Abstract
Lutein is an antioxidant that has gathered increasing attention due to its potential role in preventing or ameliorating age-related macular degeneration. Currently, it is produced from marigold oleoresin, but continuous reports of lutein-producing microalgae pose the question if those microorganisms can become an alternative source. Several microalgae have higher lutein contents than most marigold cultivars and have been shown to yield productivities hundreds of times higher than marigold crops on a per square meter basis. Microalgae and marigold are opposite alternatives in the use of resources such as land and labor and the prevalence of one or the other could change in the future as the lutein demand rises and if labor or land becomes more restricted or expensive in the producing countries. The potential of microalgae as a lutein source is analyzed and compared to marigold. It is suggested that, in the current state of the art, microalgae could compete with marigold even without counting on any of the improvements in microalgal technology that can be expected in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotenoids are a class of terpenoid pigments with a 40-carbon backbone and a large conjugated double-bond system. Carotenoids are a relevant group of compounds because of their role in photosynthesis and photoprotection (Demmig-Adams and Adams 1996, 2002), their wide applicability as food color (Delgado-Vargas et al. 2000), and their physiological roles in human tissues as antioxidants and blue light filters (Bendich and Olson 1989; Krinsky et al. 2003). Xanthophylls are a family of oxygenated carotenoids that contain hydroxyl or carbonyl groups that contribute to enhance their solubility and hence their distribution in animal tissues. Lutein ((3R,3′R,6′R)-β,ε-carotene-3,3′-diol) is a xanthophyll that, together with zeaxanthin, has gathered increasing attention on the grounds of recent studies that show how an adequate intake of this product might help to prevent or ameliorate the effects of degenerative human diseases, such as age-related macular degeneration (AMD; Carpentier et al. 2009; Chiu and Taylor 2007; Granado et al. 2003) or cataract (Arnal et al. 2009) and also in skin health (Roberts et al. 2009). Lutein, and zeaxanthin in a much smaller proportion, is the only carotenoid that is absorbed in the bloodstream after ingestion (John et al. 2002) and accumulated in the human retina and is assumed to have a protective effect due to its ability to filter out blue light and its potential to inactivate singlet oxygen and to quench active radicals thus acting as an active antioxidant.
Lutein is largely consumed as food colorant and its sales amount to $150,000,000 in the US only. In the EU, lutein extracted from higher plants using hexane or other accepted solvents is listed as E161b when used as feed additive. The use of extracts containing lutein for the formulation of nutritional supplements have gained increasing popularity for the prevention of AMD, as well as for its antioxidant properties, after the public awareness of its potential to prevent the disease. The consideration of lutein as a vitamin has also been discussed, standing on the fact that meets the three requirements to be considered an essential nutrient: (a) it cannot be synthesized by the human metabolism and must be ingested; (b) the consumption of a lutein-deprived diet has been proved to cause blindness in primates; (c) the dysfunction can be reverted by the reintroduction of lutein either dietary or as a supplement, as long as the condition does not become irreversible (Semba and Dagnelie 2003). Should lutein be eventually considered an essential nutrient or vitamin, the over 100,000,000 people at risk of developing AMD only in the western world could be systematically prescribed lutein supplements to ensure that the 5-mg recommended daily dose (Coleman and Chew 2007) is consumed.
Currently, lutein is obtained from the petals of marigold after an extraction process which yields oleoresins with varying concentrations of lutein that range from 5% to 50%, mostly in the diester form, which is roughly a half if free lutein is considered. These concentrates are the product most commonly used for the formulation of supplements. Lutein can be further purified by processes that involve saponification, further concentration, and a final recrystallization to its crystalline form (Khachik 2007). Crystalline lutein is, nevertheless, difficult to handle and, hence, is commonly sold as suspensions of the carotenoid in corn or safflower oils. These kind of purified extracts are commercial products such as FloraGLO® from Kemin Foods or Xangold® from Cognis. Pure free crystalline lutein is also available, most commonly in the form of microcapsules.
However, marigold presents drawbacks as lutein source. The flowers must be periodically harvested and the petals separated prior to extraction. The lutein content in marigold petals is variable and can be as low as 0.03% (Piccaglia et al. 1998). The result is that the production of marigold petals is a labor-intensive land-demanding process that is currently feasible in developing economies. On the other hand, lutein has also been produced synthetically, but at prices that cannot compete with marigold. The only other possible sources with a sufficient content to be considered for lutein production are certain strains of microalgae. In fact, several microalgae have been considered as potential sources of lutein/astaxanthin for several reasons: (a) its high lutein content (0.5–1.2% dry weight) compared to marigold petals; (b) petals do not have to be separated and the whole microalgal biomass is processed; (c) a homogeneous biomass is produced at a constant rate regardless of time and weather, so it lends itself better to a precisely designed extraction process; (d) valuable by-products that can be used to produce protein hydrolysates, other pigments, and even valuable lipids depending on the strain. Other lutein sources exist but because of their limited availability (crustaceans, egg yolk) or low content (corn residues) can hardly be considered.
The production of lutein from microalgae should use quite less labor than marigold but, on the other hand, would demand extensive technology and a precisely designed process that should not only be effective in recovering the lutein but also provide an appropriate valorization of the by-products, allowing to compensate for the higher cost of microalgal biomass compared to marigold petals. In this sense, the current tendency of considering microalgae as a source of lutein-rich extracts to substitute marigold oleoresin in existing processes should be revised. The extraction/purification processes should be redesigned for microalgae bearing in mind the wealth of other valuable biomolecules contained in microalgal biomass. It is also necessary to take into account that the eventual production of microalgae-derived mass market products could leave extensive amount of residual biomass that could be used for the production of lutein, among other possibilities. So the production of lutein from microalgae should be revised, envisioning the process as a part of a valorization strategy in which lutein can or cannot be the main product. To accomplish this is necessary to have a clear view of the downstream processes currently available for microalgal biomass and their applicability to the microalgae with highest lutein content while bearing in mind the economics of the global strategy.
Lutein-producing microalgae
Two main factors make a microalga a good lutein producer: the lutein content and the biomass productivity. Other factors such as the presence of a cell wall or the content of other carotenoids may be also a consideration. Adequate lutein content is a must because, otherwise, lutein extraction results will be impractical. Then, the biomass productivity, usually measured in grams of new biomass generated per culture volume unit and time (e.g., in grams per liter per hour), combines with a high content to give an outstanding producer. Most of the best producers of lutein have been compiled by Del Campo et al. (2007) and are shown in Table 1, along with the relevant factors.
Among the several microalgae that have been reported as lutein producers, only Murielopsis sp. and Scenedesmus almeriensis have been tested in growth conditions that could be considered for mass production, that is, a large-scale system working outdoors for a reasonable time span as to assume that sustained operation is feasible. With the values reported in these two cases, it is easy to see that production of lutein competitive with marigold could be achieved with either alternative. In the case reported by Del Campo et al. (2007), not only does Murielopsis attain lutein contents that easily surpass those of marigold, but a simple calculation shows that, in a year, the system is capable of producing over 65 g lutein or over 14 kg of biomass on a dry weight basis per square meter which obviously cannot be attained by a crop such as marigold specially bearing in mind that only the petals are used and that we are comparing in a dry weight basis. Still the system reported is only 55 L volume and occupies 22 m2 and the value reported is the absolute maximum found for the month of July. The scale up of such a system, basically made up of 90 m of tube with an inner diameter of 2.8 cm, poses certain technical difficulties and has a high installation cost both per volume or surface.
On the other hand, the system reported by Fernández-Sevilla et al. (2005) is also a closed tubular system of 4,000 L volume in a double-loop configuration, as shown in Fig. 1a, and uses the strain S. almeriensis, a local bloom isolated and characterized as described by Sánchez et al. (2008a, b), as the lutein producer. This is, as far as we know, the largest closed system described specifically for the production of lutein-rich microalgal biomass and one of the largest single-unit closed photobioreactors in operation in the world. Later (Fernández-Sevilla et al. 2008), the system was redesigned to increase its capacity to 28,000 L working volume, as shown in Fig. 1b, which is constituted by 10 units of 2,800 L each in fence-type configuration. In spite of the larger volume of the photobioreactors depicted in Fig. 1a and a larger tube diameter (10 cm for the 4,000-L unit and 9 cm for the fence type), the productivity of the 4,000-L system (the other is similar but not yet reported) is higher than the 50 L described by Del Campo et al. (2007), both in volume or in occupied area (Table 1). This improves greatly the economics of the production process by decreasing the installation costs. In order to make a more accurate evaluation, we will use the performance results obtained over the 1-year evaluation shown in Fig. 2. From the data presented, a year average of 6 mg g−1 can be assumed for lutein content and 0.6 g L−1 day−1 for biomass productivity which means a year average lutein productivity of 3.6 mg L−1 day−1 or 360 mg m−2 day−1 with maximums of 10 mg L−1 day−1 and 1,000 mg m−2 day−1 lutein for comparison purposes.
a 4,000-L two-loop horizontal tubular photobioreactor growing S. almeriensis (Fernández-Sevilla et al. 2005). b A later redesign is the 10-unit fence-type configuration (Fernández-Sevilla et al. 2008) totaling to 28,000 L. Both systems are built inside a greenhouse at the “Las Palmerillas” agricultural research station (El Ejido, Almeria, southern Spain, with permission of Prof. J. Pérez Parra, Fundación CAJAMAR)
Biomass productivity and lutein content in the 4,000-L tubular closed photobioreactor reported by Fernández-Sevilla et al. (2005). The symbols represent experimental values. The lines represent the estimates that have been used for the discussion
With these results in mind and taking into account that the operation of the photobioreactor is fully automated, it is again obvious that microalgae can be a source of lutein competitive with marigold, though the conditions requested for the predominance of one or the other are quite different. Marigold-based production needs land availability and cheap labor, while the microalgae-based requests technology and higher investment costs that, for growing marigold, are virtually nil. This is why currently most of the production of dry marigold petals is done in developing economies. In the near future, if lutein supplementation becomes confirmed as effective to impede or delay the progress of AMD or in the case of socioeconomic changes in the marigold-producing countries, land availability may become scarcer and labor more expensive, leaving places for more intensive, technological approaches such as microalgae-based systems.
It is difficult to comment on the possibilities of using any of the other strains reported in Table 1 because they have been investigated in laboratory conditions and particularly under continuous illumination, which makes it very difficult to extrapolate to production conditions. Anyway, Del Campo et al. (2000) considered Chlorella zofingiensis, Chlorococcum citriforme, and Neospongiococcus gelatinosum as well as Muriellopsis sp., choosing this last strain for its combination of characteristics, as exposed in their report. Chlorella sorokiniana is another microalga with a high growth rate and an interesting carotenoid profile as reported by Matsukawa et al. (2000) but has received little attention with regard to lutein production.
The approach of Shi et al. (2000, 2002, 2006) with Chlorella protothecoides is a further interesting possibility because it is potentially more scalable that photoautotrophic systems and large culture volumes may be packed in a relatively small area. The drawback of this approach is the same as its main advantage: the use of organic substrates that also promote the growth of bacteria and yeasts, microorganisms with a much higher growth rate than microalgae, and that makes a must the use of axenic cultures and the maintenance of strict sterility conditions which feasibility and economy remain to be assessed.
Improving the lutein content: stress factors
Microalgae exhibit a great metabolic plasticity in the sense that its biochemical profile can experiment profound changes in response to different environmental conditions and particularly to some so-called stress factors that can provoke dramatic changes such as the induction of the formation of cysts in Haematococcus by intense light of the increase of phycocyanins in Spirulina by ammonium. This possibility has been used to some extent for lutein by Del Campo et al. (2001) and Sánchez et al. (2008a, b), but a systematic study of the most favorable combination of stress factors has not been carried out yet. The most significant factors known to affect lutein content are illumination, measured as external irradiance (I o), pH, temperature (T), nitrogen availability and source, salinity or ionic strength, the presence of oxidizing substances, and of course, growth rate (μ). This factor is particularly difficult to study because it requests cultivation in continuous mode.
The following table shows selected information on the effect of these factors in lutein content and productivity. It must be borne in mind that any of these factors can affect either lutein content or biomass productivity in opposite ways, so that an increase in lutein content can be counterbalanced by a decrease in biomass productivity.
It is difficult to summarize the information contained in Table 2, but it seems clear that high temperature favors the accumulation of lutein particularly when it is on the brink of becoming stressing. Further temperature increases are harmful and decrease biomass productivity. A high irradiance level also seems beneficial. Although the results for indoor batch cultures of Murielopsis shown in Table 2 seem to indicate the contrary, the outdoor continuous experiments reported by the same authors for Murielopsis (Del Campo et al. 2001, 2004; Blanco et al. 2007) agree with this affirmation because the highest productivities and lutein contents are reported for the months with a higher irradiation. Sánchez et al. (2008a) also suggests that there might be a relationship between irradiance and temperature levels of external irradiance, which poses the question whether it is useful to study these factors separately or if actually the interrelationships are at least as significant as the isolated factors. In fact, the data presented in Fig. 2 shows values of lutein content for an outdoor culture of S. almeriensis consistently over 1,000 mg g−1 during May and June which we (Sánchez et al. 2008a) have been unable to reproduce in the laboratory in spite of trying to replicate indoors all the conditions taking place outdoors including the solar cycle illumination. The higher contents recorded in the outdoor experiments could be caused by the temperature decrease at night that does not take place in the laboratory experiments, which may cause a thermal shock or a luminic shock because of the lower temperature that is observed during the first hours of the morning. Another significant difference is the higher O2 concentration that takes place in the outdoor system, alone or in combination with the higher illumination and temperature during May and June.
The effect of specific growth rate, as observed in continuous or semicontinuous cultures, also seems clear in the sense that lutein tends to accumulate at low dilution rates but not enough to counter the decrease in biomass productivity that happens in these circumstances. Therefore, the systems studied are more productive in lutein when operated at the optimal dilution rate. Three things happen in a continuous culture at low dilution rate: (a) the average age of the population increases, (b) light availability decreases due to increased population density, and (c) some nutrients may become depleted. The last one is probably not the cause of the increase in lutein because the experiments in the references were nutrient-saturated in most cases. Thus, lutein just might take time to be synthesized or a low-light-induced increase in antenna size might request additional lutein, which would suggest that this pigment also plays this role in the microalgae studied.
The effect of pH is contradictory between the batch and the continuous experiments. In the former, the lutein content increases at the extremes of the range tested, while in the latter, the best results are obtained at the pH that also maximizes growth rate. In microalgae culture, pH has a special relevance because it also determines the CO2 availability and this is the difference between the batch experiments in which CO2 is continuously supplied as 1% of the aeration stream and the other two references that use a pH-controlled on-demand injection. In either case, the maximum productivity takes place at the optimum pH due to the increased biomass productivity that overrides the differences in lutein content.
The concentration of nitrogen in the culture medium, supplied as nitrate, did not caused any effect in the lutein content of the biomass, but low nitrogen concentrations decreased the biomass productivity due to nitrate depletion leading to a decrease in lutein production. It can be concluded that nitrate should be supplied in moderate excess so that growth rate is not decreased, but a nutrients excess can decrease the performance of the culture sharply, as shown by Sánchez et al. (2008a), probably because of saline stress. This is also shown in the experiments at different [NaCl] compiled in Table 2 which show little to not effect at low salinities and marked decreases for saline concentrations over 100 mM.
Finally, the last factor reviewed with a slight but positive effect in the induction stress is the addition of chemicals such as H2O2 and NaClO that, in the presence of Fe+2 ions, generates stress-inducing chemical species. The induction of oxidative stress makes sense assuming that lutein has an antioxidant protective role and particularly in heterotrophic cultures where oxidative stress is absent due to the nature of the process, although it might also be effective in phototrophic cultures. Nevertheless, the results reported are modest, although the high biomass concentrations taking place in these types of cultures bring about a significant increase in productivity.
As the revision of the literature shows, there seems to be a margin for the improvement in lutein content which is clear for Murielopsis from the report of 8 mg g−1 of Del Campo et al. (2001) and from the data presented in Fig. 2. Nevertheless, a systematic study of all the stress factors and its interactions remains to be done.
Extraction of lutein from microalgae
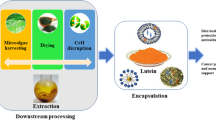
The biomass of chlorophycean microalgae differs substantially from marigold in two main aspects, its richer composition and the presence of a thick hard cell wall. Dry-milled marigold petals are usually processed with a solvent extraction to render oleoresin-containing carotenoids (typically 80% lutein and 5% zeaxanthin), mainly in ester form, and many other substances (Hojnik et al. 2008). This is usually followed by a multistep purification process that includes among others a hydrolysis step to free the hydroxylated carotenoids from the accompanying fatty acids and usually a final recrystallization that renders pure lutein/zeaxanthin. Nevertheless, lutein is rarely sold in crystalline form due to the difficulty in managing such material and for stability reasons but in oily extracts ranging from 5% to 60% (see FloraGlo from Kemin Foods and Xangold from Cognis in http://www.floraglolutein.com and http://www.cognis.com, respectively). Microalgal biomass can be processed to obtain an oleoresin-like extract with an approximate 25% lutein in free form that could be used directly for the formulation of supplements or for further purification in the same fashion as the extracts from marigold. Actually, obtaining a carotenoid-rich oleoresin from microalgae, dried or even in the form of wet paste, is a more straightforward process, as shown in Fig. 3.
The extracts obtained in that way could be subjected to already existing commercial processes to obtain pure lutein as described by Farrow and Tabenkin (1966) and could thus compete with marigold. The problem processing microalgae such as Murielopsis sp. or S. almeriensis is the necessity of a disgregation step prior to extraction to break the cell wall. This can be accomplished by a variety of procedures such as milling, ultrasound, microwave, freezing–thawing, or chemical attacks. The influence of the disgregation step, as discussed by Ceron et al. (2008) for S. almeriensis dry biomass, is summarized in the following table.
The former table shows unit operations at preparative scale and processing conditions suitable to break the cell wall of a Scenedesmus species without causing significant lutein degradation. The mortar procedure is described by Mínguez-Mosquera et al. (1992) as an analytical procedure and is supposed to give a 100% recovery and has thus being considered as a reference in Table 3, although it cannot be scaled to industrial practice. Two methods, sonication and the use of a ball mill, attain results similar to the mortar milling as long as the disgregating agent, alumina, is also used. In fact, the ball mill with alumina gives better results than the mortar and in a very short time. This is why Ceron et al. (2008) report lutein yields slightly over 100% with this method because the comparison is done against the mortar method. This summary hints that other mechanical methods should be tested, particularly on wet paste to check if the dehydration step can be skipped.
This author complements the mechanical breakage with an alkaline treatment (with 4% aqueous KOH, 40°C) that helps to complete the disgregation as well as to ionize susceptible lipids. The carotenoids can then be extracted with hexane, as happens in the procedure for marigold petals, with the advantage that the alkaline treatment converts all the lutein and zeaxanthin to their free form and retains fatty acids and chlorophylls in the aqueous phase. In this sense, Navarrete-Bolaños et al. (2005) describe pretreatments to enhance the extraction yield from marigold that could be studied for microalgae. The procedure has been carried out at a preparative scale (hundreds of grams processed) rendering an extract with 25–40% carotenoids (up to 70% lutein over total carotenoids) depending on the composition of the initial biomass (data not published), being the rest mainly constituted by sterols. Ceron et al. (2008) is the only reference that describes a full extraction procedure of lutein/zeaxanthin starting from microalgal biomass, although the final products is not pure free lutein but suspensions in olive oil. This procedure is shown in Fig. 4.
Other authors have worked on the production of carotenoid concentrates from microalgal biomass but it is not easy to find reports focused on lutein and all of them deal only with the extraction step exclusively in quantitative terms. Methods such as supercritical fluid extraction (SFE), with several modifications, or a variety of modified extraction procedures have been applied to microalgal biomass. The main techniques are summarized in Table 4; some have not been used for microalgal biomass but are included to hint their potential applicability.
Methods using solvents not appropriate for human consumption have been disregarded, such as the proposed by Li et al. (2002), and not included in the compilation for the sake of clarity. Solvent extraction with hexane is the most classic method and has been shown by Ceron et al. (2008) to be valid for microalgae biomass with high recovery and purity, although it requests multistep contact and, therefore, the necessary equipment. A natural enhancement to solvent extraction is the use of the accelerated solvent extraction (ASE, sometimes also called PLC) methodology, which uses a special type of contactor to circulate solvent at high pressure through a tightly packed bed. Those devices have become rather widespread in the last few years to perform fast extractions at the analytical scale for preconcentration and cleanup of samples. This technology has been used in different kind of biomasses and Herrero et al. (2005) applied it to the extraction of antioxidants to Spirulina that, although not specifically lutein, the chemical nature of the targeted compounds gives an idea of the low efficiency of the method for the described purposes. Another important drawback of ASE is that this technique must take place at high temperature (over 60°C and usually as high as 170°C) to lower the viscosity of the solvents, but this leads to the formation of pheophorbide from the chlorophylls in the microalgal biomass, which is a concern due to its toxicity. In any case, the extraction with solvents such as hexane or ethanol allows an easy removal of the solvent and the recovery of a high-content lutein extract. On the other hand, solvent removal is not possible for extraction techniques such as the direct extraction with vegetable oil described by Nonomura (1987). The procedure in this patent described a direct extraction performed on wet biomass by the addition of vegetable oil that is then emulsified and let to rest. No cell-breaking treatments are described, and this raises the question of the applicability of this method to microalgae with a thick cell wall such as Murielopsis or Scenedesmus, which are among the best producers. On the other hand, Nonomura (1987) claims to obtain extracts with up to 7.5% lutein (not specified if ester form) which could be interesting from the commercial standpoints if they are free from other pigments such as chlorophylls or other components such as sterols.
Extraction with supercritical CO2 (CO2-SCF) is always interesting with regard to the recovery of pharmaceutical or nutraceutical substances for its cleanness and the lack of toxicity of CO2 as a solvent. CO2-SCF has been applied to a wide variety of vegetal matter with the purpose of extracting different carotenoids with modest results in the vast majority of cases. The efficiencies of CO2-SCF in the references given in Table 4 are not absolute values, but comparisons against classic solvent extractions (e.g., in Soxhlet) carried out with solvents such as hexane, methanol, or dimethylformamide (DMF). When no cosolvent is used, CO2-SCF gives results that are much poorer than that of classic solvent extraction even for microalgae with such a weak cell wall as Dunaliela. Macías-Sánchez et al. (2009b) attempted to enhance the extraction yield of CO2-SCF with the use of ultrasound with only moderate results, similar to the enhancements obtained by Gao et al. (2009) processing marigold petals. Another drawback is that CO2-SCF tends to extract chlorophylls with an efficiency even greater than carotenoids, giving extracts heavily contaminated. This is also reported by Kitada et al. (2009) for Chlorella vulgaris. The use of cosolvent only attains moderate recovery enhancements and spoils one of the mains advantages of the CO2-SCF extraction, the instant elimination of the solvent. Thus, taking into account the cost of SCF and ASE equipment, it is difficult that those techniques can compete with solvent extraction, especially taking into account that those must be performed on dry biomass.
The selective absorption of lutein on solid phases could be a very effective separation/purification technique especially if the use of a specific phase (Shen et al. 2009) can be coupled with a contact method such as the expanded bed described by Bermejo et al. (2007) which allows the processing of raw extracts and are tolerant even to the presence of cell debris or other particulate matter which causes great problems in conventional chromatography. The last technique shown in Table 4 is a selective precipitation described by Miguel et al. (2008) who proposed the use of supercritical CO2 after a classic solvent extraction of the carotenoids. The solvent containing the carotenoids is then mixed with supercritical CO2 and the conditions of pressure and temperature adjusted to promote the precipitation of lutein. The work of Miguel et al. (2008) described experiments done with dissolutions of lutein especially prepared and not with real extracts of carotenoids obtained from vegetal sources, but still seems a promising possibility worth exploring.
In summary, currently, only extraction with solvent seems to have attained a degree of efficiency and purity sufficient to consider it as a basis for the large-scale production of lutein, although the selective adsorption in solid phase done in expanded bed contactors or the selective precipitation with supercritical CO2 would be interesting advances. Also, new solvents with obvious advantages, such as ethyl lactate, have been proposed for other vegetal matter (Ishida and Chapman 2009) and could be applied to microalgae.
Another significant improvement would be to eliminate the need of drying the microalgal biomass prior to the extraction processes. In this sense, Fernández-Sevilla et al. (2008) have proposed a modification of the procedure described by Ceron et al. (2008) aimed at the direct processing of wet biomass paste (20% dry weight) based on an extraction phase composed by hexane/ethanol/water and KOH to simultaneously accomplish the alkaline treatment for the ionization of susceptible lipids, as well as the extraction. This reduces to a minimum the number of operations and would allow the coupled design with a production photobioreactor operating in continuous mode that would eliminate the need of biomass stabilization and storage.
Application of lutein from microalgae to human health
Given the current prices of microalgal biomass, the most feasible application of microalgae as lutein producers is for pharmaceutical or nutritional products. With over 100,000,000 potential patients at risk of suffering AMD only in the eastern world, the 6 mg daily recommendation means a very large market. Lutein can be incorporated in the human diet with the consumption of green leafy vegetables, but this has several disadvantages. It requests a discipline hard to maintain for practical reasons, the amount of lutein varies in the different vegetables and even cultivars, and the most important difficulty is that the effective dose of lutein delivered to the bloodstream (bioavailability) depends on the preparation, the presence of other foods (particularly fats or fiber), and even of the patient physiology. Therefore, lutein is prescribed to patients at risk of developing AMD in the form of nutritional supplements that, taken regularly, ensure a uniform daily delivery. Pure lutein is obtained from marigold oleoresin by a patented process which includes a recrystallization step, but crystalline lutein is difficult to handle and rather susceptible to oxidation, so it is usually sold in the form of crystal suspensions in oil, usually safflower or corn, containing up to 20% weight lutein or lutein esters. These concentrates can be added to different formulas or diluted in more oil to concentrations of 10–20 mg/ml lutein to make soft gels.
Microalgae could be a source of lutein in this same fashion if the carotenoid concentrates described in the former section are purified and processed in the same way as marigold oleoresin, but another important question is if there is a way in which a oleoresin, concentrate, or even microalgal biomass can be used as a delivery form with competitive advantages over marigold products. One key factor to decide what is a good delivery form is how effective it is promoting the absorption of lutein. The more complete the absorption, the lower the dose needed and the cheaper the formulation. The effectivity with which a component is transferred from the food to the bloodstream by the digestion process is measured by the concept of bioavailability. This concept allows estimating the amount of component in the food that reaches the bloodstream and is, therefore, available to be delivered to the retina. Bioavailability comprises different steps. The first one is bioaccessibility (“digestibility”) which is defined as the amount of a food component that is released from the food matrix during digestion. Then, fat-soluble components must be incorporated into mixed micelles before absorption. Thus, the efficiency of this second step called micellization (quantities transferred into the aqueous micellar fraction) combines with digestibility to determine the bioavailability of carotenoids (Failla and Chitchumroonchokchai 2005). Interest in the bioavailability of lutein has greatly increased along with the interest on the carotenoid itself and is here revised with the aim of proposing an optimal product as a part of an optimized production process. The bioavailability of several foods has been compiled by O'Neill et al. (2001) and is compared in Table 5 with the experiments on bioavailability of lutein in microalgal biomass of S. almeriensis and lutein extracts from this microalgae in olive oil carried out by Granado-Lorencio et al. (2009).
The results indicate that, although S. almeriensis biomass is a very rich source of lutein that could supply the recommended lutein daily dose with only 0.5 g of biomass (with 1% lutein), the amount ready for absorption at the end of digestion is negligible in spite of the pretreatments given as described in Granado-Lorencio et al. (2009) and Ceron et al. (2008). Something similar happens with some of the recommended dietary sources as broccoli, lettuce, and specially, with spinach. This very low rate of transfer is probably related to the very high fiber content of these matrices (Lahaye 2006). On the other hand, the all-fat matrices prepared with extracts of S. almeriensis in olive oil resulted in very high rates of absorption. Although these kinds of formulations can also be prepared with extracts from other sources such as marigold, it seems clear that the use of a fat matrix has clear advantages as these offer the possibility of delivering a high dose of lutein with a small amount of product used and with a high repeatability compared to the ingestion of foods such as spinach or kale. It must be highlighted that the use of olive oil, in particular, as matrix has shown advantages in experiments carried out in mammals in vivo (Lakshminarayana et al. 2006).
Thus, from the point of view of bioavailability, the current production process of lutein from marigold is inefficient. This must be taken into account when designing a process for the production of lutein based on microalgae that could be competitive with marigold by ensuring an efficient and reliable absorption. The scheme shown in Fig. 5 summarizes the effect of a formulation that favors the absorption of lutein compared to common foods.
Therefore, obtaining pure lutein or even high concentration extracts is not efficient from the point of view of bioavailability. The numbers for foods such as vegetables of the commercial concentrates vary because their bioavailability will be affected by the other foods ingested in the same meal. It can be drawn from Fig. 5 that the most effective way of reliably supplying a given daily intake of lutein is to ingest in the form of an oil suspension not too concentrated. Bearing this in mind, in the next section are evaluated the prospects and feasibility of microalgae-based lutein production processes with the current knowledge and available technologies.
Conclusions and prospects
Currently, oleoresins obtained from marigold are a well-established source of lutein for different purposes. The economy of these processes heavily relays in the availability of cheap labor and in an extensive use of land that can be of bad quality and not usable for other crops (such as abrupt territories or nonleveled lands), but in this case, the crops require even more extensive labor. Although there are wide differences in the yields that can be attained from marigold cultivation (Ramesh and Singh 2008; Crnobarac et al. 2009; Diaz-Avelar et al. 2004), 13 kg flowers ha−1 day−1 is an optimistic average to take as a basis for comparison. This reduces by 85% when the petals are separated and when humidity is lost, resulting in 1.9 kg of dry petals per hectare and day, requesting up to nine cycles of soil preparation, seeding, crop raising, and harvesting. The content of lutein also varies significantly as can be seen in the comprehensive reports of Li et al. (2002) and Piccaglia et al. (1998). These authors evaluated different cultivars of Tagetes obtaining in average 420 and 170 mg lutein per 100 g of dry petals. Taking 295 mg per 100 g as appropriate and bearing in mind that the drying process and ensilage cause a 50% loss of lutein, an average productivity of approximately 3,000 mg lutein per day and hectare is obtained.
On the other hand, microalgal systems show productivities in the order of hundreds of milligrams of lutein per square meter and day (see Table 1) which translate to kilograms per hectare per day. Thus, the outdoor 50-L tubular system based in Murielopsis reported by Del Campo et al. (2004) produces an annual maximum of 1,800 g ha−1 day−1, 600 times higher that the estimated 3 g ha−1 day−1 for Tagetes. The case reported by Sánchez et al. (2008a, b) is even more significant because the productivity obtained is a year-round average of 2,900 g ha−1 day−1 operating in a pilot scale and particularly because the system has been redesigned and scaled up to 28,000 L as shown in Fig. 4. The system depicted in the figure consists of 10 × 2,800-L units in a fence-type configuration that occupies 500 m2, resulting in a culture density of 56 L m−2. The system is fully automated, including medium preparation, which is done using agriculture-grade methods, and can be operated by a single worker. A conservative estimation of the productivity of lutein of this system is 220 mg m−2 day−1 and thus 2,200 g ha−1 day−1. Therefore, this system that occupies 500 m2 would be equivalent to 36 Ha of marigold, as estimated. This highlights the advantages of microalgae in terms of labor and land occupation.
The availability of extraction methods specifically designed for microalgae such as that described by Ceron et al. (2008) is another important factor because all the industry is currently directed to marigold oleoresin as the intermediate product coming from the producing countries. This oleoresin is later processed to produce pure lutein that is then diluted in oil to give the products finally marketed to supplement producers. In this sense, the process proposed by Fernández-Sevilla et al. (2008), specifically designed for microalgae wet paste and with the aim of producing extracts of low to medium concentration directly recovered in vegetable oil and preferably in olive oil, which has been shown to be particularly adequate for the stability of lutein, is interesting. These extracts have been shown to be the best absorbed by the human digestive system and also the most suitable to ensure that the patient takes a known dose reliably and regularly.
A lot remains to be done, but the potential of microalgae seems undeniable in view of the numbers presented. Important issues will have to be solved, such as the industry inertia and the legal status of the carotenoids from microalgae, but if socioeconomics change in the producing countries, such as labor price or land availability, microalgae should be in the list of feasible alternatives.
References
Arnal E, Miranda M, Almansa I, Muriach M, Barcia JM, Romero FJ, Diaz-Llopis M, Bosch-Morell F (2009) Lutein prevents cataract development and progression in diabetic rats. Graefes Arch Clin Exp Ophthalmol 247(1):115–120
Bendich A, Olson JA (1989) Biological actions of carotenoids. FASEB J 3:1927–1932
Bermejo R, Ruiz E, Acién FG (2007) Recovery of B-phycoerythrin using expanded bed adsorption chromatography: scale-up of the process. Enzyme Microb Technol 40(4):927–933
Blanco AM, Moreno J, Del Campo JA, Rivas J, Guerrero MG (2007) Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl Microbiol Biotechnol 73:1259–1266
Carpentier S, Knaus M, Suh M (2009) Associations between lutein, zeaxanthin, and age-related macular degeneration: an overview. Crit Rev Food Sci Nutr 49(4):313–326
Ceron MC, Campos I, Sánchez JF, Acien FG, Molina E, Fernandez-Sevilla JM (2008) Recovery of lutein from microalgae biomass: development of a process for Scenedesmus almeriensis. J Agric Food Chem 56:11761–11766
Chiu CJ, Taylor A (2007) Nutritional antioxidants and age-related cataract and macular degeneration. Exp Eye Res 84:229–245
Coleman H, Chew E (2007) Nutritional supplementation in age-related macular degeneration. Curr Opin Ophthalmol 18:220–223
Crnobarac J, Jaćimović G, Marinković B, Mircov VD, Mrđa J, Babić M (2009) Dynamics of pot marigold yield formation depended by varieties and row distance. Nat Prod Commun 4(1):35–38
Del Campo JA, Moreno J, Rodriguez H, Vargas MA, Rivas J, Guerrero MG (2000) Carotenoid content of chlorophycean microalgae: factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J Biotechnol 76:51–59
Del Campo JA, Rodrıguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2001) Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J Biotechnol 85:289–295
Del Campo JA, Rodriguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2004) Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl Microbiol Biotechnol 64:848–854
Del Campo JA, Garcia-Gonzalez M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74(6):1163–1174
Delgado-Vargas F, Jiménez AR, Paredes-López O, Francis FJ (2000) Crit Rev Food Sci Nutr 40(3):173–289
Demmig-Adams B, Adams WW III (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1(1):21–26
Demmig-Adams B, Adams WW III (2002) Antioxidants in photosynthesis and human nutrition. Science 298:2149–2153
Diaz-Avelar J, Barrios JA, Jiménez B (2004) Effect of the application of acid treated biosolids on marigold (Tagetes erecta) development. Water Sci Technol 50(9):33–40
Failla M, Chitchumroonchokchai C (2005) In vitro models as tools for screening the relative bioavailabilities of provitamin A carotenoids in foods. Technical Monographs Series 3, HarvestPlus
Farrow WM, Tabenkin K (1966) Process for the preparation of lutein. US Patent 3,280,502
Fernández-Sevilla JM, Molina Grima E, Perez Parra J, Acien Fernandez FG, Magan Cañadas JJ, Friedl T (2005) Novel microalgal species and use thereof for animal and/or human consumption and in the production of carotenoids. Spanish Patent P200500374, International Application 06725770.9-1212-ES2006000072
Fernández-Sevilla JM, Acien Fernandez FG, Perez-Parra J, Magán Cañadas JJ, Granado-Lorencio F, Olmedilla B (2008) Large-scale production of high-content lutein extracts from S. almeriensis. Proceedings of the 11th International Conference on Applied Phycology, Galway, Ireland
Gao Y, Nagy B, Liu X, Simándi B, Wang Q (2009) Supercritical CO2 extraction of lutein esters from marigold (Tagetes erecta L.) enhanced by ultrasound. J Supercrit Fluids 49:345–350
Granado F, Olmedilla B, Blanco I (2003) Nutritional and clinical relevance of lutein in human health. Br J Nutr 90:487–502
Granado-Lorencio F, Herrero-Barbudo C, Acién-Fernandez FG, Molina-Grima E, Fernandez-Sevilla JM, Perez-Sacristan B, Blanco-Navarro I (2009) In vitro bioaccesibility of lutein and zeaxanthin from the microalgae Scenedesmus almeriensis. Food Chem 114:747–752
Herrero M, Martín-Álvarez PJ, Señoráns FJ, Cifuentes A, Ibáñez E (2005) Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem 93(3):417–423
Hojnik M, Skerget M, Knez Z (2008) Extraction of lutein from marigold flower petals—experimental kinetics and modelling. Lebensm-Wiss Technol 41:2008–2016
Ishida BK, Chapman MH (2009) Carotenoid extraction from plants using a novel, environmentally friendly solvent. J Agric Food Chem 57:1051–1059
John JH, Ziebland S, Yudkin P, Roe LS, Neil HAW (2002) Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet 359(9322):1969–1974
Khachik F (2007) Process for extraction and purification of lutein, zeaxanthin and rare carotenoids from marigold flowers and plants. US Patent 7,173,145
Kitada K, Machmudah S, Sasaki M, Goto M, Nakashima Y, Kumamoto S, Hasegawa T (2009) Supercritical CO2 extraction of pigment components with pharmaceutical importance from Chlorella vulgaris. J Chem Technol Biotechnol 84(5):657–661
Krinsky NI, Landrum JT, Bone RA (2003) Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 23:171–203
Lahaye M (2006) Marine algae as sources of fibres: determination of soluble and insoluble dietary fibre contents in some “sea vegetables”. J Sci Food Agric 54(4):587–594
Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V (2006) Enhanced lutein bioavailability by lyso-phosphatidylcholine in rats. Mol Cell Biochem 281:103–110
Li H, Jiang Y, Chen F (2002) Isolation and purification of lutein from the microalga Chlorella vulgaris by extraction after saponification. J Agric Food Chem 50:1070–1072
Macías-Sánchez MD, Mantell C, Rodríguez M, Martínez de la Ossa E, Lubián LM, Montero O (2005) Supercritical fluid extraction of carotenoids and chlorophyll a from Nannochloropsis gaditana. J Food Eng 66:245–251
Macías-Sánchez MD, Mantell Serrano C, Rodríguez Rodríguez M, Martínez de la Ossa E, Lubián LM, Montero O (2008) Extraction of carotenoids and chlorophyll from microalgae with supercritical carbon dioxide and ethanol as cosolvent. J Sep Sci 31:1352–1362
Macías-Sánchez MD, Mantell Serrano C, Rodríguez Rodríguez M, Martínez de la Ossa E (2009a) Kinetics of the supercritical fluid extraction of carotenoids from microalgae with CO2 and ethanol as cosolvent. Chem Eng J 150:104–113
Macías-Sánchez MD, Mantell C, Rodríguez M, Martínez de la Ossa E, Lubián LM, Montero O (2009b) Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta 77:948–952
Matsukawa R, Hotta M, Masuda Y, Chihara M, Karube I (2000) Antioxidants from carbon dioxide fixing Chlorella sorokiniana. J Appl Phycol 12:263–267
Miguel F, Martin A, Mattea F, Cocero MJ (2008) Precipitation of lutein and co-precipitation of lutein and poly-lactic acid with the supercritical anti-solvent process. Chem Eng Process 47:1594–1602
Mínguez-Mosquera I, Gandul-Rojas M, Lourdes B, Gallardo-Guerrero M (1992) Rapid method of quantification of chlorophylls and carotenoids in virgin olive oil by high-performance liquid chromatography. J Agric Food Chem 40(1):60–63
Navarrete-Bolaños JL, Rangel-Cruz CL, Jimenez-Islas H, Botello-Alvarez E, Rico-Martınez R (2005) Pre-treatment effects on the extraction efficiency of xanthophylls from marigold flower (Tagetes erecta) using hexane. Food Res Int 38:159–165
Nonomura AM (1987) Process for producing a naturally-derived carotene/oil composition by direct extraction from algae. US Patent 4,680,314
O'Neill ME, Carroll Y, Corridan B, Olmedilla B, Granado F, Blanco Y (2001) A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br J Nutr 85(4):499–507
Piccaglia R, Marotti M, Grandi S (1998) Lutein and lutein esterc ontent in different types of Tagetes patula and T. erecta. Ind Crops Prod 8:45–51
Qingxiang M, Xiang X, Yanxiang G, Qi W, Jian Z (2008) Optimisation of supercritical carbon dioxide extraction of lutein esters from marigold (Tagetes erect L.) with soybean oil as a co-solvent. Int J Food Sci Technol 43:1763–1769
Ramesh K, Singh V (2008) Effect of planting date on growth, development, aerial biomass partitioning and essential oil productivity of wild marigold (Tagetes minuta) in mid hills of Indian western Himalaya. Ind Crops Prod 27(3):380–384
Roberts RL, Green J, Lewis B (2009) Lutein and zeaxanthin in eye and skin health. Clin Dermatol 27(2):195–201
Sánchez F, Fernández JM, Acien FG, Rueda A, Perez-Parra J, Molina E (2008a) Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem 43(4):398–405
Sánchez JF, Fernández-Sevilla JM, Acién FG, Cerón MC, Pérez-Parra J, Molina-Grima E (2008b) Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Appl Microbiol Biotechnol 79(5):719–729
Semba RD, Dagnelie G (2003) Are lutein and zeaxanthin conditionally essential nutrients for eye health? Med Hypotheses 61(4):465–472
Shen Y, Hu Y, Huang K, Yin S, Chen B, Yao S (2009) Solid-phase extraction of carotenoids. J Chromatogr 1216(30):5763–5768
Shi X, Zhang X, Chen F (2000) Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzyme Microb Technol 27:312–318
Shi XM, Jiang Y, Chen F (2002) High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol Prog 18(4):723–727
Shi X, Wu Z, Chen F (2006) Kinetic modelling of lutein production by heterotrophic Chlorella at various pH and temperatures. Mol Nutr Food Res 50(8):763–768
Wei D, Chen F, Chen G, Zhang XW, Liu LJ, Zhang H (2008) Enhanced production of lutein in heterotrophic Chlorella protothecoides by oxidative stress. Sci China Ser C Life Sci 51(12):1088–1093
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández-Sevilla, J.M., Acién Fernández, F.G. & Molina Grima, E. Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol 86, 27–40 (2010). https://doi.org/10.1007/s00253-009-2420-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2420-y