Abstract
A total of 96 barrows (48 pure-bred Bama mini-pigs representing fatty genotype, and 48 Landrace pigs representing lean genotype) were randomly assigned to either a low- or adequate-protein treatment diet. The experimental period commenced at 5 weeks of age and extended to the finishing period. After euthanasia, blood and skeletal muscle samples were collected from pigs at the nursery, growing, and finishing phases. Our results indicate that the concentrations of free AAs in the plasma and muscle decreased as the age of the pigs increased. In addition, a strain × growth phase interaction (P < 0.05) was observed for the free AA pool in the plasma and muscle. The low-protein diet upregulated (P < 0.05) the mRNA levels for T1R1/T1R3 involved in glutamate binding, but downregulated (P < 0.05) the mRNA levels for PAT1, PAT2, and ASCT2, which transport neutral AAs into muscles. Bama mini-pigs had higher (P < 0.05) mRNA levels for LAT1, SNAT2, and EAAC1, but a lower (P < 0.05) mRNA level for PepT1, compared with Landrace pigs. Collectively, our findings indicate that adequate provision of dietary protein plays an important role in regulating profiles of free AA pools and expression of key AA/peptide transporters/transceptors in a genotype- and tissue-specific manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A balanced supply of dietary proteins and amino acids (AAs) is necessary for the optimal growth, development, and reproduction of animals (Deng et al. 2007a, b, 2009; Wu et al. 2010, 2011; Wu 2010, 2014), as well as enhancing feed efficiencies in livestock production and minimizing its impact on environmental health (Yin and Tan 2010; Wu et al. 2014a, b; Chen et al. 2014). Recent studies have demonstrated that AAs are not only building blocks for protein synthesis in cells and signaling molecules (Wu et al. 2010, 2012, 2013a, b, 2015; Li et al. 2011; Wang et al. 2014a, b, 2015a, b) but also regulators of gene expression (He et al. 2013; Chen et al. 2014; Zhang et al. 2013; Ren et al. 2013a, b, c, 2014a, b; Tan et al. 2011; Yang et al. 2013; Feng et al. 2014; Tang et al. 2014, Yin et al. 2014; Kong et al. 2014) and protein posttranslational regulation (Kong et al. 2012). Additionally, AAs are key precursors for the biosynthesis of hormones and low-molecular-weight nitrogenous substances (Wu 2013a, b). These physiological functions depend on the optimal concentrations of AAs and their metabolites, including peptides, polyamines, glutathione, taurine, thyroid hormones, and serotonin in the blood, cells, and tissues (Ren et al. 2013a, b; Wu et al. 2014c).
Through changes in intracellular concentrations, AAs act as signaling molecules that regulate metabolic pathways involved in muscle growth (Sancak et al. 2008) and other tissues (Brosnan and Brosnan 2013; Fernstrom 2013; San Gabriel and Uneyama 2013). This signaling function plays an important role in the regulation of skeletal muscle hypertrophy in monogastric animals through the activation of specific cell-signaling pathways (Tan et al. 2009; Yao et al. 2008). For example, the mammalian target of rapamycin (mTOR) represents a crucial kinase for protein synthesis and cell growth (Wang and Proud 2011; Zoncu et al. 2011). AA uptake requires numerous transport systems that vary in their substrate specificity, affinity, and velocity (Wu 2013a). Dietary proteins are digested in the small intestine, which releases free AAs and oligo-peptides that are absorbed by enterocytes and subsequently utilized by the liver and other organs (Davila et al. 2013; Jobgen et al. 2006). Thus, after a meal, marked changes are noted in the concentrations of free AAs in the intestinal lumen, blood plasma, and intracellular and extracellular spaces (Blachier et al. 2009).
As pig strains may vary in their capacity to recognize changes in AA concentration in body fluids, the utilization of free AAs may also differ among pig strains. The Bama mini-pig (Sus scrofa domestica) is an indigenous Chinese “fatty” strain, mainly found in Bama County of the Guangxi Province of China. Because their anatomical, physiological, and metabolic characteristics are similar to those of humans, Bama mini-pigs have been widely used in experiments for extrapolation to human metabolism and physiology (Kawaguchi et al. 2011; Liu et al. 2008). In contrast, the leaner Landrace pig has rapid growth rate and yields leaner meat, which are nutritional and commercial advantages. We hypothesized that differences between these two strains of pigs in their muscle growth, meat quality, and intermuscular adipose deposition (Liu et al. 2015) may lead to dietary protein-dependent differences in AA metabolism. The major objectives of this study were to measure free AA pools in the plasma and muscle tissues, as well as mRNA levels for AA and peptide transporters and receptors in the skeletal muscles of Bama mini-pigs and Landrace pigs fed low- or adequate-protein diets.
Materials and methods
Animals, diets, and treatments
All experiments were carried out in accordance with Chinese guidelines for animal welfare, and experimental protocols were approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, the Chinese Academy of Sciences (Yin et al. 2009). A total of 96 barrows [48 purebred Bama mini-pigs with an average initial body weight (BW) of 3.38 ± 0.96 kg, and 48 Landrace pigs with an average initial BW of 7.68 ± 0.89 kg] were fed the test diets from 5 weeks of age until they attained their finishing BW. The experiment was a 2 × 2 factorial arrangement, with two pig strains (Bama mini-pigs and Landrace pigs) and two diets [the National Research Council (NRC) diet and the Chinese conventional diet (GB)], resulting four dietary treatments (Table 1). Forty-eight piglets from each strain were randomly assigned to one of the two dietary treatments. The NRC diet, which had a higher protein level, was formulated to meet the nutrient requirements outlined by the NRC (2012), whereas the GB diet was formulated to conform to the recommendations of the Chinese National Feeding Standard for Swine (Ministry of Agriculture of the People’s Republic of China, 2004), and had a lower protein content (Table 2). The dietary AA composition, which was determined as described by Dai et al. (2014), is shown in Table 3. All pigs had free access to drinking water and their experimental diets (Yin et al. 2015). The room temperature was maintained at 25–27 °C. All pigs were fed three times a day (0800, 1300, and 1800) (Li et al. 2015).
Sample collection
Body weights for nursery, growing, and finishing phases in Landrace pigs were in the ranges of 7–20, 20–50, and 50–90 kg, respectively, whereas for Bama mini-pigs, they were in the ranges of 3–15, 15–35, and 35–55 kg, respectively (Table 1). At the end of each phase, eight pigs from each treatment group were randomly sampled. Briefly, after recording pre-slaughter BW and fasting the animals for 12 h, blood samples were obtained from the jugular vein and placed in 10 mL centrifuge tubes containing sodium heparin (14.3 USP units/mL) (Xiao 2015). The samples were then centrifuged at 900×g for 10 min at 4 °C to recover plasma, which was stored at −20 °C until analysis of free AAs was performed. The pigs were then placed under general anesthesia and killed by jugular vein injection of 4 % sodium pentobarbital solution (40 mg/kg BW). After the head, legs, tail, and viscera were removed, the carcass was split longitudinally. Samples of the longissimus dorsi (LDM) and biceps femoris (BFM) muscles on the right side of the carcass were collected immediately and visible intermuscular adipose tissue was carefully removed (Li et al. 2007; Yang et al. 2005). The samples were snap-frozen in liquid nitrogen, and stored at −80 °C for subsequent analysis (Chen et al. 2011).
Determination of free amino acids in plasma
Plasma free AA concentrations were determined as previously described (Kong et al. 2009). Briefly, 1 mL of the plasma sample and 2.5 mL of 7.5 % trichloroacetic acid solution were mixed thoroughly and centrifuged at 12,000×g and 4 °C for 15 min (Ren et al. 2014c). The supernatant fluid was collected for analysis of free AAs by an ion-exchange AA analyzer (L8800, Hitachi, Tokyo, Japan).
Determination of free amino acids in muscle
To measure the concentrations of free AAs in muscle tissue, approximately 1 g of freeze-dried muscle was homogenized in 10 mL of 10 mmol/L hydrochloric acid. The solution was adjusted to a final volume of 25 mL by adding 10 mmol/L hydrochloric acid. After centrifuging at 12,000×g for 10 min, 2 mL of the supernatant liquid was mixed with 2 mL of 8 % 5-sulfosalicylic acid. After centrifuging at 12,000×g for 10 min, the supernatant fluid was filtered through a 0.45-μm membrane before analysis of AAs using an ion-exchange AA analyzer (L8800, Hitachi, Tokyo, Japan).
RNA extraction and cDNA synthesis
Total RNA was isolated from the LDM and BFM tissues frozen in liquid N using the TRIzol reagent (Invitrogen-Life Technologies, Carlsbad, CA, USA) and treated with DNase I (Invitrogen) according to the manufacturer’s instructions. The RNA quality was confirmed with 1 % agarose gel electrophoresis and stained with 10 μg/mL ethidium bromide. The RNA had an OD260:OD280 ratio between 1.8 and 2.0 (Feng et al. 2015). The first-strand cDNA was synthesized with Oligo (dT) 20 and Superscript II reverse-transcriptase (Invitrogen), according to the manufacturer’s instructions.
Determination of mRNA levels in muscle
Primers for the selected genes were designed using the Primer 5.0 software (Table 4). Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed using the SYBR Green detection kit (TaKaRa, Japan), which contained MgCl2, dNTP, and HotStar Taq Polymerase as in our previous study (Liu et al. 2015). Briefly, an aliquot (2 μL) of a cDNA template (corresponding to 25 ng of total RNA) solution was added to a total volume of 10 μL containing 5 μL SYBR Green mix, 0.2 μL ROX Reference Dye (50 X), and 0.2 μL of either forward or reverse primers. After a pre-denaturation program (10 s at 95 °C), 40 cycles of amplification were performed (95 °C for 10 s followed by 60 °C for 20 s), followed by a melting curve program (60–99 °C with a heating rate of 0.1 °C/s and fluorescence measurement). The fluorescence signal was detected by the ABI Prism 7900 HT (Applied Biosystems, Marsiling Industrial Estate Road 3, Singapore). A melting curve was generated for each sample at the end of each run to ensure the purity of the amplified products. The amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in each sample was used to normalize the mRNA levels of the selected genes. The relative expression ratio (R) of mRNA was calculated using the following formula:
where ΔΔCt (sample − control) = (C t target gene − C t GAPDH) for the sample − (C t target gene − C t GAPDH) for the control.
RT-PCR efficiencies were determined by the amplification of a series of dilutions of cDNA according to the equation 10(−1/slope), as described by Bustin et al. (2009), and were found to be consistent between target mRNA and GAPDH. For the negative controls, cDNA was replaced with water (Wang et al. 2009).
Statistical analysis
Data were analyzed by multifactor ANOVA using the GLM procedure of SAS 9.1 for Windows (SAS Institute Inc., Cary, NC, USA), and by comparing means with Tukey’s method. The effects of pig strain, dietary protein level, physiological stage, and their interactions were all taken into account. Log transformation of variables was performed when variance of data was not homogenous among treatment groups, as assessed using the Levene’s test (Wei et al. 2012). Results are presented with means plus pooled SEM. Effects were considered statistically significant at P < 0.05. Probability values between 0.05 and 0.10 were considered to be trends.
Results
Plasma concentrations of free amino acids
As shown in Table 5, the concentrations of most AAs in the plasma were affected by the developmental stages of the pigs. As the age increased, the concentrations of Ala, Arg, Asn, Asp, Glu, Gly, Ile, Orn, Pro, Ser, Tau, and Tyr gradually decreased (P < 0.05). Plasma concentrations of 3-methylhistidine (3 M His), Cys, Gln, His, Phe, and Thr were much higher in the nursery phase (P < 0.05) than in the growing and finishing phases, but the concentration of 1 M His was lower (P < 0.05) in the nursery stage as compared to the growing and finishing phases. In contrast, the plasma concentration of α-aminobutyric acid was greater (P < 0.05) in the finishing phase than in the other two phases.
When compared to Landrace pigs, Bama mini-pigs had higher (P < 0.05) plasma concentrations of 3 M His, α-aminoadipic acid, Ile, and Val, and lower (P < 0.05) concentrations of Asp throughout the trial. In addition, Bama mini-pigs had higher (P < 0.05) plasma concentrations of Leu and Trp and a lower (P < 0.05) concentration of Cys in the nursery phase. They also had lower (P < 0.05) plasma concentrations of Ala, Cys, Gly, Met, Orn, and Ser in the growing phase, and lower (P < 0.05) concentrations of Ala, Gly, Met, and Ser but a higher (P < 0.05) concentration of Trp in the finishing phase, as compared to Landrace pigs.
Overall, the NRC diet increased (P < 0.05) plasma concentrations of 1 M His, 3 M His (except for Landrace pigs in the finishing phase), α-aminoadipic acid, and α-aminobutyric acid, as compared with the GB diet. When pigs were fed the GB diet, plasma concentrations of Gly increased (P < 0.05), especially in the finishing phase. Phase × strain interactions were observed for most AAs, notably 1 M His, Ala, Asn, Cys, Gly, His, Ile, Leu, Lys, Met, Orn, Pro, Ser, Tau, Trp, Tyr, and Val. Diet type interacted with the developmental phase for the plasma concentrations of α-aminobutyric acid, Cys, Orn, and Trp, and with the pig strain for the concentrations of Asn, Lys, Orn, Ser, Trp, and Tyr. No interactions among phase, strain, and diet were observed for any AA.
Free amino acid pools in longissimus dorsi muscle
The concentrations of free AAs in LDM are shown in Table 6. The concentrations of Ala, Asp, Glu, Gly, Orn, Pro, and Tyr in both strains of pigs decreased (P < 0.05) over time, while those of carnosine and anserine increased (P < 0.05). Most AAs decreased (P < 0.05) during the growing phase (as compared to the nursery phase), but increased (P < 0.05) at the finishing phase, including α-aminoadipic acid, Arg, GABA, Gln, Ile, Lys, Met, Phe, Ser, Thr, and Val. In contrast, as compared to the nursery phase, the concentrations of 3 M His and Asn increased (P < 0.05) during the growing phase and decreased (P < 0.05) in the finishing phase.
We found that pig strain affected (P < 0.05) the concentrations of free AAs in LDM. More specifically, Landrace pigs had a higher (P < 0.05) intramuscular Ala concentration but lower (P < 0.05) intramuscular 3 M His concentration than Bama mini-pigs throughout the trial. Landrace pigs also had higher (P < 0.05) intramuscular concentrations of Arg, Asp, Glu, Leu, Lys, Met, Phe, Ser, Thr, and Tyr in the nursery phase, of Leu and Phe in the growing phase, and of Arg in the finishing phase. Furthermore, the responses of intramuscular AA profiles to the different dietary levels of protein were dependent on pig strain.
Pigs eating the GB diet had increased (P < 0.05) α-aminoadipic acid concentration in their LDM, as compared to those eating the NRC food. The Bama mini-pigs eating the GB diet also had a greater (P < 0.05) carnosine concentration in their LDM during the growing and finishing phases. In contrast, the Gln concentrations in the LDM of Landrace pigs consuming the GB diet during the growing and finishing phases were lower (P < 0.05) than those of pigs eating the NRC diet.
Free amino acid pools in biceps femoris muscle
In BFM, the concentrations of 3 M His, anserine, and carnosine increased (P < 0.05) gradually, while those of Ala, Arg, Asn, Glu, Gly, Orn, and Pro decreased (P < 0.05) with increasing age (Table 7). The intramuscular concentrations of Asp, His, Ile, Leu, Lys, Met, Phe, Ser, Thr, Tyr, and Val in the nursery phase were higher (P < 0.05) than those in the growing and finishing phases.
Landrace pigs had higher (P < 0.05) concentrations of β-Ala, GABA, and Phe, and lower concentrations of anserine and 3 M His in BFM (P < 0.05) than Bama mini-pigs throughout the trial. In addition, as compared to Bama mini-pigs, Landrace pigs had higher (P < 0.05) intramuscular concentrations of Ala, Arg, Leu, Asn, Gly, Lys, Met, Pro, Ser, Thr, and Tyr in the nursery phase, of Asn, Gly, Met, and Pro in the growing phase, and of Tyr in the finishing phase.
Overall, the contrasting protein contents of the NRC and GB diets affected intramuscular AA pools in the two strains of pigs differently. However, the NRC diet increased (P < 0.05) intramuscular His concentration regardless of pig strain, as well as the intramuscular concentrations of Ala and Gly in both strains of pigs (except for Bama mini-pigs in the finishing phase).
mRNA levels for AA-sensing genes in muscle
As shown in Table 8, the mRNA levels for Calpain-3 in the LDM decreased (P < 0.05) as age increased. The mRNA level for mTOR increased (P < 0.05) in the growing phase, but decreased (P < 0.05) in the finishing phase, as compared to the nursery phase. In the LDM of Bama mini-pigs, the mRNA level for mTOR was higher (P < 0.05), while that for calpain-3 (especially in the growing and finishing phases) was lower (P < 0.05) than in Landrace pigs. A strain × phase interaction was observed (P < 0.05) for the mRNA level of Calpain-3.
The mRNA level for mTOR in BFM increased (P < 0.05) over time. Landrace pigs had higher (P < 0.05) mRNA levels in BFM associated with calpain-3 and T1R3 (especially in the nursery and finishing phases) than Bama mini-pigs. Furthermore, the NRC diet enhanced (P < 0.05) the mRNA level for calpain-3 but reduced (P < 0.05) that for T1R3 in BFM, when compared to the GB diet. Strain × phase interactions were observed (P < 0.05) for mRNA levels for T1R1 and T1R3 in BFM.
mRNA levels for AA and peptide transporters in muscle
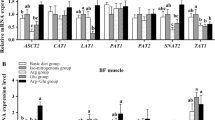
Table 9 shows that, with advancing age, SNAT2 mRNA levels in skeletal muscle increased (P < 0.05), while b0,+ transporter mRNA levels decreased (P < 0.05). Compared to the nursery phase, the PAT1 mRNA level increased (P < 0.05) during the growing phase and decreased (P < 0.05) in the finishing phase. In contrast, the mRNA levels for PAT2 and transporter EAAC1 decreased (P < 0.05) during the growing phase and increased (P < 0.05) in the finishing phase, when compared to the nursery phase. When strain differences were compared, the mRNA levels for SNAT2, EAAC1, and PAT1 in LDM were higher (P < 0.05), while those for the oligopeptide transporter PepT1 and AA transporter b0,+ were lower (P < 0.05) in Bama mini-pigs than in Landrace pigs. All pigs fed the NRC diet had higher (P < 0.05) mRNA levels for PAT1, LAT1, and PepT1 than those fed the GB diet. A strain × diet interaction (P < 0.05) was observed for the mRNA level of PAT2, and strain × phase interactions (P < 0.05) were observed for SNAT2, b0,+, and EAAC1 mRNA levels in LDM.
As shown in Table 10, the mRNA levels for b0,+ and PepT1 transporters in BFM increased (P < 0.05) with age. The mRNA levels for PAT1 and SNAT2 increased (P < 0.05) during the growing phase and decreased (P < 0.05) in the finishing phase, when compared to the nursery phase. The mRNA levels for LAT1 and ASCT2 decreased (P < 0.05) in the growing phase and increased (P < 0.05) in the finishing phase, when compared to the nursery phase. In contrast to Landrace pigs, Bama mini-pigs had higher (P < 0.05) mRNA levels for LAT1 and ASCT2 in the nursery and finishing phases, as well as mRNA levels for PepT1 in nursery and growing phases, and lower (P < 0.05) mRNA levels for PepT1 in the finishing phase. The NRC diet enhanced (P < 0.05) the mRNA levels for LAT1 and ASCT2 to a greater extent than the GB diet. Strain × diet interactions (P < 0.05) were observed for mRNA levels corresponding to LAT1 and b0,+, and strain × phase interactions (P < 0.05) were observed for mRNA levels corresponding to ASCT2, SNAT2, and PepT1 in BFM.
Discussion
The small intestine is a major site of AA catabolism in humans and animals (Swaid et al. 2013; Wang et al. 2008). In this organ, enterocytes utilize AAs for ATP production, protein synthesis, and generation of various metabolites that exert physiological effects locally and in peripheral tissues (Blachier et al. 2013). Intestinal metabolism plays an important role in the entry of dietary AAs into the portal circulation as well as the plasma pattern of AAs (Riedijk et al. 2007; Wu 1998). In pigs, the capacity of the intestines to catabolize AAs can vary with age and the time post feeding, which results in fluctuating AA concentrations in the portal blood over time (Blachier et al. 2013). In the present study, the concentrations of plasma AAs were the highest during the nursery phase for both pig strains, regardless of diet, and then declined markedly as age increased.
Plasma AA concentrations are affected by a variety of factors, including intracellular protein synthesis and degradation in tissues, AA transport and metabolism, and intestinal microbiota activity (Blachier et al. 2007; Dai et al. 2011, 2012, 2013). There is evidence that the pig small intestine extensively catabolizes AA in a segment-dependent manner (Dai et al. 2015; Yang et al. 2014) such that nearly 50 % of total dietary AA (the sum of all AA) do not enter the portal circulation. Based on results of the present study, we suggest that an overall increase in AA catabolism in the pig small intestine of occurs with age. Substantial differences in several plasma AAs were observed between Bama mini-pigs and Landrace pigs. The plasma concentrations of the branched-chain amino acids (BCAAs), namely Ile, Leu, and Val, in Landrace pigs were much lower than those in Bama mini-pigs, especially during the nursery and finishing phases. The decrease in the circulating concentrations of AAs in Landrace pigs may be secondary to increased catabolism in the small intestine, skeletal muscle, and other tissues, since BCAAs provide α-amino groups for the endogenous synthesis of glutamine, especially in skeletal muscle (Wu 2009; Yoneda et al. 2009). In addition, Leu activates the Ser/Thr protein kinase mTOR signaling pathway that upregulates protein synthesis and cell growth (Duan et al. 2015). We also found that Bama mini-pigs had higher concentrations of 3 M His than Landrace pigs throughout the trial. In some species such as cattle (Houweling et al. 2012), an increase in the circulating concentration of 3 M His is a useful indicator of muscle protein degradation. If this is also true for growing swine, our findings suggest that Bama mini-pigs may have a greater rate of muscle proteolysis and a lower rate of AA deposition in muscule protein. Further studies are needed to test this hypothesis.
Concentrations of free AA in tissues reflect the nutritional status of an animal (He et al. 2012; Sales et al. 2013), as protein synthesis is regulated by intracellular AA concentration (Miyazaki and Esser 2009). In addition, free AAs are essential for tissue growth because they regulate protein synthesis and catabolism to favor net protein deposition in tissues, especially skeletal muscle (Burrin et al. 1995). In the present study, concentrations of most free AA in the muscles of Landrace pigs were higher than those in Bama mini-pigs, which may contribute to dynamic protein turnover and muscle growth in this lean pig strain.
Biological sensing of AA in vivo plays a key role in coupling changes in whole-body protein and AA metabolism, which allows appropriate physiological responses. Receptors for umami taste and sweet taste are closely related to each other (San Gabriel and Uneyama 2013). The umami taste receptor T1R1/T1R3 mediates the response to umami ligands, such as monosodium glutamate. In the present study, T1R1/T1R3 expression was higher in the BFM muscle of pigs fed the GB diet than in those fed the NRC diet, indicating that a low-protein diet upregulates the gene expression of the umami taste receptor. In addition, pig genotype interacted with the developmental stage regarding the mRNA levels corresponding to T1R1/T1R3 receptors. It remains to be determined whether changes in mRNA levels for these receptors can be translated into changes in their protein abundances.
Previous studies have indicated that AA-induced activation of mTORC1 is developmentally regulated in skeletal muscle (Suryawan and Davis 2010; Suryawan et al. 2013), and indeed the present study shows that as age increased, the expression of mTOR in BFM also increased. Additionally, expression of mTOR in LDM was higher in the growing phase but lower in the finishing phase. The calpain system plays an important role in myofibrillar protein degradation. Muscle growth and postmortem tenderization of meat are highly related to the degree of proteolysis, and therefore, the calpain system activity affects muscle growth and meat tenderness (Tait et al. 2014). The decreased expression of calpain-3 with increasing age might indicate a lower level of tenderness as the animals growing. Results of this investigation showed that the calpain-3 mRNA level in Landrace pigs was higher than that in Bama mini-pigs, suggesting improved meat tenderness in the Landrace strain.
Recent studies have indicated that AA transporters not only act as nutrient transporters, but also as nutrient signaling components responsible for the activation of mTORC1, which activates protein translation (Heublein et al. 2010; Nicklin et al. 2009; Pinilla et al. 2011). Our results showed that the strain of pig affected the mRNA levels for AA transporters. In particular, the mRNA levels for SNAT2, EAAC1, and PAT1 in LDM, and LAT1 and ASCT2 in BFM were higher, while the mRNA levels for PepT1 (especially in the finishing phase) were lower in Bama mini-pigs, compared with Landrace pigs. In addition, an increasing number of studies have demonstrated that large neutral AAs (BCAAs and aromatic AAs) in the plasma are taken up by muscle cells via the large neutral AA transporter LAT1 (Suryawan et al. 2013), which is crucial for platelet-derived growth factor-induced vascular smooth muscle growth (Liu et al. 2004). SNAT2 transports glutamine into the cell for the LAT1-CD98 bi-transport system to export Gln and increase the influx of large neutral AA such as Leu (Baird et al. 2009). Both LAT1 and SNAT2 are related to the activation of the mTOR signaling pathway (Nicklin et al. 2009; Pinilla et al. 2011). EAAC1 is also a key transporter for glutamate (Fu et al. 2013). Upregulation of muscular AA transporters allows for greater uptake and accumulation of AAs in muscle tissue and, therefore, enhances lean protein deposition (Nishimura and Naito 2008). Accordingly, Bama mini-pigs may have the potential to transport more neutral AA and glutamate, but fewer oligopeptides than Landrace pigs, because PepT1 is a proton-dependent transporter for di- and tri-peptides (Daniel 2004). Further investigation is required to test this hypothesis.
The growth and development of pigs involves not only changes in weight and shape, but also alterations in chemical composition and physiological functions (Blachier et al. 2013; Wu et al. 2004; Hu et al. 2015). In the present study, the mRNA levels of most AA transporters increased with age, such as SNAT2 in LDM, and b0,+ AT and PepT1 transporters in BFM. Similarly, Feng et al. (2008) showed that mRNA levels for b0,+ AT and y+ LAT1 transporters in crossbred growing pigs increased with age. In contrast, the mRNA levels for PAT1 in both LDM and BFM increased during the growing phase and decreased during the finishing phase, while the mRNA levels for PAT2 and EAAC1 in LDM and LAT1 and ASCT2 in BFM decreased during the growing phase but increased during the finishing phase. These differences may be due to changes in AA requirements in response to physiological alterations in animals (Hou et al. 2015; Wu et al. 2013). We also found that the mRNA level for the b0,+ AT transporter decreased in LDM but increased in BFM with advancing age. The system b0,+ is an antiporter that takes up cationic AAs and Cys in exchange for neutral AAs (Chen et al. 2009; Wang et al. 2013). Differences in muscle-subtype may explain the variation in expression of this AA transporter.
Food intake stimulates muscle protein synthesis, which is triggered by the postprandial rise in AAs (Suryawan and Davis 2011). Diets with high levels of AAs may further stimulate protein synthesis, as found in our study in which the NRC diet increased mRNA levels for AA transporters, including PAT1, LAT1, and PepT1 in LDM and of LAT1 and ASCT2 in BFM. Thus, dietary protein affects the growth performance and excretion of dietary nitrogen in pigs (He et al. 2015). According to previous studies (Wu 1998, 2011; Kong et al. 2009), higher levels of nutrients, especially AAs, that enter the portal vein from the small intestine can promote tissue protein synthesis in animals. Therefore, the NRC diet that had a higher level of protein may improve the absorption of dietary AAs and may also directly regulate the metabolism of absorbed nutrients through a signal transduction mechanism. In this regard, it is noteworthy that expression of the proton-assisted AA transporters PAT1 and PAT2 are affected by dietary protein intake. PAT1 and PAT2 are not only responsible for the transport of a variety of small neutral AAs (Goberdhan et al. 2005), but they also have the capability to act as transceptors (Goberdhan 2010) to affect muscle protein metabolism.
In summary, the genetic background and dietary level of protein intake markedly affected free AA concentrations in pig plasma and skeletal muscle, as well as mRNA levels for key AA receptors and transporters in skeletal muscle. These effects of genotype and diet varied with the developmental stage of the animals. Collectively, our findings provide a molecular basis for future development of effective nutritional strategies to increase nutrient utilization in pig production.
References
Baird FE, Bett KJ, MacLean C et al (2009) Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol-Endoc M 3:E822–E829
Blachier F, Mariotti F, Huneau JF et al (2007) Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 4:547–562
Blachier F, Boutry C, Bos C et al (2009) Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 3:814s–821s
Blachier F, Wu G, Yin Y et al (2013) Developmental amino acid metabolism in the pig small and large intestine epithelial cells. In: Nutritional and physiological functions of amino acids in pigs. F. Blachier, G. Wu, and Y. Yin eds. Springer (Wien), pp 59–74
Brosnan JT, Brosnan ME (2013) Glutamate: a truly functional amino acid. Amino Acids 45:413–418
Burrin DG, Davis TA, Ebner S et al (1995) Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 5:593–599
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 4:611–622
Chen LX, Li P, Wang JJ et al (2009) Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids 1:143–152
Chen RJ, Yin YL, Pan J, Gao YL, Li TJ (2011) Expression profiling of IGFs and IGF receptors in piglets with intrauterine growth restriction. Livestock Science 136:72–75
Chen S, Liu S, Zhang F, Ren WK, Li Z, Yin J, Duan JL, Peng YY, Liu G, Yin YL, Wu GY (2014) Effects of dietary l-glutamine supplementation on specific and general defense responses in mice immunized with inactivated Pasteurella multocida vaccine. Amino Acids. Amino Acids 46:2365–2375
Dai ZL, Wu GY, Zhu WY (2011) Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 16:1768–1786
Dai ZL, Li XL, Xi PB et al (2012) Regulatory role for l-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 43:233–244
Dai ZL, Li XL, Xi PB et al (2013) L-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 45:501–512
Dai ZL, Wu ZL, Jia SC et al (2014) Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J Chromatogr B 964:116–127
Dai ZL, Wu ZL, Hang SQ et al (2015) Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol Hum Reprod 21:389–409
Daniel H (2004) Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66:361–384
Davila AM, Blachier F, Gotteland M et al (2013) Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res 1:95–107
Deng D, Huang RL, Li TJ, Wu GY, Xie MY, Tang ZR, Kang P, Zhang YM, Fan MZ, Kong XF, Ruan Z, Xiong H, Deng ZY, Yin YL (2007a) Nitrogen balance in barrows fed low-protein diets supplemented with essential amino acids. Livestock Science 109:220–223
Deng D, Li AK, Chu WY, Huang RL, Li TJ, Kong XF, Liu ZJ, Wu GY, Zhang YM, Yin YL (2007b) Growth performance and metabolic responses in barrows fed low-protein diets supplemented with essential amino acids. Livestock Science 109:224–227
Deng D, Yao K, Chu WY, Li TJ, Huang RL, Yin YL, Liu HQ, Zhang JS, Wu GY (2009) Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low-protein diet. J Nutr Biochem 20:544–552
Duan YH, Li FN, Liu HN et al (2015) Nutritional and regulatory roles of leucine in muscle growth and fat reduction. Front Biosci-Landmark 20:796–813
Feng DY, Zhou XY, Zuo JJ et al (2008) Segmental distribution and expression of two heterodimeric amino acid transporter mRNAs in the intestine of pigs during different ages. J Sci Food Agr 6:1012–1018
Feng Z, Zhou XL, Wu F, Yao K, Kong XF, Li TJ, Blachier F, Yin YL (2014) Both dietary supplementation with monosodium l-glutamate and fat modify circulating and tissue amino acid pools in growing pigs, but with little interactive effect. PLoS One 9:e84533
Feng ZM,Li TJ, Wu L, Xiao DF, Blachier F, Yin YL (2015) Monosodium l-glutamate and/or dietary fat differently modifies the composition of the intestinal microbiota in growing pigs. Obesity Facts 8:87–100
Fernstrom JD (2013) Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids 45:419–430
Fu DZ, Yang HS, Kong XF et al (2013) Molecular cloning and expression profiling of excitatory amino acid carrier 1 in suckling Huanjiang mini-piglets with large or small body weight at birth. Mol Biol Rep 4:3341–3350
Gang Chen, Zhang Jun, Zhang Yuzhe, Liao Peng, Li Tiejun, Chen Lixiang, Yin Yulong, Wang Jinquan, Guoyao Wu (2014) Oral MSG administration alters hepatic expression of genesfor lipid and nitrogen metabolism in suckling piglets. Amino Acids 46:245–250
Goberdhan DCI (2010) Intracellular amino acid sensing and mTORC1-regulated growth: new ways to block an old target? Curr Opin Invest Dr 12:1360–1367
Goberdhan DCI, Meredith D, Boyd CAR et al (2005) PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development 10:2365–2375
He Q, Ren P, Kong X et al (2012) Comparison of serum metabolite compositions between obese and lean growing pigs using an NMR-based metabonomic approach. J Nutr Biochem 2:133–139
He LQ, Yang HS, Hou YQ, Li TJ, Fang J, Zhou XH, Yin YL, Wu L, Nyachoti M, Wu GY (2013) Effects of dietary l-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids 45:383–391
He LQ, Wu L, Xu ZQ et al (2015) Low-protein diets affect ileal amino acid digestibility and gene expression of digestive enzymes in growing and finishing pigs. Amino Acids. doi:10.1007/s00726-015-2059-1
Heublein S, Kazi S, Ogmundsdottir MH et al (2010) Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene 28:4068–4079
Hou YQ, Yin YL, Wu G (2015) Dietary essentiality of “nutritionally nonessential amino acids” for animals and humans. Exp Biol Med. doi:10.1177/1535370215587913
Houweling M, van der Drift SGA, Jorritsma R et al (2012) Technical note: quantification of plasma 1- and 3-methylhistidine in dairy cows by high-performance liquid chromatography-tandem mass spectrometry. J Dairy Sci 6:3125–3130
Hu SD, Li XL, Rezaei R et al (2015) Safety of long-term dietary supplementation with l-arginine in pigs. Amino Acids 47:925–936
Jobgen WS, Fried SK, Fu WI et al (2006) Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17:571–588
Kawaguchi H, Miyoshi N, Miura N et al (2011) Microminipig, a non-rodent experimental animal optimized for life science research: novel atherosclerosis model induced by high fat and cholesterol diet. J Pharmacol Sci 2:115–121
Kong XF, Yin YL, He QH et al (2009) Dietary supplementation with Chinese herbal powder enhances ileal digestibilities and serum concentrations of amino acids in young pigs. Amino Acids 4:573–582
Kong XF, Tan B, Yin YL et al (2012) L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem 9:1178–1183
Kong XF, Wang X, Yin YL, Li XL, Gao HJ, Bazer F, Wu GY (2014) Putrescine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Biol Reprod 91(106):1–10
Li LL, Yin YL, Liu YH, Hou DX, Hou ZP, Yang CB, Yang XJ (2007) Intramuscular administration of zinc metallothionein to preslaughter stressed pigs improves anti-oxidative status and porl quality. Asian-Austr J Animal Sci 20:761–767
Li FN, Yin YL, Tan B et al (2011) Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 5:1185–1193
Li FN, Duan YF, Li YF, Yin YL, geng MM, Oladele OA, Kim SW, Yin YL (2015) Effects of dietary n-6:n-3 PUFA ratio on fatty acid composition, free amino 2 acid profile and gene expression of transporters in finishing pigs British Journal of Nutrition 113, 739–748
Liu XM, Reyna SV, Ensenat D et al (2004) Platelet-derived growth factor stimulates LAT1 gene expression in vascular smooth muscle: role in cell growth. FASEB J 18:768–770
Liu Y, Zeng BH, Shang HT et al (2008) Bama miniature pigs (sus scrofa domestica) as a model for drug evaluation for humans: comparison of in vitro metabolism and in vivo pharmacokinetics of lovastatin. Comp Med 6:580–587
Liu YY, Li FN, He LQ et al (2015) Dietary protein intake affects expression of genes for lipid metabolism in porcine skeletal muscle in a genotype-dependent manner. Br J Nutr 113:1069–1077
Ministry of Agriculture of the People’s Republic of China (2004) Feeding Standard of Swine (GB, NY/T 65-2004). China Agriculture Press, Beijing
Miyazaki M, Esser KA (2009) Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J Appl Physiol 4:1367–1373
National Research Council (NRC) (2012) Nutrient requirements of swine. National Academy Press, Washington
Nicklin P, Bergman P, Zhang BL et al (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 3:521–534
Nishimura M, Naito S (2008) Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab Pharmacokinet 1:22–44
Pinilla J, Aledo JC, Cwiklinski E et al (2011) SNAT2 transceptor signalling via mTOR: a role in cell growth and proliferation? Front Biosci 3:1289–1299
Ren W, Li Y, Yin Y et al (2013a) Structure, metabolism and functions of amino acids: An overview. In: Nutritional and physiological functions of amino acids in pigs, F. Blachier, G. Wu and Y. Yin Eds, Springer (Wien), pp 91–108
Ren WK, Liu SP, Chen S et al (2013b) Dietary l-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids 4:947–955
Ren WK, Yin J, Zhu XP, Liu G, Li NZ, Peng YY, Yin YL (2013c) Glutamine on intestinal inflammation: a mechanistic perspective. Euro J Inflamm 11:13–24
Ren WK, Chen S, Yin J, Duan JL, Li TJ, Liu G, Feng ZM, Tan BE, Yin YL, Wu GY (2014a) Dietary Arginine Supplementation of Mice Alters the Microbial Population and Activates Intestinal Innate Immunity. J Nutr 144:568–579
Ren WK, Chen S, Yin J, Duan JL, Li TJ, Liu G, Feng ZM, Tan BE, Yin YL, Wu GY (2014b) Dietary l-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids. Amino Acids 46:2403–2413
Ren WK, Yin J, Wu MM, Liu G, Yang G, Xion X, Su DD, Wu L, Li TJ, Chen S, Duan JL, Yin YL, Wu GY (2014c) Serum amino acids profile and the beneficial effects of l-arginine or l-glutamine supplementation in dextran sulfate sodium colitis. PLoS One 9:e88335
Riedijk MA, Stoll B, Chacko S et al (2007) Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc Natl Acad Sci USA 9:3408–3413
Sales F, Pacheco D, Blair H et al (2013) Muscle free amino acid profiles are related to differences in skeletal muscle growth between single and twin ovine fetuses near term. SpringerPlus 1:1–9
San Gabriel A, Uneyama H (2013) Amino acid sensing in the gastrointestinal tract. Amino Acids 45:451–461
Sancak Y, Peterson TR, Shaul YD et al (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 5882:1496–1501
Suryawan A, Davis TA (2010) The abundance and activation of mTORC1 regulators in skeletal muscle of neonatal pigs are modulated by insulin, amino acids, and age. J Appl Physiol 5:1448–1454
Suryawan A, Davis TA (2011) Regulation of protein synthesis by amino acids in muscle of neonates. Front Biosci (Landmark Ed) 16:1445–1460
Suryawan A, Nguyen HV, Almonaci RD et al (2013) Abundance of amino acid transporters involved in mTORC1 activation in skeletal muscle of neonatal pigs is developmentally regulated. Amino Acids 3:523–530
Swaid F, Sukhotnik I, Matter I et al (2013) Dietary glutamine supplementation prevents mucosal injury and modulates intestinal epithelial restitution following acetic acid induced intestinal injury in rats. Nutr Metab:1–9
Tait RG, Shackelford SD, Wheeler TL et al (2014) μ-Calpain, calpastatin, and growth hormone receptor genetic effects on preweaning performance, carcass quality traits, and residual variance of tenderness in Angus cattle selected to increase minor haplotype and allele frequencies. J Anim Sci 2:456–466
Tan B, Yin YL, Liu ZQ et al (2009) Dietary l-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 1:169–175
Tan B, Yin YL, Liu ZQ et al (2011) Dietary l-arginine supplementation differentially regulates expression of lipid-metabolic genes in porcine adipose tissue and skeletal muscle. J Nutr Biochem 5:441–445
Wang XM, Proud CG (2011) mTORC1 signaling: what we still don’t know. J Mol Cell Biol 4:206–220
Wang JJ, Chen LX, Li P et al (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 6:1025–1032
Wang WC, Shi CY, Zhang JS et al (2009) Molecular cloning, distribution and ontogenetic expression of the oligopeptide transporter PepT1 mRNA in Tibetan suckling piglets. Amino Acids 4:593–601
Wang WC, Blachier F, Fu DZ et al (2013) Ontogenic expression of the amino acid transporter b0, + AT in suckling Huanjiang piglets: effect of intra-uterine growth restriction. Brit J Nutr 5:823–830
Wang WW, Dai ZL, Wu ZL et al (2014a) Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46:2037–2045
Wang WW, Wu ZL, Lin G et al (2014b) Glycine stimulates protein synthesis and inhibits oxidative stress in pig small-intestinal epithelial cells. J Nutr 144:1540–1548
Wang H, Zhang C, Wu G et al (2015a) Glutamine enhances tight-junction protein expression and modulates CRF signaling in the jejunum of weanling piglets. J Nutr 145:25–31
Wang H, Ji Y, Wu G et al (2015b) L-Tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. J Nutr 145:1156–1162
Wei JW, Carroll RJ, Harden KK et al (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wu GY (1998) Intestinal mucosal amino acid catabolism. J Nutr 8:1249–1252
Wu GY (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu GY (2010) Functional amino acids in growth, reproduction, and health. Adv Nutr 1:31–37
Wu G (2013a) Amino Acids: Biochemistry and Nutrition. CRC Press, Boca Raton, Florida
Wu G (2013b) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu GY (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol 5:34
Wu G, Knabe DA, Kim SW (2004) Arginine nutrition in neonatal pigs. J Nutr 134:2783S–2783S
Wu G, Bazer FW, Burghardt RC et al (2010) Impacts of amino acid nutrition on pregnancy outcome in pigs: Mechanisms and implications for swine production. J Anim Sci:E195–E204
Wu X, Yin YL, Li TJ, Wang L, Ruan Z, Liu ZQ, Hou YQ (2010b) Dietary protein, energy and arginine affect LAT1 expression in forebrain white matter differently. Animal 4:1518–1521
Wu G, Bazer FW, Johnson GA et al (2011) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu X, Zhang J, Liu ZQ, Li TJ, Yin YL (2012) Effects of oral supplementation with glutamate or combination of glutamate and N-carbamylglutamate on intestinal mucosa in piglets. J Anim Sci 90:337–339
Wu X, Shu XG, Xie CY, Li TJ, Hu J, Yin YL, Deng ZY (2013) The acute and chronic effects of monosodium l-glutamate on serum iron and total iron-binding capacity in the jugular. Artery and vein of pigs. Biol Trace Elem Res 153:191–195
Wu G, Wu ZL, Dai ZL et al (2013a) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Wu X, Xie C, Yin YL, Li FN, Li TJ, Huang RL, Deng ZY (2013b) Effect of l-arginine on HSP70 expression in liver in weanling piglets. BMC Veterinary Research 9:63
Wu G, Fanzo J, Miller DD et al (2014a) Production and supply of high-quality food protein for human consumption: sustainability, challenges and innovations. Ann NY Acad Sci 1321:1–19
Wu G, Bazer FW, Cross HR (2014b) Land-based production of animal protein: impacts, efficiency, and sustainability. Ann NY Acad Sci 1328:18–28
Wu G, Bazer FW, Dai ZL et al (2014c) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Wu X, Xie CY, Zhang YZ, Fan ZY, Yin YL, Blachier F (2015) Glutamate–glutamine cycle and exchange in the placenta–fetus unit during late pregnancy. Amino Acids 47:45–53
Xiao,H, Wu MM, Shao FY, Tan BE, Li TJ Ren WK, Yin J,. Wang Q. He H, Yin YL, Hou RQ (2015) Metabolic profiles in the response to supplementation with composite antimicrobial peptides in piglets challenged with deoxynivalenol. J Anim Sci. doi:10.2527/jas2014-8229
Tang YL, Tan BE, Xiong X, L FN, Ren WK, Kong XF, Qiu W, Hardwidge P, Yin YL (2014) Methionine deficiency reduces autophagy and accelerates death in intestinal epithelial cells infected with enterotoxigenic Escherichia coli. Amino Acids. DOI 10.1007/s00726-014-1781-4
Yang CB, Li AK, Yi YL, Huang RL, Li TJ, Li LL, Liao YP, Deng ZY, Zhong HY, Yang XJ, Fan MZ (2005) Effects of dietary supplementation of cysteamine on growth performance, carcass quality, serum hormones and gastric ulcer in finishing pigs. J Sci Food Agric 85:1947–1952
Yang HS, Fu DZ, Kong XF et al (2013) Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets. J Anim Sci 6:2740–2748
Yang YX, Dai ZL, Zhu WY (2014) Important impacts of intestinal bacteria on utilization of dietary amino acids in pigs. Amino Acids 46:2489–2501
Yao K, Yin YL, Chu WY et al (2008) Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr 5:867–872
Yin YL and Tan BE (2010) Manipulation of dietary nitrogen, amino acids and phosphorus to reduce environmental impact of swine production and enhance animal health. J Food Agri Environ 8:447–462
Yin FG, Liu YL, Yin YL et al (2009) Dietary supplementation with Astragalus polysaccharide enhances ileal digestibilities and serum concentrations of amino acids in early weaned piglets. Amino Acids 2:263–270
Yin J, Ren WK, Duan JL, Wu L, Chen S, Li TJ, Yin YL, Wu GY (2014) Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids 46:883–892
Yin J, Duan LJ, Cui ZJ, Ren WK, Li TJ, Yin YL (2015) Hydrogen peroxide-induced oxidative stress activates NF-kB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 5:15479–15486
Yoneda J, Andou A, Takehana K (2009) Regulatory roles of amino acids in immune response. Curr Rheumatol Rev 4:252–258
Zhang J, Yin YJ, Shu X, Li TJ, Li FN, Tan BE, Wu ZL, Wu GY (2013) Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids 45:1169–1177
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Bio 1:21–35
Acknowledgments
The present work was jointly supported by grants from the National Basic Research Program of China (No. 2012CB124704 and 2013CB127305), National Nature Science Foundation of China (31372325, 31270044), K.C. Wong Education Foundation (Hong Kong), and Texas A&M AgriLife Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Y., Kong, X., Li, F. et al. Co-dependence of genotype and dietary protein intake to affect expression on amino acid/peptide transporters in porcine skeletal muscle. Amino Acids 48, 75–90 (2016). https://doi.org/10.1007/s00726-015-2066-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2066-2