Abstract
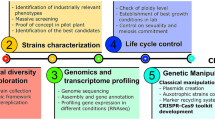
Strains belonging to the yeast species Kluyveromyces marxianus have been isolated from a great variety of habitats, which results in a high metabolic diversity and a substantial degree of intraspecific polymorphism. As a consequence, several different biotechnological applications have been investigated with this yeast: production of enzymes (β-galactosidase, β-glucosidase, inulinase, and polygalacturonases, among others), of single-cell protein, of aroma compounds, and of ethanol (including high-temperature and simultaneous saccharification-fermentation processes); reduction of lactose content in food products; production of bioingredients from cheese-whey; bioremediation; as an anticholesterolemic agent; and as a host for heterologous protein production. Compared to its congener and model organism, Kluyveromyces lactis, the accumulated knowledge on K. marxianus is much smaller and spread over a number of different strains. Although there is no publicly available genome sequence for this species, 20% of the CBS 712 strain genome was randomly sequenced (Llorente et al. in FEBS Lett 487:71–75, 2000). In spite of these facts, K. marxianus can envisage a great biotechnological future because of some of its qualities, such as a broad substrate spectrum, thermotolerance, high growth rates, and less tendency to ferment when exposed to sugar excess, when compared to K. lactis. To increase our knowledge on the biology of this species and to enable the potential applications to be converted into industrial practice, a more systematic approach, including the careful choice of (a) reference strain(s) by the scientific community, would certainly be of great value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Taxonomic history of the present species Kluyveromyces marxianus
Kluyveromyces marxianus was first described in 1888 by E.C. Hansen, which at that time was named Saccharomyces marxianus after Marx, the person who originally isolated this yeast from grapes (Lodder and Kreger-van Rij 1952). In their monograph, Lodder and Kreger-van Rij (1952) describe ten strains of S. marxianus, among which a particular strain labeled Zygosaccharomyces marxianus, which had been deposited at the Centraalbureau voor Schimmelcultures (CBS) in 1922 by H. Schnegg, was arbitrarily chosen as the type strain. This corresponds to the present CBS 712 strain. Some differences among the ten mentioned strains were already pointed out at that time, regarding the formation of pseudomycelium, and the capacities of assimilating and fermenting lactose. Rotting leaves of sisal, sewage of a sugar factory, and “Lufthefe” (aerated yeast) are other habitats from which the strains of S. marxianus had been isolated. Already in 1939, Sacchetti had observed that inulin is fermented by S. marxianus (Lodder and Kreger-van Rij 1952). Although it was already recognized at that time that S. marxianus and Saccharomyces fragilis, which had been isolated from kefir in 1909 by Jörgensen, were very closely related, they were considered distinct species (Lodder and Kreger-van Rij 1952). In 1951, Luh and Phaff affirmed that S. fragilis “is the only yeast species capable of attacking pectin” (Lodder and Kreger-van Rij 1952). Yoghurt, soft cheese, a lung with tuberculosis, Koumiss (a beverage made of fermented mare’s milk), a human lesion of tonsils and the pharynx, and feces are other habitats from which the 11 strains belonging to the S. fragilis species had been obtained from (Lodder and Kreger-van Rij 1952).
Mainly due to differences in spore and ascus morphology, in the capacity of fermenting and oxidizing different sugars, and in the occurrence of hybridization between strains, when compared to true Saccharomyces yeasts, there was a need to reclassify the former species S. fragilis and S. marxianus, besides Saccharomyces lactis, into a new taxon (van der Walt 1970). In 1956, van der Walt described the new genus Kluyveromyces, the type species of which was Kluyveromyces polysporus (van der Walt 1956). Later, it was found that the latter yeast had very similar properties to the three above-mentioned species, and consequently, they were all reclassified into the genus Kluyveromyces, which encompassed 18 species in the second edition of The Yeasts, a taxonomic study (Lodder 1970). Additional habitats from which strains had been isolated include Bantu Beer, milk of a mastitic cow, asthmatic expectoration, and maize meal (van der Walt 1970). Again, Kluyveromyces fragilis was considered closely related but still separate from K. marxianus, mainly due to the former’s high capacity of fermenting lactose. Dairy products and human and animal lesions were the prevalent origin of strains in the K. fragilis taxon (van der Walt 1970).
In the third edition of The Yeasts, a taxonomic study (Kreger-van Rij 1984), the genus Kluyveromyces was divided into 11 species. On the basis of interfertility, the taxon K. marxianus was organized into seven varieties, which are able to readily hybridize (van der Walt and Johannsen 1984). Concomitantly, the former species K. fragilis and K. lactis disappeared.
In the most recent edition of The Yeasts, a taxonomic study (Kurtzman and Fell 1998), the chapter on the Kluyveromyces genus includes 15 species. The seven varieties within the K. marxianus species, proposed in the previous edition of the monograph, were eliminated by considering them as either independent species (e.g., K. lactis and K. dobzhanskii) or synonyms of K. lactis or K. marxianus (Lachance 1998).This is due to the examination of the genetic structure of populations, in combination with hybridization ability, as criteria for classification. Consequently, the former species or varieties Kluyveromyces bulgaricus, K. cicerisporus, K. fragilis, and K. wikenii could not be considered distinct from K. marxianus (Lachance 1998).
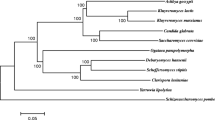
Since the biological concept of species cannot be applied to homothallic organisms, such as the majority of yeasts in the Kluyveromyces taxon, any classification is always based on arbitrary criteria, which have changed along time, as discussed above. Since the development of rapid and efficient gene sequencing tools, it became natural to utilize gene sequences as the criterion for the comparison and classification of microorganisms into the different taxa. Rather than performing single gene comparisons, the most recent reports on the taxonomy of Kluyveromyces yeasts employ multigene sequence analyses for elucidating the phylogeny of the different strains. Using this strategy, Kurtzman and Robnett (2003) showed that the species described by Lachance (1998) in the Kluyveromyces genus are actually distributed into six clades, indicating the polyphyly of this group of yeasts. This is mainly due to the previous criteria employed in classification, such as ascus morphology (in this particular case, ascus deliquescence), which are inadequate as phylogenetic descriptors (Kurtzman 2003; Kurtzman and Robnett 2003). It has been proposed that genera should be circumscribed according to the phylogenetically defined clades, rather than on phenotypic analyses (Kurtzman and Robnett 2003). As a result of this, the number of species in the Kluyveromyces genus decreased to six and the species K. marxianus is proposed as the conserved type species (Kurtzman 2003; Lachance 2007). The type species of the originally described Kluyveromyces genus (van der Walt 1956), namely, K. polysporus, has been reclassified into the newly proposed Vanderwaltozyma genus (Kurtzman 2003; Lachance 2007).
Biochemistry, metabolism, and physiology
It should be noted that the great majority of studies published on K. marxianus have not aimed at looking into its biochemistry, metabolism, or physiology. Most of the works that are publicly available explored potential applications of this organism (see “Biotechnological applications”), without investigating what takes place at the intracellular level. Typically, the yeast cells have been cultivated on a specific substrate, and measurements have been carried out in such a way that only the concentrations of a substrate and of a product, besides the cell concentration, are determined. In what concerns physiology, carbon balances are very rarely looked at, meaning that it is only possible to have a rough macroscopic picture of the cellular reactions and, hence, of the organism’s physiology.
Since the 1970’s, a number of studies has been published on biochemical and metabolic aspects of different K. marxianus strains (a summary is presented in Table 1). Some studies were actually aimed at identifying suitable classification methods for K. marxianus, which has always been a challenging task. These include the observation that, in contrast to S. cerevisiae, ergosterol is the only sterol present in K. marxianus; the use of microcalorimetry for identification purposes; and the characterization of coenzyme Q and monosaccharide patterns of cell walls (Table 1).
In other cases, K. marxianus was simply used as the source of specific compounds, which were the actual focus of research (mainly enzymes). Examples of this kind of study include those on fructose-1,6-bisphosphatase, uridine diphosphate (UDP) glucose-4-epimerase, an acid phosphatase, an amine oxidase, protein phosphatases, carboxypeptidases, and aminopeptidases (Tables 1 and 2). A number of transport studies were also carried out, namely, on the transport of sugars (lactose, glucose, fructose, galactose, and xylose) and organic acids (lactic and malic acids) (Table 1). Finally, a few metabolic studies were carried out with the aim of characterizing some non-transport aspects of K. marxianus, such as the absence of complex I in the respiratory chain, the regulation of adenilate cyclase by Ras proteins, the functional characterization of Mig1p (involved in glucose repression), analysis of cell-wall composition, presence of efflux pumps and their role in drug resistance, and the regulation of the nicotinamide adenine dinucleotide phosphate (oxidized form) (NADP+)-dependent glutamate dehydrogenase (Table 1). In many cases, these studies were performed in parallel with other yeasts, mainly with S. cerevisiae.
In terms of biochemical studies on enzymes that have industrial interest, K. marxianus has been used as a source of inulinase, β-galactosidase, β-glucosidase, and endopolygalacturonases (Table 2). Besides these, some less widespread enzymes with potential industrial application, such as protein phosphatases, carboxypeptidases, and aminopeptidases have also been investigated more recently (Table 2).
In the 1970s, physiological studies focusing on the influence of some common environmental factors on the growth of K. marxianus started to appear in the literature, as a reflection of the eventual interest in using this yeast for industrial applications. Chassang-Douillet et al. (1973) presented the first clear physiological comparison of K. marxianus and S. cerevisiae, carried out using synthetic media and demonstrating that the so-called glucose effect was absent in K. marxianus, as opposed to S. cerevisiae. Later studies reported on the effects of pH (de Sánchez and Castillo 1980), ethanol concentration (Bajpai and Margaritis 1982), and sugar concentration (Margaritis and Bajpai 1983) on the growth kinetics of K. marxianus. Importantly, K. marxianus started to be included in comparative biochemical and physiological studies on yeast in general, such as those related to catabolite repression (Eraso and Gancedo 1984), sensitivity toward toxins (Sukroongreung et al. 1984), and growth inhibition by fatty acids (Viegas et al. 1989).
The use of defined synthetic media combined with chemostat cultivations for quantitative physiological studies started around the 1990s, with works focusing on the regulation of respiration and fermentation and on the so-called Crabtree-effect in yeasts (van Urk et al. 1990; Verduyn et al. 1992). It was then quantitatively shown that K. marxianus presents a strong Crabtree-negative character, since no ethanol production was observed after a glucose pulse applied to respiring cells, in contrast to what is commonly observed with S. cerevisiae and even with K. lactis, to a lesser extent (Kiers et al. 1998). This was later confirmed by Bellaver et al. (2004). Castrillo and Ugalde (1993) showed that when oxidoreductive metabolism sets in in K. marxianus, as a function of increasing glycolytic flux, the maximum respiratory capacity of the cells has not yet been achieved, which is in contrast with the situation in S. cerevisiae, in which the onset of respirofermentative metabolism coincides with the achievement of its maximum respiratory capacity. From these studies, K. marxianus was classified as facultatively fermentative and Crabtree-negative (van Dijken et al. 1993). It is important to note that it cannot grow under strictly anaerobic conditions and that the occurrence of ethanol formation is almost exclusively linked to oxygen limitation (Visser et al. 1990; van Dijken et al. 1993; Bellaver et al. 2004). More recently, Blank et al. (2005) showed that K. marxianus presents the highest tricarboxylic acid cycle flux during batch growth on glucose among the 14 hemiascomycetous yeasts studied within the Génolevures consortium (Souciet et al. 2000).
Other physiological studies report on various issues, such as flocculation (Fernandes et al. 1992, 1993), the influence of CO2 on the survival of K. marxianus (Isenschmid et al. 1995), the influence of the specific growth rate on the morphology of the NRRLy2415 strain, which displays significant growth in pseudo-hyphal form (O’Shea and Walsh 2000), the effects of increased air pressure on the biomass yield of K. marxianus (Pinheiro et al. 2000), the response of K. marxianus to oxidative agents such as hydrogen peroxide (Pinheiro et al. 2002), and the macromolecular composition of K. marxianus cells as a function of the specific growth rate (Fonseca et al. 2007). The later data can be particularly useful for metabolic flux analysis studies.
One important aspect on the physiology of K. marxianus is the fact that significantly different growth parameters, such as μ max and Y x/s, have been reported not only for different strains within the species but also for the same strain when investigated in different laboratories (Fonseca et al. 2007).
From the data in Tables 1 and 2 (and also in Table 3), it can be observed that the number of strains that have been investigated is quite large, and many of them were not obtained directly from the main culture collections worldwide. If, on the one hand, this leads to an interesting metabolic diversity and to several potential applications, as described below in this review, it makes it difficult, on the other hand, to gain fundamental knowledge on the metabolism and physiology of this yeast. In this sense, it would be necessary that researchers started using a reduced number of strains (chosen from key culture collections), similarly to the way in which the K. lactis community has been using the CBS 2359 strain (Lachance 1998; Fukuhara 2006). This would allow the development of efficient molecular genetic tools for K. marxianus (probably starting with genome sequencing), which are the basis for performing systematic studies that will finally lead to a better understanding of the biology of this species. A possibility would be to choose one or two strains with characteristics that have given K. marxianus a clear advantage over other yeasts: thermotolerance, high growth rate, absence of fermentative metabolism upon sugar excess, and a broad substrate spectrum. For making this choice, an approach as the one reported by van Dijken et al. (2000) could be followed.
Recombinant DNA technology
As with any (potential) industrial organism, rational genetic manipulation is one of the most efficient ways of optimizing process yield and/or productivity. In some cases, the application of recombinant DNA (rDNA) technology may even become a prerequisite for a successful industrial process, either to increase product titer and/or purity to levels at which the process becomes economically feasible or to render the producing host capable of synthesizing a heterologous compound. This kind of activity has been well known as metabolic engineering, which is now a consolidated discipline (Stephanopoulos et al. 1998). rDNA technology is also an invaluable technique for genetic and physiological studies, which in turn are essential for increasing our understanding of K. marxianus.
Already more than two decades ago, transformation methods for inserting foreign DNA into K. marxianus have been developed. Das et al. (1984) constructed a plasmid called pGL2, containing the kanamycin resistance gene as a dominant selectable marker, and the KARS2 autonomously replicating sequence of K. lactis. They showed that the transformation method of intact cells with alkali cations, originally developed for Saccharomyces cerevisiae by Ito et al. (1983), also worked in the strain K. fragilis C21. However, the transformation efficiency was rather low.
A breakthrough in molecular biology research of Kluyveromyces yeasts was the discovery of the pKD1 plasmid in the species Kluyveromyces drosophilarum (Falcone et al. 1986). The 4.8-kb, 1.65 μm pKD1 plasmid proved to have a similar organization but different sequences and host specificities, when compared to other already known plasmids, such as the 2 μ plasmid of Saccharomyces yeasts (Chen et al. 1986). In contrast to the latter, pKD1 can be maintained stable in K. lactis, but not in S. cerevisiae, in the absence of selective pressure (Bianchi et al. 1987). Later, it was shown that the insertion of the kanamycin resistance gene, the URA3 gene of S. cerevisiae, a replication origin for E. coli, and the ampicillin resistance gene into pKD1 rendered a shuttle plasmid that could be transformed and maintained in K. marxianus strains CBS 6556 and CBS 712, though still with low-transformation efficiencies (Chen et al. 1989). This is in accordance with the fact that ARS and centromere sequences of K. lactis work in K. marxianus and vice versa (Das et al. 1984; Iborra and Ball 1994). Thus, pKD1-based plasmids have become the most common choice for inserting foreign DNA sequences into K. marxianus (Bergkamp et al. 1993b; Bartkevičiute et al. 2000; Zhang et al. 2003).
Iborra (1993) reported for the first time transformation efficiencies in the order of hundreds to thousands of transformants per microgram of DNA with K. marxianus, either with the lithium method (Ito et al. 1983) or using electroporation (Meilhoc et al. 1990). Similar results were obtained more recently by Zhang et al. (2003).
Besides requiring efficient vectors and transformation protocols, foreign gene expression also depends on the promoter and eventually a signal sequence for directing the synthesized protein into the extracellular environment, which usually facilitates downstream operations. For this purpose, Bergkamp et al. (1993a) used the promoter and prepro-signal sequence of the INU1 (inulinase) gene to successfully direct heterologous expression and secretion of α-galactosidase in K. marxianus, with dramatically higher efficiencies when compared to the use of classical S. cerevisiae promoters, such as PGK.
With the INU1 promoter, heterologous gene expression can be fine-tuned by choosing the appropriate carbon source. Another regulated promoter that was successfully used in K. marxianus is the tetracycline repressible promoter (Pecota and da Silva 2005).
Strong, constitutive promoters for driving heterologous gene expression have also been described, such as that of a purine-cytosine permease gene (Ball et al. 1999). Instead of fine-tuning foreign gene expression according to promoter strength or induction properties, Pecota et al. (2007) developed an insertion cassette that enables multicopy integration of a precise number of gene copies into K. marxianus with recycling of the selection marker.
Auxotrophic mutants of K. marxianus, for their use in transformation experiments, have been reported for leucine, uracil, histidine, or triptophane requirement (Bergkamp et al. 1991, 1993b; Basabe et al. 1996; Hong et al. 2007). Dominant markers applicable for the selection of K. marxianus transformants include at least the kanamycin, the aureobasidin A, and the nurseothricin resistance genes (Das et al. 1984; Hashida-Okado et al. 1998; Goldstein and McCusker 1999; Steensma and Ter Linde 2001; Ribeiro et al. 2007). Recycling of the marker gene for multiple gene disruptions can be performed in K. marxianus in the same way as in S. cerevisiae and K. lactis with the Cre-loxP system (Güldener et al. 1996; Ribeiro et al. 2007).
A number of genes have been cloned and sequenced in different K. marxianus strains, and the most relevant ones are indicated in Table 3.
Biotechnological applications
When evaluating the yeast K. marxianus for biotechnological applications, it is impossible not to consider other most popular species, mainly S. cerevisiae and K. lactis. The former is probably the most employed biocatalyst in the biotechnological industry and a model organism in biological studies, whereas the latter has been chosen as a model Crabtree-negative, lactose-utilizing organism (Lachance 1998; Fukuhara 2006). The fact that several K. marxianus strains have obtained the generally-regarded-as-safe (GRAS) status, similarly to S. cerevisiae and K. lactis (Hensing et al. 1995) indicates that this aspect does not impose any disadvantage for the former, when compared to the latter yeasts, in terms of process approval by regulatory agencies. The fact that K. lactis was chosen by the scientific community as the model organism in the Kluyveromyces genus, and not K. marxianus, led not only to a much better understanding of its physiology (for reviews, see e.g., Schaffrath and Breunig 2000; Wolf et al. 2003; Breunig and Steensma 2003 and the whole issue no. 3, vol. 6 of FEMS Yeast Research), and to the full sequencing of its genome (Dujon et al. 2004) but also to the development of several applications, including the expression of more than 40 heterologous proteins (van Ooyen et al. 2006). This is due, to a great extent, to the fact that researchers have used, from the beginning, a very small number of K. lactis isolates (Fukuhara 2006), which has not been the case in the K. marxianus species.
The development of biotechnological applications with K. marxianus has been motivated by a number of advantages it has when compared to K. lactis. These include at least the fact that it can grow on a broader variety of substrates and at higher temperatures, its higher specific growth rates, and the lesser tendency to produce ethanol it has when exposed to sugar excess (Rouwenhorst et al. 1988; Steensma et al. 1988; Bellaver et al. 2004; see also “Biochemistry, metabolism, and physiology”).
One very important aspect of the ecology of K. marxianus should be taken into account when considering its biotechnological utilization: Individuals have been isolated from an enormous variety of habitats (see “Taxonomic history of the present species Kluyveromyces marxianus ”).
The obvious consequence is that the metabolic diversity is broad, and hence, potential biotechnological applications of K. marxianus strains are manifold. A summary of the most explored applications with this yeast follows.
Although several yeasts have been reported for the production of aroma compounds, only a few of these can find industrial application due to their GRAS status (Medeiros et al. 2000, 2001). Kluyveromyces sp. produce aroma compounds such as fruit esters, carboxylic acids, ketones, furans, alcohols, monoterpene alcohols, and isoamyl acetate in liquid fermentation (Scharpf et al. 1986; Fabre et al. 1995). Of all these compounds, 2-phenyl ethanol (2-PE), with rose petals aroma, is the most important commercially (Welsh et al. 1989; Leclercq-Perlat et al. 2004). Natural 2-PE has a high-value (approximately US $1,000 kg−1) serving a current world market of approximately 7,000 tons per annum (data from 1990; Etschmann et al. 2002). This alcohol presents sensorial characteristics that influence the quality of the wine, distilled drinks, or fermented foods. It is also found in fresh beer and is added to various industrial food products such as ice creams, bullets, non-alcoholic drinks, gelatines, puddings, and bubble gums (Wittmann et al. 2002). The influences of the carbon source (Fabre et al. 1998; Medeiros et al. 2000), aeration rate (Medeiros et al. 2001), media composition (Etschmann et al. 2004), and cultivation conditions (Etschmann and Schrader 2006) on the aroma production using K. marxianus were studied.
K. marxianus possesses the natural ability to excrete enzymes. This is a desired property for cost-efficient downstream processing of low- and medium-value enzymes (Hensing et al. 1994). The enzymes that hydrolyze pectic substances are known as pectic enzymes, pectinases, or pectinolytic enzymes (Wimborne and Rickard 1978). Pectinases are industrially used in the extraction and clarification of fruit juices (i.e., grape and apple; Schwan et al. 1997; Blanco et al. 1999). Other interesting applications are related to the maceration of vegetables, oil extraction, and formulation of animal feed using complex mixtures with cellulases to make the nutritional assimilation easier (Blanco et al. 1999). K. marxianus has considerable economic advantages over Aspergillus as an endo-PG source, even without genetic improvement of the strains (Harsa et al. 1993). Thus, the use of PGs from K. marxianus has attracted considerable interest (Garcia-Garibay et al. 1987b; Harsa et al. 1993; Donaghy and McKay 1994). Moreover, no other pectinolytic enzyme, besides PG, was reported to be secreted by K. marxianus CCT 3172 in the culture media, which should facilitate the process to produce pure enzyme (Schwan et al. 1997).
In K. marxianus, the pectinolytic enzymes are only produced during exponential growth, but almost all PG is secreted in the start of the stationary phase (Schwan and Rose 1994; Schwan et al. 1997; Serrat et al. 2004). Among the cultivation parameters, dissolved oxygen was reported to be the key in the production of both biomass and endo-PG (Wimborne and Rickard 1978; Garcia-Garibay et al. 1987a). High rates and yields of biomass production require high oxygenation levels that, however, repress endo-PG induction (Wimborne and Rickard 1978). It was reported that K. marxianus exhibited pectolytic ability when it was grown without shaking and under anaerobic conditions, and no activity was found at high aeration rates (Barnby et al. 1990; Schwan and Rose 1994; García-Garibay et al. 1987a).
The effect of temperature was assessed on both growth and endo-PG production in combination with the effect of dissolved oxygen (Schwan and Rose 1994). Temperature was also reported to have no direct effect on the synthesis of this enzyme but influenced the growth rate and had an indirect effect due to changes in oxygen solubility (Cruz-Guerrero et al. 1999). Addition of pectin in an aerobic culture in a fermenter was reported to derepress the production of the enzyme (Garcia-Garibay et al. 1987a). However, whereas some authors did not find any effect of pectin addition to the medium in an endo-PG producing strain (Schwan and Rose 1994; Schwan et al. 1997), others reported the enhancement of pectinase production by this yeast when pectin was added (Wimborne and Rickard 1978; Lim et al. 1980; Cruz-Guerrero et al. 1999). K. marxianus CCT 3172 was able to break down pectin but required a usable source of carbon and energy to elaborate pectinolytic activity (Schwan and Rose 1994). It had a strong endo-PG activity between pH 4–6 with pH 5 as optimum (Schwan et al. 1997). Furthermore, the type of pectinase excreted by this strain was pointed as a feasible alternative to fungal production due to the lower broth viscosity, which can make downstream operations easier (Almeida et al. 2003a).
Lactose-intolerance can be circumvented by removing lactose from the diet or by converting this sugar into glucose and galactose with β-d-galactosidase (Rajoka et al. 2003). It was reported that only approximately 2% of the recognized yeast species are capable of fermenting lactose (Barnett et al. 1983), among which strains within the Kluyveromyces genus can be found. In a screening performed with yeast strains belonging to different genera, only two cultures of K. fragilis and Ferrissia fragilis showed β-galactosidase activity (Fiedurek and Szczodrak 1994). Lactose is considered the primary inducer of β-d-galactosidase synthesis (Furlan et al. 2000) and production of lactase by K. marxianus using cheese whey as a nutrient source has been investigated by several authors (Sonawat et al. 1981; Nunes et al. 1993).
During the stationary phase of growth, the β-d-galactosidase activity of K. marxianus CBS 712 and CBS 6556 remained approximately constant (Rech et al. 1999). In contrast, a reduction of β-d-galactosidase activity was reported by other authors in the stationary phase (Mahoney et al. 1975). The highest β-d-galactosidase activity of K. marxianus CBS 712 and CBS 6556 was reported to be at 37°C, decreasing quickly at temperatures above 40°C (Rech et al. 1999), while in K. marxianus IMB3, the enzyme is optimally active at 50°C (Barron et al. 1995a). On the other hand, the β-galactosidase of K. marxianus CBS 6556 is more stable than the corresponding enzymes of other strains, when stored at low temperatures, e.g., 4°C (Rech et al. 1999; Itoh et al. 1982; Brady et al. 1995). When different substrates were investigated for β-galactosidase production by K. marxianus, lactose supported the highest enzyme activities (Rajoka et al. 2003; Rajoka et al. 2004).
Inulinase is an enzyme that cleaves fructose molecules from inulin. Its expression is induced by inulin or sucrose, and the enzyme can be excreted to the culture medium or remain associated to the cell wall (Rouwenhorst et al. 1988; Barranco-Florido et al. 2001). K. marxianus has been widely studied for inulinase production, aiming at the production of fructose syrup from inulin (Cruz-Guerrero et al. 1995).
Pessoa and Vitolo (1999) obtained the highest inulinase activities with the K. marxianus DSM 70106 strain, using inulin as the carbon source. Growth of K. marxianus on sucrose also proceeds via the action of an extracellular inulinase (Hensing et al. 1994), which is repressed when growth is not sucrose-limited (Rouwenhorst et al. 1988; Parekh and Margaritis 1985; Grootwassink and Hewitt 1983).
K. marxianus has also been proposed as a source of: (1) oligonucleotides, used as flavour enhancers in food products; (2) oligosaccharides, used as prebiotics; and (3) oligopeptides, immuno stimulators added to dairy products that are released in the wort after whey protein proteolysis (Belem et al. 1997; Belem and Lee 1998, 1999). Recent studies have shown the potential of K. marxianus FII 510700 biomass as an alternative source to S. cerevisiae for yeast autolysates (Lukondeh et al. 2003a), alkali-insoluble glucans (Lukondeh et al. 2003c), and a natural bioemulsifier (Lukondeh et al. 2003b).
In the field of bioremediation, processes using K. marxianus were developed for the removal of copper ions (II) with molasses as a nutrients source (Aksu and Dönmez 2000). Lead (II) uptake by K. marxianus from contaminated molasses had negative effects on cell growth. Nevertheless, the decrease in biomass formation did not lead to decreased lead (II) uptake; on the contrary, the biosorption ability was higher at higher initial lead (II) concentrations (Skountzou et al. 2003).
Ethanol production at elevated temperatures has received much attention because of the potential cost savings, which could be obtained by continuous evaporation of ethanol from the broth under reduced pressure (Hacking et al. 1984; Gough et al. 1996, 1997, 1998; Banat et al. 1998). This topic was recently reviewed for yeasts in general, including K. marxianus. The advantages described, besides the energy savings due to reduced cooling costs, were higher saccharification and fermentation rates, continuous ethanol removal, and reduced contamination (Banat et al. 1998). However, the temperature increase has a negative effect on ethanol yield and also reduces the cell viability (Anderson et al. 1986; Ballesteros et al. 1991). K. marxianus was reported to produce alcohol at temperatures above 40°C and to have a maximum growth temperature of 47°C (Anderson et al. 1986), 49°C (Hughes et al. 1984), or even 52°C (Banat et al. 1992). Lower ethanol tolerance was observed when K. marxianus was compared to S. cerevisiae, and this was correlated with the activity of the plasma membrane ATPase (Rosa and Sa-Correia 1992; Fernanda and Sa-Correia 1992). Hacking et al. (1984) screened yeast strains for their ability to ferment glucose to ethanol at high temperatures. The tolerance of all species seemed to decrease with temperature, but in general, Kluyveromyces strains were more thermotolerant than Saccharomyces, which in turn can produce higher ethanol yields. Anderson et al. (1986) compared K. marxianus strains isolated from sugar mills and CBS strains for ethanol production at high temperatures. The CBS strains produced the same ethanol amounts as the new isolates but with lower cell viability and higher cultivation time. Sakanaka et al. (1996) reported the fusion of a thermotolerant strain of K. marxianus with a high ethanol producing strain of S. cerevisiae; however, their fermentative capacity was severely impaired and the fusants’ thermostability was lower than for either of the parental cells.
While Schwan and Rose (1994) reported that ethanol production in galactose-containing medium was not as high as when glucose was the carbon source, Duvnjak et al. 1987 found that galactose was a better carbon source for ethanol production than glucose; however, the strains employed in both works were different. The conversion of xylose into ethanol by K. marxianus was already reported some time ago (Margaritis and Bajpai 1982).
Different process strategies have been used for ethanol production with K. marxianus: batch cultures with elevated substrate concentrations (Grubb and Mawson 1993; Barron et al. 1996), fed-batch production (Ferrari et al. 1994; Gough et al. 1998; Love et al. 1996), continuous system (Love et al. 1998), membrane recycle bioreactors (Tin and Mawson 1993), two-stage fermentation (Hack et al. 1994; Banat et al. 1996), immobilization with β-galactosidase (Hahn-Hägerdal 1985), calcium-alginate-immobilized cells (Bajpai and Margaritis 1987a,b; Marwaha et al. 1988; Nolan et al. 1994; Riordan et al. 1996; Barron et al. 1996; Brady et al. 1996, 1997a,b, 1998; Ferguson et al. 1998; Gough and Mchale 1998), cells immobilized in poly(vinyl alcohol) cryogel beads (Gough et al. 1998), or in Kissiris, a mineral glass foam derived from lava (Nigam et al. 1997; Love et al. 1996, 1998), extractive fed batch cultures (Jones et al. 1993), simultaneous saccharification and fermentation processes with added enzymes (Barron et al. 1995b, 1996, 1997; Boyle et al. 1997; Nilsson et al. 1995; Ballesteros et al. 2002a,b, 2004; Kádár et al. 2004) or by cloning of heterologous cellulase genes (Hong et al. 2007), and the use of mixed cultures (Ward et al. 1995).
Cheese whey contains lactose and a protein fraction sufficiently rich in essential amino acids. Cheese whey cultivations with K. marxianus have been proposed, with promising results, as a means of reducing the pollution caused by this industrial waste stream (Ghaly and Singh 1989; Giec and Kosikowski 1992; Harden 1996; Aktas et al. 2005) and/or to produce single-cell protein (Giec and Kosikowski 1992; Ben-Hassan et al. 1992, Ben-Hassan and Ghaly 1995; Belem and Lee 1999; Schultz et al. 2006; Ghaly and Kamal 2004). Aerobic cultures of microorganisms in cheese whey can reduce up to 90–95% of its BOD (Grubb and Mawson 1993), resulting in bioingredients of high added value for the food industry (Belem et al. 1997).
Other potential applications of the yeast K. marxianus, which can be found in the literature include its use as baker’s yeast (Caballero et al. 1995) and as an anticholesterolemic agent (Yoshida et al. 2004). The cellular components involved in the hypocholesterolemic activity of K. marxianus were further examined (Yoshida et al. 2005).
Last but not the least, K. marxianus has been investigated as a host for the production of heterologous proteins. In general, yeasts are capable of performing some post-translational modifications of proteins, such as glycosylation and/or other modifications required for optimal biological activity and stability (Hensing et al. 1995). S. cerevisiae has been the most commonly used yeast host for the production of heterologous proteins (Romanos et al. 1992; Gellissen and Hollenberg 1997; Porro et al. 2005). Nevertheless, this yeast has some drawbacks, such as its strong aerobic fermentation behavior and a tendency to hyperglycosylate secreted glycoproteins (Hensing et al. 1994). K. lactis has also been used for the production of heterologous proteins (van den Berg et al. 1990; Panuwatsuk and da Silva 2002; Bartkevičiute and Sasnauskas 2003; van Ooyen et al. 2006). K. marxianus, which is phylogenetically close to K. lactis, is supposed to have a similar capacity for synthesis and secretion of high molecular weight proteins (Wésolowski-Louvel et al. 1996). Some examples showing that heterologous protein production in this yeast is possible have been reported in the literature (Bergkamp et al. 1993a; Bartkevičiute et al. 2000; Zhang et al. 2003). More recently, Pecota et al. (2007) successfully expressed lactate dehydrogenase activity in K. marxianus, using an integrative multi-copy system, resulting in lactate production by this yeast. Hong et al. (2007) expressed thermostable endo-β-1,4-glucanase, cellobiohydrolase, and β-glucosidase, also making use of an integrative system, generating a strain capable of converting cellulosic materials into ethanol. Although these studies demonstrate that the heterologous proteins expressed in K. marxianus were functional, the capacity of K. marxianus to perform post-translational modifications of heterologous proteins still remains to be investigated.
References
Abranches J, Morais PB, Rosa CA, Mendonça-Hagler LC, Hagler AN (1997) The incidence of killer activity and extracellular proteases in tropical yeast communities. Can J Microbiol 43:328–336
Aksu Z, Dönmez G (2000) The use of molasses in copper (II) containing wastewaters: effects on growth and copper (II) bioaccumulation properties of Kluyveromyces marxianus. Proc Biochem 36:451–458
Aktas N, Boyacı IH, Mutlu M, Tanyolac A (2005) Optimization of lactose utilization in deproteinated whey by Kluyveromyces marxianus using response surface methodology (RSM). Biores Technol 97:2252–2259
Almeida C, Branyik T, Moradas-Ferreira P, Teixeira J (2003a) Continuous production of pectinase by immobilized yeast cells on spent grains. J Biosci Bioeng 96:513–518
Amrane A, Prigent Y (1996) Behaviour of the yeast Kluyveromyces marxianus var. marxianus during its autolysis. Antonie van Leeuwenhoek 69:267–272
Anderson PJ, McNeil K, Watson K (1986) High-efficiency carboidrate fermentation to ethanol at temperatures above 40°C by Kluyveromyces marxianus var. marxianus isolated from sugar mills. Appl Environ Microbiol 51:1314–1320
Bacci Júnior M, Siqueira CG, Antoniazi SA, Ueta J (1996) Location of the b-galactosidase of the yeast Kluyveromyces marxianus var. marxianus ATCC 10022. Antonie van Leeuwenhoek 69:357–361
Bajpai P, Margaritis A (1982) Ethanol inhibition kinetics of Kluyveromyces marxianus grown on Jerusalem artichoke juice. Appl Environ Microbiol 44:1325–1329
Bajpai P, Margaritis A (1987a) Kinetics of ethanol production by immobilized Kluyveromyces marxianus cells at varying sugar concentrations of Jerusalem artichoke juice. Appl Microbiol Biotechnol 26:447–449
Bajpai P, Margaritis A (1987b) The effect of temperature and pH on ethanol production by free and immobilized cells of Kluyveromyces marxianus grown on Jerusalem artichoke extract. Biotechnol Bioeng 30:306–313
Ball MM, Raynal A, Guerineau M, Iborra F (1999) Construction of efficient centromeric, multicopy and expression vectors for the yeast Kluyveromyces marxianus using homologous elements and the promoter of a purine-cytosine-like permease. J Mol Microbiol Biotechnol 1:347–353
Ballesteros I, Ballesteros M, Cabanas A, Carrasco J, Martin C, Negro MJ, Saez F, Saez R (1991) Selection of thermotolerant yeasts for simultaneous saccharification and fermentation (SSF) of cellulose to ethanol. Appl Biochem Biotechnol 28:307–315
Ballesteros I, Oliva JM, Negro MJ, Manzanares P, Ballesteros M (2002a) Simultaneous saccharification and fermentation process for converting the cellulosic fraction of olive oil extraction residue into ethanol. Grasas y Aceites 53:282–288
Ballesteros M, Oliva JM, Manzanares P, Negro MJ, Ballesteros I (2002b) Ethanol production from paper material using a simultaneous saccharification and fermentation system in a fed-batch basis. World J Microbiol Biotechnol 18:559–561
Ballesteros M, Oliva JM, Negro MJ, Manzanares P, Ballesteros I (2004) Ethanol from lignocellulosic materials by a simultaneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Proc Biochem 39:1843–1848
Banat IM, Nigam P, Marchant R (1992) Isolation of thermotolerant, fermentative yeasts growing at 52°C and producing ethanol at 45°C and 50°C. World J Microbiol Biotechnol 8:259–263
Banat IM, Singh D, Marchant R (1996) The use of a thermotolerant fermentative Kluyveromyces marxianus IMB3 yeast strain for ethanol production. Acta Biotechnol 16:215–223
Banat IM, Nigam P, Singh D, Marchant R, McHale AP (1998) Ethanol production at elevated temperatures and alcohol concentrations: Part I - Yeasts in general. World J Microbiol Biotechnol 14:809–821
Barnby FM, Morpeth FF, Pyle DL (1990) Endopolygalacturonase production from Kluyveromyces marxianus. Resolution, purification, and partial characterisation of the enzyme. Enzyme Microb Technol 12:891–897
Barnett JA, Payne RW, Yarrow D (1983) Yeasts: characteristics and identification. Cambridge University Press, Cambridge
Barranco-Florido E, García-Garibay M, Gómez-Ruiz L, Azaola A (2001) Immobilization system of Kluyveromyces marxianus cells in barium alginate for inulin hydrolysis. Proc Biochem 37:513–519
Barron N, Marchant R, McHale L, McHale AP (1995a) Partial characterization of b-glucosidase activity produced by Kluyveromyces marxianus IMB3 during growth on cellobiose containing media at 45°C. Biotechnol Lett 17:1047–1050
Barron N, Marchant R, McHale L, McHale AP (1995b) Studies on the use of a thermotolerant strain of Kluyveromyces marxianus in simultaneous saccharification and ethanol formation from cellulose. Appl Microbiol Biotechnol 43:518–520
Barron N, Marchant R, McHale L, McHale AP (1996) Ethanol production from cellulose at 45°C using a batch-fed system containing alginate-immobilized Kluyveromyces marxianus IMB3. World J Microbiol Biotechnol 12:103–104
Barron N, Mulholland H, Boyle M, McHale AP (1997) Ethanol production by Kluyveromyces marxianus IMB3 during growth on straw-supplemented whiskey distillery spentwash at 45°C. Bioproc Eng 17:383–386
Bartkevičiute D, Sasnauskas K (2003) Studies of yeast Kluyveromyces lactis mutations conferring super-secretion of recombinant proteins. Yeast 20:1–11
Bartkevičiūtė D, Šiekštelė R, Sasnauskas K (2000) Heterologous expression of the Kluyveromyces marxianus endopolygalacturonase gene (EPG1) using versatile autonomously replicating vector for a wide range of host. Enzyme Microb Technol 26:653–656
Basabe L, Cabrera N, Yong V, Menéndez J, Delgado JM, Rodríguez L (1996) Isolation and characterization of mutants as an approach to a transformation system in Kluyveromyces marxianus. Curr Genet 30:89–92
Beezer AE, Newell RD, Tyrrell HJ (1979) Characterisation and metabolic studies of Saccharomyces cerevisiae and Kluyveromyces fragilis by flow microcalorimetry. Antonie van Leeuwenhoek 45:55–63
Belem MAF, Lee BH (1998) Production of bioingredients from Kluyveromyces marxianus grown on whey: an alternative. Crit Rev Food Sci Nut 38:565–598
Belem MAF, Lee BH (1999) Fed-batch fermentation to produce ologonucleotides from Kluyveromyces marxianus grown on whey. Proc Biochem 34:501–509
Belem MAF, Gibbs BF, Lee BH (1997) Enzymatic production of ribonucleotides from autolysates of Kluyveromyces marxianus grown on whey. J Food Sci 62:851–857
Bellaver LH, de Carvalho NMB, Abrahão-Neto J, Gombert AK (2004) Ethanol formation and enzyme activities around glucose-6-phosphate in Kluyveromyces marxianus CBS 6556 exposed to glucose or lactose excess. FEMS Yeast Res 4:691–698
Ben-Hassan RM, Ghaly AE (1995) Continuous production of single-cell protein from cheese whey lactose using Kluyveromyces fragilis. Trans ASAE 38:1121–1127
Ben-Hassan RM, Ghaly AE, Ben-Abdallah N (1992) Metabolism of cheese whey lactose by Kluyveromyces fragilis for energy and growth under batch condition. Appl Biochem Biotechnol 33:97–116
Bergkamp RJ, Geerse RH, Verbakel JM, Musters W, Planta RJ (1991) Cloning and disruption of the LEU2 gene of Kluyveromyces marxianus CBS 6556. Yeast 7:963–970
Bergkamp RJM, Bootsmas TC, Toschka HY, Mooren ATA, Kox L, Verbakel JMA, Geerse RH, Planta RJ (1993a) Expression of an a-galactosidase gene under control of the homologous inulinase promoter in Kluyveromyces marxianus. Appl Microbiol Biotechnol 40:309–317
Bergkamp RJ, Geerse RH, Verbakel JM, Planta RJ (1993b) Cloning and sequencing of the URA3 gene of Kluyveromyces marxianus CBS 6556. Yeast 9:677–681
Bhattacharjee H, Bhaduri A (1992) Distinct functional roles of two active site thiols in UDPglucose 4-epimerase from Kluyveromyces fragilis. J Biol Chem 267:11714–11720
Bianchi MM, Falcone C, Re CX, Wéslowski-Louvel M, Frontali L, Fukuhara H (1987) Transformation of the yeast Kluyveromyces lactis by new vectors derived from the 1.6 mm circular plasmid pKD1. Curr Genet 12:185–192
Blanco P, Sieiro C, Villa TG (1999) Production of pectic enzymes in yeasts. FEMS Microbiol Lett 175:1–9
Blank LM, Lehmbeck F, Sauer U (2005) Metabolic-flux and network analysis in fourteen hemiascomycetous yeasts. FEMS Yeast Res 5:545–558
Boyle M, Barron N, McHale AP (1997) Simultaneous saccharification and fermentation of straw to ethanol using the thermotolerant yeast strain Kluyveromyces marxianus IMB 3. Biotechnol Lett 19:49–51
Brady D, Marchant R, McHale L, McHale AP (1995) Isolation and partial characterization of b-galactosidase activity produced by a thermotolerant strain of Kluyveromyces marxianus during growth on lactose-containing media. Enzyme Microb Technol 17:696–699
Brady D, Nigam P, Marchant R, McHale L, McHale AP (1996) Ethanol production at 45°C by Kluyveromyces marxianus IMB3 immobilized in magnetically responsive alginate matrices. Biotechnol Lett 18:1213–1216
Brady D, Nigam P, Marchant R, McHale AP (1997a) Ethanol production at 45°C by alginate-immobilized Kluyveromyces marxianus IMB3 during growth on lactose-containing media. Bioproc Eng 16:101–104
Brady D, Nigam P, Marchant R, Singh D, McHale AP (1997b) The effect of Mn2+ on ethanol production from lactose using Kluyveromyces marxianus IMB3 immobilized in magnetically responsive matrices. Bioproc Eng 17:31–34
Brady D, Logan SR, McHale AP (1998) The effect of soluble alginate and calcium on b-galactosidase activity produced by the thermotolerant, ethanol-producing yeast strain Kluyveromyces marxianus IMB3. Bioproc Eng 18:101–104
Breunig KD, Steensma HY (2003) Kluyveromyces lactis: genetics, physiology, and application. In: de Winde JH (ed) Functional genetics of industrial yeasts, Topics in current genetics, vol. 2. Springer-Verlag, Berlin Heidelberg New York, pp 171–205
Büschges R, Bahrenberg G, Zimmermann M, Wolf K (1994) NADH: ubiquinone oxidoreductase in obligate aerobic yeasts. Yeast 10:475–479
Caballero R, Olguín P, Cruz-Guerrero A, Gallardo F, García-Garibay M, Gómez-Ruiz L (1995) Evaluation of Kluyveromyces marxianus as baker’s yeast. Food Res Int 28:37–41
Carvalho-Silva M, Spencer-Martins I (1990) Modes of lactose uptake in the yeast species Kluyveromyces marxianus. Antonie van Leeuwenhoek 57:77–81
Cassart JP, Ostling J, Ronne H, Vandenhaute J (1997) Comparative analysis in three fungi reveals structurally and functionally conserved regions in the Mig1 repressor. Mol Gen Genet 255:9–18
Castrillo JI, Ugalde UO (1993) Patterns of energy metabolism and growth kinetics of Kluyveromyces marxianus in whey chemostat culture. Appl Microbiol Biotechnol 40:386–393
Chassang-Douillet A, Ladet J, Boze H, Galzy P (1973) Respiratory metabolism of Kluyveromyces fragilis van der Walt. Z Allg Mikrobiol 13:193–199
Chen XJ, Saliola M, Falcone C, Bianchi MM, Fukuhara H (1986) Sequence organization of the circular plasmid pKD1 from the yeast Kluyveromyces drosophilarum. Nucleic Acids Res 14:4471–4481
Chen XJ, Bianchi MM, Suda K, Fukuhara H (1989) The host range of the pKD1-derived plasmids in yeast. Curr Genet 16:95–98
Corpillo D, Valetti F, Giuffrida MG, Conti A, Rossi A, Finazzi-Agrò A, Giunta C (2003) Induction and characterization of a novel amine oxidase from the yeast Kluyveromyces marxianus. Yeast 20:369–379
Cruz-Guerrero A, García-Peña I, Bárzana E, García-Garibay M, Gómez-Ruiz L (1995) Kluyveromyces marxianus CDBB-L-278: a wild inulinase hyperproducing strain. J Ferm Bioeng 80:159–163
Cruz-Guerrero A, Bárzana E, García-Garibay M, Gómez-Ruiz L (1999) Dissolved oxygen threshold for the repression of endo-polygalacturonase production by Kluyveromyces marxianus. Proc Biochem 34:621–624
Das S, Kellermann E, Hollenberg CP (1984) Transformation of Kluyveromyces fragilis. J Bacteriol 158:1165–1167
de Bruijne AW, Schuddemat J, van den Broek PJA, van Steveninck J (1988) Regulation of sugar transport systems of Kluyveromyces marxianus: the role of carbohydrates and their catabolism. Biochim Biophys Acta 939:569–576
de Morais MA Jr (2003) The NADP+-dependent glutamate dehydrogenase of the yeast Kluyveromyces marxianus responds to nitrogen repression similarly to Saccharomyces cerevisiae. Braz J Microbiol 34:334–338
de Sánchez SB, Castillo FJ (1980) Effect of pH on the growth of Kluyveromyces fragilis on deproteinized whey. Acta Cient Venez 31:24–26
Donaghy JA, McKay AM (1994) The use of K1uvveromyces fragilis for the extraction of orange peel pectins. J Appl Bacteriol 76:506–510
Dujon B, Sherman D, Fischer G et al (2004) Genome evolution in yeasts. Nature 430:35–44
Duvnjak Z, Houle C, Mok KL (1987) Production of ethanol and biomass from various carbohydrates by Kluyveromyces fragilis. Biotechnol Lett 9:343–346
Eraso P, Gancedo JM (1984) Catabolite repression in yeasts is not associated with low levels of cAMP. Eur J Biochem 141:195–198
Etschmann MMW, Schrader J (2006) An aqueous-organic two-phase bioprocess for efficient production of the natural aroma chemicals 2-phenylethanol and 2-phenylethylacetate with yeast. Appl Microbiol Biotechnol 71:440–443
Etschmann MMW, Bluemke W, Sell D, Schrader J (2002) Biotechnological production of 2-phenylethanol. Appl Microbiol Biotechnol 59:1–8
Etschmann MMW, Sell D, Schrader J (2004) Medium optimization for the production of the aroma compound 2-phenylethanol using a genetic algorithm. J Mol Catal B: Enzym 29:187–193
Fabre CE, Duviau VJ, Blanc PJ, Goma G (1995) Identification of volatile flavour compounds obtained in culture of Kluyveromyces marxianus. Biotechnol Lett 17:1207–1212
Fabre CE, Blanc PJ, Goma G (1998) Production of 2-phenylethyl alcohol by Kluyveromyces marxianus. Biotechnol Prog 14:270–274
Falcone C, Saliola M, Chen XJ, Frontali L, Fukuhara H (1986) Analysis of a 1.6-micron circular plasmid from the yeast Kluyveromyces drosophilarum: structure and molecular dimorphism. Plasmid 15:248–252
Ferguson P, Mulholland H, Barron N, Brady D, McHale AP (1998) Sucrose-supplemented distillery spent-wash as a medium for production of ethanol at 45°C by free and alginate-immobilized preparations of Kluyveromyces marxianus IMB3. Bioproc Eng 18:257–259
Fernanda R, Sa-Correia I (1992) Ethanol tolerance and activity of plasma membrane ATPase in Kluyveromyces marxianus and Saccharomyces cerevisiae. Enzyme Microb Technol 14:23–27
Fernandes PA, Keen JN, Findlay JBC, Moradas-Ferreira PA (1992) Protein homologous to glyceraldehyde-3-phosphate dehydrogenase is induced in the cell wall of a flocculant Kluyveromyces marxianus. Biochim Biophys Acta 1159:67–73
Fernandes PA, Sousa M, Moradas-Ferreira P (1993) Flocculation of Kluyveromyces marxianus is induced by a temperature upshift. Yeast 9:859–866
Fernandes PA, Sena-Esteves M, Moradas-Ferreira P (1995) Characterization of the glyceraldehyde-3-phosphate dehydrogenase gene family from Kluyveromyces marxianus - polymerase chain reaction single-strand conformation polymorphism as a tool for the study of multigenic families. Yeast 11:725–733
Ferrari MD, Loperena L, Varela H (1994) Ethanol production from concentrated whey permeate using a fed-batch culture of Kluyveromyces fragilis. Biotechnol Lett 16:205–210
Fiedurek J, Szczodrak J (1994) Selection of strain, culture conditions and extraction procedures for optimum production of b-galactosidase from Kluyveromyces fragilis. Acta Microbiol Pol 43:57–65
Fonseca A, Spencer-Martins I, van Uden N (1991) Transport of lactic acid in Kluyveromyces marxianus: evidence for a monocarboxylate uniport. Yeast 7:775–780
Fonseca GG, Gombert AK, Heinzle E, Wittmann C (2007) Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res 7:422–435
Fukuhara H (2006) Kluyveromyces lactis - A retrospective. FEMS Yeast Res 6:323–324
Furlan SA, Schneider ALS, Merkle R, Carvalho-Jonas MD, Jonas R (2000) Formulation of a lactose-free, low-cost culture medium for the production of b-D-galactosidase by Kluyveromyces marxianus. Biotechnol Lett 22:589–593
Garcia-Garibay M, Gómez-Ruiz L, Bárzana E (1987a) Studies on the simultaneous production of single-cell protein and endo-polygalacturonase from Kluyveromyces fragilis. Biotechnol Lett 9:411–416
Garcia-Garibay M, Torres J, López-Munguía-Canales A, Casas LT (1987b) Influence of oxygen transfer rate on b-galactosidase production from Kluyveromyces marxianus. Biotechnol Lett 9:417–420
Gasnier B (1987) Characterization of low- and high-affinity glucose transports in the yeast Kluyveromyces marxianus. Biochim Biophys Acta 903:425–433
Gellissen G, Hollenberg CP (1997) Application of yeasts in gene expression studies: A comparison of Saccharomyces cerevisiae, Hansenula polymorpha and Kluyveromyces lactis—a review. Gene 190:87–97
Ghaly AE, Singh RK (1989) Pollution potential reduction of cheese whey through yeast fermentation. Appl Biochem Biotechnol 22:181–203
Ghaly AE, Kamal M (2004) Submerged yeast fermentation of acid cheese whey for protein production and pollution potential reduction. Water Res 38:631–644
Giec A, Kosikowski FV (1992) Activity of lactose fermenting yeasts in producing biomass from concentrated whey permeates. J Food Sci 47:1892–1894
Gonçalves JA, Castillo FJ (1982) Partial Purification and characterization of b-D-galactosidase from Kluyverornyces rnarxianus. J Dairy Sci 65:2088–2094
Gough S, McHale AP (1998) Continuous ethanol production from molasses at 45°C using alginate-immobilized Kluyveromyces marxianus IMB3 in a continuous-flow bioreactor. Bioproc Eng 19:33–36
Gough S, Flynn O, Hack CJ, Marchant R (1996) Fermentation of molasses using a thermotolerant yeast, Kluyveromyces marxianus IMB3: simplex optimization of media supplements. Appl Microbiol Biotechnol 46:187–190
Gough S, Brady D, Nigam P, Marchant R, McHale AP (1997) Production of ethanol from molasses at 45°C using alginate-immobilized Kluyveromyces marxianus IMB3. Bioproc Eng 16:389–392
Gough S, Barron N, Zubov AL, Lozinsky VI, McHale AP (1998) Production of ethanol from molasses at 45°C using Kluyveromyces marxianus IMB3 immobilized in calcium alginate gels and poly(vinyl alcohol) cryogel. Bioproc Eng 19:87–90
Grootwassink JWD, Hewitt GM (1983) Inducible and constitutive formation of b-fructofuranosidase (inulase) in batch and continuous cultures of the yeast Kluyveromyces marxianus. J Gen Microbiol 129:31–41
Grubb CF, Mawson AJ (1993) Effects of elevated solute concentrations on the fermentation of lactose by Kluyveromyces marxianus Y-113. Biotechnol Lett 15:621–626
Goldstein AL, McCusker JH (1999) Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541–1553
Güldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH (1996) A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res 24:2519–2524
Hack CJ, Banat IM, Singh D, Marchant R (1994) Ethanol production by a strain of Kluyveromyces marxianus at elevated temperatures in various bioreactor configurations. In: Proceedings of Conference on Fermentation Physiology, Pub. Institution of Chemical Engineers, Brighton, pp 7–9
Hacking AJ, Taylor IWF, Hanas CM (1984) Selection of yeasts able to produce ethanol from glucose at 40°C. Appl Microbiol Biotechnol 19:361–363
Hahn-Hägerdal B (1985) Comparison between immobilized Kluyveromyces fragilis and Saccharomyces cerevisiae coimmobilized with b-galactosidase, with respect to continuous ethanol production from concentrated whey permeate. Biotechnol Bioeng 27:914–916
Harden TJ (1996) The reduction of BOD and production of biomass from acid whey by Kluyveromyces marxianus. Food Aust 48:456–457
Harsa S, Zaror CA, Pyle DL (1993) Production of polygalacturonases from Kluyveromyces marxianus fermentation—preliminary process design and economics. Proc Biochem 28:187–195
Hashida-Okado T, Ogawa A, Kato I, Takesako K (1998) Transformation system for prototrophic industrial yeasts using the AUR1 gene as a dominant selection marker. FEBS Lett 425:117–122
Hensing M, Vrouwenvelder H, Hellinga C, Baartmans R, van Dijken JP (1994) Production of extracellular inulinase in high-cell-density fed-batch cultures of Kluyveromyces marxianus. Appl Microbiol Biotechnol 42:516–521
Hensing MC, Rouwenhorst RJ, Heijnen JJ, van Dijken JP, Pronk JT (1995) Physiological and technological aspects of large-scale heterologous-protein production with yeasts. Antonie van Leeuwenhoek 67:261–279
Holloway P, Subden RE (1993) The isolation and nucleotide sequence of the pyruvate decarboxylase gene from Kluyveromyces marxianus. Curr Genet 24:274–277
Hong J, Wang Y, Kumagai H, Tamaki H (2007) Construction of thermotolerant yeast expressing thermostable cellulase genes. J Biotechnol 130:114–123
Hughes DB, Tudrosaen NJ, Moye CJ (1984) The effect of temperature on the kinectics of ethanol production by a thermotolerant strain of Kluyveromyces marxianus. Biotechnol Lett 6:1–6
Huo K, Li Y (1995) Cloning and expression of Kluyveromyces fragilis LAC4 gene. Sci China B 38:1332–1340
Iborra F (1993) High efficiency transformation of Kluyveromyces marxianus by a replicative plasmid. Curr Genet 24:181–183
Iborra F, Ball MM (1994) Kluyveromyces marxianus small DNA fragments contain both autonomous replicative and centromeric elements that also function in Kluyveromyces lactis. Yeast 10:1621–1629
Isenschmid A, Marison IW, von Stockar U (1995) The influence of pressure and temperature of compressed CO2 on the survival of yeast cells. J Biotechnol 39:229–237
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Itoh T, Susuki M, Adachi S (1982) Production of b-galactosidase from lactose-fermenting yeasts. Agric Biol Chem 46:899–904
Jia J, Wheals A (2000) Endopolygalacturonase genes and enzymes from Saccharomyces cerevisiae and Kluyveromyces marxianus. Curr Genet 38:264–270
Jolivet P, Bergeron E, Benyair H, Meunier JC (2001) Characterization of major protein phosphatases from selected species of Kluyveromyces. Comparison with protein phosphatases from Yarrowia lipolytica. Can J Microbiol 47:861–870
Jones TD, Havard JM, Daugulis AJ (1993) Ethanol production from lactose by extractive fermentation. Biotechnol Lett 15:871–876
Kádár ZS, Szengyel ZS, Réczey K (2004) Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Ind Crops Prod 20:103–110
Kataoka M, Kotaka A, Thiwthong R, Wada M, Nakamori S, Shimizu S (2004) Cloning and overexpression of the old yellow enzyme gene of Candida macedoniensis, and its application to the production of a chiral compound. J Biotechnol 114:1–9
Kiers J, Zeeman AM, Luttik M, Thiele C, Castrillo JI, Steensma HY, van Dijken JP, Pronk JT (1998) Regulation of alcoholic fermentation in batch and chemostat cultures of Kluyveromyces lactis CBS 2359. Yeast 14:459–469
Kim WH, Chung JH, Back JH, Choi J, Cha JH, Koh HY, Han YS (2003) Molecular cloning and characterization of an NADPH quinone oxidoreductase from Kluyveromyces marxianus. J Biochem Mol Biol 36:442–449
Kreger-van Rij NJW (1984) The yeasts: a taxonomic study, 3rd edn. Elsevier, Amsterdam
Künkel W, May R (1976) Alcohol dehydrogenase (ADH) in yeast cells. I. Cytoplasmic, mitochondrial and nuclear ADH in Saccharomyces carlsbergensis and Kluyveromyces fragilis. Z Allg Mikrobiol 16:529–536
Kurtzman CP (2003) Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia, Vanderwaltozyma and Zygotorulaspora. FEMS Yeast Res 4:233–245
Kurtzman CP, Fell JW (eds) (1998) The yeasts: a taxonomic study, 4th edn. Elsevier, Amsterdam
Kurtzman CP, Robnett CJ (2003) Phylogenetic relationships among yeasts of the ‘Saccharomyces complex’ determined from multigene sequence analyses. FEMS Yeast Res 3:417–432
Lachance MA (1998) Kluyveromyces van der Walt emend. van der Walt. In: Kurtzman CP, Fell JW (eds) The yeasts: a taxonomic study, 4th edn. Elsevier, Amsterdam, pp 227–247
Lachance MA (2007) Current status of Kluyveromyces systematics. FEMS Yeast Res 7:642–645
Ladrière JM, Delcour J, Vandenhaute J (1993) Sequence of a gene coding for a cytoplasmic alcohol dehydrogenase from Kluyveromyces marxianus ATCC 12424. Biochim Biophys Acta 1173:99–101
Ladrière JM, Georis I, Guerineau M, Vandenhaute J (2000) Kluyveromyces marxianus exhibits an ancestral Saccharomyces cerevisiae genome organization downstream of ADH2. Gene 255:83–91
Laloux O, Cassart JP, Delcour J, van Beeumen J, Vandenhaute J (1991) Cloning and sequencing of the inulinase gene of Kluyveromyces marxianus var. marxianus ATCC 12424. FEBS Lett 289:64–68
Leclerc M, Chemardin P, Arnaud A, Ratomahenina R, Galzy P, Gerbaud C, Raynal A, Guérineau M (1987) Comparison of the properties of the purified beta-glucosidase from the transformed strain of Saccharomyces cerevisiae TYKF2 with that of the donor strain Kluyveromyces fragilis Y610. Biotechnol Appl Biochem 9:410–422
Leclercq-Perlat MN, Corrieu G, Spinnler HE (2004) Comparison of volatile compounds produced in model cheese medium deacidified by Debaryomyces hansenii or Kluyveromyces marxianus. J Dairy Sci 87:1545–1550
Lim J, Yamasaki Y, Suzuki Y, Ozawa J (1980) Multiple forms of endo-polygalacturonase from Saccharomyces fragilis. Agric Biol Chem 44:473–480
Llorente B, Malpertuy A, Blandin G, Artiguenave F, Wincker P, Dujon B (2000) Genomic exploration of the hemiascomycetous yeasts: 12. Kluyveromyces marxianus var. marxianus. FEBS Lett 487:71–75
Lodder J (1970) The yeasts: a taxonomic study, 2nd edn. NHPC, Amsterdam
Lodder J, Kreger-van Rij NJW (1952) The yeasts: a taxonomic study. NHPC, Amsterdam
Love G, Nigam P, Barron N, Singh D, Marchant R, McHale AP (1996) Ethanol production at 45°C using preparations of Kluyveromyces marxianus IMB3 immobilized in calcium alginate and kissiris. Bioproc Eng 15:275–277
Love G, Gough S, Brady D, Barron N, Nigam P, Singh D, Marchant R, McHale AP (1998) Continuous ethanol fermentation at 45°C using Kluyveromyces marxianus IMB3. Immobilized in calcium alginate and kissiris. Bioproc Eng 18:187–189
Lukondeh T, Ashbolt NJ, Rogers PL (2003a) Evaluation of Kluyveromyces marxianus as a source of yeast autolysates. J Ind Microbiol Biotechnol 30:52–56
Lukondeh T, Ashbolt NJ, Rogers PL (2003b) Evaluation of Kluyveromyces marxianus FII 510700 grown on a lactose-based medium as a source of a natural bioemulsifier. J Ind Microbiol Biotechnol 30:715–720
Lukondeh T, Ashbolt NJ, Rogers PL (2003c) Confirmation of an alkali-insoluble glucans from Kluyveromyces marxianus cultivated on a lactose-based medium. World J Microbiol Biotechnol 19:349–355
Mahoney RR, Nickerson TA, Whitaker JR (1975) Selection of strain, growth conditions and extraction procedures for optimum production of lactase from Kluyveromyces fragilis. J Dairy Sci 58:1620–1629
Majumdar S, Bhattacharjee H, Bhattacharyya D, Bhaduri A (1998) UDP-galactose 4-epimerase from Kluyveromyces fragilis: reconstitution of holoenzyme structure after dissociation with parachloromercuribenzoate. Eur J Biochem 257:427–433
Margaritis A, Bajpai P (1982) Direct fermentation of D-xylose to ethanol by Kluyveromyces marxianus strains. Appl Environ Microbiol 44:1039–1041
Margaritis A, Bajpai P (1983) Effect of sugar concentration in Jerusalem artichoke extract on Kluyveromyces marxianus growth and ethanol production. Appl Environ Microbiol 45:723–725
Martins DB, de Souza CG Jr, Simões DA, de Morais MA Jr (2002) The b-galactosidase activity in Kluyveromyces marxianus CBS6556 decreases by high concentrations of galactose. Curr Microbiol 44:379–382
Marwaha SS, Kennedy JF, Sehgal VK (1988) Simulation of process conditions of continuous ethanol fermentation of whey permeate using alginate entrapped Kluyveromyces marxianus NCYC-179 cells in a packed-bed reactor system. Proc Biochem 23:17–22
Medeiros ABP, Pandey A, Freitas RJS, Christen P, Soccol CR (2000) Optimization of the production of aroma compounds by Kluyveromyces marxianus in solid-state fermentation using factorial design and response surface methodology. Biochem Eng J 6:33–39
Medeiros ABP, Pandey A, Christen P, Fontoura PSG, de Freitas RJS, Soccol CR (2001) Aroma compounds produced by Kluyveromyces marxianus in solid state fermentation on a packed bed column bioreactor. World J Microbiol Biotechnol 17:767–771
Meilhoc E, Masson JM, Teissié J (1990) High efficiency transformation of intact yeast cells by electric field pulses. J Biotechnol 8:223–227
Molnár O, Prillinger H, Lopandic K, Weigang F, Staudacher E (1996) Analysis of coenzyme Q systems, monosaccharide patterns of purified cell walls, and RAPD-PCR patterns in the genus Kluyveromyces. Antonie van Leeuwenhoek 70:67–78
Mukherji S, Bhaduri A (1992) An essential histidine residue for the activity of UDPglucose 4-epimerase from Kluyveromyces fragilis. J Biol Chem 267:11709–11713
Neves L, Oliveira R, Lucas C (2004) Yeast orthologues associated with glycerol transport and metabolism. FEMS Yeast Res 5:51–62
Nguyen TH, Fleet GH, Rogers PL (1998) Composition of the cell walls of several yeast species. Appl Microbiol Biotechnol 50:206–212
Nigam P, Banat IM, Singh D, McHale AP, Marchant R (1997) Continuous ethanol production by thermotolerant Kluyveromyces marxianus IMB3 yeast immobilized on mineral kissiris at 45°C. World J Microbiol Biotechnol 13:283–288
Nilsson U, Barron N, McHale L, McHale AP (1995) The effects of phosphoric and pretreatment on conversion of cellulose to ethanol at 45°C using the thermotolerant yeast Kluyveromyces marxianus IMB3. Biotechnol Lett 17:985–988
Nolan AM, Barron N, Brady D, McAree T, Smith D, McHale L, McHale AP (1994) Ethanol production at 45°C by an alginate-immobilized strain of Kluyveromyces marxianus following growth on glucose-containing media. Biotechnol Lett 16:849–852
Nunes MFA, Massaguer S, Alegre RM (1993) Produção e propriedades de b-galactosidase de Kluyveromyces marxianus NRRL Y-2415. Rev Farm Bioquim Univ S Paulo 29:25–30
Oberyé EH, Maurer K, Mager WH, Planta RJ (1993) Structure of the ABF1-homologue from Kluyveromyces marxianus. Biochim Biophys Acta 1173:233–236
O’Shea DG, Walsh PK (2000) The effect of culture conditions on the morphology of the dimorphic yeast Kluyveromyces marxianus var. marxianus NRRLy 2415: a study incorporating image analysis. Appl Microbiol Biotechnol 53:316–322
Panuwatsuk W, da Silva NA (2002) Evaluation of pKD1-based plasmid systems for heterologous protein production in Kluyveromyces lactis. Appl Microbiol Biotechnol 58:195–201
Parekh S, Margaritis A (1985) Inulinase (b-fructofuranosidase) production by Kluyveromyces marxianus in batch culture. Appl Microbiol Biotechnol 22:446–448
Pecota DC, da Silva NA (2005) Evaluation of the tetracycline promoter system for regulated gene expression in Kluyveromyces marxianus. Biotechnol Bioeng 92:117–123
Pecota DC, Rajgarhia V, da Silva NA (2007) Sequential gene integration for the engineering of Kluyveromyces marxianus. J Biotechnol 127:408–416
Penman CS, Duffus JH (1974) Ergosterol is the only sterol in Kluyveromyces fragilis. Antonie van Leeuwenhoek 40:529–531
Pessoa Jr A, Vitolo M (1999) Inulinase from Kluyveromyces marxianus: culture medium composition and enzyme extraction. Braz J Chem Eng 16:237–245
Pinheiro R, Belo I, Mota M (2000) Air pressure effects on biomass yield of two different Kluyveromyces strains. Enzyme Microb Technol 26:756–762
Pinheiro R, Belo I, Mota M (2002) Oxidative stress response of Kluyveromyces marxianus to hydrogen peroxide, paraquat and pressure. Appl Microbiol Biotechnol 58:842–847
Porro D, Sauer M, Branduardi P, Mattanovich D (2005) Recombinant protein production in yeasts. Mol Biotechnol 31:245–259
Postma E, van der Broek PJA (1990) Continuous-culture study of the regutation of glucose and fructose transport in Kluyveromyces marxianus CBS 6556. J Bacteriology 172:2871–2876
Prudêncio C, Sansonetty F, Sousa MJ, Côrte-Real M, Leão C (2000) Rapid detection of efflux pumps and their relation with drug resistance in yeast cells. Cytometry 39:26–35
Queirós O, Casal M, Althoff S, Moradas-Ferreira P, Leão C (1998) Isolation and characterization of Kluyveromyces marxianus mutants deficient in malate transport. Yeast 14:401–407
Rajoka MI, Khan S, Shahid R (2003) Kinetics and regulation studies of the production of b-galactosidase from Kluyveromyces marxianus grown on different substrates. Food Technol Biotechnol 41:315–320
Rajoka MI, Latif F, Khan S, Shahid R (2004) Kinetics of improved productivity of b-galactosidase by a cycloheximide-resistant mutant of Kluyveromyces marxianus. Biotechnol Lett 26:741–746
Ramirez-Zavala B, Mercado-Flores Y, Hernandez-Rodriguez C, Villa-Tanaca L (2004a) Purification and characterization of a lysine aminopeptidase from Kluyveromyces marxianus. FEMS Microbiol Lett 235:369–375
Ramirez-Zavala B, Mercado-Flores Y, Hernandez-Rodriguez C, Villa-Tanaca L (2004b) Purification and characterization of a serine carboxypeptidase from Kluyveromyces marxianus. Int J Food Microbiol 91:245–252
Ray S, Mukherji S, Bhaduri A (1995) Two tryptophans at the active site of UDP-glucose 4-epimerase from Kluyveromyces fragilis. J Biol Chem 270:11383–11390
Raynal A, Guerineau M (1984) Cloning and expression of the structural gene for b-glucosidase of Kluyveromyces fragilis in Escherichia coli and Saccharomyces cerevisiae. Mol Gen Genet 195:108–115
Raynal A, Gerbaud C, Francingues MC, Guerineau M (1987) Sequence and transcription of the b-glucosidase gene of Kluyveromyces fragilis cloned in Saccharomyces cerevisiae. Curr Genet 12:175–184
Rech R, Cassini CF, Secchi AR, Ayub MAZ (1999) Utilization of protein-hydrolyzed chesse whey for the production of b-galactosidase by Kluyveromyces marxianus. J Ind Microbiol Biotechnol 23:91–96
Ribeiro O, Gombert AK, Teixeira JA, Domingues L (2007) Application of the Cre-loxP system for multiple gene disruption in the yeast Kluyveromyces marxianus. J Biotechnol 131:20–26
Riordan C, Love G, Barron N, Nigam P, Marchant R, McHale L, McHale AP (1996) Production of ethanol from sucrose at 45°C by alginate-immobilized preparations of the thermotolerant yeast strain Kluyveromyces marxianus IMB3. Biores Technol 55:171–173
Romanos MA, Scorer CA, Clare JJ (1992) Foreign gene expression in yeast: a review. Yeast 8:423–488
Rosa FM, Sa-Correia I (1992) Ethanol tolerance and activity of plasma membrane ATPase in Kluyveromyces marxianus and Saccharomyces cerevisiae. Enzyme Microb Technol 14:23–27
Rouwenhorst RJ, Visser LE, van der Baan AA, Scheffers WA, van Dijken JP (1988) Production, distribution, and kinetic properties of inulinase in continuous culture of Kluyveromyces marxianus CBS 6556. Appl Environ Microbiol 54:1131–1137
Rouwenhorst RJ, Hensing M, Verbakel J, Scheffers WA, van Dijken JP (1990a) Structure and properties of the extracellular inulinase of Kluyveromyces marxianus CBS 6556. Appl Environ Microbiol 56:3337–3345
Rouwenhorst RJ, Ritmeester WS, Scheffers WA, van Dijken JP (1990b) Localization of inulinase and invertase in Kluyveromyces species. Appl Environ Microbiol 56:3329–3336
Sakanaka K, Yan W, Kishida M, Sakai T (1996) Breeding a fermentative yeast at high temperature using protoplast fusion. J Ferment Bioeng 81:104–108
Schaffrath R, Breunig KD (2000) Genetics and molecular physiology of the yeast Kluyveromyces lactis. Fung Genet Biol 30:173–190
Scharpf LG, Seitz EW, Morris JA, Farbood MI (1986) Generation of flavor and odor compounds through fermentation processes. In: Parliament TH, Croteau R (eds) Biogeneration of aroma, American Chemical Society, Washington, DC, vol. 317, pp 323–346
Schultz N, Chang L, Hauck A, Reuss M, Syldatk C (2006) Microbial production of single-cell protein from deproteinized whey concentrates. Appl Microbiol Biotechnol 69:515–520
Schwan RF, Rose AH (1994) Polygalacturonase production by Kluyveromyces marxianus: effect of medium composition. J Appl Bacteriol 76:62–67
Schwan RF, Cooper RM, Wheals AE (1997) Endopolygalacturonase secretion by Kluyveromyces marxianus and other cocoa pulp-degrading yeasts. Enzyme Microb Technol 21:234–244
Serrat M, Bermudez RC, Villa TG (2004) Polygalacturonase and ethanol production in Kluyveromyces marxianus—potential use of polygalacturonase in foodstuffs. Appl Biochem Biotechnol 117:49–64
Šiekštelė R, Bartkevičiūtė D, Sasnauskas K (1999) Cloning, targeted disruption and heterologous expression of the Kluyveromyces marxianus endopolygalacturonase gene (EPG1). Yeast 15:311–322
Skountzou P, Soupioni M, Bekatorou A, Kanellaki M, Koutinas AA, Marchant R, Banat IM (2003) Lead(II) uptake during baker’s yeast production by aerobic fermentation of molasses. Proc Biochem 38:1479–1482
Sonawat HM, Agrawal A, Dutta SM (1981) Production of b-galactosidase from Kluyveromyces fragilis grown on whey. Folia Microbiol 26:370–376
Souciet J-L, Artiguenave MAF, Blandin G, Bolotin-Fukuhara M, Bon E, Brottier P et al (2000) Genomic exploration of the hemiascomycetous yeasts: 1. A set of yeast species for molecular evolution studies. FEBS Letters 487:3–12
Stambuk BU, Franden MA, Singh A, Zhang M (2003) D-Xylose transport by Candida succiphila and Kluyveromyces marxianus. Appl Biochem Biotechnol 108:255–263
Steensma HY, Ter Linde JJ (2001) Plasmids with the Cre-recombinase and the dominant nat marker, suitable for use in prototrophic strains of Saccharomyces cerevisiae and Kluyveromyces lactis. Yeast 18:469–472
Steensma HY, de Jongh FCM, Linnekamp M (1988) The use of electrophoretic karyotypes in the classification of yeasts: Kluyveromyces marxianus and K. lactis. Curr Genet 14:311–317
Stephanopoulos GN, Aristidou AA, Nielsen J (1998) Metabolic Engineering Principles and Methodologies. Academic Press, San Diego
Sukroongreung S, Schappert KT, Khachatourians GG (1984) Survey of sensitivity of twelve yeast genera toward T-2 toxin. Appl Environ Microbiol 48:416–419
Tin CSF, Mawson AJ (1993) Ethanol production from whey in a membrane recycle bioreactor. Proc Biochem 28:217–221
Ternan NG, McMullan G (2000) The utilization of 4-aminobutylphosphonate as sole nitrogen source by a strain of Kluuyveromyces fragilis. FEMS Microbiol Lett 184:237–240
Ternan NG, McMullan G (2002) Iminodiacetate and nitrilotriacetate degradation by Kluyveromyces marxianus IMB3. Biochem Biophys Res Comm 290:802–805
Toyoda Y, Sy J (1984) Purification and phosphorylation of fructose-1,6-bisphosphatase from Kluyveromyces fragilis. J Biol Chem 259:8718–8723
van den Berg JA, van der Laken KJ, van Ooyen AJJ, Renniers TCHM, Rietveld K et al (1990) Kluyveromyces as a host for heterologous gene expression. Expression and secretion of prochymosin. Bio/Techno 8:135–139
van den Broek PJ, de Bruijne AW, van Steveninck J (1987) The role of ATP in the control of H+-galactoside symport in the yeast Kluyveromyces marxianus. Biochem J 242:729–734
van der Walt JP (1956) Kluyveromyces- a new yeast genus of the Endomycetales. Antonie van Leeuwenhoek 22:265–272
van der Walt JP (1970) Kluyveromyces van der Walt emend. van der Walt. In: Lodder J (ed) The yeasts: a taxonomic study, 2nd edn. NHPC, Amsterdam, pp 316–378
van der Walt JP, Johannsen E (1984) Kluyveromyces van der Walt emend. van der Walt. In: Kreger-van Rij NJW (ed) The yeasts: a taxonomic study, 3rd edn. Elsevier, Amsterdam, pp 224–251
van Dijken JP, Weusthuis RA, Pronk JT (1993) Kinetics of growth and sugar consumption in yeasts. Antonie van Leeuwenhoek 63:343–352
van Dijken JP, Bauer J, Brambilla L et al (2000) An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enz Microb Technol 26:706–714
van Leeuwen CC, Postma E, van den Broek PJ, van Steveninck J (1991) Proton-motive force-driven D-galactose transport in plasma membrane vesicles from the yeast Kluyveromyces marxianus. J Biol Chem 266:12146–12151
van Ooyen AJ, Dekker P, Huang M, Olsthoorn MM, Jacobs DI, Colussi PA, Taron CH (2006) Heterologous protein production in the yeast Kluyveromyces lactis. FEMS Yeast Res 6:381–392
van Urk H, Voll WSL, Scheffers WA, van Dijken JP (1990) Transient-state analyses of metabolic fluxes in Crabtree-positive and Crabtree-negative yeasts. Appl Environ Microbiol 56:281–287
Verduyn C, Postma E, Scheffers WA, van Dijken JP (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501–517
Verzotti E, Geymonat M, Valetti F, Lanzetti L, Giunta C (1994) In the budding yeast Kluyveromyces marxianus, adenylate cyclase is regulated by Ras protein(s) in vitro. Yeast 10:993–1001
Viegas CA, Rosa MF, Sá-Correia I, Novais JM (1989) Inhibition of yeast growth by octanoic and decanoic acids produced during ethanolic fermentation. Appl Environ Microbiol 55:21–28
Visser W, Scheffers WA, Batenburg-van der Vegte WH, van Dijken JP (1990) Oxygen requirements of yeasts. Appl Environ Microbiol 56:3785–3792
Yoda K, Ko JH, Nagamatsu T, Lin Y, Kaibara C, Kawada T, Tomishige N, Hashimoto H, Noda Y, Yamasaki M (2000) Molecular characterization of a novel yeast cell-wall acid phosphatase cloned from Kluyveromyces marxianus. Biosci Biotechnol Biochem 64:142–148
Ward C, Nolan AM, O’Hanlon F, McAree T, Barron N, McHale L, McHale AP (1995) Production of ethanol at 45°C on starch-containing media by mixed cultures of the thermotolerant, ethanol-producing yeast Kluyveromyces marxianus IMB3 and the thermophilic filamentous fungus Talaromyces emersonii CBS 813.70. Appl Microbiol Biotechnol 43:408–411
Wésolowski-Louvel M, Breunig KD, Fukuhara H (1996) Kluyveromyces lactis: genetics, biochemistry and molecular biology of non-conventional yeast. Springer-Verlag, Berlin Heidelberg New York
Welsh FW, Murray WD, Williams RE (1989) Microbiological and enzymatic production of flavor and fragrance chemicals. Crit Rev Biotechnol 9:105–169
Wimborne MP, Rickard PAD (1978) Pectinolytic activity of Saccharomyces fragilis cultured in controlled environments. Biotechnol Bioeng 20:231–242
Wittmann C, Hans M, Bluemke W (2002) Metabolic physiology of aroma-producing Kluyveromyces marxianus. Yeast 19:1351–1363
Wolf K, Breunig K, Barth G (eds) (2003) Non-conventional yeasts in genetics, biochemistry and biotechnology: practical protocols. Springer-Verlag, Berlin Heidelberg New York
Workman WE, Day DF (1984) The cell wall-associated inulinase of Kluyveromyces fragilis. Antonie van Leeuwenhoek 50:349–353
Yoshida Y, Yokoi W, Wada Y, Ohishi K, Ito M, Sawada H (2004) Potent hypocholesterolemic activity of the yeast Kluyveromyces marxianus YIT 8292 in rats fed a high cholesterol diet. Biosci Biotechnol Biochem 68:1185–1192
Yoshida Y, Yokoi W, Ohishi K, Ito M, Naito E, Sawada H (2005) Effects of the cell wall of Kluyveromyces marxianus YIT 8292 on the plasma cholesterol and fecal sterol excretion in rats fed on a high-cholesterol diet. Biosci Biotechnol Biochem 69:714–723
Zhang J, Yuan H, Wen T, Xu F, Di Y, Huo K, Li YY (2003) Cloning of the KcURA3 gene and development of a transformation system for Kluyveromyces cicerisporus. Appl Microbiol Biotechnol 62:387–391
Acknowledgements
Grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Brazil), Deutscher Akademischer Austausch Dienst (DAAD) (Germany), and Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Brazil) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fonseca, G.G., Heinzle, E., Wittmann, C. et al. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl Microbiol Biotechnol 79, 339–354 (2008). https://doi.org/10.1007/s00253-008-1458-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1458-6