The naturally occurring Malic acid is L-form, whereas L- and D-malic acid are prepared synthetically as a racemic mixture . Malic acid can be formed in metabolic cycles in both the TCA and glyoxalate cycles; it offers cells with carbon skeletons or energy. Because the current chemical synthetic method makes a racemic mixture of L- and D-malic acid, biorefinery of malic acid remains superior in terms of stereo-selectivity . Malic acid can be mainly produced by fungal species. However, when fungal fermentation reaches the pilot or industrial scale, some difficulties will need to be overcome, such as oxygen transfer and especially morphological problems. Potential toxin formation is another question. An alternative way to produce L-malic acid is biorefinery by well-characterized microorganisms, such as yeast and E. coli .
Downstream processes require separation and purification of the 1,4-diacid products, which remains the main economic hurdles in the commercialization of bio-based production. Downstream unit operations of the C4 dicarboxylic acids from the fermentation broth have much more similarities . Adsorption, precipitation methods and in situ product recovery strategies are widely applied to downstream processes. Separation and purification techniques, including precipitation, extraction , adsorption , electrodialysis and membrane separation , have been effectively used for the recovery of bio-based C4 dicarboxylic acids. One challenge to successful separation of 1,4-diacids from crude broths is how to apply purification technologies on the industrial-scale that have good productivity and yields as those in the laboratory. For years, these technological challenges are considered as frontline issues of an economically -sustainable biorefinery .
2 Upstream Processing
In spite of many benefits and the growing attention to biotechnology, common commercial routines in biorefinery processes are constrained by time and cost. Various methods and tools are essential to the natural or engineered strains required in which scientists and engineers to develop effective production processes.
2.1 Microbial Producers
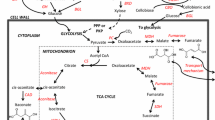
Most prokaryotic and eukaryotic microorganisms produce succinic, malic, and fumaric acids as naturally major metabolic intermediates as shown in Fig. 8.2. Different kinds of genetic changes and modification have been made to wild type microorganisms, and many engineered strains have been retained in the current 1,4-diacid production in bioreactors. Host strain engineering research is carried out with the following objectives: (1) to decrease by-products , (2) to improve both redox availability and energy efficiency , (3) to recognize the blocks to the target formation through diagnostic research.
Succinic acid can be made as a fermentation end-product or as an intermediate of the central metabolism via the reductive TCA branch [9]. Traditional microorganisms for succinic acid production are well known as Anaerobiospirillum succiniciproducens , Actinobacillus succinogenes , Mannheimia succiniciproducens and recombinant Escherichia coli strains (Table 8.1) [10,11,12]. Theoretically, two molecules of succinic acid are synthesized from one molecule of monosaccharide anaerobically. Carbon dioxide consumption (one mole CO2 per mole succinic acid) makes the synthesis environmentally-friendly [13, 14]. Corynebacterium glutamicum is a quick-growing microorganism used for a long period in industrial production of organic acids , amino acids , and nucleic acids. C. glutamicum, can produce organic acids such as lactic , succinic, and acetic acid using glucose as a carbon source with high-density cells and high productivity [15]. Besides C. glutamicum, genome sequence of Saccharomyces cerevisiae is clearly and physiologically well characterized, and it can make 1,4-diacids at very low pH cultivation conditions. Efforts to genetically modify S. cerevisiae for succinic acid production have been reported [16]. Genetic improvement and industrial utilization of S. cerevisiae have been established, which makes S. cerevisiae suitable for the succinic acid refinery.
An inclusive range of microorganisms produces fumaric acid as a naturally occurring intermediate in the TCA branch, or as a product in the urea cycle. Succinate dehydrogenase can convert succinate to fumaric acid by oxidation . Fumarase can convert fumarate to malate . Some microorganisms make fumaric acid as the fermentative product. Particularly, fungi are recognized as fumaric acid producers [17]. Fumaric acid producers are normally identified as Rhizopus, Cunninghamella , Mucor , and Circinella species, among which Rhizopus species (Table 8.2) is one of the best-known producers. Rhizopus species can yield fumaric acid in both aerobic and anaerobic cultivation conditions. However, it should be noted that not all R. oryzae species are able to make fumaric acid. R. oryzae can be categorized into two categories: type I merely forms fumaric acid, with little or no lactic acid production; type II forms lactic acid with little or no fumaric acid production [18].
Natural malic acid producers at high production levels such as Aspergillus flavus, are presently disqualified as a result of special growth requests and some mycotoxins. Because A. oryzae is a close relative or even one ecotype of A. flavus, the high-level malic acid production with a GRAS status (generally regarded as safe) can be integrated with current large-scale production capability [19]. Genetic engineering has been used to improve malic acid production capacity of fungi, bacteria and yeasts. Table 8.3 summarizes several representative microorganisms used for malic acid production .
2.2 Metabolic Engineering Toward Higher Yield
As intermediates of the TCA cycle , succinic, fumaric and malic acids are end products if sugar or glycerol are used as the carbon source [20]. Many pathways are used for these three 1,4-diacids fermentation such as the reductive branch of the TCA cycle, the oxidative TCA cycle, or the glyoxylate pathway. Figure 8.3 shows the TCA pathway and reductive carboxylation pathway to succinic, fumaric and malic acids accumulation of E. coli , and succinic acid production pathway of A. succinogenes . The formation of one mole of succinic acid through the reductive pathway is considered to fix one mole of CO2 and consume two moles of nicotinamide adenine dinucleotide hydride (NADH) . One mole of glucose can furnish two moles of NADH by the glycolytic pathway . Consequently, the redox balance as well as high yield is thoroughly linked to the level of NADH. Thus, the current metabolic fluxes and regulation tools require enhanced schemes of optimized metabolic engineering of E. coli for succinic acid production. Systematic engineering has been applied to develop succinic acid biorefineries [20,21,22].
TCA cycle pathway and reductive carboxylation pathway to succinic, fumaric, and malic acids accumulation of E. coli : 1 pyruvate formate lyase (PFL); 2 lactate dehydrogenase (LDH); 3 ack-pta; 4 alcohol dehydrogenase (ADH); 5 phosphoenolpyruvate carboxylase (PEPC) ; 6 pyruvate carboxylase (PC) ; 7 malate dehydrogenase (MDH); 8 fumarase; 9 citric synthesis; 10 malic enzyme ; 11 isocitric lyase (LCL); 12 fumarate reductase (FRD); 13 glucose phosphotransferase. Succinic acid production pathway by A. succinogenes : 1 phosphoenolpyruvate carboxykinase (PEPCK); 2 malate dehydrogenase (MDH); 3 FRD; 4 pyruvate kinase (PK); 5 pyruvate oxidase/reductase; 6 acetate kinase (AK); 7 alcohol dehydrogenase (ADH); 8 LDH
A. succinogenes , A. succiniciproducens , M. succiniciproducens are recognized as natural producers. Many efforts have been put afford to improve the yield by fermentation engineering [23]. Because E. coli , C. glutamicum, and S. cerevisiae are not natural producers, fully genetic engineered pathways are required for improving facilities to produce succinic acid. In the improvement of metabolic engineering , many strategies have been taken for host strains’ application. Several attempts have been made to overcome the existing limits of host strains, that include introduction of an ATP-dependent glucose transport system, knockout of ADHE or LDH encoding genes that leading to by-product formation pathway, positive change of the ATP formation and control of pyruvate accumulation [24]. Better understanding of metabolic circumstance of native succinic acid strains and heterologous host strains is critical to pursue economic and biotechnological production of succinic acid. Much work needs to be done to realize the preferred target.
Internal redox balance for example, enhances overexpression of key enzymes, and amends pathways of by-product formation as one approach that leads to high levels of production. Taking overexpression of genes that code for anaplerotic enzymes in the resultant strain C. glutamicum ΔldhA-pCRA717 for example, when pyruvate carboxylase activity is enhanced, the production rate of succinic acid by the mutant strain is 1.5-fold higher than that of the control parental strain. When the dry cell weight of the mutant strain reached 50 g/L, succinic acid could be produced as well with the recurrent addition of Na2CO3 and sugar under oxygen deprivation. Succinic acid titers reached almost 150 g/L, and the yield was over 0.9 g/g with a productivity of over 3 g/L/h [25].
The usage of S. cerevisiae has been intensively investigated for the production of ethanol . Because succinic acid is not normally produced at high levels in S. cerevisiae, metabolic engineering provides an important strategy to increase succinic acid formation. Many studies so far focus on approaches using the TCA cycle in the route of oxidative or glyoxylate shunt leading to synthetic succinic acid. At present, the highest level succinic acid fermentation is 43 g/L, with a productivity of 0.45 g/L/h in S. cerevisiae (Reverdia) [26]. Yan et al. [27] modified the S. cerevisiae pathway for succinic acid manufacture. The engineered S. cerevisiae could produce 6.17 ± 0.34 g/L of succinic acid via the fabricated path. The succinic acid production increased to over 8 g/L when GPD1 was deleted. After regulation by biotin and urea, succinic acid titer was nearly 10 g/L with a yield of over 0.3 mol/mol glucose. By suitable CO2 supplement in the bioreactor, the genetic modified strain could produce almost 13 g/L succinic acid with a yield of over 0.2 mol/mol glucose. The whole fermentation process was conducted at pH 3.8. Thus, the planned engineering scheme is effective for the production of free acid at low pH values. The ability to grow and produce succinic acid at low pH is a good economic advantage of yeast strains compared with other succinic acid producing microorganisms.
C3 together with C1 mechanism relating to the CO2 fixation is catalyzed by pyruvate carboxylase under aerobic cultivation conditions, which explains the high yields and productivity in fumaric acid production by microorganisms [6]. This type of CO2 fixation process can lead to the formation of oxaloacetic acid; thus, intermediates in C4 citrate cycle can be reserved for biorefinery during aerobic cultivation conditions. When the nitrogen resource becomes restrictive, the growth phase ends , while utilization of the glucose and CO2 fixation processes continue, which makes C4 1,4-diacids continue to accumulate. Theoretically, the maximal yield is two moles of fumaric acid per mole of glucose consumed. Reductive pyruvate carboxylation results in two moles of CO2 fixation. Strains cannot produce ATP for the purposes of maintenance and transport if the reductive pyruvate carboxylation is the only pathway . Consequently, the TCA cycle is also active during fumaric acid production [17]. Metabolic engineering has been conducted to fumaric acid production by non-fumaric acid producers. The chief difficulty of fumaric acid fermentation by Rhizopus species is their morphology control in bioreactor. The Rhizopus species are less genetically accessible than S. cerevisiae and E. coli .
For the route from glucose to malic acid , adenosine triphosphate (ATP) is neutral. This process can lead to the fixation of one mole of CO2 per mole of malic acid. For this case, the maximum theoretical yield is two moles malic acid per mole of glucose [8]. This kind of cytosolic pathway is usually denoted as the reductive tricarboxylic acid (rTCA) pathway, which is utilized by many Aspergillus species that natively produce malic acid. Yeast synthesizes malic acid by either cytosolic or mitochondrial pathway. The enzymes that are intricate in the malic acid metabolic pathway have an impact on the production amount of malic acid such as fumarase overexpression in the TCA cycle, malate dehydrogenase overexpression in the reductive TCA cycle, the malate synthase in glyoxylate pathway, the pyruvate carboxylase in the reductive pathway, and even the cytosolic malate dehydrogenase. Nakayama et al. characterized malic acid production mechanism in sake yeast , and found high-level production of malic acid by yeast could be realized by the suppression of mitochondrial activity [28]. In bacteria T. fusca muC, the synthesis pathway of malic acid was shown to be derive of from phosphoenolpyruvate to oxaloacetate , followed by the reduction process from oxaloacetate to malate. The yield of malic acid by the engineered T. fusca muC-16 was increased by 47.9% compared with the parent strain muC. The muC-16 strain can grow on 100 g/L cellulose with 62.76 g/L malic acid in batch fermentation [29]. In another attempt, the phosphoenolpyruvate (PEP) carboxykinase of M. succiniciproducens MBEL55E was overexpressed in recombinant E. coli . The final malic acid production was about 10 g/L after only 12 h aerobic fermentation [23].
2.3 Cofactor Engineering of Strains
Metabolic engineering of the 1,4-diacid producer can be used to debottleneck the 1,4-diacid pathway, improve product transportation, and enhance paths straightly involved in the 1,4-diacid manufacture [30]. However, the carbon flux shift to the 1,4-diacid route is insufficient to govern the formation of some metabolite by-products , although it is frequently used to disallow pathways involved in by-product formation. Moreover, optimization of the yield and productivity can be realized by arrangement of the genetic operations.
Evolution coupled with genetic modification is performed to grow overproducing strains for succinic acid biorefineries. To keep the redox or ATP stability , Singh et al. cloned a gene encoding the ATP-generating PEPCK enzyme, to eliminate the pathway for forming ethanol and acetate to improve succinic acid production in the ldhA, pflB, ptsG mutant strain . The recombinant strains can make succinic acid with a 60% increased production compared with the control E. coli [31]. In engineered E. coli KLPPP, phosphoenolpyruvate carboxykinase was overexpressed with the deletion of lactate dehydrogenase, phosphotransacetylase–acetate kinase, pyruvate formate lyase, and pyruvate oxidase genes. The recombinant strain showed a high conversion yield of succinic acid on galactose (1.2 mol/mol) compared to glucose (0.48 mol/mol). The concentration of succinic acid was over 22 g/L, and the molar yield was about 1.1 mol/mol total sugar [32]. One type of E. coli strain with ptsG mutation and homologous or heterologous (cyanobacterial) ppc gene expression was engineered for production of succinic acid. Oxaloacetate was the precursor of succinic acid. As a result of ppc gene overexpression, the PEP carboxylase activity was enhanced, and then the PEP carboxylation ability of producing oxaloacetate was improved. The recombinant strain had good performance in succinic acid fermentation , especially using glucose and xylose mixtures as the carbon source [33].
It is not easy to produce fumaric acid via genetic modified microorganisms. From another perspective, it provides possible chance or solution for improving fumaric acid fermentation by recombinant strains. In the study of Xu et al., both malate dehydrogenase (RoMDH) and fumarase (RoFUM1) originating from R. oryzae was introduced into S. cerevisiae [34]. In their study, when pyruvate carboxylase was overexpressed, a novel cytosolic fumaric acid biosynthetic pathway was reconstructed.
E. coli can produce fumaric acid. However, carbon flux to acetyl-CoA, acetate , formate, and succinate must be restricted or blocked. Besides E. coli and Pichia pastoris, Rhizopus spp. has also been genetically modified. However, most Rhizopus species are unaffected by antibiotics, such as ampicillin, chloramphenicol and streptomycin. Uracil auxotrophic isolates of Rhizopus spp. can usually be utilized by genetic changes; however, it is not easy to isolate the target. Compared with E. coli or S. cerevisiae, there are seldom effective genetic methods available for Rhizopus spp. Thus, it has been largely unexplored for fumaric acid production via genetic alteration of Rhizopus spp. There is one disadvantage of fumaric acid production by E. coli : the cultivation has to be conducted at near-neutral pH because the acidic circumstances prohibit the growth of organisms. A low-pH fermentative process would be advantageous especially for separation processing. Thus, many Rhizopus and S. cerevisiae species would be useful host organisms, which are tolerant of acidic conditions.
As production hosts, Aspergillus offers several prospective advantages when scaling up, one of which is that the strains can be genetically modified to produce high levels of malic acid . A general genetic tool always comprises available markers, selective promoters , and gene knockout/transformation methods. Most importantly, a number of species’ genome sequences should be annotated. High-throughput screening methods can be used to evaluate established mutagenesis systems [35, 36]. Besides S. cerevisiae and E. coli , there is no native replicating extrachromosomal vectors; thus, the unstable artificial ones are usually selected and developed. However, engineered A. oryzae has been engineered by overexpression of native genes for improvement of malic acid production. In this way, rTCA pathway was conjunct with a C4 dicarboxylic acid transporter. Malic acid productivity increased 27%. By A. oryzae NRRL 3488 overexpressing three genes , the final malic acid titer was 154 g/L in 164 h. The productivity was 0.94 g/L/h, and the conversion yield of glucose was almost 1.4 mol/mol, which was a very high malic acid production in the literature [37].
3 Fermentation Process Engineering
Optimization and enhancement of fermentation process is determined by technical maturity and economics . Fermentation performance dictates the whole strategy and effectiveness of the biorefinery process [38]. Main performance factors including concentration, yield, productivity , and process efficiency , whereas techno-economic analysis can provide a direct data-driven understanding of the fermentation cost.
Titer is defined as the fermentative 1,4-diacid concentration in the fermentation broth . Titer is normally stated in the unit of g/L (grams of 1,4-diacid/liter of fermentation broth). High fermentative titers result in importantly cheap costs from the upstream to the downstream stage with less energy and substance consumption. Productivity is normally stated in the unit of g/L/h (grams product/liter of fermentation broth/time), which is defined as the production rate of 1,4-diacid per bioreactor volume. The productivity factor controls the overall fermentative volume for the achievable capacity goal. An increase in productivity decreases the number or volume of requisite fermenters and otherwise reduces the related operation capital. Productivity improvement of a designed plant can help increase the plant capacity utilization. Yield is defined as the conversion efficiency from one or more feedstock fraction to the target 1,4-diacid product. The unit is g/g sugar or mol/mol sugar. The yield answers the question of the number of feedstocks required for a particular amount of the 1,4-diacid production. The theoretical maximum to the product yield is determined by the stoichiometry of product pathway and the connected metabolic pathways . Because a fraction of the feedstock is necessary for metabolic growth and energetic systems maintenance, the maximum theoretical yield is usually unachievable. Fermentations with low yields not only increase the cost of net feedstock, but also raise the downstream costs because vacant feedstocks or surplus by-products must be separated from the crude broth .
Fermentation process engineering involves assessing many process parameters, such as media proposal , seed cultivation and inoculum , pH and temperature control, gas transfer, time control and feedstock feeding approach. Some scale-dependent factors, such as partial pressure of CO2 for anaerobic cultivation , and agitation speed , are studied to assess the producers’ sensitivity for commercial-scale conditions , which is necessary for guaranteeing probable performance across scales. Sometimes, different engineered strains are used for different scale-up conditions in developing a process on the industrial scale.
Table 8.4 summarizes common feed strategies for succinic, fumaric and malic acids production. Process engineering approaches can be used to change the fermentative efficiency , but usually this is not enough. Media components and fermentation control are the straightforward methods to obtain a high-level manufacture of 1,4-diacids. As the seed and preferred fermentation ensilage constantly have a range of nutrient components, the effect of one single component and the relationship between different constituents need careful study and experimental design. Factor screening can be performed by some methods such as response surface methodology (RSM) , Plackett-Burman design (PBD) , or central composite design (CCD) , to find the most significant aspects.
3.1 Production of Succinic, Fumaric and Malic Acids Acid from Sugar
Most research on 1,4-diacid fermentation uses sugars as the feedstock. Glucose is always used as one type of carbon source in most organisms. Use of glucose as a carbon source in cells is by either aerobic or anaerobic fermentation. All of these processes follow from an earlier metabolic pathway named glycolysis. The first step of glycolysis is the glucose phosphorylation by hexokinase, which creates glucose 6-phosphate .
Except glucose, many other monosaccharides , disaccharides , even polysaccharides are generally used in succinic acid production. Effect of various sugars on the fermentation of succinic acid has been broadly tested [13,14,15]. For the production of fumaric acid, glucose is not the only appropriate monosaccharide for Rhizopus strains [39]. Xylose , fructose , can be used and converted as well , but the yields are relatively low . Among the disaccharides, maltose can be transformed into fumaric acid while lactose is slow, and sucrose cannot yield fumaric acid. Fumaric acid can be produced by R. arrhizus NRRL 2582 using starch because the strain has amylase . The performance of the fermentation is not efficient as that with glucose as the carbon source, but it is still cost effective [40]. Carbon sources for malic acid fermentation include starch, molasses , and the cellulosic hydrolytes such as crop stock hydrolyte. If the fermentation can be scaled-up, a green process would be realized for crop stock conversion, which can be used in food or chemical fields. Joint production of malic acid and succinic acid is possible with a sugar tolerant yeast named Zygosaccharomyces rouxii V19. The maximum produced amount of malic acid is about 75 g/L, and the glucose consumption yield is about 33 8% yield with 0.5% supplement of glutamic acid , when a little succinic acid and malic acid are added to the media [36].
3.2 Alternative Substrates from Lignocellulosic Biomass
There is a major focus worldwide on conversion of renewable lignocellulosic biomass to platform chemicals [41]. It is forecast in 2025 that nearly 30% of the raw materials used for chemical production will be from biomass resources [42]. The effective exploitation of renewable sources can play a key role in the economic viability of cellulosic 1,4-diacids. Figure 8.4 shows cellulosic C4 diacids production processes. Lignocellulose is the main structure of plants that consists of cellulose, hemicellulose and lignin (Fig. 8.4) whereas cellulosic feedstocks should be non-food based crop residues , wood residues, dedicated energy crops, and industrial wastes . It is therefore, a big challenge to transform the sugars derived from these feedstocks into 1,4-diacids since there is much variability in their cellulosic content. The conversion processes usually contain five major steps: biomass type, biomass pretreatment , choice or production of saccharolytic enzymes-cellulases and hemicellulases , fermentation of hexoses or pentoses , and downstream recovery . New technologies can provide a broad range of residual feedstocks that are suitable for sustainable 1,4-diacids fermentation with less costs or by-products [43].
An eco-friendly process for conversion of polysaccharides to monomeric sugars is via hydrolysis enzymes, i.e., hemicellulases , which promote cellulose hydrolysis by exposing the cellulose fibers . Nearly 50 years have passed since the hydrolysis process of cellulose was conducted by cellulolytic enzymes [44]. After pretreatment by different techniques, biomass can be used as the feedstocks using various saccharification and fermentation strategies. Separate hydrolysis and fermentation (SHF) is used widely as a separate hydrolysis and fermentation process marked as a traditional two-step procedure. Firstly, the lignocellulose is hydrolysed by the special enzymes. Secondly, the reducing sugars produced are fermented to 1,4-diacids.
Simultaneous saccharification and fermentation (SSF) trials have been conducted on the saccharification of the lignocellulosic biomass and the subsequent conversion of the sugars to 1,4-diacids. Biomass, enzymes and fermentation microbe were placed in the same reactor. A successful SSF process relies on many factors such as pH, substrate , temperature , agitation, and feed strategy. Simultaneous saccharification and co-fermentation (SSCF) has been known as one of the possible choices for xylose -rich lignocellulosic materials conversion. Numerous genetically engineered microorganisms have been used to test SSCF for end products glucose and xylose. Efficient process design plays an important role. When a single unit is used and several different operations are performed for these integration processes, it is possible for the engineer to increase the performance efficiency of the coupled process [45, 46].
The problem with hydrolysates fermentation by E. coli is that the pentose in hydrolysis mixtures is usually delayed that tends to incomplete consumption [47, 48]. If the preferred feedstock, such as glucose, has a certain concentration in the media, the passage and metabolism of other sugars can be inhibited until the glucose is exhausted in many cases. However, fermentation of xylose to succinate can be enhanced via ATP supply in metabolically engineered E. coli K12 [49]. A mutant strain E. coli AFP184 can simultaneously consume xylose and glucose for succinic acid fermentation [50]. Moreover, detoxification is usually necessary to decrease many toxic components that are formed in the pretreatment process , although the extra detoxification increases operation cost. Efficient fermentation process for making succinic acid from corn stalk hydrolysates can be realized by a ptsG mutant E. coli [51,52,53,54,55,56]. Fungi can grow in hydrolysates, which originate from the acid hydrolysis of corn straw . Through a two-stage corn straw utilization strategy for making fumaric acid by R. oryzae , the final fumaric acid production can reach about 28 g/L, with 0.35 g/g yield and 0.33 g/L/h productivity , respectively [40].
Solid-state fermentation by filamentous fungi, particularly direct bioconversion or coupled hydrolysis and fermentation from lignocellulosic feedstock , has potential to be an important biotechnology for fumaric, and malic acid production [57]. Aspergillus species can make malic acid from thin stillage [58]. Li et al. reported that Rhizopus Delemar can produce malic acid at high levels from corn straw hydrolytes [59]. Consequently, malic acid yield can be improved by the regulation of the metabolic network of the host strain . R. Delemar can make over 120 g/L malic acid after 60 h fermentation. Zou et al. reported that a novel A. pullulans can make polymalic acid (PMA) and malic acid from hydrolysates of xylose and corncob . The results showed that fed-batch cultivation with xylose in a five-Liter fermenter achieved a high PMA titer (about 80 g/L) with about 91 g/L of malic acid after hydrolysis. The maximum PMA productivity was 0.52 g/L/h [60].
Biomass derived bio-oil have been investigated intensively as an alternative of diesel and gasoline fuels. Production of bio-oil via pyrolysis /thermochemical conversion of biomass has attracted much attention. Bio-oil has a high energy density and has potentials for partial replacement of fuels. However, the oxygen content of bio-oil is as high as 50 wt/wt%, the water content of bio-oil is 15–30 wt/wt%, and the C/H ratio is high; thus, it cannot be used directly as transportation fuel . Deep deoxygenation is regarded as one of key upgrading technologies, which is critical to the treatment of bio-oil. Adding water can help separate bio-oil into two fractions , an aqueous fraction (AP-bio-oil) together with a heavy organic fraction. The heating value of the organic fraction is always bigger than the value of crude bio-oil as a result of its low oxygen content. So, it is a nice method to recovery bio-oil from the aqueous phase and the organic phase before it is upgraded. However, there are many different components in AP-bio-oil. The AP-bio-oil is able to offer many carbon sources and some nitrogen sources to support microbe growth. The transgenic E. coli named MG-PYC can live in modified M9 media, which contains 20 v/v% AP-bio-oil, and produces 0.38 g/L succinic acid. When glucose at a concentration of 4 g/L in the media is used by addition of 20 v/v% AP-bio-oil, succinic acid production increased from 1 to 2.4 g/L. When the carbon source was replaced by enzymatic hydrolysates of corn stock, the final succinic acid production was 10 g/L. This media by addition of 212.5 v/v% AP-bio-oil can result in about 12 g/L succinic acid concentration [61]. The paper industry produces paper solid waste (PSW) as part of its processing pulp . PSW can be used for fumaric acid production. The filamentous fungus R. oryzae 1526 can make fumaric acid through submerged and solid state fermentation . The results showed that the PPSW can provide carbon source and trace elements for the growth of the fungus R. oryzae and fumaric acid can be formed during fermentation [62, 63].
There are carbohydrates in lignocellulose , but it is difficult to use, because the pretreatment process is expensive and the conversion rate to bioproducts is low. However, with the development and improvement of pretreatment technology and microbial metabolic engineering , lignocellulose is regarded as an important biomaterial resource for value-added chemical production. Lignocellulose is more competitive as substrate than sugar or starch for 1,4-diacid production in the future. Cellulose can be effectively dissolved by ionic liquids (ILs) in mild conditions in recent studies. In this process, the linkage between cellulose, hemicelluloses and other components are broken. In addition, pretreatment by ILs can destroy the crystallinity of cellulose, which renders the biomaterials more susceptible to the hydrolysis process by enzymes . Among them, most types of wood such as hardwood and softwood can completely dissolve in 1-allyl-3-methylimidazolium chloride (AmimCl). Various biomass pretreated by ILs has been studied for production of biochemicals or biofuels . In the study of Wang et al. [64], both pinewood and corn stover were pretreated by AmimCl and then used as a media for succinic acid production. Results confirmed that cellulose can be effectively extracted from pinewood, and pinewood was in degraded into a uniform pulp with the help of AmimCl. The enzymatic hydrolysis rate of pinewood extraction reached over 72%. Moreover, corn stover can be effectively treated by AmimCl pretreatment integrated with either team explosion or hot compressed water . Extract of pinewood can make over 20 g/L succinic acid. The average yield was about 0.4 g/g biomass. Workflow calculations revealed that pinewood pretreated with ILs can reach a 57% theoretical yield of succinic acid.
Macroalgae are regarded as “Third generation biomass”, and are viable renewable feedstocks for biochemicals or biofuels. Compared to many land-based crop materials, macroalgae show many attractive features: (i) land is not required for the cultivation of macroalgae, (ii) fertilizers are not required and (iii) freshwater is not needed so that it does not compete with food -crops. Moreover, due to lack of hemicellulose and lignin, few pretreatment steps are needed to release fermentative sugars, which can indeed reduce the operation costs. In the study of Bai et al., the engineered E. coli BS002 was constructed to make succinic acid from Laminaria japonica (a typical macroalgae, Fig. 8.5a) [65]. After several pretreatment steps coupled with enzymatic hydrolysis , L. japonica hydrolysates can produce about 10.3 g/L glucose and 10.1 g/L mannitol. L. japonica hydrolysates were used for succinic acid fermentation by the engineered E. coli BS002 as the substrate . Finally, about 18 g/L succinic acid was made from the hydrolysates by a 72 h dual-phase-fermentation strategy to obtain a yield of 1.24 mol/mol total sugar, which was 73% of the maximum theoretical yield. Succinic acid was similarly produced by engineered E. coli KLPPP using red macroalgae (Palmaria palmate, Fig. 8.5b). The P. palmata hydrolysates were full of glucose and galactose, which were used effectively for succinic acid fermentation . The production of succinic acid was about 22 g/L, and the yield was 1.13 mol/mol total sugars, which was 66% of the maximum theoretical yield. These results demonstrate macroalgae has novel and economical features for the production of biochemical [32, 65].
3.3 Cultivation Strategies with High Production Levels
Simple anaerobic cultivation does not facilitate cell growth and metabolism of many microbes such as E. coli . Dual-phase fermentation strategy, which is defined as aerobic cell growth followed by anaerobic fermentation, has been developed to enhance the cell mass accumulation and subsequent 1,4-diacid production [9]. Engineered E. coli strain was conducted in the dual-phase fermentation process. Cell mass was a significant signal for the time of transition from aerobic to anaerobic phase. Great cell density in the bioreactor by the DO-control strategy is usually used to improve the cell mass of the cell during the aerobic stage. In a dual-phase fermentation process in bioreactor, initial aerobic growth of E. coli SD121 facilitates the subsequent anaerobic production of succinic acid by ptsG mutation. The final concentration of succinic acid was about 60 g/L with a yield of 0.87 g/g sugar [33]. Jiang et al. studied the effect of growth phase feeding methods on succinic acid production by E. coli AFP111 [66]. The physiological state of the aerobically grown cells was critical for the subsequent anaerobic fermentation of succinic acid. For example, some environmental and physiological factors of the aerobic phase played a key role in the improvement of succinic acid production. A novel membrane-bioreactor-electrodialysis system was built for succinic acid production with high concentration, productivity and yield. In this system, ultrafiltration membrane was applied to attach fermentation and separation process, and to clarify the fermentation broth . Under the optimized conditions, biomass concentration and succinic acid concentration were over 40 g/L and 14 g/L, respectively, which were 20 times higher compared with the batch cultivation process in the study of Meynial-Salles et al. [67]. More in situ product recovery strategies are discussed in Sect. 8.4.3.
Up until now, for Rhizopus strains , fermentation processes described have made 126 g/L fumaric acid with 1.38 g/L/h productivity and the conversion yield of glucose was 0.97 g/g. This required optimization factors such as pH, aeration, and carbonate/CO2 supply [7, 17]. Limitations of the Rhizopus strains are pH tolerance and morphology . Especially, the accessibility for genetic engineering and versatility to the alternative carbon sources are other limitations. The energetic mechanism of fumaric acid exportation by Rhizopus should be carefully investigated for host strains. In a SSF process with starchy materials (the total sugar concentration of cornstarch was 100 g/L.), 2-deoxyglucose-resistant mutant strains of R. Oryzae can make as high as 44 g/L fumaric acid. During the SSF process with degermed corn powder (100 g/L initial total sugar) by DG-3, the maximum fumaric acid concentration was about 33 g/L with 0.44 g/L/h productivity [39].
Malic acid is usually made by a “two-step fermentation” process. Fumaric acid is made and then converted to malic acid by fumarate hydratase. In the synthesis pathway , malic acid can be catalyzed from fumaric acid synthesized absolutely via a chemical synthetic method. A one-step-fermentation is always an optimal strategy since it makes malic acid using normal sugars as carbon resource . Furthermore, CO2 fixation also remains in the fermentation process of malic acid. During fermentation process by Saccharomyces spp., L-malic acid can be made by the fumarate pathway that was catalyzed by cytosolic fumarase or mitochondrial fumarase, or by oxaloacetic acid that was catalyzed by malate dehydrogenase (MDH), but the titer was as low as 0.5–1 g/L [35]. A continuous system was set up for L-malic acid conversion from fumaric acid in a novel micro-reactor with surface-immobilized fumarase, which indicated the possibilities for micro-reactor-based biotransformation [68].
4 Downstream Processing
It is critical to decrease the production cost of the biorefinery process so as to compete with traditional petrochemical production. In general, over 60% of the total production cost (TPC) is made by the downstream processing whose target is absolute separation of the products from the crude broth [69]. Solid/liquid separation is usually the first downstream processing unit operation carried out by centrifugation or microfiltration, commonly followed by ultrafiltration to separate cells or cell fragments, proteins and other solid compounds from the supernatant broth. Different unit operations have been used, for example, precipitation processes with calcium hydroxide or ammonia, traditional membrane separation processes such as electrodialysis , and predispersed solvent extraction or reactive extraction . Adsorption and crystallization unit operations are usually used for the final purification of the isolated 1,4-diacids. These unit operations are described and their features are discussed with their possible application for the industrial separation of C4 1,4-diacids crystals.
4.1 Main Separation Unit Operations
The classical manufacturing approach for the separation of carboxylic acids from crude fermentation broth is a precipitation process using calcium oxide or calcium hydroxide. With the help calcium oxide or calcium hydroxide , the calcium salts of 1,4-diacid can be filtered from the aqueous broth and then reacted with sulfuric acid . By this means, the free 1,4-diacid is purified . By-product is a large amount of gypsum. It is a remarkable fact that many other organic acids in the broth will be precipitated together with succinic acid , fumaric acid and malic acid at the same time [69, 70]. Precipitation with ammonia has been widely studied for succinic acid separation.
Ion-exchange remain the main operation for separation and purification of 1,4-diacids from aqueous broths . Ion-exchange is mainly used for the removal of residual anions or cations. Generally, ion-exchange steps are usually applied in extraordinary purification stages as selectivity is not high enough, thus, the product yields remain low.
Liquid–liquid extraction is a widely-used technique in the separation of fermentation-based 1,4-diacids on the lab scale. However, liquid–liquid extraction is not widely applied on the large-scale as most traditional extraction reagents show unfavorable distribution coefficients for different 1,4-diacids. To improve the extraction yield and the selectivity, many researchers use different reactive components for the liquid–liquid extraction of 1,4-diacids from an aqueous phase , such as amines, which can dissolve in miscible organic solvents. Amines have a great affinity for negatively charged components because of their high basicity, which makes them appropriate for the extraction. The pre-treatment of fermentation broth as well as the preparation of succinic acid ammonium using ammonia in the re-extraction can be used to improve the extraction yield of succinic acid produced by microbial fermentation. The amine reacts with the 1,4-diacids at the interface between the organic phase and aqueous phase , which leads to the reaction of amine–1,4-diacid–complexes. These complexes are then solubilized into the organic phase. The mechanism consists of proton transfer and ion pair construction relying on the types of amines and the organic solvents. Predispersed solvent extraction (PDSE) is a novel method for separating solutes from extremely dilute solution by solvent extraction quickly. The use of colloidal liquid aphrons (CLAs, micron-sized solvent droplets surrounded by a thin aqueous film) in predispersed solvent extraction may ameliorate the problems such as emulsion formation, reduction of interfacial mass transfer and low interfacial mass transfer areas in solvent extraction process.
Electrodialysis is a favored method in the food field for removal of citric acid from juices. The application of electrodialysis for downstream separation of 1,4-diacids has been investigated on the lab-scale [69]. Shortcomings of electrodialysis are its high energy consumption , the cost of the membrane material, and the low selectivity and capacity. Another question is the problem of binary ions, which are hard to treat via the electrodialysis membrane. Membrane fouling is another problem presently in the continuous electrodialysis process.
Membrane-based technology, together with centrifugation, has been generally used for the removal of solids from liquid solutions. This process can result in low operation cost and energetic consumption. Investigators have reported the application of filtration in the separation of proteins , acids, and other natural organic compounds which is a feasible strategy for clarifying complex solid–liquid mixture systems. Moreover, several membrane systems can be conducted on fermentative systems to recycle strain cells and obtain product. In integrated systems, permeant can be drawn off at times, which can reduce the concentrations of the target products around cells. In integrated systems, separation is coupled with fermentation without coagulant, so it is widely used in the centrifugation process.
As one of the oldest but effective processes for the preparation of 1,4-diacid crystals, crystallization process can be used as a final purification step. As succinic, fumaric and malic acids are solid forms in room temperature , direct crystallization might provide the desired target in solid form without extra unit operations . However, the product yield is low because many salts of 1,4-diacids remain as residuals in the crystal broth. The low-purity product is hard to use as monomer for many polymerization reactions. To obtain a high purity (>99%) of 1,4-diacids, further purification process is needed to remove residual ionic impurities .
Examination of small differences on the molecular-scale structure of organic acids has had an effect on understanding the mechanisms of adsorption . Hwang et al. studied the adsorption behavior of C4-dicarboxylic acids at a hematite /water interface [72], and indicated that orientation of the two carboxylic groups and pK a values of the organic acids substantially affected the adsorption density as well as the position and characteristics of the pH adsorption edges. Succinic acid has higher molecular flexibility. Fumaric acid with transconfiguration appears to bind to hematite mainly as a deprotonated outer-sphere complex using only one carboxylic group.
4.2 Separation and Purification from the Crude Broth
Most of the proposed process schemes described above for the separation of 1,4-diacids from crude broth do not have sufficiently high (ca. 95%) selectivity. Since the final purification results determine one important cost factor, the production of by-products such as acetic acid, formic acid, pyruvic acid in the TCA branch should be reduced to a minimum level by metabolic engineering of the microbe producers and optimization of the cultivation processes. The contaminants of the post-fermentation broth usually include left-over glycerol , enzymes, other proteins , salts and other acids formed in the process of microbial cultivation and metabolism . Thus, the final standard of bioconversion success is determined by the purification process. Figure 8.6 shows batch and fed-batch succinic acid fermentation processes simulated by software SuperPro Designer. It can be seen that unit operations of the downstream process are numerous and important .
One effective unit operation can influence the entire recovery yield of the downstream process. In crystallization , carboxylic acids show varied distribution between dissociated and free-acid forms at various pH values. Undissociated 1,4-diacids have various solubilities . Li et a.l [73] found that the solubility of free succinic acid was less than 30 g/L at 4 °C and pH 2.0. In this condition, the other by-product acids, such as lactic , acetic and formic acids, were totally water miscible. So succinic acid crystallization from fermentation broth was realized at 4 °C and at low pH, and by-product acids remained in the solution. By this one-step recovery strategy, succinic acid could be selectively crystallized with 70% yield and 90% purity, respectively.
Ultrafiltration can be another important unit to clarify the succinic acid fermentation broths [74]. Various ultrafiltration membranes such as PES (100 kDa, PES 30 kDa, PES 10 kDa) and RC (10 kDa) have been tested and the results show ultrafiltration is achievable for succinic acid from fermentation broth [75], for which 99.6% microorganism cells could be removed from the broth and proteins could be separated well. The highest removal rate of proteins was 87% for membrane PES10 kDa. A clear permeate was obtained after ultrafiltration compared to centrifugation. Study of membrane fouling mechanisms in ultrafiltration revealed that many are fouled by concentration polarization or the cake layer by resistance-in-series model. Hermia’s model as well as its four individual sub-models was used to analyze the predominant fouling mechanism. Results indicated that the fouling of RC and PES (30 kDa) was controlled by the blocking mechanism, while PES (100 kDa) was controlled by the intermediate blocking and PES (10 kDa) was controlled by the cake layer.
The most common method used for fumaric acid capture is precipitation . CaCO3 suspension has always been used as a neutralizing reagent as its Ca2+ salt slowly dissolves during the period of fumaric acid production. CaCO3 also results in viscosity and foaming problems as a result of the less solubility of calcium fumarate. As a result of the higher solubility of sodium fumarate and magnesium fumarate, fumarate production using neutralizing reagents, such as Na2CO3 or MgCO3 can lead to alternative downstream operations. If CaCO3 acts as a neutralizer, the broth is firstly filtered to remove the cells, and then acidified to obtain the acid crystals. When Na2CO3 or MgCO3 is used, heating is avoided because the cell might be possibly reused.
Membrane separation can be regarded as one of the recovery steps in the process of removal of organic acid from simulated or actual fermentation broths [70]. Staszak et al. evaluated concentration and separation of fumaric acid from simulated and actual fermentation broth by ceramic membranes [71]. They showed that the retention of fumaric salt rose with the increasing pH values in the feed solution. The retention of uncharged chemicals such as fumaric acid or glycerol, were less than 2%.
Nanofiltration and bipolar electrodialysis in a hybrid system has been proposed to obtain fumaric acid from fermentation broths [76], which showed that fumaric acid can be isolated and concentrated efficiently, with no additional costs related to waste generation and regeneration . Adsorption can be used as an alternative method for recovering the low-concentration fumaric acid in the filtrate after crystallization. Acetone was applied to effectively desorb fumaric acid from the column packed with activated carbon . The desorption process was followed with acetone removal by evaporation and washing with water to produce highly purified fumaric acid crystals. Both activated carbon and acetone can be recovered and reused in the repeated adsorption − desorption process [77].
Different configurations of microbial electrolysis desalination cell have been examined for the production of malic acid . The microbial electrolysis desalination and chemical-production cell (MEDCC) has a less internal resistance . And among the reactors, the anode -biofilm population had a high Geobacter percentage [78]. MEDCC as a new system for malic acid production was successfully coupled to biological electrodialysis with bipolar membranes . The specific electricity usage of the MEDCC was around 10% to 30% of the electricity consumption in this electrodialysis process [79].
4.3 In Situ Product Recovery (ISPR)
Many integrated biotechnology platforms have been developed for sustainable processes [69, 70]. Inhibition caused by accumulative acidic products is a chief cause for small production of a target product in fermentation broths . The concentration of the product can be increased by a method called in situ product removal strategy and coupled systems. Table 8.5 summarizes integrated bioprocesses for the production of succinic acid . The significance of the integrated process is understood as “good integration = high efficiency ”. Initial assistance from process engineering concerning commercially viable proposal principles is a vibrant element of many technological platforms . Successful platforms of process integration can provide streamlined workflows for the “design-build-check-learn” cycles, which not only encourage multidisciplinary collaboration, but also fundamental understanding of biological ISPR processes [1].
An appropriate ISPR technique should be selected according to the physical and chemical properties of the target products and the process fermentation process. A. succinogenes 130Z can make succinic acid in a batch fermentation process. The batch cultivation is effective carried out with alkaline anion-exchange adsorbents as the solid pH neutralizing reagent. Alkaline anion exchange adsorbent is used for in situ removal of succinic acid from the broth and the hydroxyl groups of the acids react with the alkaline anion exchange adsorbent at the same time [80]. Meynial-Salles et al. established a novel fermentation-membrane-separation system that produced a concentrated succinic acid broth with over 80 g/L titers by the strain A. succiniciproducens [67]. For this system, fouling of membranes as well as flux throughput in the modules should be carefully monitored . Li et al. [81] applied an ISPR technique to A. succinogenes fed-batch fermentation (Fig. 8.7, ISPR unit coupled in yellow frame). Expanded bed adsorption using anion exchange adsorbents was used to directly remove succinic acid from the bioreactor efficiently. The united fermentation-separation system could eliminate inhibitory effects of succinic acid for continuous high level production. The coupled process could prolong cell growth cycle and reduce the number of downstream steps. The final succinic acid production was over 140 g/L, and the conversion yield was about 0.5 g/g glucose. However, the adsorption coupled fermentation generally yielded a huge amount of wastewater and eluting/regeneration reagents. A unique membrane separation based fermentation system was set up for succinic acid production with less water and energy consumption [82]. In this combined system, product inhibition was improved by in situ recovering acids and reloading the fresh broth . Cell density remained high for a longer time up to 130 h, and succinic acid production rose to over 70 g/L. Succinic acid could be crystallized with 90% recovery . HPLC and 1H NMR analyses showed that the purity of the separated solid succinic acid surpassed 99%.
Fumarate salts can cause inhibitory effects on fermentation to some extent [83]. Production of soluble sodium or potassium fumarate can inhibit cell growth and metabolism when the titers of fumarate reach about 40 g/L by R. arrhizu. It is clear that free fumaric acid can inhibit strain production more seriously than dissociated sodium fumarate. When the pH is low, produced fumaric acid by the fungi inertly diffuses back over the plasma membrane , thus reducing the intracellular pH until the fermentation becomes terminated. In an integrated process of fermentation and crystallization , upon cooling and seeding, fumaric acid can directly crystallize from fermentation broths which can actually lead to an extreme decrease in the consumption of neutralizing base required during fermentation and of acidification in the product recovery process [84, 85]. However, one difficulty of in situ separation via Rhizopus species is the morphological problem of the fungi clumps, which are inclined to grow on the bioreactor walls and even on the stirrers. Morphology problems lead to separation unit constraints. Thus, much effort is put into improving morphology of the fungi producers.
ISPR processes have shown greatly benefit in improving production, recovery yield and productivity of the fermentation processes. Compared to the in situ precipitation strategy, the efficiencies of many other separation units are not high, and some are much more complicated. The industrial achievability of ISPR processes remains to be recognized systematically, and thorough knowledge of the integration process needs further investigation for application .
In summary, Table 8.6 compares common separation methods and unit operations used for 1,4-diacid purification in downstream processes .
5 Final Remarks
5.1 Techno-Economics Challenges
Design and realization of an adequate process for the production of 1,4-diacids from fermentation broth depends not only on the scalability and robustness, but also on the overall recovery yield and total costs. A suitable factory scheme must embody a plan that can make a product with profit. Cost sensitivity as well as many other factors can allow for optimization of plant factors to minimize the production cost. This sensitivity analysis can provide information about the range of possible outcomes and about the extent to which the outcome responds to thinkable variations in uncertain parameters. Although many biomass-derived C4 1,4-diacid production processes are successful on the lab-scale, it is still challenging for large-scale operations. Much effort is necessary for developing a thorough understanding of biomass, stable engineered strain and unit operations enhancement in upstream and downstream processes . Researchers will need to focus on biomass conversion with competitive and sustainable approaches.
Process simulation is challenging and critical for the understanding of the bio-based process designs of C4 1,4-diacid production from biomass. Taking techno-economics of biomass pretreatments as an example, one economical pretreatment approach for a feedstock may not be suitable for another type of biomass, even though the bioresources belong to the same species. Hindrances of the current pretreatment methods contain inadequate recovery of cellulose or lignin, accumulation of by-products which result in inhibitory effect on the end-product fermentation, high chemical/energy consumption , and unwanted by-product production. Most of the techno-economic studies are based on process simulations for C4 1,4-diacid production from biomass to make acid-soluble lignin content and produce fermentative sugars (glucose, xylose or arabinose ) .
5.2 Conclusions and Future Outlook
The scientific literature provides many cases of microorganisms modified to make C4 1,4-diacids. Traditionally, the gentle way to the commercialization stage is realized by typical mutagenesis, fermentation process, separation and purification . The fully integrated technology platform leverages a realistic methodology to strain improvement coupled to the next generation tools in synthetic biology, systematic engineering, high-throughput screening (HTS) , fermentation enhancement , and efficient separation tools. Developing methods, skills, and data bases that can be quickly integrated and used to improve a potential production platform are necessary. The traditional petrochemical synthetic methods mainly depend on crude oil as the feedstock. Delivery periods and costs, available storage factory, weather and international markets , availability of cash and economy can all affect the price of oil. Nevertheless, presently, both WTI crude oil price and Brent crude oil price are about US $45, which are much less than the crude oil prices in 2008. Concerning global energy security and the environmental sustainability , a safe and reliable biorefinery plant for platform chemical production will attract growing attention as an alternative to the traditional petrochemical factory.
The market availability for succinic acid and its derivatives is predicted to be 245,000 tons per year [20]. Actually, petrochemical industry dominates most commercial succinic acid production. Extraordinarily, the noteworthy development in the economically competitive route of succinic acid production from renewable biomass has being carried out years ago. Presently, industrial plant of succinic acid from natural feedstocks has been realized in the world [3]. Right now, succinic acid is being produced commercially through the fermentation of glucose from renewable feedstock by several companies. Commercial biotechnological succinic acid production is reported by Requette and Bioamber. In Spain, Succinity is a joint venture between BASF SE and Corbion Purac., which produces 10,000 tons succinic acid per year. In USA, Myriant, together with ThyssenKrupp Uhde, has a 13,600 t/y capacity. A joint venture between DNP Green Technology, ARD, and Mitsui & Co. in France, named BioAmber, built a demonstration plant with 3000 t/y capacity in 2015, whose biorefinery processes for producing succinic acid will lead to a total reduction in CO2 emissions and a 60% reduction of energy consumption (www.bio-amber.com). “Bio-succinic acid makes the big time.” On March, 2016, BioAmber announced that she has successfully started up its first commercial-scale platform for bio-based succinic acid production, bringing a reliable source of the versatile bio-based material to the market (www.icis.com). In Italy, there is also a 10,000 t/y succinic acid capacity in Reverdia [26], which is a joint venture between Royal DSM N.V. and Roquette Frères. Organisms used by these manufacturers include Basfia succiniciproducens , E. coli , S. cerevisiae , et al. According to a survey report from MarketsandMarkets (M&M, an international market research and management advisory company established in 2001) [86], the market of succinic acid is anticipated to grow at a speed of 18.7% from 2011 to 2016. The worldwide market of succinic acid in terms of profits is evaluated to be worth over $182 million in 2010, and is likely to be worth $496 million by 2016. Asia-Pacific is the third succinic acid consuming region and will probable to be the largest rising market in the future due to the strong demand from developing countries such as India and China. The increasing demand of succinic acid is promoting scientists and engineers to develop cost effective synthesis routes to support its overgrowing markets .
Commercially fumaric acid is presently only produced by catalytic isomerization from petroleum-derived maleic acid, resulting in a conversion yield of over 90%. Maleic anhydride can be transformed into maleic acid through the catalytic oxidation of hydrocarbons including benzene, butene and butane. According to Roa Engel et al. [6], annual production of maleic anhydride is crudely 1.8 million tons, 3% of which is used for the refining of fumaric acid. The amount of fumaric acid produced is roughly 90,000 tons per year. Prices of the petroleum-based fumaric acid are estimated to be about $1–2 per kg or $0.7–0.8 per lb. The rough price is nearly 10% higher than the price of maleic anhydride, which is used as the raw material.
Malic acid has a prospective market . It is a crude material for the manufacture of polymalic acid (PMA). PMA is a new biodegradable polymer similar to polylactic acid, however, it relies on the economic and process feasibility of the manufacture. Many bulk retailers report the prices of malic acid at about $0.9 per kg [57]. Racemic malic acid can be made by the double hydration of maleic anhydride, and the U.S. production capacity was about 5000 tons in 2000 [87]. Chiral resolution of the racemic mixture gives the (S)-enantiomer that can be selectively obtained from the fumaric acid fermentation process. The production of L-malic acid has already been industrialized. At present, many companies pay attention to the L-malic acid production as it is an intermediate -volume compound. Its annual world production is roughly 40,000 tons [7]. It is assessed that the demand request of L-malic acid could be up to 200,000 tons per year. The main global companies producing malic acid include Bartek (Canada), CIA Quimica (Mexico), Croda colloids/Tate & Lyle PLC (UK), Fuso/Kawasaki Kasei/Kyowo Hakko Kogyo (Japan), Lonza (Switzerland), Merck KGaA (Germany), Rifa Industrial/Yongsan Chemicals (Korea), Thirumalai Chemicals (India), and Nanjing Kokhai Biotechnical/Changzhou Changmao (China). Presently, the raw materials for the synthetic processes are derived from crude oil or natural gas. Nevertheless, the increased demand for food and drug safety will compete with industrial routes in the not too distant future. Microbial production is a potential option for economic manufacture of C4 1,4-diacids.
References
Barton NR, Burgard AP, Burk MJ, Crater JS, Osterhout RE, Pharkya P, Steer BA, Sun J, Trawick JD, Van Dien SJ, Yang TH, Yim H. An integrated biotechnology platform for developing sustainable chemical processes. J Ind Microbiol Biotechnol. 2015;42:349–60.
Dumeignil F, Capron M, Katryiok B, Wojcieszak R, Löfberg A, Girardon JS, Desset S, MArin MA, Duhamel LJ, Paul S. Biomass-derived platform molecules upgrading through catalytic processes: yielding chemicals and fuels. J Jpn Petrol Inst. 2015;58:257–73.
Becker J, Lange A, Fabarius J, Wittmann C. Top value platform chemicals: bio-based production of organic acids. Curr Opin Biotechnol. 2015;36:168–75.
Jang YS, Kim B, Shin JH, Choi YJ, Choi S, Song CW, Lee J, Park HG, Lee SY. Bio-based production of C2–C6 platform chemicals. Biotechnol Bioeng. 2012;109:2437–59.
McKinlay JB, Vieille C, Zeikus JG. Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol. 2007;76:727–40.
Roa Engel CA, Straathof AJJ, Zijlmans TW, van Gulik WM, van der Wielen LAM. Fumaric acid production by fermentation. Appl Microbiol Biotechnol. 2008;78:379–89.
Xu Q, Li S, Huang H, Wen JP. Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol Adv. 2012;30:1685–96.
Liu YH, Song JN, Tan TW, Liu L. Production of fumaric acid from L-malic acid by solvent engineering using a recombinant thermostable fumarase from Thermus thermophilus HB8. Appl Biochem Biotechnol. 2015;175:2823–31.
Thakker C, Martínez I, San KY, Bennett GN. Succinate production in Escherichia coli. Biotechnol. 2012;7:213–24.
Cheng KK, Zhao XB, Zeng J, Zhang JA. Biotechnological production of succinic acid: current state and perspectives. Biofuels Bioprod Biorefin. 2012;6:302–18.
Cao YJ, Zhang RB, Sun C, Cheng T, Liu YH, Xian M. Fermentative succinate production: an emerging technology to replace the traditional petrochemical processes. Biomed Res Int. 2013;11:1–12.
Song H, Lee SY. Production of succinic acid by bacterial fermentation. Enzym Microb Technol. 2006;39:352–61.
Beauprez JJ, Mey MD, Soetaert WK. Microbial succinic acid production: natural versus metabolic engineered producers. Process Biochem. 2010;45:1103–14.
Li J, Zheng XY, Fang XJ, Liu SW, Chen KQ, Jiang M, Wei P, Ouyang PK. A complete industrial system for economical succinic acid production by Actinobacillus succinogenes. Bioresour Technol. 2011;102:6147–52.
Bechthold I, Bretz K, Kabasci S, Kopitzky R, Springer A. Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem Eng Technol. 2008;31:647–54.
Pinazo JM, Domine ME, Parvulescu V, Petru F. Sustainability metrics for succinic acid production: a comparison between biomass-based and petrochemical routes. Catal Today. 2015;239:17–24.
Roa Engel CA, van Gulik WM, Marang L, van der Wielen LAM, Straathof AJJ. Development of a low pH fermentation strategy for fumaric acid production by Rhizopus oryzae. Enzym Microb Technol. 2011;48:30–47.
Wang XY, Chen J, Quinn PJ. Reprogramming microbial metabolic pathways. Dordrecht: Springer; 2012. p. 225–40.
Knuf C, Nookaew I, Brown SH, McCulloch M, Berry A, Nielsen J. Investigation of malic acid production in Aspergillus oryzae under nitrogen starvation conditions. Appl Environ Microbiol. 2013;79:6050–8.
Chen XZ, Zhou L, Tian KM, Kumar A, Singh S, Prior BA, Wang ZX. Metabolic engineering of Escherichia coli: a sustainable industrial platform for bio-based chemical production. Biotechnol Adv. 2013;31:1200–23.
Hong KK, Kim JH, Yoon JH, Park HM, Choi SJ, Song GH, Lee JC, Yang YL, Shin HK, Kim JN, Cho KH, Lee JH. O-Succiny-L-homoserine-based C4-chemical production: succinic acid, homoserine lactone, γ-butyrolactone, γ-butyrolactone derivatives, and 1,4-butanediol. J Ind Microbiol Biotechnol. 2014;41:1517–24.
Yang L, Lübeck M, Ahring BK, Lübeck PS. Enhanced succinic acid production in Aspergillus saccharolyticus by heterologous expression of fumarate reductase from Trypanosoma brucei. Appl Microbiol Biotechnol. 2016;100:1799–809.
Moon SY, Hong SH, Kim TY, Lee SY. Metabolic engineering of Escherichia coli for the production of malic acid. Biochem Eng J. 2008;40:312–20.
Cheng KK, Wang GY, Zeng J, Zhang JA. Improved succinate production by metabolic engineering. Biomed Res Int J. 2013;3:1–12.
Okino S, Noburyu R, Suda M, Jojima T, Inui M, Yukawa H. An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl Microbiol Biotechnol. 2008;81(3):459–64.
Ahn JH, Jang YS, Lee SY. Production of succinic acid by metabolically engineered microorganisms. Curr Opin Biotechnol. 2016;42:54–66.
Yan DJ, Wang CX, Zhou JM, Liu YL, Yang MH, Xing JM. Construction of reductive pathway in Saccharomyces cerevisiae for effective succinic acid fermentation at low pH value. Bioresour Technol. 2014;156:232–9.
Nakayama S, Tabata K, Oba T, Kusumoto K, Mitsuiki S, Kadokura T, Nakazato A. Characteristics of the high malic acid production mechanism in Saccharomyces cerevisiae sake yeast strain No. 28. J Biosci Bieng. 2012;114:281–5.
Deng Y, Mao Y, Zhang XJ. Metabolic engineering of a laboratory-evolved Thermobifida Fusca muC strain for malic acid production on cellulose and minimal treated lignocellulosic biomass. Biotechnol Prog. 2016;32:14–20.
Kamm B. Production of platform chemicals and synthesis gas from biomass. Angew Chem Int Ed. 2007;46:5056–8.
Singh A, Cher Soh K, Hatzimanikatis V, Gill RT. Manipulating redox and ATP balancing for improved production of succinate in E. coli. Metab Eng. 2011;13:76–81.
Olajuyin AM, Yang MH, Liu YL, Mu TZ, Tian JN, Adaramoye OA, Xing JM. Efficient production of succinic acid from Palmaria palmata hydrolysate by metabolically engineered Escherichia coli. Bioresour Technol. 2016;214:653–9.
Wang D, Li Q, Yang MH, Zhang YJ, Su ZG, Xing JM. Efficient production of succinic acid from corn stalk hydrolysates by a recombinant Escherichia coli with ptsG mutation. Process Biochem. 2011;46:365–71.
Xu G, Liu L, Chen J. Reconstruction of cytosolic fumaric acid biosynthetic pathways in Saccharomyces cerevisiae. Microb Cell Factories. 2012;24:11–24.
Yéramian N, Chaya C, Suárez Lepe JA. L-(−)-malic acid production by Saccharomyces spp. during the alcoholic fermentation of wine (1). J Agric Food Chem. 2007;55:912–9.
Kazuya Taing OT. Production of malic and succinic acids by sugar-tolerant yeast Zygosaccharomyces rouxii. Eur Food Res Technol. 2007;224:343–7.
Brown SH, Bashkirova L, Berka R, Chandler T, Doty T, McCall K, McCulloch M, McFarland S, Thompson S, Yaver D, Berry A. Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of L-malic acid. Appl Microbiol Biotechnol. 2013;97:8903–12.
Morais AR, Lukasik RB. Green chemistry and the biorefinery concept. Sustain Chem Process. 2013;1:18.
Deng YF, Li S, Xu Q, Gao M, Huang H. Production of fumaric acid by simultaneous saccharification and fermentation of starchy materials with 2-deoxyglucose-resistant mutant strains of Rhizopus oryzae. Bioresour Technol. 2012;107:363–7.
Xu Q, Li S, Fu YQ, Tai C, Huang H. Two-stage utilization of corn straw by Rhizopus oryzae for fumaric acid production. Bioresour Technol. 2010;101:6262–4.
Girisuta B, Kalogiannis KG, Dussan K, Leahy JJ, Hayes MHB, Stefanidis SD, Michailof CM, Lappas AA. An integrated process for the production of platform chemicals and diesel miscible fuels by acid-catalyzed hydrolysis and downstream upgrading of the acid hydrolysis residues with thermal and catalytic pyrolysis. Bioresour Technol. 2012;126:92–100.
Yabushita M, Kobayashi H, Fukuoka A. Catalytic transformation of cellulose into platform chemicals. Appl Catal B-Environ. 2014;145:1–9.
Kiran EU, Trzcinski AP, Ng WJ, Liu Y. Enzyme production from food wastes using a biorefinery concept. Waste Biomass Valor. 2014;5:903–17.
Isikgora FH, Becer CR. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem-UK. 2015;6:4497–559.
Kıran EU, Trzcinskia AP, Liu Y. Platform chemical production from food wastes using a biorefinery concept. J Chem Technol Biotechnol. 2014;90:1364–79.
Menon V, Rao M. Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energ Combust. 2012;38:522–50.
Bradfield MFA, Mohagheghi A, Salvachúa D, Smith H, Black BA, Dowe N, Beckham GT, Nicol W. Continuous succinic acid production by Actinobacillus succinogenes on xylose-enriched hydrolysate. Biotechnol Biofuels. 2015;8:181–97.
Bradfield MFA, Nicol W. Continuous succinic acid production from xylose by Actinobacillus succinogenes. Bioprocess Biosyst Eng. 2016;39:233–44.
Liu R, Liang LY, Chen KQ, Ma JF, Jiang M, Wei P, Ouyang PK. Fermentation of xylose to succinate by enhancement of ATP supply in metabolically engineered Escherichia coli. Appl Microbiol Biotechnol. 2012;94(4):959–68.
Andersson C, Hodge D, Berglund KA, Rova U. Effect of different carbon sources on the production of succinic acid using metabolically engineered Escherichia coli. Biotechnol Prog. 2007;23:381–8.
Salvachúa D, Mohagheghi A, Smith H, MFA B, Nicol W, Black BA, Biddy MJ, Dowe N, Beckham GT. Succinic acid production on xylose-enriched biorefinery streams by Actinobacillus succinogenes in batch fermentation. Biotechnol Biofuels. 2016;9:28–42.
Corona-González RI, Varela-Almanza KM, Arriola-Guevara E, Martínez-Gómez AJ, Pelayo-Ortiz C, Toriz G. Bagasse hydrolyzates from Agave tequilana as substrates for succinic acid production by Actinobacillus succinogenes in batch and repeated batch reactor. Bioresour Technol. 2016;205:15–23.
Zhao Y, Cao WJ, Wang Z, Zhang BW, Chen KQ, Ouyang PK. Enhanced succinic acid production from corncob hydrolysate by microbial electrolysis cells. Bioresour Technol. 2016;202:152–7.
Tan JP, Jahim JM, Wu TY, Harun S, Kim BH, Mohammad AW. Insight into biomass as a renewable carbon source for the production of succinic acid and the factors affecting the metabolic flux toward higher succinate yield. Ind Eng Chem Res. 2014;53:16123–34.
Akhtar J, Ani Idris A, Aziz RA. Recent advances in production of succinic acid from lignocellulosic biomass. Appl Microbiol Biotechnol. 2014;98:987–1000.
Cok B, Tsiropoulos I, Roes AL, Patel MK. Succinic acid production derived from carbohydrates: an energy and greenhouse gas assessment of a platform chemical toward a bio-based economy. Biofuel Bifprod Bior. 2014;8:16–29.
Mondala AH. Direct fungal fermentation of lignocellulosic biomass into itaconic, fumaric, and malic acids: current and future prospects. J Ind Microbiol Biotechnol. 2015;42:487–506.
West TP. Malic acid production from thin stillage by Aspergillus species. Biotechnol Lett. 2011;33:2463–7.
Li XJ, Liu Y, Yang Y, Zhang H, Wang HL, Wu Y, Zhang M, Sun T, Cheng JS, Wu XF, Pan LJ, Jiang ST, Wu HW. High levels of malic acid production by the bioconversion of corn straw hydrolyte using an isolated Rhizopus Delemar strain. Biotechnol Bioproc E. 2014;19:478–92.
Zou X, Yang J, Tian X, Guo MJ, Li ZH, Li YZ. Production of polymalic acid and malic acid from xylose and corncob hydrolysate by a novel Aureobasidium pullulans YJ 6–11 strain. Process Biochem. 2016;51:16–23.
Wang CX, Thygesen A, Liu YL, Li Q, Yang MH, Dang D, Wang Z, Wan YH, Lin WG, Xing JM. Bio-oil based biorefinery strategy for the production of succinic acid. Biotechnol Biofuels. 2013;6:74.
Das RK, Brar SK, Verma M. Potential use of pulp and paper solid waste for the bio-production of fumaric acid through submerged and solid state fermentation. J Clean Prod. 2016;112:4435–44.
Zhang X, Wang X, Shanmugam KT, Ingram LO. L-malate production by metabolically engineered Escherichia coli. Appl Environ Microbiol. 2011;77:427–34.
Wang CX, Yan DJ, Li Q, Sun W, Xing JM. Ionic liquid pretreatment to increase succinic acid production from lignocellulosic biomass. Bioresour Technol. 2014;172:283–9.
Bai B, Zhou JM, Yang MH, Liu YL, Xu XH, Xing JM. Efficient production of succinic acid from macroalgae hydrolysate by metabolically engineered Escherichia coli. Bioresour Technol. 2015;185:56–61.
Jiang M, Liu SW, Ma JF, Chen KQ, Yu L, Yue FF, Xu B, Wei P. Efficient production of succinic acid from macroalgae hydrolysate by metabolically engineered Escherichia coli. Appl Environ Microbiol. 2015;76(4):1298–300.
Meynial-Salles I, Dorotyn S, Soucaille P. A new process for the continuous production of succinic acid from glucose at high yield, titer, and productivity. Biotechnol Bioeng. 2008;99:129–35.
Gorazd Stojkovič G, Plazl I, Žnidaršič-Plazl P. L-Malic acid production within a microreactor with surface immobilised fumarase. Microfluid Nanofluid. 2011;10:627–35.
Cheng KK, Zhao XB, Zeng J, Wu RC, Xu YZ, Liu DH, Zhang JA. Downstream processing of biotechnological produced succinic acid. Appl Microbiol Biotechnol. 2012;95:841–50.
Curcio E, Profio GD, Drioli E. Recovery of fumaric acid by membrane crystallization in the production of L-malic acid. Sep Purif Technol. 2003;33:63–73.
Staszak K, Woźniak M, Sottek M, Karaś Z, Prochaska K. Removal of fumaric acid from simulated and real fermentation broth. J Chem Technol Biotechnol. 2015;90:432–40.
Hwang YS, Lenhart JJ. Adsorption of C4-dicarboxylic acids at the hematite/water interface. Langmuir. 2008;24:13934–43.
Li Q, Wang D, Wu Y, Li WL, Zhang YJ, Xing JM, Su ZG. One step recovery of succinic acid from fermentation broths by crystallization. Sep Purif Technol. 2010;72:294–300.
Wang CX, Li Q, Tang H, Zhou W, Yan DJ, Xing JM, Wan YH. Clarification of succinic acid fermentation broth by ultrafiltration in succinic acid bio-refinery. J Chem Technol Biotechnol. 2013;88:444–8.
Wang CX, Li Q, Tang H, Yan DJ, Zhou W, Xing JM, Wan YH. Membrane fouling mechanism in ultrafiltration of succinic acid fermentation broth. Bioresour Technol. 2012;116:366–71.
Woźniak MJ, Prochaska K. Fumaric acid separation from fermentation broth using nanofiltration (NF) and bipolar electrodialysis (EDBM). Sep Purif Technol. 2014;125:179–86.
Zhang K, Zhang LJ, Yang ST. Fumaric acid recovery and purification from fermentation broth by activated carbon adsorption followed with desorption by acetone. Ind Eng Chem Res. 2014;53:12802–8.
Liu GL, Zhou Y, Luo HP, Cheng X, Zhang RD, Teng WK. A comparative evaluation of different types of microbial electrolysis desalination cells for malic acid production. Bioresour Technol. 2015;198:87–93.
Liu GL, Luo HP, Wang HH, Wang BW, Zhang RD, Chen SS. Malic acid production using a biological electrodialysis with bipolar membrane. J Membr Sci. 2014;471:179–84.
Li Q, Li WL, Wang D, Liu BB, Tang H, Yang MH, Liu Q, Xing JM, Su ZG. pH neutralization while succinic acid adsorption onto anion-exchange resins. Appl Biochem Biotechnol. 2010;160(2):438–45.
Li Q, Wang D, Hu GY, Xing JM, Su ZG. Integrated bioprocess for high-efficiency production of succinic acid in an expanded-bed adsorption system. Biochem Eng J. 2011;56:150–7.
Wang CX, Ming W, Yan DJ, Zhang CC, Yang MH, Liu YL, Zhang Y, Guo BH, Wan YH, Xing JM. Novel membrane-based biotechnological alternative process for succinic acid production and chemical synthesis of bio-based poly (butylene succinate). Bioresour Technol. 2014;156:6–13.
Engel CR, Straathof A, Van Gulik W, Van Der Wielen L. Integration of fermentation and crystallisation to produce fumaric acid. New Biotechnol. 2009;25S:S173.
Chen ST, Duh SC, Wang KT. Facile synthesis of L-malic acid by a consecutive enzymatic reaction. Biotechnol Lett. 1994;16:355–8.
Prochaska K, Woźniak-Budych MJ. Recovery of fumaric acid from fermentation broth using bipolar electrodialysis. J Membr Sci. 2014;469:428–35.
MarketsandMarkets. Succinic acid market by applications & geography—global trends & forecasts (2011–2016); 2012.
Miltenberge K. Hydroxycarboxylic acids, aliphatic. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim; 2000.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Li, Q., Xing, J. (2017). Production of 1,4-Diacids (Succinic, Fumaric, and Malic) from Biomass. In: Fang, Z., Smith, Jr., R., Qi, X. (eds) Production of Platform Chemicals from Sustainable Resources. Biofuels and Biorefineries. Springer, Singapore. https://doi.org/10.1007/978-981-10-4172-3_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-4172-3_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4171-6
Online ISBN: 978-981-10-4172-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)