Abstract
The objective of this study was to evaluate effects of dietary l-lysine on the intestinal mucosa and expression of cationic amino acid transporters (CAT) in weaned piglets. Twenty-eight piglets weaned at 21 days of age (Duroc × Landrace × Yorkshire; 6.51 ± 0.65 kg body weight) were assigned randomly into one of the four groups: Zein + LYS (zein-based diet + 1.35 % supplemental lysine), Zein − LYS (zein-based diet), NF (nitrogen-free diet), and CON (basal diet). The experiment lasted for 3 weeks, during which food intake and body weight were recorded. At the end of the trial, blood was collected from the jugular vein of all pigs, followed by their euthanasia. Dietary supplementation with lysine enhanced villus height and crypt depth in the jejunum (P < 0.05). Jejunal mRNA levels for the b0,+-AT, y+LAT1 and CAT1 genes were greater (P < 0.05) in the Zein + LYS group than in the control, and the opposite was observed for CAT1. Dietary content of lysine differentially affected intestinal CAT expression to modulate absorption of lysine and other basic amino acids. Thus, transport of these nutrients is a key regulatory step in utilization of dietary protein by growing pigs and lysine in the diet influences the expression of amino acid transporters in the small intestine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Establishing optimal requirements for amino acids (AA) is critically important for maximizing performance of weaned piglets (Kim et al. 2005). Diets for piglets should be formulated to meet requirements for both nutritionally essential and non-essential AA in an ideal pattern (Wu et al. 2013). Lysine is typically the first limiting AA in swine diets (Gatrell et al. 2013; Wu 2010a) and, therefore, it is important to understand how dietary lysine is absorbed by the small intestine of piglets (He et al. 2009; Li et al. 2009, 2011a, b; Wu et al. 2009).
Amino acids are transported by neutral, acidic, or basic AA transporters (Kanai and Endou 2003; Seow et al. 2004; Wu 2013a). Cationic amino acid transporters (CAT) are widely distributed in tissues and play an important role in the transport of arginine, histidine, lysine, and ornithine to regulate their homeostasis (Russell et al. 2003). The different carrier proteins mediating transport of cationic AA include Na+-independent systems y+, y+L, b0,+, and b+, as well as the Na+-dependent system B0,+ (Mann et al. 2003; Wu et al. 2009). There are many reports which indicated that the importance of CAT in the small intestine to maintain homeostasis of basic AA and overall protein nutrition in the body (Bröer et al. 2000).
Complex relationships exist among neutral and cationic AA transporters (François et al. 2004). However, no studies have examined effects of dietary intake of l-lysine on expression of CAT in the small intestine of developing pigs, particularly in response to the feeding of zein as the exclusive source of protein. Therefore, the present study was conducted with weaned pigs to fill in this gap of knowledge and also to evaluate effects of dietary l-lysine intake on growth performance, as well as intestinal mucosal morphology and CAT expression.
Materials and methods
The experimental design and procedures of this study were reviewed and approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, The Chinese Academy of Sciences.
Animals and dietary treatment
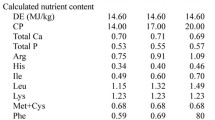
Twenty-eight piglets (offspring of Duroc × Landrace × Yorkshire) with body weight (BW) of 6.51 ± 0.65 kg (mean ± SD) were weaned at 21 days of age and were assigned randomly to one of the four dietary treatments (7 pigs/treatment). The young pigs were housed individually in cages and were allowed for a 1-week period of adaptation before being weighed and randomly allocated to dietary treatments (Table 1). Dietary treatments consisted of a basal diet (CON) formulated to meet nutrient specifications for weaned piglets (NRC 1998), two diets containing zein (Wujiang Bache Drug Company, Jiangsu, China) supplemented with 1.35 % lysine (the Zein + LYS group) or without lysine (the Zein − LYS group), and a N-free (NF) diet (Table 1). The zein-containing diets were formulated to contain similar nutrient levels (except for lysine in the Zein − LYS diet) to those in the CON group, but the sources of dietary protein were different from the CON group (Table 1). Zein contained the following nutrients (%, as-fed basis): dry matter, 94.73; crude protein, 85.2; Lys, 0.08; His, 1.91; Arg, 1.43; Val, 2.90; Ile, 3.00; and Leu, 19.2. Pigs were fed their respective diets for 3 weeks, during which feed intake and BW were measured weekly.
Tissue sample collection
At the end of the trial when pigs were 42-day-old, blood was collected from their jugular vein, followed by euthanasia, as described previously (Geng et al. 2011; Yin et al. 2010c). After euthanasia, the entire intestine and viscera were rapidly removed and dissected free of mesenteric attachments and placed on a smooth, cold surface tray (Hou et al. 2012; Wang et al. 2008). Intestinal segments (10 cm) were obtained from the proximal jejunum and the distal ileum, which were then thoroughly flushed with sterile saline (Yang et al. 2011; Zhou et al. 2011), immediately frozen in liquid nitrogen, and stored at −80 °C for analysis of gene expression or fixed in 10 % neutral buffered formalin for examination of intestinal morphology.
Intestinal morphology
Formalin-fixed jejunum and ileum samples were embedded in paraffin; cross sections of the segments were cut approximately 5 mm thick using a microtome and stained with hematoxylin and eosin (Wu et al. 1996). In each section, villus height and crypt depth were measured using a light microscope with a computer-assisted morphometric system (Yao et al. 2011). Villus height was defined as the distance from the villus tip to the crypt mouth, and crypt depth from the crypt mouth to the base (Zhou et al. 2011).
RNA extraction and cDNA synthesis for b0+-AT, CAT1, and y + LAT1 in the intestine
Intestinal and liver tissue samples were pulverized in liquid nitrogen. Total RNA was isolated from about 100 mg of the powder using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) and treated with DNase I (Invitrogen) according to the manufacturer’s instructions. Total RNA was reversed into cDNA using a SuperScript First-Strand Synthesis System kit (Invitrogen). Polymerase chain reaction amplification was performed in a total volume of 50 μl including Taq DNA polymerase and specific primers. Primers for b0,+-AT, CAT-1, y+LAT-1, and β-actin were designed with Primer 5.0 (Wang et al. 2009c). The following sequences of PCR primer pairs are used: forward:5′-GAACCCAAGACCACAAATC-3′, reverse: 5′-ACCCAGTGTCGCAAGAAT-3′ for b0,+-AT (180 bp); forward: 5′-CATCAAAAACTGGCAGCTCA-3′, Reverse: 5′-TGGTAGCGATGCAG TCAAAG-3′ for CAT-1 (185 bp); forward: 5′-TTTGTTATGCGGAACTGG-3′, reverse: 5′-AAAGGTGATGGCAATGAC-3′ for y+LAT1 (180 bp); forward: 5′-GGATGCAGA AGGAGAT CACG-3′, reverse: 5′-ATCTGCTGGAAGGTGGACAG-3′ for β-actin (145 bp).
Quantification of mRNA levels was performed using real-time PCR analysis
The quality of RNA was assessed by 1 % agarose gel electrophoresis, and stained with 10 μg/mL ethidium bromide. In all samples, RNA had an OD260:OD280 ratio between 1.8 and 2.0. Synthesis of the first strand of cDNA was performed using Oligo(dT)-20 and Superscript II reverse-transcriptase (Invitrogen). Primers for porcine b0,+-AT, CAT-1, and y+LAT1 cDNA sequences were designed to generate amplification products. β-actin was used as an internal reference gene to normalize target gene transcript levels. Real-time PCR was performed using the SYBR Green detection kit, containing MgCl2, dNTP, and Hotstar Taq polymerase. An aliquot (1 μL) of cDNA template solution was added to a total volume of 10 μL containing 5 μL of the SYBR Green mix, and 0.4 μL each of forward and reverse primers. The following protocol was used: (i) pre-denaturation program (30 s at 95 °C); (ii) amplification and quantification program, repeated 40 cycles (5 s at 95 °C, 30 s at 60 °C); and (iii) a melting curve program (extension at 72 °C). The relative quantification of gene amplification by RT-PCR was performed using cycle threshold (Ct) values (Fu et al. 2006). The comparative Ct value method was employed to quantitate mRNA levels for b0,+-AT, CAT-1 and y+LAT-1 relative to those for β-actin as described by Yin et al. (2011).
Statistical analysis
Data are presented as the mean ± SEM. One-way analysis of variance was used to analyze statistically experimental data using SAS 8.0 (Yin et al. 2004; Zhou et al. 2011). Differences among treatment means were determined by the Student–Newman–Keuls multiple comparison (Wei et al. 2012). Probability values <0.05 were taken to indicate statistical significance.
Results
Effects of diets with different l-lysine levels on growth performance
There were no differences (P > 0.05) in initial BW among the four groups of piglets (Table 2). However, compared with the control group, pigs fed the other three diets had lower (P < 0.05) final BW, lower feed intake, and lower growth performance. Moreover, the final BW of pigs in the Zein − LYS group was the lowest among all the study animals. In particular, the average daily gain (ADG) and average daily feed intake (ADFI) differed among the four dietary treatments (P < 0.05). Compared with the NF group, ADG, and ADFI in the Zein + LYS and Zein − LYS groups were decreased substantially (P < 0.05). Pigs in the CON group had normal growth, but the ADG was negative in the Zein + LYS, Zein − LYS and NF groups (Table 2).
Effects of diets with different l-lysine levels on intestinal morphology
Data on intestinal morphology are summarized in Fig. 1a and representative staining of jejunal and ileal mucosae is shown in Fig. 1b. In the control group, the jejunum and ileum in pigs fed the Zein + LYS diet had higher villus height (P < 0.05), whereas the ileal villus height in the NF fed pigs was similar to that in the control pigs. Pigs fed the Zein + LYS diet had the deepest crypts compared to pigs in all other treatments, whereas pigs fed the Zein − LYS had the shallowest crypts (P < 0.01). There were no differences (P > 0.05) in crypt depth between NF and the control piglets (Fig. 1a). Mucosal thickness in the jejunum was not influenced by dietary treatment. In the ileum, there were significant differences in villus height, mucosal thickness, and crypt depth between the control group and each of the other treatment groups (P < 0.05). Compared with control group, villus height in pigs fed the Zein + LYS and NF diets was decreased by 35.1 and 42.8 % (P < 0.05), respectively. Jejunal mucosal thickness was reduced by 40.8 % (P < 0.05) in NF fed pigs, compared with the control group. Pigs in the NF diet had a lower crypt depth than those fed the Zein − LYS diet (Fig. 1b).
a Effects of diets with different levels of l-lysine on intestinal morphology. Villus height (V–H), mucosal thickness (M-T), and crypt depth (C-D) were measured. Values are expressed as mean ± SEM, n = 7. Mean values with different letters differ (P < 0.05). Pigs in the Zein + LYS group were fed zein plus added l-lysine. Pigs in the Zein − LYS Group were fed zein without l-lysine supplementation. Pigs in the NF group were fed a nitrogen-free diet. Pigs in the control group were fed a basal diet. b Representative staining of the jejunal and ileum mucose in piglets. A was the Zein + LYS group, B was the Zein − LYS group, C was the NF group, D was the control group. Villus height, mucosal thickness and crypt depth from animals had a simple columnar epithelium smoothly covering the underlying lamina propria, as seen in the sections for the jejunum and the ileum
Expression of b0,+-AT, y+LAT1, and CAT1 mRNA in the small intestine of piglets fed different diets
Changes in b0,+-AT, y + LAT1, and CAT1 mRNA levels in the small intestine of piglets are shown in Table 3. The abundance of b0,+-AT, y+LAT-1, and CAT-1 mRNA in the jejunum was higher (P < 0.05) in the control group than in the other three treatment groups, except for y+LAT-1 in pigs fed the Zein + LYS diet. In the treatment groups other than the control group, pigs fed the Zein + LYS diet had greater (P < 0.05) values for b0,+-AT and y+LAT-1 than pigs fed the Zein − LYS and NF diets. No differences were detected between the Zein − LYS and NF dietary treatment. There were significant differences (P < 0.01) among treatment groups for the CAT-1 mRNA abundance. Especially, the expression of CAT-1 mRNA abundance in Zein + LYS group was the lowest. Compared with the NF group, pigs fed the Zein + LYS and Zein − LYS diets had 61.8 and 29.4 % lower levels of intestinal CAT-1 mRNA, respectively. Interestingly, b0,+-AT and y+LAT-1 mRNA were detected mostly in the jejunum (Table 2).
In the ileum, there were no difference (P > 0.05) in the abundance of b0,+-AT mRNA levels among dietary treatments (Table 3). However, y+LAT-1 or CAT-1 mRNA levels in the control group were different from the three other groups (P < 0.05). Compared with the control, y+LAT-1 and CAT-1 mRNA abundances in the Zein + LYS, Zein − LYS and NF groups were lower (P < 0.05). No differences were detected in y+LAT-1 or CAT-1 mRNA levels among pigs fed the Zein + LYS, Zein − LYS and NF diets (P > 0.05).
Expression of b0,+-AT, y + LAT1, and CAT1 mRNA in the different intestinal segments of pigs
Data on b0,+-AT, y + LAT1, and CAT1 mRNA levels in different segments of the small intestine are shown in Figs. 2, 3, and 4. The abundance of b0,+-AT mRNA (Fig. 2) differed among the four groups (P < 0.05); however, there were no differences among dietary treatments in the ileum (P > 0.05). In the jejunum, the abundance of b0,+-AT in pigs fed the Zein + LYS diet was higher (P < 0.05) compared to those fed the Zein − LYS and NF diets, but values were similar between the Zein − LYS and NF groups. Likewise, jejunal y+LAT1 mRNA abundance in the Zein + LYS group was the highest, and there were significant differences among the four groups (P < 0.05) (Fig. 3). Additionally, y+LAT1 mRNA abundance differed in the ileum (P < 0.05), and was higher in the Zein + LYS group than in the Zein − LYS and NF groups. CAT1 mRNA abundance was significantly influenced (P < 0.05) by dietary treatment such that values for the NF group were higher than those for the Zein + LYS and Zein − LYS groups (Fig. 4).
Abundance of b0+,-AT mRNA in the small intestine. Values are expressed as mean ± SEM, n = 7. Mean values with different letters differ (P < 0.05). Pigs in the Zein + LYS group were fed zein plus added l-lysine. Pigs in the Zein − LYS Group were fed zein without l-lysine supplementation. Pigs in the NF group were fed a nitrogen-free diet. Pigs in the control group were fed a basal diet
Abundance of y+LAT1 mRNA in the small intestine. Values are expressed as mean ± SEM, n = 7. Mean values with different letters differ (P < 0.05). Pigs in the Zein + LYS group were fed zein plus added l-lysine. Pigs in the Zein − LYS Group were fed zein without l-lysine supplementation. Pigs in the NF group were fed a nitrogen-free diet. Pigs in the control group were fed a basal diet
Expression abundance of CAT1 mRNA in the small intestine. Values are expressed as mean ± SEM, n = 7. Mean values with different letters differ (P < 0.05). Pigs in the Zein + LYS group were fed zein plus added l-lysine. Pigs in the Zein − LYS Group were fed zein without l-lysine supplementation. Pigs in the NF group were fed a nitrogen-free diet. Pigs in the control group were fed a basal diet
Discussion
Amino acids are essential nutrients for tissue protein synthesis and other metabolic functions in animals (Guo et al. 2008; Teng et al. 2010; Wang et al. 2005; Wu 2009, 2010b). In this study, we used zein as a protein source. Zein, a natural protein in corn, is severely deficient in basic AA (particularly lysine), compared with common feedstuffs for animal diets (Li et al. 2011a). Our results indicate that, compared with the NF and Zein − LYS groups, dietary supplementation with lysine influenced expression of the genes for transport of basic AA to affect intestinal morphology and regulate growth performance. Thus, an ideal dietary pattern for the weaned piglets can increase the efficiency of protein synthesis (Deng et al. 2010; Li et al. 2011b; Rezaei et al. 2013a, b); otherwise, feeding diets deficient in lysine will increase the oxidation of other AA to CO2, water, ammonia, and urea (Li et al. 2008; Wu 2010b). In the present study, growth performance of piglets in the Zein + LYS and Zein − LYS groups was lower than that in the NF group, which might be caused by different sources of diets. Notably, zein was the only protein source that may be imbalanced in the composition of AA (Table 1). Meanwhile, there are reports that excess AA are usually not stored in the body and are subjected to irreversible loss via oxidation and production of nitrogenous metabolites (Deng et al. 2010; Gattas and Silva 2012; Yin et al. 2010a, b, c) and, therefore, AA must be supplied daily through the diet (Wu 2009). Furthermore, growth performance of piglets feeding nitrogen-free diet can maintain weight gain in a short-term, but the components of the gain is likely fat rather than protein. In the NF group, the diet contained a large amount of corn starch which could provide energy and precursors for lipid synthesis.
The small intestine has a main function for nutrient absorption and transport. A normal structure of the small-intestinal mucosa is necessary for optimal growth as well as nutrient digestion and absorption (Rezaei et al. 2011; Sukhotnik et al. 2005; Yao et al. 2012). The present study shows that dietary supplementation with lysine attenuated an increase in villus height and crypt depth in the jejunum, but in the ileum lysine supplementation decreased mucosal thickness, villus height and crypt depth. Some studies have shown that degradation of lysine and other basic AA in the small intestine plays a role in modulating the intestinal villus, crypt, and mucosal thickness (Wang et al. 2009b) because their metabolites are essential for DNA and protein synthesis (Blachier et al. 2007; Tan et al. 2009; Wu et al. 2009). Because intestinal epithelial cells do not degrade lysine (Chen et al. 2007, 2009), microbes in the lumen of the intestine are likely responsible for lysine catabolism in the gut (Dai et al. 2010, 2012a, b). Furthermore, because weaning stress causes intestinal atrophy and dysfunction (Gu et al. 2002; Hampson 1986; Wang et al. 2009a), lysine nutrition plays a key role in maintaining gut integrity in weaned piglets (Wu et al. 2009). Thus, results of the current study provide an additional line of evidence that dietary lysine is necessary for maintaining intestinal mucosal integrity, particularly under stressful conditions such as weaning. Additionally, our findings also support that notion that a proper balance among AA (including both nutritionally essential and non-essential AA) in diets crucial for maximizing efficiency of animal production (Ren et al. 2012; Tan et al. 2012; Wang et al. 2013; Wu 2013a, b).
Nutrient content of a diet, especially AA, can affect AA transport by animal tissues (Stein et al. 1987; Wolfram et al. 1984). Dietary AA is absorbed via their transporter systems on the mucosal side of the small intestine. Several recent reports have suggested that diets with different lysine levels have different effects on the expression of basic AA transporters in the small intestinal mucosa of weanling piglets (Chen and Liou 2012; Deng et al. 2010; Zhi et al. 2010). Results of the present study indicate that the abundance of b0,+-AT, y+LAT-1, and CAT-1 mRNA in the jejunum was influenced by dietary content of lysine. For example, in pigs fed the Zein + LYS diet, the abundance of b0,+-AT and y+LAT-1 was highest, suggesting that dietary intake of lysine affects intestinal expression of these AA transporters. Interestingly, intestinal CAT1, expression was reduced in pigs fed the Zein + LYS diet. Thus, b0,+-AT and y+LAT-1 are expressed in the intestinal mucosa of young pigs, especially in the jejunum, but CAT1 mRNA is largely reduced in response to dietary lysine deficiency. Under nutritionally stress conditions, lysine deficiency may lead to an increase in expression of some AA transporters. Likewise, feeding a diet with imbalanced AA or a N-free diet, abundance of some AA transporters may be enhanced. It is evident that nutrient deficiency may activate the body’s own protection system to maintain animal normal growth. For example, Stein et al. (1987) reported that dietary supplementation of lysine could improve absorptive capacity for basic AA. Furthermore, substrate concentration can modulate transport of nutrients in the gastrointestinal tract (Zhi et al. 2010). In general, feeding diets with high protein or AA content may increase transport capacity for some AA in the small intestine. This improvement is mainly due to an increase in the relative levels of mRNA abundance for AA transporters (Furuya and Graciano 2012; Garcia-Villalobos and Morales-Trejo 2012; Hernández et al. 2012).
Absorption and transport of basic AA is a complex physiological process that is influenced by many factors, including diet and hormones. However, the factors that play key roles in these processes are not yet well understood. Nonetheless, results of the current study reveal that a diet deficient in lysine has different effects on CAT transporters in the piglet small intestine. Dietary content of lysine differentially affects intestinal CAT expression to modulate absorption of lysine and other AA. Thus, transport of these nutrients is a key regulatory step in utilization of dietary protein by growing pigs. Whereas the findings of this study could help advance our knowledge of AA nutrition, further research will be required to elucidate the underlying mechanisms.
Abbreviations
- AA:
-
Amino acids
- CAT:
-
Cationic amino acid transporters
References
Blachier F, Mariotti F, Huneau JF et al (2007) Effects of amino acid derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 33:547–562
Bröer A, Wagner CA, Lang F et al (2000) The heterodimeric amino acid transporter 4F2hc/y + LAT2 mediates arginine efflux in exchange with glutamine. Biochem J 349:787–795
Chen TS, Liou SY (2012) Amino acids with basic amino side chain accelerate ability of polyphenolic compounds. Food Chem 134:9–14
Chen LX, Yin YL, Jobgen WS et al (2007) In vitro oxidation of essential amino acids by intestinal mucosal cells of growing pigs. Livest Sci 109:19–23
Chen LX, Li P, Wang JJ et al (2009) Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids 37:143–152
Dai ZL, Zhang J, Wu G et al (2010) Utilization of amino acids by bacteria from the pig small intestine. Amino Acids 39:1201–1215
Dai ZL, Li XL, Xi PB et al (2012a) Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 42:1597–1608
Dai ZL, Li XL, Xi PB et al (2012b) Regulatory role for l-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 43:233–244
Deng JP, Yang F, Yin YL et al (2010) Effects of digestible lysine levels on growth performance, serum metabolites and carcass composition in barrows. J Food Agric Environ 8:514–518
François V, EllenI C, Carsten W et al (2004) CATs and HATs: the SLC7 family of amino acid transporter. Eur J Physiol 447:532–542
Fu WJ, Hu J, Spencer T et al (2006) Statistical models in assessing fold changes of gene expression in real-time RT-PCR experiments. Comput Biol Chem 30:21–26
Furuya WM, Graciano TS (2012) Digestible lysine requirement of Nile tilapia fingerlings fed arginine-to-lysine-balanced diets. Rev Bras Zootecn 41:485–490
Garcia-Villalobos H, Morales-Trejo A (2012) Effects of dietary protein and amino acid levels on the expression of selected cationic amino acid transporters and serum amino acid concentration in growing pigs. Arch Anim Nutr 66:257–270
Gatrell SK, Berg LE, Barnard JT et al (2013) Tissue distribution of indices of lysine catabolism in growing swine. J Anim Sci 91:238–247
Gattas GF, Silva CD (2012) Dietary digestible lysine levels in diets for barrows from 60 to 100 days of age. Rev Bras Zootecn 41:91–97
Geng MM, Li TJ, Kong XF et al (2011) Reduced expression of intestinal N-acetylglutamate synthase in suckling piglets: a novel molecular mechanism for arginine as a nutritionally essential amino acid for neonates. Amino Acids 40:1513–1522
Gu XH, Li DF, She RP (2002) Effect of weaning on small intestinal structure and function in the piglet. Archiv für Tierernaehrung 56:275–286
Guo HX, Heinamaki J, Yliruusi J (2008) Stable aqueous film coating dispersion of Zero. J Colloid Interf Sci 322:478–484
Hampson DJ (1986) Alterations in piglet small intestinal structure at weaning. Res Vet Sci 40:32–40
He QH, Kong XF, Wu GY et al (2009) Metabolomic analysis of the response of growing pigs to dietary l-arginine supplementation. Amino Acids 37:199–208
Hernández F, López M, Martínez S et al (2012) Effect of low-protein diets and single sex on production performance, plasma metabolites, digestibility, and nitrogen excretion in 1- to 48-day-old broilers. Poultry Sci 91:683–692
Hou YQ, Wang L, Zhang W et al (2012) Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 43:1233–1242
Kanai Y, Endou H (2003) Functional properties of multispecific amino acid transporters and their implications to transporter mediated toxicity. J Toxicol Sci 28:1–17
Kim SW, Wu G, Baker DH (2005) Ideal protein and dietary amino acid requirements for gestating and lactating sows. Pig News Inf 26:89–99
Li TJ, Dai QZ, Yin YL et al (2008) Dietary starch sources affect net portal appearance of amino acids and glucose in growing pigs. Animal Consortium 2:723–729
Li XL, Bazer FW, Gao HJ et al (2009) Amino acids and gaseous signaling. Amino Acids 37:65–78
Li XL, Rezaei R, Li P et al (2011a) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Li FN, Yin YL, Tan BE et al (2011b) Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 41:1185–1193
Mann EG, David LY, Sobrevia L (2003) Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83:183–252
National Research Council (NRC) (1998) Swine Nutrient requirements. National Academy of Science, Washington, DC
Ren W, Yin YL, Liu G et al (2012) Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 42:2089–2094
Rezaei R, Knabe DA, Li XL et al (2011) Enhanced efficiency of milk utilization for growth in surviving low-birth-weight piglets. J Anim Sci Biotech 2:73–83
Rezaei R, Knabe DA, Tekwe CD et al (2013a) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Rezaei R, Wang WW, Wu ZL et al (2013b) Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotech 4:7
Russell H, Taylor PM, Hundal HS (2003) Amino acid transporters: roles in amino acid sensing and signaling in animal cells. Biochem J 373:1–18
Seow HF, Stefan B, Angelika B et al (2004) Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet 36:1003–1007
Stein ED, Chang SD, Diamond JM (1987) Comparison of different dietary amino acids as inducers of intestinal amino acid transporter. Am J Physiol 274:232–239
Sukhotnik I, Habib H, Jorge M et al (2005) Oral arginine improves intestinal recovery following ischemia–reperfusion injury in rat. Pediatr Surg Int 21:191–196
Tan B, Yin YL, Liu ZQ et al (2009) Dietary l-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 37:169–175
Tan BE, Li XG, Wu G et al (2012) Dynamic changes in blood flow and oxygen consumption in the portal-drained viscera of growing pigs receiving acute administration of l-arginine. Amino Acids 43:2481–2489
Teng L, Lu CH, Zhu LG et al (2010) The biodegradation of zein in vitro and in vivo and its application in implants. Pharm Sci Tech. doi:10.1208/s12249-010-9565-y
Wang HJ, Lin ZX, Liu XM et al (2005) Heparin-loaded zero microsphere film and hemocompatibility. J Control Release 105:120–131
Wang JJ, Chen LX, Li P et al (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Wang XQ, Ou DY, Yin JD et al (2009a) Proteomic analysis reveals altered expression of proteins related to glutathione metabolism and apoptosis in the small intestine of zinc oxide-supplemented piglets. Amino Acids 37:209–218
Wang WW, Qiao SY, Li DF (2009b) Amino acids and gut function. Amino Acids 37:105–110
Wang WC, Gu WT, Tang XF et al (2009c) Molecular cloning, tissue distribution and ontogenetic expression of the amino acid transporter b0,+ cDNA in the small intestine of Tibetan suckling piglets. Comp Biochem Physiol 154:157–164
Wang WW, Wu ZL, Dai ZL et al (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids. doi:10.1007/s00726-013-1493-1
Wei JW, Carroll RJ, Harden KK et al (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wolfram S, Giering H, Scharrer E (1984) Na+-gradient dependence of basic amino acid transporter into rat intestinal brush border membrane vesicles. Biochem Physiol 78:475–480
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2010a) Recent advances in swine amino acid nutrition. J Anim Sci Biotech 1:49–61
Wu G (2010b) Functional amino acids in growth, reproduction and health. Adv Nutr 1:31–37
Wu G (2013a) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton, p 503
Wu G (2013b) Functional amino acids in nutrition and health. Amino Acids. doi:10.1007/s00726-013-1500-6
Wu G, Meier SA, Knabe DA (1996) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126:2578–2584
Wu G, Bazer FW, Davis TA et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu G, Wu ZL, Dai ZL et al (2013) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Yang CB, Albin DM, Wang ZR et al (2011) Apical Na+-D-glucose co-transporter 1 (SGLT1) activity and protein abundance are expressed along the jejunal crypt–villus axis in the neonatal pig. Am J Physiol Gastrointest Liver Physiol 300:60–70
Yao K, Li TJ, Huang RL et al (2011) Dietary l-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br J Nutr 105:703–709
Yao K, Yin YL, Li XL et al (2012) Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids 42:2491–2500
Yin YL, Huang RL, de Lange CFM et al (2004) Effect of including purified Jack Bean lectin in a casein based diet on apparent and true ileal amino acid digestibility in growing pigs. Anim Sci 79:283–291
Yin FG, Zhang ZZ, Huang J et al (2010a) Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. Br J Nutr 103:1404–1412
Yin YL, Huang RL, Li TJ et al (2010b) Amino acid metabolism in the portal-drained viscera of young pigs: effects of dietary supplementation with chitosan and pea hull. Amino Acids 39:1581–1587
Yin YL, Yao K, Liu ZJ et al (2010c) Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486
Yin FG, Yin YL, Li TJ (2011) Developmental changes of serum amino acids in suckling piglets. J Food Agric Environ 9:322–327
Zhi AM, Zuo JJ, Zhou XY (2010) The Influence of different lysine concentration on the cationic amino acid transporter mRNA expression of porcine IEC. Chinese Agric Sci Bull 26:6–11
Zhou XH, Wu X, Yin YL et al (2011) Preventive oral supplementation with glutamine and arginine has beneficial effects on the intestinal mucosa and inflammatory cytokines in endotoxemic rats. Amino Acids. doi:10.1007/s00726-011-1137-2
Acknowledgments
This research was jointly supported by grants from National Basic Research Program of China (2013CB127301), National Natural Science Foundation of China (31272463, 31110103909), Hunan strategy emerging industry science research project (2011GK4061), the Chinese Academy of Sciences Visiting Professorship for Senior International Scientists Grant No. 2011T2S15), and Texas A&M AgriLife Research Hatch project (H-8200).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, L., Yang, H., Hou, Y. et al. Effects of dietary l-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids 45, 383–391 (2013). https://doi.org/10.1007/s00726-013-1514-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1514-0