Abstract
Carotenoids are a diverse group of isoprenoid pigments that play crucial roles in plants, animals, and microorganisms, including body pigmentation, bio-communication, precursors for vitamin A, and potent antioxidant activities. With their potent antioxidant activities, carotenoids are emerging as molecules of vital importance in protecting against chronic degenerative disease, such as aging, cancer, cataract, cardiovascular, and neurodegenerative diseases. Due to countless functions in the cellular system, carotenoids are extensively used in dietary supplements, food colorants, aquaculture and poultry feed, nutraceuticals, and cosmetics. Moreover, the emerging demand for carotenoids in these vast areas has triggered their industrial-scale production. Currently, 80%–90% of carotenoids are produced synthetically by chemical synthesis. However, the demand for naturally produced carotenoids is increasing due to the health concern of synthetic counterparts. This article presents a review of the industrial production of carotenoids utilizing a number of diverse microbes, including microalgae, bacteria, and fungi, some of which have been genetically engineered to improve production titers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids, a subfamily of a most diverse class of isoprenoids (also called “terpenoids”) pigments that are primarily synthesized by all photosynthetic organisms, including plants, microalgae (predominantly by the genus of Chlorella, Scenedesmus, Botryococcus, Dunaliella, and Haematococcus), some non-photosynthetic bacteria (e.g., Agrobacterium aurantiacum and Halobacterium salinarium), and fungi (e.g., Blakeslea trispora, Mucor circinelloides, and Xanthophyllomyces dendrorhous). Also, in recent years, in several species of aphids and adelgids, spider mites, and gall midges de novo carotenoid biosynthesis have been revealed [9, 34]. Most carotenoids are derivatives of tetraterpenoid (C40) pigment phytoene, biosynthesized by the condensation of two geranylgeranyl diphosphate (C20; GGDP) molecules, catalyzed by a key enzyme phytoene synthase (PSY or CrtB). The remaining carotenoids are derived from a phytoene by undergoing various enzymatic reactions of desaturation, isomerization, cyclization, hydroxylation, epoxidation, and ketolation.
In photosynthetic organisms, carotenoids and chlorophylls (bacteriochlorophyll in bacteria) are ubiquitous and functional components of the photosynthetic organelles and play an important role in light harvesting and photoprotection by dissipation of excess energy. In non-photosynthetic bacterium (e.g., phylum Deinococcus–Thermus) and fungi, carotenoids play a key role as potent photoprotective and stress-protecting agents [3]. Similarly, carotenoids are essential for the normal health and behavior of animals. Carotenoids play a vital role in the cellular machinery, from antioxidant to gene regulation and apoptosis. Among the functions of carotenoids in animals, provitamin A, antioxidant, and anticancer activities are the most significant. Also, owing to these biological activities, carotenoids are widely used in food, feed, nutraceuticals, and cosmetics. The emerging demand of carotenoids in these vast areas has triggered their industrial-scale production. This article presents a review of the progress made thus far in carotenoids production from microbial consortia, including microalgae, bacteria, and fungi. Many reviews previously discussed the different aspects of microbial carotenoids production and their applications [8, 20, 33, 50, 68]. Borowitzka et al. [8] summarized the potential sources and existing high-value products from microalgae used for feed (aquaculture pigmentation), food (nutritional supplements), and cosmetics. Gong and Bassi [20] reviewed the current technologies involved in microalgal carotenoids production, including downstream processing. Mata-Gómez et al. [33] reviewed various factors influencing the production of carotenoid in yeast, focusing on carbon source, light, temperature, aeration, and metal ions and salts (Ba, Ca, Fe, Mg, Zn, and Co). Schmidt et al. [50] reviewed the potential of Phaffia rhodozyma yeast for the biotechnological production of astaxanthin in a cost-effective way in comparison with other natural sources, including Haematococcus pluvialis, fungi, and transgenic plants. Ye and Bhatia [68] reviewed the metabolic pathway engineering approach for optimization of carbon flux and carotenogenesis for production of lycopene, zeaxanthin, and astaxanthin in non-carotenogenic yeast and bacterial cells. In addition, Rodríguez-Sáiz et al. [42] reviewed the low-cost raw materials and fermentation parameters for improved productivity of astaxanthin with X. dendrorhous.
Considering the previous reviews which are focused on various aspects of microbial carotenoids production and applications, the current review attempts to update and summarize all the critical elements of carotenoids production from algae, bacteria, and fungi. In addition to the carotenoids production using homologous and heterologous systems, their biosynthesis and chemistry, their impotence in photosynthetic and non-photosynthetic microbes, applications and marketing potential, and cost of production are discussed for a better understanding of the industrial significance of microbial platforms for carotenoids production.
Chemistry and biosynthesis of carotenoids
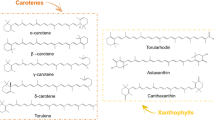
To date, nearly 721 carotenoids have been identified [36]. Based on their structure, these carotenoids can be divided into two major groups: (1) hydrocarbon carotenoids are referred to as carotenes (such as phytoene, lycopene, α-carotene, and β-carotene) and (2) oxygenated derivatives of hydrocarbon carotenoids are known as xanthophylls (e.g., lutein, canthaxanthin, and astaxanthin). According to their functions, carotenoids can be either primary or secondary. Primary carotenoids, including violaxanthin, antheraxanthin, and zeaxanthin (collectively called VAZ), lutein, neoxanthin, and β-carotene, are associated with structural and functional components in the photosynthetic apparatus and primarily function as light-harvesting pigments. The secondary carotenoids such as astaxanthin, canthaxanthin are produced at high levels (dispensed in oily droplets) under abiotic stress conditions and play a significant role in cell protection. Carotenoids can be further classified into provitamin A carotenoids (e.g., α-carotene and β-carotene) and non-provitamin A carotenoids, the latter of which cannot be converted to retinal (e.g., lycopene, lutein, and astaxanthin) because they lack the non-substituted β-ionone ring structure. Apart from these classes, apocarotenoids and C50 carotenoids (e.g., decaprenoxanthin) are other categories of carotenoids. Apocarotenoids are enzymatic products of carotenoids by carotenoid cleavage dioxygenases (CCDs) via singlet oxygen 1O2 attack. Biologically and commercially important apocarotenoids include visual pigment vitamin A, retinoids, and retinoic acid, plant hormone abscisic acid (ABA) (and strigolactone, crocin, crocetin, and picrocrocin, the red pigment of the stigmata of Crocus sativus), annatto pigment bixin (from seeds of Bixa orellana), β-apo-8-carotenal (food colorant E160, found in spinach and citrus fruits), and aromatic volatile fragrance compounds α-ionone and β-ionone [5, 48]. Microbes such as Corynebacterium glutamicum are unique in producing the C50 carotenoid decaprenoxanthin and its glycosylated forms [45].
Universally, in all the carotenoids producing organisms, carotenoids are biosynthesized using the isoprenoid precursor’s isopentenyl pyrophosphate (IPP; C5) and its allylic isomer dimethylallyl pyrophosphate (DMAPP). The IPP and DMAPP are derived either from the 2-C-methyl-d-erythritol 4-phosphate (MEP) [also known as 1-deoxyxylulose-5-phosphate (DXP)] pathway (as the first intermediate) or by mevalonate (MVA) pathways. The most photosynthetic organisms, including microalgae and plants, predominantly utilize the MVA pathway, whereas, bacteria and fungi derive IPP and DMAPP primarily via the MEP pathway. The major steps of MVA and the MEP pathways are outlined in Fig. 1.
The initial steps of the MEP pathway are catalyzed by DXP synthase (DXS) in plants and Dxs in microbes. These are the key enzymes that provide sufficient flux of carotenoid precursors for enhanced biosynthesis. Similarly, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase from an MVA pathway is crucial for enhancing the biosynthesis of isoprenoid precursors. Thus, these enzymes are largely targeted for enhanced biosynthesis of carotenoids. IPP and its isomer DMAPP are condensed to generate geranyl pyrophosphate (GPP; C10) and elongated to farnesyl pyrophosphate (FPP; C15) and geranylgeranyl diphosphate (GGPP; C20) (Fig. 1). In the further downstream pathway, the first C40-carotenoid 15-(Z)-phytoene is synthesized by PSY (syn. CrtB). A sequence of desaturation by phytoene desaturase (PDS, CrtI) and isomerization of 15-(Z)-phytoene produce the first physiologically important carotenoids, lycopene.
Lycopene (ψ,ψ-carotene) serves as a common intermediate for the biosynthesis of almost all the other C40 carotenoids. Cyclization of the ends of the lycopene chain (linear ψ end group) by lycopene β- (LCY-β) or lycopene ε-cyclase (LCY-ε) produces carotenoids with β- or ε-rings, respectively [43]. A genetically and physiologically controlled molecular synergism between β- and ε-cyclases plays a substantial regulatory role in modulating the flux towards the production of β-carotene on one side and α-carotene on the other side (Fig. 2) [11]. α-carotene contains one ε-ring and one β-ring (β,ε-carotenoids); thus, the conversion of lycopene to α-carotene requires both LCY-ε (syn. CrtYE or CrtL-e) and LCY-ß (syn. CrtY, CrtYB or CrtZ). On the other hand, in the case of β-carotene (β,β-Carotenoids), cyclization of both β-rings is catalyzed by LCY-ß. In the further downstream pathways, the enzymatic activity of various ketolase and hydrolase induces diverse carotenoids, including lutein, zeaxanthin, and astaxanthin (Fig. 2). It is interesting to note that carotenoids with two ε-rings are rarely found in nature, wherein a single LCY-ε adds two ε-rings to lycopene to form ε-carotene (ε,ε-carotene). Lactucaxanthin (ε,ε-carotene -3,3′-diol) and its stereoisomer tunaxanthin are the most studied examples of carotenoids with two ε-rings, found in lettuce and fish Tilapia nilotica, respectively [47]. It is also interesting to note that β-carotene possesses the highest provitamin A activity of 100% due to the presence of two unmodified β-ionone rings, whereas α-carotene possesses a provitamin A activity of 53% (one unmodified β-ring), and lycopene lacks provitamin A activity because of the absence of a β-ionone ring. Similarly, the modification of the β-ionone ring (e.g., hydroxylation in the case of zeaxanthin and keto-hydroxylation in the case of astaxanthin) causes the complete loss of provitamin A activity.
The carotenoids biosynthetic enzymes among plants, algae, bacteria, and fungi (Crt-type) share significant sequence homology. However, their functions show considerable diversity. For example, in fungi, the enzymatic activities of PSY and LCY-β are encoded by one fusion gene, CrtYB. Similarly, the single desaturase CrtI gene from red yeast Sporidiobolus pararoseus can effectively catalyze four- and five-step desaturation and isomerization steps to from lycopene from phytoene. The carotenoids biosynthesis enzymes from plants, algae, bacteria, and fungi were reviewed in [15, 30]. The major enzymes from plants and microbes are illustrated in Figs. 1 and 2.
Significance of carotenoids in photosynthetic and non-photosynthetic microbes
The unique structure of carotenoids imparts several biological functions in plants, animals, and microbes, including photosynthesis, photoprotection, coloration, and cell signaling. The characteristic yellow, red or orange color of carotenoids is attributed to the presence of a polyene chain (–C=C–)n with 8–13 conjugated carbon–carbon double bonds that function as a chromophore [48]. Moreover, the high reducing potential of the polyene chain provides the effective neutralization of reactive oxygen species (ROS), including lipid peroxyl radical (ROO−) by delivering a resonance-stabilized carbon-centered peroxyl radical (e.g., ROO-lycopene) [19].
In photosynthetic organisms, chlorophylls and carotenoids, especially VAZ pigments and β-carotene, form the indispensable components of both the photosystem I (PSI) and PSII reaction centers of oxygenic photosynthetic apparatus, and function as potent antioxidants and light-collecting pigments [46]. Similarly, in non-photosynthetic bacteria (e.g., phylum Deinococcus–Thermus) and fungi, carotenoids play the key role of potent photoprotective and stress‑protecting agents by scavenging the ROS, while serving as a precursor for the biosynthesis of other biologically active metabolites, such as retinal, trisporoids (in Mucorales) and sporopollenins [3]. Likewise, carotenoids are involved in the cellular resistance mechanisms of Deinococcus–Thermus bacteria to environmental stresses. These are extremophilic bacteria known for their absolute resistance to extreme environmental conditions of high temperature, radiation, oxidation, and desiccation. Recent investigations suggested that the unique structure of carotenoids deinoxanthin and thermozeaxanthin found in Deinococcus radiodurans and Thermus thermophiles, respectively, provides resistance to these extreme stresses [59]. With several other mechanisms of ROS scavenging, DNA protection, and functional interactions with membrane proteins, the hydrophobic–hydrophilic–hydrophobic structure of thermozeaxanthin consisting of zeaxanthin, glucose, and branched fatty acids provides unique stability to the lipid bilayer for growth at high temperatures [59].
The cleavage product of carotenoids, known as apocarotenoids, such as ABA, strigolactone, and dihydroactinidiolide, also plays vital physiological roles in the flavor, fragrances, signaling, and many aspects of growth and development of plants and microbes [11].
Economic importance and marketing potential of carotenoids
Owing to their potent antioxidant, Vitamin A, anti-inflammatory, and cell signaling activities, remarkable progress has been made in the last decade in the production carotenoids to meet the exceptionally high demands for dietary supplements, food colorants, pharmaceuticals, animal feed, and cosmetics. In 2017, the global market value for carotenoids was estimated at nearly USD 1.5 billion, with an estimated growth to $2.0 billion in 2022, and with a compound annual growth rate of (CAGR) of 5.7% for the period of 2017–2022 [4]. The capsanthin, astaxanthin, β-carotene, lutein, annatto, lycopene, and canthaxanthin together share nearly 90% of the total market value (Fig. 3). Animal feed (poultry and aquatic animals) is the most prominent sector, accounting for 41% of the total revenue share, followed by food and dietary supplements. Capsanthin alone contributed USD300 million in 2017 (20% of the total market) and was projected to reach USD385 million by 2022 at a CAGR of 5.1%. Similarly, the contribution by the astaxanthin market was recorded as USD288.7 million and is estimated to reach USD426.9 million by 2022 at a CAGR of 8.1% [4].
Health benefits of carotenoids
ROS, including singlet oxygen (1Δg), hydroxyl radical (•OH), and superoxide (•O2−), play a crucial role in the development of chronic degenerative diseases such as aging, cancer, cataract and cardiovascular (CVD), and neurodegenerative diseases. The consumption of a diet rich in carotenoids provides significant protection against the development of the disease, because of the potent antioxidant properties of carotenoids. With varying levels, all carotenoids function as inhibitors of ROS-induced lipid peroxidation, lipopolysaccharide (LPS)-induced superoxide production, peroxide-induced cytotoxicity, low-density lipoprotein (LDL) oxidation, and plasma levels of 12-hydroxy and 15-hydroxy fatty acids [24, 41, 61], which probably facilitate minimization of the pathogenesis of atherosclerosis. Apart from the potent antioxidant activities, the anti-inflammatory and immunomodulatory activities of carotenoids have received significant attention for their active role in preventing oxidative stress, cancer, and CVD [25]. The significance of carotenoids in human health is illustrated in Fig. 3.
Among the carotenoids, xanthophyll carotenoids bearing terminal carbonyl groups (–C=O) conjugated to the polyene backbone (e.g., ketocarotenoids astaxanthin and canthaxanthin) work as superior ROS scavengers and antioxidants, without pro-oxidative effects [23]. The presence of carbonyl groups in conjugation with their polyene backbone helps to stabilize the carbon-centered radicals more efficiently than the polyene backbone alone. Additionally, the α-hydroxy (–OH) and ketone (–C=O) groups on each ring of the astaxanthin molecule accomplish a higher hydrophilic nature than other carotenoids. Moreover, the anchoring of polar rings of astaxanthin on both sides of the cell membrane effectively protects the lipids from peroxidation [1].
Numerous epidemiologic and interventional studies have demonstrated that an adequate intake of carotenoids protects from chronic degenerative disease. Lutein has been shown to attenuate the neuroinflammation in LPS-activated BV-2 microglia through inhibiting p38 kinase, c-Jun N-terminal kinase (JNK), and Akt kinase stimulated nuclear transcription factor NF-κB activation. Lutein has also been shown to promote extracellular signal-regulated kinase 1/2 (ERK-1/2)-induced NF-E2-related factor 2 (Nrf2) activation, suggesting that the potent role of lutein plays in controlling inflammation-related neurodegenerative disorders [66]. Similarly, lycopene can potentially inhibit tumor metastasis by slowing down cell-cycle progression, mediated by promoting the E-cadherin and β-catenin expression, and by decreasing the matrix metalloproteinase, proliferating cell nuclear antigen (PCNA), and insulin-like growth factor-1 [62]. Lycopene has shown to potentially inhibit the proliferation of diverse cancer cell lines originating from the human colon, liver, breast, prostate, and lymphocyte [62]. Using a placebo-controlled study, the consumption of lycopene (15 mg of lycopene/day for 8 weeks) was found beneficial for improving endothelial function and minimizing oxidative stress, by increased LDL particle size, preventing early atherosclerosis, and decreasing blood pressure and lipid peroxidation [25].
Lycopene exhibits potent hypolipidemic activities through molecular mechanisms that are either the same as statins by direct inhibition of HMG-CoA reductase, a rate-limiting enzyme in the cholesterol biosynthetic pathway, or distinct from that of statins via cholesterol homeostasis, mediated through down-regulation of proprotein convertase subtilisin/kexin type 9 mRNA synthesis. Additionally, lycopene-ameliorated inflammation stimulated the expression of PCSK-9 via suppressing the expression of inflammatory markers [53]. Similarly, lycopene consumption has other health benefits, including prevention of leukocyte and prostate tissue DNA damage, increased gap junctional intercellular communication, improved bone strength by reducing bone resorption, and protection from type 2 diabetes by enhancing the glucose homeostasis [58, 62].
In young healthy females, dietary astaxanthin is also found to favorably modulate the immune response and biomarkers of oxidative DNA damage by enhancing the mitogen-induced lymphoproliferation, natural killer cell cytotoxic activity, and by decreasing plasma concentrations of plasma C-reactive protein [38].
Lutein and its isomer, zeaxanthin, accumulate in the macula and suppress both the ROS and reactive nitrogen species (RNS)-mediated oxidative stress in the eye tissues, thus reducing the risk of cataracts, age-related macular degeneration (AMD), and other eye diseases [32]. In addition, treatment with astaxanthin (topical or oral administration) can effectively prevent UVA-associated photoaging, such as wrinkling or skin sagging by downregulating matrix metalloproteinase-1 (MMP-1), which is responsible for wrinkling and sagging by degradation of dermal components, elastin, and collagen [52]. Thus, because of its potent antioxidant and anti-aging functions, astaxanthin is widely used in cosmetics.
Capsanthin and β-carotene suppress the formation of ROS in H2O2-treated rat liver epithelial cells and minimize the detrimental effects of the H2O2-mediated inhibition of gap junction intercellular communication, thus lowering the risk of the harmful effects of oxidative stress [24].
In our recent study, among the four xanthophyll carotenoids, 9-(Z) neoxanthin was found to be most effective for the reduction of cell viability of lung (A549) and cervical (HeLa) cancer cells, with IC50 values of 7.5 and 3.8 μM, respectively. Therefore, the regular intake of 9-(Z) neoxanthin might play a beneficial role in minimizing cervical and lung cancer risks [47].
In contrast to the potent antioxidant activities, some carotenoids are reported to possess prooxidant effects under specific physiological conditions. For example, in a randomized, double-blinded, placebo-controlled α-tocopherol, β-carotene cancer prevention (ATBC) study among 29,133 Finnish male smokers (average intensity of 18 cigarettes daily) aged 50–69 years, 50 mg daily α-tocopherol for 6.1 years decreased the risk of prostate cancer, whereas 20 mg β-carotene increased the risk of lung cancer with an overall 8% higher mortality [60]. While the precise mechanism of the prooxidant effects of β-carotene is still unknown, a prevailing hypothesis suggests that the oxidation products of β-carotene that result from components of tobacco smoke in the presence of the relatively high partial pressure of oxygen in the lung are mainly responsible. Consistent evidence demonstrates that β-carotene supplementation can increase lung cancer risk and overall mortality in tobacco smokers. Thus, β-carotene supplementation should be avoided by smokers.
Carotenoid-based commercial products
Considering the potent health beneficial properties, several carotenoid-based commercial products as supplements are available on the market for (1) provitamin A activity; (2) potent antioxidant and lipid peroxidation inhibiting activity; (3) bone health, skin health (anti-aging), physical stamina (sports nutrition), vision, and immune system enhancing; (4) cancer, cardiovascular, neuronal, and gastrointestinal protection, and v) animal nutrition (particularly for poultry and fish) [1, 5, 8]. The major carotenoids-based commercial products available on the market are outlined in Fig. 4.
In recent years, striking red–orange coloration of ornamental aquaculture animals, such as freshwater crustaceans and fishes, has become a popular marketing factor and growing trend [2]. Carotenoids, mainly astaxanthin and canthaxanthin, are supplied as dietary supplements to enhance skin coloration. In addition to the coloration, carotenoids potentially improve the health and reproduction of ornamental animals, including enhanced embryonic and larval development, maturation, boosted immune response, and photoprotection [2, 70]. Because of the extensive application, the aquaculture feed is the most prominent sector that accounts for the significant revenue generated by astaxanthin and other carotenoids.
The carotenoid market is rapidly growing as a result of numerous applications currently being made. Thus, in the future, research on natural carotenoid sources will most likely expand into other dimensions. A large number of natural and synthetic carotenoids based products, mainly animal feed and dietary supplements, have been commercialized. However, extensive research is needed to explore the beneficial health properties at the cellular and molecular levels.
Microbial production of commercially vital carotenoids
Using microalgal platforms, several commercially vital carotenoids are currently under industrial production, whereas the production of carotenoids exploiting metabolic pathway engineering of bacteria and fungi is mostly under developmental phases. Commercial production using high carotenoids accumulating microalgae such as Dunaliella salina (β-carotene; using open pond technology), H. pluvialis (Astaxanthin) and Chlorella zofingiensis (Canthaxanthin), Scenedesmus spp. (Lutein), Botryococcus braunii (Echinenone), Phaeodactylum tricornutum (Fucoxanthin) has been established [4]. Similarly, fungus B. trispora (β-carotene, lycopene), M. circinelloides (β-carotene), yeast X. dendrorhous (syn. Phaffia rhodozyma; astaxanthin), bacterium Gordonia jacobea (canthaxanthin), and C. glutamicum (C50 carotenoid Decaprenoxanthin) are currently exploited for the large-scale production of carotenoids [14]. Also, fungus such as Fusarium sporotrichioides, Neurospora crassa, bacterium Paracoccus carotinifaciens, A. aurantiacum, and Halobacterium salinarium are emerging as new promising sources of carotenoids. In addition to the natural carotenoids producing microbes, several non-carotenogenic microbes, such as Blakeslea trispora, Saccharomyces cerevisiae, X. dendrorhous, and Escherichia coli, have been genetically engineered to produce commercially vital carotenoids, including astaxanthin, β-carotene, lycopene, and canthaxanthin.
Advantages of microbial platforms for carotenoids production
Presently, nearly 80–90% of the carotenoids market is fulfilled by synthetic sources, manufactured by chemical synthesis. However, in recent years, the demand for naturally produced carotenoids, especially using carotenoid plants and microbes, has been increasing due to the health concern of synthetic carotenoids. Natural carotenoids are mainly extracted from plant sources. For example, lutein is extracted from the flowers of Tagetes erecta, lycopene from tomato fruit, β-carotene from carrot and the fruit of palm oil, capsanthin from red pepper, and bixin from annatto (B. orellana) seeds [49]. However, carotenoids production using microbes, primarily using microalgae D. salina and H. pluvialis, has been initiated for the large-scale production of β-carotene and astaxanthin, respectively [8]. The carotenoids production of microbial platforms has several advantages over plants. Fernández-Sevilla et al. [16] suggested that with evidence from less land, water, nutrients (nitrogen, phosphorus, and potassium) and labor requirement, significantly 5–10 times higher growth rate, and year-round harvesting, microalga can be economically used for the commercial production of lutein, superior to the conventionally used natural source marigold flower petals, the harvesting of which needs comparatively more land and labor, and is seasonal. Additionally, the lutein concentration of dehydrated marigold petals (0.02–2.8 g/100 g) is found to be equivalent to microalgae biomass (0.24–0.74 g/kg) [31]. Moreover, operating factors (e.g., stress-driven adaptive process) affecting the contents of lutein and other carotenoids in microbes can be further optimized for enhanced production. Also, the cost of the microbial carotenoids production can be significantly reduced by utilizing the agro-industrial wastes as low-cost substrates [17].
Harnessing the potential of carotenoids producing microbes
Microalgae species belonging to genus Dunaliella, Haematococcus, and Scenedesmus are predominantly studied to produce carotenoids and other valuable metabolites. Fernández-Sevilla [16] reviewed the biotechnological production of lutein from microalgae belonging to genus Muriellopsis, Scenedesmus, Chlorella, Chlorococcum, and Neospongiococcus and suggested that these microalgae can be used for the commercial production of lutein economically, and are superior to the conventionally used natural source marigold flower petals.
The substrate composition and concentration, the cultivation and stress parameters, and the addition of metabolic precursors and inhibitors need to be precisely optimized for the efficient and economical production of desired carotenoids. Major studies of the optimization of carotenoids production utilizing microbial platforms are highlighted in Table 1. Using a one-step 50 L outdoor tubular photobioreactor, the dilution rate (0.7 L/day), nitrate concentration (0.5 mmol/g day) in the feed medium, and irradiance (2500 mE/m2 s) for the highest continuous production of biomass (0.7 g/L day) and astaxanthin (8.0 mg/L day, 1.3% DCW) from microalga H. pluvialis were optimized [18]. Nitrate limitation was identified as the key factor triggering the accumulation of astaxanthin in a photobioreactor. Also, astaxanthin content and productivity were linearly proportional to the average irradiance on the reactor surface (1000–2500 mE/m2 s) and irradiance inside the culture (200–800 mE/m2 s). In another study, the high-titer production of astaxanthin (350 mg/L or 4.1 mg/g DCW) by the semi-industrial illuminated fermentation processes (800 L scale) of X. dendrorhous was achieved using white light, and by ultraviolet light [13]. Saenge et al. [44] optimized the carbon/nitrogen (C/N) ratio of 85, glycerol concentration of 9.5%, and surfactants (Tween 20 at 0.15%) for efficient bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids utilizing yeast Rhodotorula glutinis in a stirred tank bioreactor with controlled pH (6.0) and aeration rate (2 vvm; L of air passing through 1 L of medium/min). In fed-batch fermentation, the highest carotenoids production of 135.25 mg/L was obtained. Surfactants (Span 20 at 0.2%) are reported to decrease the mycelial length, which helps in disperse the growth of B. trispora, resulting in the enhanced production of β-carotene from 138 mg/L to 251 mg/L [12]. Also, the enhanced cell permeability of yeast cells in the presence of Span 20 (0.2%) permits the exchange of mating stimulators between the opposite type strains.
Microbial production of lycopene can be promoted with the addition of inhibitors that suppress the downstream pathway of β-carotene biosynthesis by inhibiting the lycopene cyclase mediated chain cyclization process. Hernández-Almanza et al. [22] reviewed the various inhibitors, such as imidazole, ketoconazole, nicotine, piperidine, 2-amino-6-methylpyridine, and creatinine used in the concentration of 20–500 ppm for the enhanced production of lycopene (up to 100% of total carotenoids) by B. trispora, Dietzia natronolimnaea, R. glutinis, and many other microbes.
The utilization of agro-industrial waste is a novel approach for the microbial production of carotenoids. Frengova and Beshkova [17] reviewed the biosynthesis of major carotenoids astaxanthin, β-carotene, γ-carotene, torulene, and torularhodin in Phaffia and Rhodotorula yeast. They suggested that agro-industrial by-products such as sugarcane and beet molasses can be utilized as low-cost alternatives to carbon and nitrogen sources for microbial carotenoids production, which could significantly reduce the manufacturing cost and minimize the problems related to their safe disposal.
The massive biosynthesis of secondary carotenoid astaxanthin in Haematococcus under stress conditions (‘‘green’’ to ‘‘red’’ transition) coincide with enhanced lipid and oil globule protein metabolism to from cytoplasmic lipid droplets [27, 51]. The lipid droplet sub-compartment functions as a sink, which helps in the efficient sequestration of astaxanthin. During stress, the lipid metabolism of Haematococcus shifts from the synthesis of membrane lipid to the storage of neutral lipids (such as triacylglycerols) which form the core of the lipid droplets. The cytoplasmic lipid droplets help in the effective sequestration of astaxanthin, thus preventing the feedback inhibition of biosynthesis.
To achieve the economic viability for the carotenoid production from microalgal platforms, increasing the productivity of biomass is a prerequisite. In this regard, light-driven metabolism and the low photosynthetic efficiency of microalgae in photobioreactors are the main hurdles [64]. To improve the efficiency of sunlight-to-biomass conversion in the microalgal biofactories that generally occur with a significantly lower efficiency (35%–80%), several strategies have been successfully attempted [65], which include (1) engineering of the light-harvesting antenna to reduce non-photochemical quenching losses and light attenuation; (2) engineering photosynthetic electron transport with increased numbers or activity of Cytb6f and plastoquinone complexes present in the photosynthesis apparatus in order to overcome kinetic constraints that result in energy losses; (3) engineering of the energy/reductant sink capacity of Calvin–Benson cycle; and (4) engineering of Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and the construction of synthetic chloroplastic photorespiratory bypasses have been found to be beneficial in terms of achieving higher biomass productivity.
Heterologous carotenoid production in microbes
Different multigene pathways, including MEP and MVA pathways, carotenoids biosynthetic pathway, and other apocarotenoids and ketocarotenoids biosynthetic pathways, have been genetically engineered or introduced in heterologous systems for industrially important carotenoid production. Similar to the carotenoids production using carotenogenic microbes, several parameters and production strategies have been investigated for the efficient and economical production of the desired carotenoid utilizing heterologous systems. For example, as outlined in Fig. 5, several strategies have been used to enhance the carotenoid production in E. coli. In the first step, carotenoid biosynthesis is improved by increasing supplies of the essential precursors (pyruvate and glyceraldehyde-3-phosphate) of the MEP pathway. Second, the metabolic flux of IPP and DMAPP is overproduced by the overexpression of key isoprenoid genes of the MEP pathway, including 1-deoxy-d-xylulose- 5-phosphate synthase (Dxs) and isopentenyl diphosphate isomerase (Idi). Third, the key channels consuming the carotenoid precursors were silenced or inhibited. Fourth, enhanced production of essential cofactors adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) through overexpression of key genes of the tricarboxylic acid (TCA) cycle (e.g., α-ketoglutarate dehydrogenase and ATP synthesis (e.g., ATP synthase), which fulfill the energy requirements of biosynthetic events. The major events of genetic engineering of microorganisms for the heterologous production of carotenoids are summarized in Table 2.
Yoon et al. [71] investigated and optimized several parameters for the combinatorial expression of the prokaryotic mevalonate pathway for the enhanced production of β-carotene (465 mg/L) in E. coli DH5α utilizing a glycerol concentration of 2% (w/v). The top (portions supplying mevalonate from acetyl-CoA) and bottom MVA pathways (IPP from mevalonate) from Enterococcus faecalis and Streptococcus pneumoniae, respectively, were found to be the most efficient for the production of β-carotene, compared to E. coli as it harbors these top and bottom MVA pathways from other microbes, including Staphylococcus aureus, Streptococcus pyogenes, and S. cerevisiae. Similarly, among various E. coli strains (DH5α, S17-1, MG1655, XL1-Blue, and BL21) and carbon sources (glucose, galactose, glycerol, xylose, and maltose), the DH5α was found to be most efficient for β-carotene production using glycerol as the carbon source. These observations suggested that the readily metabolizable carbon sources are not favorable for β-carotene production. Moreover, glycerol is massively produced as a by-product in the biodiesel manufacturing process which can be used as an inexpensive and eco-friendly alternative for the large-scale production of carotenoids. In another study, in metabolically engineered E. coli cultures, harboring high-copy number plasmids that were grown with inoculum at the stationary growth phase showed lower accumulation of lycopene [26]. Conversely, cultures derived using low-copy plasmid yielded high amounts of lycopene irrespective of inoculum state. A temperature shift (37 → 25 °C) of high cell density fed-batch cultures further increased lycopene productivity, with the highest lycopene yield of 260 mg/L for 60 h. The segregational or structural instability of large size and high-copy-number plasmids is a key factor limiting recombinant gene expression. Thus, low-copy plasmid and early growth stage inoculum are shown to be most appropriate for the enhanced production of lycopene in E. coli. Also, the selection of appropriate cultivation temperatures is a critical factor in both lycopene production and biomass productivity.
In a significant improvement over existing metabolic engineering techniques, Wang et al. [63] applied multiplex automated genome engineering (MAGE) to optimize the MEP pathway in E. coli to produce fivefold higher lycopene within 3 days. Using the MAGE, ribosome binding site (RBS) of 24 genes, including Dxs, Dxr, Idi, and farnesyl diphosphate synthase (IspA), is optimized, as well as various genes, including glutamate dehydrogenase (gdhA) and phosphoglycerate mutase (YtjC) knockouts by the introduction of nonsense mutations from secondary pathways. Interestingly, silencing of gdhA increases the lycopene production but causes a 32% reduction in growth rate. Among several isolated mutants, the optimization of Dxs and Idi showed lycopene accumulation (9 mg/g DCW). Since the higher accumulation of hydrophobic molecules, such as lycopene exert stress on the cells, the additional optimization of RNA polymerase subunit sigma factor (rpoS; a critical stress response regulator) and Dxr, along with the inactivation of YtjC, improved the growth rate and lycopene accumulation by increasing stress resistance.
Sun et al. [54] attempted to create a consolidated bioprocessing (CBP) organism for biomass utilization and conversion. S. cerevisiae strain integrated with an artificial ten-gene pathway including d-xylose utilization genes consisting of xylose reductase (Xr), d-xylulokinase (Xks), and xylitol dehydrogenase (Xdh) from Scheffersomyces stipites. Also, five zeaxanthin biosynthetic pathway genes, including GGPP synthase (CrtE), CrtB, CrtI, CrtY, and CrtZ from Erwinia uredovora, were integrated to synthesize zeaxanthin directly from birchwood xylan to utilize d-xylose. Moreover, among the 14 constitutive promoters characterized, promoter TEF1p was found to be the strongest under glucose-limited conditions, resulting in the highest zeaxanthin production from xylan with a titer of 0.74 mg/L.
A sufficient supply of cofactors (ATP and NADPH) and isoprenoid precursors is necessary for the engineering of β-carotene synthesis modules. Zhao et al. [73] investigated and optimized complete β-carotene synthetic pathway in E. coli by engineering five key pathways, including β-carotene synthesis, MEP, and three central metabolic pathways of ATP synthesis, TCA cycle, and pentose phosphate pathway (PPP) to increase precursor (DMAPP and IPP) and cofactor (ATP and NADPH) supplies. Engineering MEP pathways (Dxs and Idi) resulted in a 3.5-fold increase of β-carotene accumulation, while engineering β-carotene synthesis pathways from Gram-negative bacterium Pantoea agglomerans resulted in the additional 3.4-fold increase. The highest β-carotene yields of 21, 17, and 39% were recorded after modulating a single gene of ATP synthesis (ATP synthase), PPP (Transaldolase B) and TCA modules (α-ketoglutarate dehydrogenase), respectively. Combined engineering of TCA and PPP pathways showed a significant synergistic effect on the enhanced accumulation of β-carotene, leading to a 64% higher yield of β-carotene over parental strain. Fed-batch fermentation employing best strain CAR005 produced 2.1 g/L β-carotene (74-fold yield increase over the wild-type) with a yield of 60 mg/g DCW. Interestingly, the combined modulation of TCA and PPP modules showed a synergistic effect for enhanced production of β-carotene, whereas the combined modulation of TCA and ATP synthesis modules was not beneficial. Thus, it was proposed that increasing the supply of NADPH is more beneficial than increasing ATP for improving β-carotene production.
Reyes et al. [40] utilized oxidative stress using cyclic H2O2 shocking as a driving force (adaptive evolution) for the 100% higher production of β-carotene (12 mg/g DCW) from S. cerevisiae, which was engineered to produce carotenoids using heterologous genes from another yeast, X. dendrorhous. Furthermore, some oxidative stress-resistant mutants isolated from H2O2 challenged cultures were found to produce 300% more β-carotene (18 mg/g DCW) than their parental strain. The transcriptome of the isolated hyper-producing mutant suggested an increased expression of HMG-CoA biosynthetic enzymes, which is the substrate for the crucial rate-limiting step in IPP production.
Li et al. [29] optimized the substrate channeling (coordinated expression) of CLY β- (CrtY) and β-carotene 3-hydroxylase (CrtZ; a rate-limiting enzyme) in E. coli for extraordinary zeaxanthin production (11.95 mg/g DCW), which was significantly higher than that of the natural zeaxanthin producer D. salina (6 mg/g DCW). Substrate channeling is a metabolic engineering approach optimized so that no intermediate in the pathway accumulates toxic levels. In this process, first enzyme CrtY acts on the lycopene and transporting the product (β-carotene) to a neighboring cascade enzyme CrtZ, resulting in higher production of zeaxanthin. For the coordinated expression of these two enzymes to produce zeaxanthin from lycopene, the introduction of tunable intergenic regions (TIGR) was found to be more efficient than the fusion of protein-mediated substrate channeling. Also, to catalyze the rate-limiting step for zeaxanthin production, CrtZ from Pantoea ananatis was found to be superior to Pantoea agglomerans and H. pluvialis.
In addition to the enhanced supply of precursors and biosynthetic intermediates, down-regulation or inhibiting the synthesis of ergosterol and squalene can drive the entire metabolic flux towards carotenoids biosynthesis. Overexpressing the HMG-CoA reductase gene and adding an ergosterol synthesis inhibitor (100 mg/L ketoconazole) in the recombinant S. cerevisiae showed 135.1% and 15.6% increments of β-carotene concentration, respectively [67]. Moreover, the combination of overexpressing the HMG-CoA reductase gene and adding ketoconazole resulted in 206.8% increment of pigment content (6.29 mg/g DCW). Similarly, the modulation of CrtYE and carotenoid cleavage dioxygenase (CCD1) is investigated to produce valuable apocarotenoids, α- and β- ionone, retinal, and retinol [72].
The genetic engineered approaches have shown promising results to accumulate the significantly high amount of carotenoids in carotenogenic and non-carotenogenic microbes. However, enhanced accumulation has been shown to exert stress on the bacterial cells, because of the hydrophobic nature of carotenoids [55]. Also, carotenoids are prone to degradation by cellular oxidants. Thus, the overexpression of stress factors (σ factors) such as rpoS, rpoD, and sigA has shown improved lycopene accumulation by altering the cellular machinery of cells towards improved oxidative stress tolerance, increasing the stress resistance and pleiotropic effects involving significant direct metabolic associations with thiamine and pyridoxal biosynthesis (thiamine is necessary as a cofactor for DXP) and other enzymes of the MEP pathway [56]. Considering these observations, the modification of cellular stress regulators can play a crucial role in enhancing the production of carotenoids. Also, despite the significant advantages of non-native carotenoid output in microbes, the large-scale and cost-effective carotenoids production is currently quite challenging.
Cost of microbial carotenoids production
The majority of the commercial market, especially for feed and cosmetics, is occupied by synthetic carotenoids. The cost of the chemical synthesis of carotenoids is generally less than that of natural pigments. However, for human consumption, natural carotenoids are preferred. The cost studies indicated that the natural production of astaxanthin in economically feasible and competitive with synthetic astaxanthin, which has a production cost of USD1000/Kg [28]. In a study in China [28], the production cost of astaxanthin using H. pluvialis has estimated as USD718/Kg, with a production cost of microalgae biomass of only USD18/Kg using low-cost photobioreactors and raceway ponds. A raceway pond is a shallow recirculating flow channel in which paddle wheels are used to drive the continuous flow of microalgal biomass suspended in the culture medium. It was estimated that if the same production facilities were established in the USA, the cost of labor would increase the cost of astaxanthin to approximately USD600/Kg, compared to USD120/Kg in China. Similarly, the cost of production can be 4.5 times higher if only photobioreactors are used for the production. However, the running and facilities establishment costs can be reduced significantly using raceway ponds, since the maintenance and consumables cost of raceway ponds are only about 5% of that of photobioreactors [63]. The higher contamination is the primary limitation in the outdoor raceway ponds cultivation of Haematococcus cultivation. Thus, the photobioreactors are used to provide vegetative cells for raceway ponds. Also, raceway ponds are operated in batch mode for a short period to avoid contamination.
Summary and future directions
To date, a total of 594 prokaryotes have been characterized for native carotenoids biosynthesis. However, validated pathways have been illustrated in only 5% of organisms [36]. The identification of the genetic and molecular basis of carotenoids accumulations in these microbes can potentially help in the development of suitable strategies for the enhanced and efficient production of carotenoids. Among the microbes, microalgae H. pluvialis are most widely explored for the economically vital carotenoid astaxanthin. The economically feasible commercial production has been achieved using the appropriate stress and low-cost bioreactors. Moreover, the cost of the microbial carotenoids production can be significantly reduced by utilizing agro-industrials wastes as a low-cost and eco-friendly carbons source, which can solve the problems of their safe disposal. Further research is needed in the context of utilizing agro-industrials wastes for biomass and carotenoids production.
Sufficient evidence suggests that in H. pluvialis cytoplasmic lipid droplets help in the effective sequestration of secondary ketocarotenoid astaxanthin. In the future, in addition to targeting biosynthetic pathways for enhanced production, approaches to improve sink capacity need to be found for the higher production of carotenoids. Similarly, species-specific cultivation methods should be optimized to harness the maximum potential of microbes.
The metabolically engineered E. coli and other non-carotenogenic microbes have shown exceptional performance for the massive production of commercially vital carotenoid. In some cases, with the use of efficient engineering approaches on the proper strain, innovative bioreactor designs, and optimized cultivation conditions, carotenoids production has been observed to be higher than that of native carotenogenic microbes. The modification of σ factors has shown promising results for enhanced production of lycopene in bacteria. In the future, carotenoids regulation by these factors can be investigated in detail. Similarly, the cost of carotenoids production using genetically engineered microbes, the environmental impact of carotenoids production, appropriateness for human consumption, and other factors need to be investigated.
References
Ambati RR, Phang S-M, Ravi S, Aswathanarayana RG (2014) Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs 12:128–152. https://doi.org/10.3390/md12010128
Auerswald L, Gäde G (2008) Simultaneous extraction of chitin and astaxanthin from waste of lobsters Jasus lalandii, and use of astaxanthin as an aquacultural feed additive. Afr J Mar Sci 30:35–44. https://doi.org/10.2989/AJMS.2008.30.1.4.454
Avalos J, Carmen Limón M (2015) Biological roles of fungal carotenoids. Curr Genet 61:309–324. https://doi.org/10.1007/s00294-014-0454-x
BBC Research (2018) The global market for carotenoids, FOD025F. Available via DIALOG. https://www.bccresearch.com/title of subordinate document, Accessed 25 Oct 2018
Berman J, Zorrilla-López U, Farré G et al (2015) Nutritionally important carotenoids as consumer products. Phytochem Rev 14:727–743. https://doi.org/10.1007/s11101-014-9373-1
Beuttler H, Hoffmann J, Jeske M et al (2011) Biosynthesis of zeaxanthin in recombinant Pseudomonas putida. Appl Microbiol Biotechnol 89:1137–1147. https://doi.org/10.1007/s00253-010-2961-0
Bhataya A, Schmidt-Dannert C, Lee PC (2009) Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem 44:1095–1102. https://doi.org/10.1016/j.procbio.2009.05.012
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756. https://doi.org/10.1007/s10811-013-9983-9
Bryon A, Kurlovs AH, Dermauw W et al (2017) Disruption of a horizontally transferred phytoene desaturase abolishes carotenoid accumulation and diapause in Tetranychus urticae. PNAS 114:E5871–E5880. https://doi.org/10.1073/pnas.1706865114
Campenni L, Nobre BP, Santos CA et al (2013) Carotenoid and lipid production by the autotrophic microalga Chlorella protothecoides under nutritional, salinity, and luminosity stress conditions. Appl Microbiol Biotechnol 97:1383–1393. https://doi.org/10.1007/s00253-012-4570-6
Cazzonelli CI, Pogson BJ (2010) Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci 15:266–274
Choudhari SM, Ananthanarayan L, Singhal RS (2008) Use of metabolic stimulators and inhibitors for enhanced production of β-carotene and lycopene by Blakeslea trispora NRRL 2895 and 2896. Bioresour Technol 99:3166–3173. https://doi.org/10.1016/j.biortech.2007.05.051
de la Fuente JL, Rodríguez-Sáiz M, Schleissner C et al (2010) High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous. J Biotechnol 148:144–146. https://doi.org/10.1016/j.jbiotec.2010.05.004
Dufossé L (2017) Current carotenoid production using microorganisms. In: Singh OV (ed) Bio-pigmentation and biotechnological implementations. John Wiley and Sons, Inc., Hoboken, USA, pp 87–106
Farré G, Sanahuja G, Naqvi S et al (2010) Travel advice on the road to carotenoids in plants. Plant Sci 179:28–48. https://doi.org/10.1016/j.plantsci.2010.03.009
Fernández-Sevilla JM, Acién Fernández FG, Molina Grima E (2010) Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol 86:27–40. https://doi.org/10.1007/s00253-009-2420-y
Frengova GI, Beshkova DM (2009) Carotenoids from Rhodotorula and Phaffia: yeasts of biotechnological importance. J Ind Microbiol Biotechnol 36:163–180. https://doi.org/10.1007/s10295-008-0492-9
García-Malea MC, Acién FG, Del Río E et al (2009) Production of astaxanthin by Haematococcus pluvialis: taking the one-step system outdoors. Biotechnol Bioeng 102:651–657. https://doi.org/10.1002/bit.22076
Giri AK, Rawat JK, Singh M et al (2015) Effect of lycopene against gastroesophageal reflux disease in experimental animals. BMC Complement Altern Med 15:110. https://doi.org/10.1186/s12906-015-0631-6
Gong M, Bassi A (2016) Carotenoids from microalgae: a review of recent developments. Biotechnol Adv 34:1396–1412. https://doi.org/10.1016/j.biotechadv.2016.10.005
Grama BS, Chader S, Khelifi D et al (2014) Induction of canthaxanthin production in a Dactylococcus microalga isolated from the Algerian Sahara. Bioresour Technol 151:297–305. https://doi.org/10.1016/j.biortech.2013.10.073
Hernández-Almanza A, Montañez J, Martínez G et al (2016) Lycopene: progress in microbial production. Trends Food Sci Technol 56:142–148. https://doi.org/10.1016/j.tifs.2016.08.013
Jackson H, Braun CL, Ernst H (2008) The chemistry of novel xanthophyll carotenoids. Am J Cardiol 101:50D–57D. https://doi.org/10.1016/j.amjcard.2008.02.008
Kim J-S, Lee W-M, Rhee HC, Kim S (2016) Red paprika (Capsicum annuum L.) and its main carotenoids, capsanthin and β-carotene, prevent hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Chem Biol Interact 254:146–155. https://doi.org/10.1016/j.cbi.2016.05.004
Kim JY, Paik JK, Kim OY et al (2011) Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis 215:189–195. https://doi.org/10.1016/j.atherosclerosis.2010.11.036
Kim S-W, Kim J-B, Ryu J-M et al (2009) High-level production of lycopene in metabolically engineered E. coli. Process Biochem 44:899–905. https://doi.org/10.1016/j.procbio.2009.04.018
Lemoine Y, Schoefs B (2010) Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res 106:155–177. https://doi.org/10.1007/s11120-010-9583-3
Li J, Zhu D, Niu J et al (2011) An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol Adv 29:568–574. https://doi.org/10.1016/j.biotechadv.2011.04.001
Li X-R, Tian G-Q, Shen H-J, Liu J-Z (2015) Metabolic engineering of Escherichia coli to produce zeaxanthin. J Ind Microbiol Biotechnol 42:627–636. https://doi.org/10.1007/s10295-014-1565-6
Liang M-H, Zhu J, Jiang J-G (2017) Carotenoids biosynthesis and cleavage related genes from bacteria to plants. Crit Rev Food Sci Nutr 19:1–20. https://doi.org/10.1080/10408398.2017.1322552
Lin J-H, Lee D-J, Chang J-S (2015) Lutein production from biomass: marigold flowers versus microalgae. Bioresour Technol 184:421–428. https://doi.org/10.1016/j.biortech.2014.09.099
Manayi A, Abdollahi M, Raman T et al (2016) Lutein and cataract: from bench to bedside. Crit Rev Biotechnol 36:829–839. https://doi.org/10.3109/07388551.2015.1049510
Mata-Gómez LC, Montañez JC, Méndez-Zavala A, Aguilar CN (2014) Biotechnological production of carotenoids by yeasts: an overview. Microb Cell Fact 13:12. https://doi.org/10.1186/1475-2859-13-12
Moran NA, Jarvik T (2010) Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328:624–627. https://doi.org/10.1126/science.1187113
Nasri Nasrabadi MR, Razavi SH (2010) Use of response surface methodology in a fed-batch process for optimization of tricarboxylic acid cycle intermediates to achieve high levels of canthaxanthin from Dietzia natronolimnaea HS-1. J Biosci Bioeng 109:361–368. https://doi.org/10.1016/j.jbiosc.2009.10.013
Nupur LNU, Vats A, Dhanda SK et al (2016) ProCarDB: a database of bacterial carotenoids. BMC Microbiol 16:96. https://doi.org/10.1186/s12866-016-0715-6
Papp T, Csernetics A, Nagy G et al (2013) Canthaxanthin production with modified Mucor circinelloides strains. Appl Microbiol Biotechnol 97:4937–4950. https://doi.org/10.1007/s00253-012-4610-2
Park JS, Chyun JH, Kim YK et al (2010) Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab 7:18. https://doi.org/10.1186/1743-7075-7-18
Prabhu S, Rekha PD, Young C-C et al (2013) Zeaxanthin production by novel marine isolates from coastal sand of India and its antioxidant properties. Appl Biochem Biotechnol 171:817–831. https://doi.org/10.1007/s12010-013-0397-6
Reyes LH, Gomez JM, Kao KC (2014) Improving carotenoids production in yeast via adaptive laboratory evolution. Metab Eng 21:26–33. https://doi.org/10.1016/j.ymben.2013.11.002
Riccioni G (2009) Carotenoids and cardiovascular disease. Curr Atheroscler Rep 11:434–439
Rodríguez-Sáiz M, de la Fuente JL, Barredo JL (2010) Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl Microbiol Biotechnol 88:645–658. https://doi.org/10.1007/s00253-010-2814-x
Ruiz-Sola MÁ, Rodríguez-Concepción M (2012) Carotenoid biosynthesis in Arabidopsis: a colorful pathway. Arabidopsis Book 10:e0158. https://doi.org/10.1199/tab.0158
Saenge C, Cheirsilp B, Suksaroge TT, Bourtoom T (2011) Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem 46:210–218. https://doi.org/10.1016/j.procbio.2010.08.009
Saini RK, Keum Y-S (2017) Progress in microbial carotenoids production. Indian J Microbiol 57:129–130. https://doi.org/10.1007/s12088-016-0637-x
Saini RK, Keum Y-S (2018) Significance of genetic, environmental, and pre- and postharvest factors affecting carotenoid contents in crops: a review. J Agric Food Chem 66:5310–5324. https://doi.org/10.1021/acs.jafc.8b01613
Saini RK, Moon SH, Gansukh E, Keum Y-S (2018) An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chem 266:56–65. https://doi.org/10.1016/j.foodchem.2018.05.104
Saini RK, Nile SH, Park SW (2015) Carotenoids from fruits and vegetables: chemistry, analysis, occurrence, bioavailability and biological activities. Food Res Int 76. Part 3:735–750. https://doi.org/10.1016/j.foodres.2015.07.047
Sandmann G (2015) Carotenoids of biotechnological importance. In: Schrader J, Bohlmann J (eds) Biotechnology of isoprenoids. Springer International Publishing, Switzerland, pp 449–467
Schmidt I, Schewe H, Gassel S et al (2011) Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl Microbiol Biotechnol 89:555–571. https://doi.org/10.1007/s00253-010-2976-6
Solovchenko AE (2015) Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynth Res 125:437–449. https://doi.org/10.1007/s11120-015-0156-3
Suganuma K, Nakajima H, Ohtsuki M, Imokawa G (2010) Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J Dermatol Sci 58:136–142. https://doi.org/10.1016/j.jdermsci.2010.02.009
Sultan Alvi S, Ansari IA, Khan I et al (2017) Potential role of lycopene in targeting proprotein convertase subtilisin/kexin type-9 to combat hypercholesterolemia. Free Radic Biol Med 108:394–403. https://doi.org/10.1016/j.freeradbiomed.2017.04.012
Sun J, Shao Z, Zhao H et al (2012) Cloning and characterization of a panel of constitutive promoters for applications in pathway engineering in Saccharomyces cerevisiae. Biotechnol Bioeng 109:2082–2092. https://doi.org/10.1002/bit.24481
Sun T, Miao L, Li Q et al (2014) Production of lycopene by metabolically-engineered Escherichia coli. Biotechnol Lett 36:1515–1522. https://doi.org/10.1007/s10529-014-1543-0
Taniguchi H, Henke NA, Heider SAE, Wendisch VF (2017) Overexpression of the primary sigma factor gene sigA improved carotenoid production by Corynebacterium glutamicum: application to production of β-carotene and the non-native linear C50 carotenoid bisanhydrobacterioruberin. Metab Eng Comm 4:1–11. https://doi.org/10.1016/j.meteno.2017.01.001
Thawornwiriyanun P, Tanasupawat S, Dechsakulwatana C et al (2012) Identification of newly zeaxanthin-producing bacteria isolated from sponges in the Gulf of Thailand and their zeaxanthin production. Appl Biochem Biotechnol 167:2357–2368. https://doi.org/10.1007/s12010-012-9760-2
Thies F, Mills LM, Moir S, Masson LF (2017) Cardiovascular benefits of lycopene: fantasy or reality? Proc Nutr Soc 76:122–129. https://doi.org/10.1017/S0029665116000744
Tian B, Hua Y (2010) Carotenoid biosynthesis in extremophilic deinococcus-thermus bacteria. Trends Microbiol 18:512–520. https://doi.org/10.1016/j.tim.2010.07.007
Virtamo J, Taylor PR, Kontto J et al (2014) Effects of α-tocopherol and β-carotene supplementation on cancer incidence and mortality: 18-year postintervention follow-up of the alpha-tocopherol, beta-carotene cancer prevention study. Int J Cancer 135:178–185. https://doi.org/10.1002/ijc.28641
Visioli F, Artaria C (2017) Astaxanthin in cardiovascular health and disease: mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct 8:39–63. https://doi.org/10.1039/C6FO01721E
Viuda-Martos M, Sanchez-Zapata E, Sayas-Barberá E et al (2014) Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: a review. Crit Rev Food Sci Nutr 54:1032–1049. https://doi.org/10.1080/10408398.2011.623799
Wang HH, Isaacs FJ, Carr PA et al (2009) Programming cells by multiplex genome engineering and accelerated evolution. Nature 460:894–898. https://doi.org/10.1038/nature08187
Wichuk K, Brynjólfsson S, Fu W (2014) Biotechnological production of value-added carotenoids from microalgae: emerging technology and prospects. Bioengineered 5:204–208. https://doi.org/10.4161/bioe.28720
Wobbe L, Remacle C (2015) Improving the sunlight-to-biomass conversion efficiency in microalgal biofactories. J Biotechnol 201:28–42. https://doi.org/10.1016/j.jbiotec.2014.08.021
Wu W, Li Y, Wu Y et al (2015) Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol Nutr Food Res 59:1663–1673. https://doi.org/10.1002/mnfr.201500109
Yan G, Wen K, Duan C (2012) Enhancement of β-carotene production by over-expression of HMG-CoA reductase coupled with addition of ergosterol biosynthesis inhibitors in recombinant Saccharomyces cerevisiae. Curr Microbiol 64:159–163. https://doi.org/10.1007/s00284-011-0044-9
Ye VM, Bhatia SK (2012) Pathway engineering strategies for production of beneficial carotenoids in microbial hosts. Biotechnol Lett 34:1405–1414. https://doi.org/10.1007/s10529-012-0921-8
Yeh T-J, Tseng Y-F, Chen Y-C et al (2017) Transcriptome and physiological analysis of a lutein-producing alga Desmodesmus sp. reveals the molecular mechanisms for high lutein productivity. Algal Res 21:103–119. https://doi.org/10.1016/j.algal.2016.11.013
Yi X, Li J, Xu W et al (2015) Shrimp shell meal in diets for large yellow croaker Larimichthys croceus: effects on growth, body composition, skin coloration and anti-oxidative capacity. Aquaculture 441:45–50. https://doi.org/10.1016/j.aquaculture.2015.01.030
Yoon S-H, Lee S-H, Das A et al (2009) Combinatorial expression of bacterial whole mevalonate pathway for the production of beta-carotene in E. coli. J Biotechnol 140:218–226. https://doi.org/10.1016/j.jbiotec.2009.01.008
Zhang C, Chen X, Lindley ND, Too H-P (2018) A “plug-n-play” modular metabolic system for the production of apocarotenoids. Biotechnol Bioeng 115:174–183. https://doi.org/10.1002/bit.26462
Zhao J, Li Q, Sun T et al (2013) Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab Eng 17:42–50. https://doi.org/10.1016/j.ymben.2013.02.002
Acknowledgements
This paper was supported by KU research professor program of Konkuk University, Seoul, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Saini, R.K., Keum, YS. Microbial platforms to produce commercially vital carotenoids at industrial scale: an updated review of critical issues. J Ind Microbiol Biotechnol 46, 657–674 (2019). https://doi.org/10.1007/s10295-018-2104-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2104-7