Abstract
Little is known about effects of dietary glutamine supplementation on specific and general defense responses in a vaccine-immunized animal model. Thus, this study determined roles for dietary glutamine supplementation in specific and general defense responses in mice immunized with inactivated Pasteurella multocida vaccine. The measured variables included: (1) the production of pathogen-specific antibodies; (2) mRNA levels for pro-inflammatory cytokines, toll-like receptors and anti-oxidative factors; and (3) the distribution of P. multocida in tissues and the expression of its major virulence factors in vivo. Dietary supplementation with 0.5 % glutamine had a better protective role than 1 or 2 % glutamine against P. multocida infection in vaccine-immunized mice, at least partly resulting from its effects in modulation of general defense responses. Dietary glutamine supplementation had little effects on the production of P. multocida-specific antibodies. Compared to the non-supplemented group, dietary supplementation with 0.5 % glutamine had no effect on bacterial burden in vivo but decreased the expression of major virulence factors in the spleen. Collectively, supplementing 0.5 % glutamine to a conventional diet provides benefits in vaccine-immunized mice by enhancing general defense responses and decreasing expression of specific virulence factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recent years have witnessed growing interest in the immunology of functional amino acids, such as arginine (Ren et al. 2012a, b, 2013i, 2014a, b), proline (Ren et al. 2013b, c), glutamine (Boutry et al. 2012; Ren et al. 2014b, 2013g), glycine (Wang et al. 2013), as well as tryptophan, methionine and cysteine (Hou et al. 2012; Le Floc’h et al. 2011; Li et al. 2007). Available evidence shows that glutamine supplementation regulates defense responses in immune-compromised host (Ren et al. 2013d, f, 2014b). Effective defense responses, including both general and specific immune responses, are critical for the clearance of pathogens, as well as for protection against unwanted responses (Ren et al. 2013h).
Glutamine actively participates in inter-organ metabolism of nitrogen and carbon in mammals (Curthoys and Watford 1995), regulates cell signaling pathways and metabolism (Chiu et al. 2012; Dai et al. 2013), and is a major energy substrate for lymphocytes and macrophages (Wu et al. 1991a, b, c). This amino acid is also capable of regulating gene expression and cell signaling pathways in various cell types, including immunocytes (Li et al. 2007). Results of recent compelling studies have indicated that glutamine regulates the general defense responses, including the production of cytokines (Ren et al. 2013e), the function of immune cells (Yeh et al. 2004; Lai et al. 2004), and the expression of innate immune regulators, e.g., like toll-like receptors (Ren et al. 2013e) in various animal models. However, little is known about effects of dietary glutamine on general or specific defense responses in an animal model immunized with a vaccine. We have reported that arginine or proline boosts antibody production in vaccine-immunized mice (Ren et al. 2013i, b). These findings indicate that glutamine may affect specific immune responses, such as clearance of pathogens and the expression of major virulence factors in vivo (Ren et al. 2013e). Thus, it is possible that glutamine plays an important role in regulating antibody production in vaccine-immunized hosts. However, direct evidence for supporting this hypothesis is missing.
Therefore, this study was conducted to determine effects of graded dosages of glutamine supplementation on general and specific defense responses in mice pre-immunized with inactivated P. multocida vaccine. Moreover, we analyzed the burden of P. multocida, and expression of its major virulence factors in control and glutamine-supplemented animals.
Materials and methods
Preparation of the bacterium and inactivated vaccine
The P. multocida serotype A (CQ2) strain used in the present study was isolated from the lung tissue of clinically infected cattle, which was dead with pneumonia. The inactivated vaccine was prepared according to previous studies (Ren et al. 2013c, i).
Experimental design
One hundred fifty female KM mice (body weight 18–22 g) were obtained from Laboratory Animal Center of Third Military Medical University, Chongqing, China. The mice were housed in a pathogen-free mouse colony (temperature, 20–30 °C; relative humidity, 45–60 %; lighting cycle, 12 h/day) and had free access to food and drinking water. Animals were randomly divided into one of five groups (n = 30 per group): (1) mice that received dietary supplementation with 0.5 % l-glutamine (Ajinomoto Inc., Tokyo, Japan) (basal diet + 0.5 % l-glutamine) from day 0 and immunized with inactivated vaccines at a dosage of 4 × 108 CFU on days 8 and 13 (Vaccine-0.5 % Gln group); (2) mice that received dietary supplementation with 1.0 % l-glutamine (basal diet + 1.0 % l-glutamine) from day 0 and immunized with inactivated vaccines at a dosage of 4 × 108 CFU on days 8 and 13 (Vaccine-1.0 % Gln group); (3) mice that received dietary supplementation with 2.0 % l-glutamine (basal diet + 2.0 % l-glutamine) from day 0 and immunized with inactivated vaccines at a dosage of 4 × 108 CFU on days 8 and 13 (Vaccine-2.0 % Gln group); (4) mice that were immunized with inactivated vaccines at a dosage of 4 × 108 CFU on days 8 and 13 (Vaccine group); (5) mice that received administration of the same volume of phosphate-buffered saline on days 8 and 13 (control group). The dosage and the time for glutamine treatment were determined according our previous studies (Ren et al. 2013a, f, 2014b). At day 18, all of the mice were challenged by an intraperitoneal injection of P. multocida serotype A (CQ2) at the dosage of 4.4 × 105 CFU (2LD50). The content of glutamine and other amino acids in the basal diet was measured and presented in previous papers (Ren et al. 2012b, 2013d, f). The time and the dosage for vaccine immunization, as well as the time and the dosage for challenge with P. multocida, were chosen on the basis of previous studies (Ren et al. 2013c, e, i). Ten mice in each group were used to calculate survival rate. The others were killed to collect the heart, liver, spleen, lung and kidney at 12 h post-infection for bacterial counting and further molecular analysis. Serum was obtained at 12 h post-infection for determination of cytokine levels and antibody titers. This study was performed according to the guidelines of the Laboratory Animal Ethical Commission of the Southwest University.
Counting of bacteria
The number of viable bacteria in the heart, liver, spleen, lung and kidney was measured by homogenizing tissues in saline, plating serial dilutions on the Martin broth agar, and counting CFU after 16 h of growth at 37 °C (Ren et al. 2013e).
Analysis of serum cytokines
Serum levels of tumor necrosis factor (TNF)-alpha were measured using an ELISA kit in accordance with the manufacturer’s instructions (Cusabio Biotech Company Limited) (Ren et al. 2013i).
Quantitative real-time RT-PCR
Total RNA was isolated from the liquid nitrogen–frozen spleen using TRIZOL regents (Invitrogen, USA) and then treated with DNase I (Invitrogen, USA) according to the manufacturer’s instructions. Primers were designed with Primer 5.0 according to the mouse gene sequence or synthesized according to the previous reports. Primers were reported in a previous study (Ren et al. 2013e) or are shown in Table 1. Beta-actin was used as an internal control to normalize target gene transcript levels. For determining the expression of virulence factors, the 16S rRNA was used as the reference gene (Ren et al. 2013e). Real-time PCR was performed according to our previous study (Ren et al. 2014a).
Analysis of antibodies
Enzyme-linked immunosorbent assays were used for the detection of antibodies according to previous studies (Ren et al. 2013c, h, i). Serum levels of IgG1 and IgG2a were determined by IgG1 Isotyping Enzyme Immunometric Assay (EIA) and IgG2a Isotyping EIA kits, respectively (Innovating Concepts Laboratories, Inc. Mira Loma, CA).
Statistical analysis
All statistical analyses were performed using SPSS 17.0 software (SPSS, Inc.). Data are expressed as means with their standard errors. Multiple comparisons were performed using the one-way ANOVA (Wei et al. 2012). Survival rates of mice were evaluated using Kaplan–Meier analysis. Differences were considered significant at P < 0.05.
Results
Survival rates
The survival rates were calculated every day after infection with P. multocida serotype A. All mice were dead in the control group within 3 days post-infection, whereas the number of dead mice for the Vaccine, Vaccine-0.5 % Gln, Vaccine-1.0 % Gln, and Vaccine-2.0 % Gln groups was 6, 4, 5 and 8, respectively. The survival rates of mice were analyzed by Kaplan–Meier analysis. As shown in Fig. 1, dietary 0.5 % glutamine supplementation conferred better protection on mice against infection than 1 or 2 % glutamine.
Survival rates in mice supplemented with or without glutamine. Mice were immunized with inactivated Pasteurella multocida vaccine and fed diets supplemented with 0.0 % glutamine (Vaccine group), 0.5 % glutamine (vaccine-0.5 % Gln group), 1.0 % glutamine (Vaccine-1.0 % Gln group), or 2.0 % glutamine (Vaccine-2.0 % Gln group), or mice were immunized with PBS as the control group (n = 10 per group). 1, Control; 2, Vaccine; 3, Vaccine-0.5 % Gln; 4, Vaccine-1.0 % Gln; 5, Vaccine-2.0 % Gln
Bacterial burden
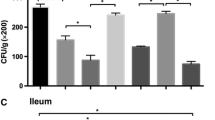
As shown in Fig. 2A, there was no difference in bacterial burden in the lung among vaccine-immunized and control groups. In the spleen, the bacterial burden in the vaccine-immunized group was lower (P < 0.05) than that in the control group, while little difference was found among the 0.5, 1 and 2 % Gln groups (Fig. 2B). In the heart, bacterial burden in the Vaccine and Vaccine-0.5 % Gln groups was lower (P < 0.01) than those in the Vaccine-1.0 % Gln, Vaccine-2.0 % Gln, and control groups (Fig. 2C). In the liver, vaccine immunization increased (P < 0.01) bacterial burden, as compared to the control group (Fig. 2D). In the kidney, bacterial burden in the Vaccine-1.0 % Gln group was higher (P < 0.01) than that in the other groups, while no difference was found among the Vaccine, Vaccine-0.5 % Gln and control groups (Fig. 2E). Collectively, dietary 0.5 % glutamine supplementation decreases the bacterial burden compared to the higher dosage of glutamine supplementation.
Bacterial burdens in different tissues. A Bacterial burden in the lung was calculated from different groups at 12 h post Pasteurella multocida infection. B Bacterial burden in the spleen. C Bacterial burden in the heart. D Bacterial burden in the liver. E Bacterial burden in the kidney. Mice were immunized with inactivated Pasteurella multocida vaccine and fed diets supplemented with 0.0 % glutamine (Vaccine group), 0.5 % glutamine (Vaccine-0.5 % Gln group), 1.0 % glutamine (Vaccine-1.0 % Gln group), or 2.0 % glutamine (Vaccine-2.0 % Gln group), or mice were immunized with PBS as the control group. Data are mean ± SEM, n = 6, a–b mean values sharing different superscripts within each item differ (P < 0.05); A-C mean values sharing different superscripts within each item differ (P < 0.01)
Serum antibody titres
Serum antibody titres against P. multocida serotype A were measured after mice were challenged with P. multocida serotype A. Although glutamine supplementation increased (P < 0.05) the OD value of antibody (Fig. 3A), there was no difference in serum antibody titers among glutamine-supplemented and vaccine groups (Fig. 3B). Further analysis did not reveal differences in serum levels of IgG1 and IgG2A among glutamine-supplemented and vaccine groups (Fig. 3C). However, vaccine immunization increased (P < 0.05) serum levels of IgG1, IgG2A, and antibody titers, compared to the control group (Fig. 3). Given the observation that dietary 0.5 % glutamine supplementation had a better protective effect and decreased bacterial burden, compared to higher dosage of glutamine supplementation, the mRNA levels for CD28, CD80, CD86, and interleukin (IL)-5, which play important role in antibody production, were analyzed in the spleen among the Vaccine, Vaccine-0.5 % Gln and control groups. Glutamine supplementation increased (P < 0.05) the mRNA expression of CD28, but decreased (P < 0.05) the mRNA expression of CD86, compared to the vaccine group (Fig. 4A, C). However, glutamine supplementation did not affect the mRNA expression of CD80 and IL-5, compared with those in the vaccine group (Fig. 4B, D). Collectively, dietary glutamine supplementation did not influence the production of pathogen-specific antibodies.
Serum antibody levels. A The OD value of serum antibody in different groups. B Serum antibody titers in different groups. C Serum level of IgG1. D Serum level of IgG2A. Mice were immunized with inactivated Pasteurella multocida vaccine and fed diets supplemented with 0.0 % glutamine (Vaccine group), 0.5 % glutamine (Vaccine-0.5 % Gln group), 1.0 % glutamine (Vaccine-1.0 % Gln group), or 2.0 % glutamine (Vaccine-2.0 % Gln group), or mice were immunized with PBS as the control group. Data are mean ± SEM, n = 6, a–c mean values sharing different superscripts within each item differ (P < 0.05)
mRNA expressions of antibody promoters in different groups. A mRNA expressions of CD28 in the spleen. B mRNA expressions of CD80 in the spleen. C mRNA expressions of CD86 in the spleen. D mRNA expressions of IL-5 in the spleen. Mice were immunized with inactivated Pasteurella multocida vaccine and fed diets supplemented with 0.0 % glutamine (Vaccine group), 0.5 % glutamine (Vaccine-0.5 % Gln group), or mice were immunized with PBS as the control group. Data are mean ± SEM, n = 6, a–c mean values sharing different superscripts within each item differ (P < 0.05)
Pro-inflammatory cytokines
No difference was detected in the splenic mRNA levels for pro-inflammatory cytokines, including IL-1 beta, IL-6, IL-8 and TNF-alpha, between control and vaccine groups (Fig. 5). Dietary glutamine supplementation decreased (P < 0.05) the mRNA expression of IL-6 and IL-8 in the spleen, compared to the Vaccine group (Fig. 5B, C). However, little difference was found in the expression of IL-1 beta and TNF-alpha in the spleen between Vaccine and Vaccine-0.5 % Gln groups (Fig. 5A, D). There were no differences in serum levels of TNF-alpha between Vaccine and Vaccine-0.5 %Gln group (data not shown).
mRNA expressions of pro-inflammatory cytokines in different groups. A mRNA expressions of IL-1 beta in the spleen. B mRNA expressions of IL-6 in the spleen. C mRNA expressions of IL-8 in the spleen. D mRNA expressions of TNF-alpha in the spleen. Mice were immunized with inactivated Pasteurella multocida vaccine and fed diets supplemented with 0.0 % glutamine (Vaccine group), 0.5 % glutamine (Vaccine-0.5 % Gln group), or mice were immunized with PBS as the control group. Data are mean ± SEM, n = 6, a–b mean values sharing different superscripts within each item differ (P < 0.05)
Anti-oxidative parameters
As shown in Fig. 6, vaccine immunization affected the mRNA expression of anti-oxidative factors in the spleen, including catalase (CAT), superoxide dismutases (CuZnSOD), and NQ, compared to the control groups. Dietary supplementation with 0.5 % glutamine decreased (P < 0.05) the mRNA expression of glutathione peroxidase 1 (GPx-1) and CuZnSOD in the spleen, while did not affect the expression of CAT and NQ in the spleen, compared with those in the vaccine group (Fig. 6).
mRNA expressions of anti-oxidative factors in different groups. A mRNA expressions of GPx-1 in the spleen. B mRNA expressions of CAT in the spleen. C mRNA expressions of CuZnSOD in the spleen. D mRNA expressions of NQ in the spleen. Mice were immunized with inactivated Pasteurella multocida vaccine and fed diets supplemented with 0.0 % glutamine (Vaccine group), 0.5 % glutamine (Vaccine-0.5 % Gln group), or mice were immunized with PBS as the control group. Data are mean ± SEM, n = 6, a–b mean values sharing different superscripts within each item differ (P < 0.05)
Toll-like receptors
Like anti-oxidative factors, vaccine immunization affected the mRNA expression of TLR-2, 4, 6, and 9 (Fig. 7). Compared to the vaccine group, dietary supplementation with 0.5 % glutamine increased (P < 0.05) the mRNA levels for TLR-6, 8 and 9 in the spleen, but had little effects on others in the spleen (Fig. 7).
mRNA expressions of TLRs in different groups. A mRNA expressions of TLR-2 in the spleen. B mRNA expressions of TLR-4 in the spleen. C mRNA expressions of TLR-5 in the spleen. D mRNA expressions of TLR-6 in the spleen. E mRNA expressions of TLR-7 in the spleen. F mRNA expressions of TLR-8 in the spleen. G mRNA expressions of TLR-9 in the spleen. Mice were immunized with inactivated Pasteurella multocida vaccine and fed diets supplemented with 0.0 % glutamine (Vaccine group), 0.5 % glutamine (Vaccine-0.5 % Gln group), or mice were immunized with PBS as the control group. Data are mean ± SEM, n = 6, a–c mean values sharing different superscripts within each item differ (P < 0.05)
Virulence factors
As shown in Fig. 8, vaccine promoted (P < 0.05) the mRNA expression of virulence factors in the spleen, including ompA, omph, pm0442 and pfhB2, compared to the control group. Compared to the vaccine group, dietary supplementation with 0.5 % glutamine decreased (P < 0.05) mRNA levels for omph, plpE, pfhB2 and hasR, and did not affect the mRNA levels of other virulence factors (Fig. 8).
mRNA expressions of virulence factors of Pasteurella multocida in different groups. A mRNA expressions of ompA in the spleen. B mRNA expressions of ompH in the spleen. C mRNA expressions of pm0442 in the spleen. D mRNA expressions of pm0979 in the spleen. E mRNA expressions of plpE in the spleen. F mRNA expressions of pfhB2 in the spleen. G mRNA expressions of hasR in the spleen. Mice were immunized with inactivated Pasteurella multocida vaccine and fed diets supplemented with 0.0 % glutamine (Vaccine group), 0.5 % glutamine (Vaccine-0.5 % Gln group), or mice were immunized with PBS as the control group. Data are mean ± SEM, n = 6, a–b mean values sharing different superscripts within each item differ (P < 0.05)
Discussion
Glutamine is the most abundant amino acid in the plasma of humans and many other animals (Wu et al. 2013a). The role of this amino acid in immune responses has received much attention over the past 25 years (Wu et al. 1991d, 1992; Wu 2013b). In this study, a lower dosage of supplemental glutamine (0.5 %) has better protection against P. multocida serotype A than higher dosages of glutamine supplementation (1 and 2 %). The possible reason is a higher dosage of glutamine supplementation increases the bacterial burden of P. multocida and the expression of its virulence factors in mice (Ren et al. 2013e), while affecting the function of organism through excessive production of ammonia (Rezaei et al. 2013; Wu et al. 2014). Indeed, in the current study, we have observed a higher burden of bacteria in the heart, liver and kidney after dietary supplementation with 1 or 2 % Gln, compared to 0.5 % glutamine supplementation. Because dietary supplementation with 0.5 % glutamine can protect mice from P. multocida, the mechanism whereby glutamine confers this benefit was investigated. Thus, we measured variables, including specific defense responses (e.g., antibody production) and general defense responses (e.g., bacterial burden, innate immunity, anti-oxidative response and the expression of virulence factors). We used the spleen for analysis because it is a major site for defense responses in most mammals, including mice, pigs, and humans (Bronte and Pittet 2013).
Similar to a finding of our previous study (Ren et al. 2013e), dietary supplementation with 0.5 % glutamine does not affect bacterial burden in the lung, spleen, heart, liver and kidney of mice. Pathogen-specific antibodies in the serum are useful indicators of specific immune defenses and provide the host with protection against the pathogen (Dorner and Radbruch 2007). In this work, different dosages of glutamine supplementation have little effects on the serum titers of P. multocida-specific antibodies or on the serum levels of IgG1 and IgG2A. The data on the mRNA levels for some positive regulators, including CD28, B7 and IL-5, in the spleen also support this conclusion. Although previous investigations have reported that glutamine supplementation increases the levels of serum IgG and IgA (Zhong et al. 2012; Zhou et al. 2012), these antibodies participate in general, rather than pathogen-specific, defense responses. In support of this view, we have observed that glutamine supplementation increases the production and transportation of secretory IgA by the small intestine (our unpublished data). Thus, like l-arginine (Ren et al. 2013i) and l-proline (Ren et al. 2013c; Wu et al. 2011a), glutamine exerts an important role in the function of the small intestine. It is noteworthy that these amino acids are inter-convertible in most mammals, including mice, pigs and humans (Wu et al. 2004, 2009; Wu 2009; Xi et al. 2011). Arginine, glutamine and proline may have separate and synergic effects on the immune system. Thus, effects of these and other amino acids on immune responses can be an objective indicator of their dietary requirements by animals (Hou et al. 2013; Wu et al. 2013b).
Results from the analysis of mRNA levels for pro-inflammatory cytokines and TLRs in the spleen indicate that glutamine supplementation affects the general defense response in vaccine-immunized mice. This is surprising because glutamine has been reported to enhance general immune responses in animals, including pigs (Wu et al. 2013c), mice (Ren et al. 2013a), and cows (Caroprese et al. 2013). Of note, mRNA levels for GPx-1 and CuZnSOD were lower in the spleen of glutamine-supplemented mice, compared to the control group. This result suggests a reduction in oxidative stress in response to glutamine supplementation. Similarly, numerous well-designed investigations have consistently indicated that glutamine increases anti-oxidative capacity in mice (Ren et al. 2014b; Cruzat et al. 2014), rats (Xu et al. 2014), pigs (Wu et al. 2011b, 2013c; Wang et al. 2008), and sheep (Sawant et al. 2014; Washburn et al. 2013). Furthermore, we found that dietary supplementation with 0.5 % glutamine decreased the expression of virulence factors of P. multocida, as we previously reported (Ren et al. 2013e). However, the underlying mechanisms are largely unknown and need to be elucidated in future studies. It would be of interest to determine whether glutamine modulates the expression of virulence factors in animals challenged with pathogens other than P. multocida, because the virulence factor is the main mediator of infection and disease (Guttman and Finlay 2008, 2009).
In conclusion, dietary supplementation with 0.5 % glutamine has beneficial effects on the protection against P. multocida infection in mice pre-immunized with the inactivated P. multocida vaccine. This role of glutamine may result from the moderation of general defense responses and the expression of virulence factors of P. multocida. However, glutamine supplementation has little effects on the pathogen-specific defense response. To our knowledge, this is the first study to explore the effects of glutamine supplementation on specific and general defense responses in vaccine-immunized mice. Our results help to understand the physiological and immunological roles of glutamine as a truly functional amino acid in animal and human nutrition.
Abbreviations
- CAT:
-
Catalase
- CuZnSOD:
-
Superoxide dismutases
- GPx-1:
-
Glutathione peroxidase 1
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- NQ:
-
1,4-Naphthoquinone
References
Boutry C, Matsumoto H, Bos C et al (2012) Decreased glutamate, glutamine and citrulline concentrations in plasma and muscle in endotoxemia cannot be reversed by glutamate or glutamine supplementation: a primary intestinal defect? Amino Acids 43:1485–1498
Bronte V, Pittet MJ (2013) The spleen in local and systemic regulation of immunity. Immunity 39:806–818
Caroprese M, Albenzio M, Marino R et al (2013) Dietary glutamine enhances immune responses of dairy cows under high ambient temperature. J Dairy Sci 96:3002–3011
Chiu M, Tarditos S, Barilli A et al (2012) Glutamine stimulates mTORC1 independent of the cell content of essential amino acids. Amino Acids 43:2561–2567
Cruzat VF, Bittencourt A, Scomazzon SP et al (2014) Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia. Nutrition 30:602–611
Curthoys NP, Watford M (1995) Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 15:133–159
Dai ZL, Li XL, Xi PB et al (2013) l-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 45:501–512
Dorner T, Radbruch A (2007) Antibodies and B cell memory in viral immunity. Immunity 27:384–392
Guttman JA, Finlay BB (2008) Subcellular alterations that lead to diarrhea during bacterial pathogenesis. Trends Microbiol 16:535–542
Guttman JA, Finlay BB (2009) Tight junctions as targets of infectious agents. Biochim Biophys Acta 1788:832–841
Hou YQ, Wang L, Zhang W et al (2012) Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 43:1233–1242
Hou YQ, Wang L, Yi D et al (2013) N-Acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids 45:513–522
Lai YN, Yeh SL, Lin MT et al (2004) Glutamine supplementation enhances mucosal immunity in rats with gut-derived sepsis. Nutrition 20:286–291
Le Floc’h N, Otten W, Merlot E (2011) Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 41:1195–1205
Li P, Yin YL, Li DF et al (2007) Amino acids and immune function. Br J Nutr 98:237–252
Ren WK, Liu G, Li TJ et al (2012a) Dietary supplementation with arginine and glutamine confers a positive effect in porcine circovirus-infected pig. J Food Agric Environ 10:485–490
Ren WK, Yin YL, Liu G et al (2012b) Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 42:2089–2094
Ren W, Li Y, Yu X et al (2013a) Glutamine modifies immune responses of mice infected with porcine circovirus type 2. Br J Nutr 110:1053–1060
Ren W, Wu M, Luo W et al (2013b) Dietary supplementation with proline confers a positive effect in both porcine circovirus-infected pregnant and non-pregnant mice. Br J Nutr 110:1492–1499
Ren W, Zou L, Ruan Z et al (2013c) Dietary l-proline supplementation confers immunostimulatory effects on inactivated Pasteurella multocida vaccine immunized mice. Amino Acids 45:555–561
Ren WK, Liu SP, Chen S et al (2013d) Dietary l-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids 45:947–955
Ren WK, Luo W, Wu MM et al (2013e) Dietary l-glutamine supplementation improves pregnancy outcome in mice infected with type-2 porcine circovirus. Amino Acids 45:479–488
Ren WK, Yin J, Zhu XP et al (2013f) Glutamine on Intestinal Inflammation: a mechanistic perspective. Eur J Inflamm 11:315–326
Ren WK, Yu R, Liu G et al (2013g) DNA vaccine encoding the major virulence factors of Shiga toxin type 2e (Stx2e)-expressing Escherichia coli induces protection in mice. Vaccine 31:367–372
Ren WK, Zou LX, Li NZ et al (2013h) Dietary arginine supplementation enhances immune responses to inactivated Pasteurella multocida vaccination in mice. Br J Nutr 109:867–872
Ren W, Chen S, Yin J, Duan J, Li T, Liu G, Feng Z, Tan B, Yin Y, Wu G (2014a) Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr 144:988–995
Ren W, Yin J, Wu M et al (2014b) Serum amino acids profile and the beneficial effects of l-arginine or l-glutamine supplementation in dextran sulfate sodium colitis. PLoS One 9(2):e88335
Rezaei R, Wang WW, Wu ZL et al (2013) Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotech 4:7
Sawant OB, Ramadoss J, Hankins GD et al (2014) Effects of l-glutamine supplementation on maternal and fetal hemodynamics in gestating ewes exposed to alcohol. Amino Acids. doi:10.1007/s00726-014-1751-x
Wang J, Chen L, Li P et al (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Wang WW, Wu ZL, Dai ZL et al (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Washburn SE, Sawant OB, Lunde ER et al (2013) Acute alcohol exposure, acidemia or glutamine administration impacts amino acid homeostasis in ovine maternal and fetal plasma. Amino Acids 45:543–554
Wei JW, Carroll RJ, Harden KK et al (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2013a) Amino acids: biochemistry and nutrition. CRC, Boca Raton
Wu G (2013b) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G, Field CJ, Marliss EB (1991a) Glutamine and glucose metabolism in rat splenocytes and mesenteric lymph node lymphocytes. Am J Physiol Endocrinol Metab 260:E141–E147
Wu G, Field CJ, Marliss EB (1991b) Elevated glutamine metabolism in splenocytes from spontaneously diabetic BB rats. Biochem J 274:49–54
Wu G, Field CJ, Marliss EB (1991c) Glutamine and glucose metabolism in thymocytes from normal and spontaneously diabetic BB rats. Biochem Cell Biol 69:801–808
Wu G, Field CJ, Marliss EB (1991d) Glucose and glutamine metabolism in rat macrophages: enhanced glycolysis and unaltered glutaminolysis in spontaneously diabetic BB rats. Biochim Biophys Acta 1115:166–173
Wu G, Field CJ, Marliss EB (1992) Enhanced glutamine and glucose metabolism in cultured rat splenocytes stimulated by phorbol myristate acetate plus ionomycin. Metabolism 41:982–988
Wu G, Knabe DA, Kim SW (2004) Arginine nutrition in neonatal pigs. J Nutr 134:2783S–2790S
Wu G, Bazer FW, Davis TA et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu G, Bazer FW, Burghardt RC et al (2011a) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu G, Bazer FW, Johnson GA et al (2011b) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Bazer FW, Satterfield MC et al (2013a) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Wu G, Wu ZL, Dai ZL et al (2013b) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Wu L, Wang W, Yao K et al (2013c) Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. PLoS One 8(7):e69502
Wu G, Bazer FW, Dai ZL et al (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Xi P, Jiang Z, Zheng C et al (2011) Regulation of protein metabolism by glutamine: implications for nutrition and health. Front Biosci (Landmark Ed) 16:578–597
Xu CL, Sun R, Qiao XJ et al (2014) Protective effect of glutamine in intestinal injury and bacterial community in rats exposed to hypobaric hypoxia environment. World J Gastroenterol 20:4662–46674
Yeh SL, Lai YN, Shang HF et al (2004) Effects of glutamine supplementation on innate immune response in rats with gut-derived sepsis. Br J Nutr 91:423–429
Zhong X, Li W, Huang X et al (2012) Effects of glutamine supplementation on the immune status in weaning piglets with intrauterine growth retardation. Arch Anim Nutr 66:347–356
Zhou Y, Zhang P, Deng G et al (2012) Improvements of immune status, intestinal integrity and gain performance in the early-weaned calves parenterally supplemented with L-alanyl-l-glutamine dipeptide. Vet Immunol Immunopathol 145:134–142
Acknowledgments
This research was jointly supported by National Basic Research Project (2013CB127301) MATS-Beef Cattle Yak system (CARS-38), National Natural Science Foundation of China (31272463), and Texas A&M AgriLife Research (H-82000).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Chen and S. Liu contributed equally to the present study.
Rights and permissions
About this article
Cite this article
Chen, S., Liu, S., Zhang, F. et al. Effects of dietary l-glutamine supplementation on specific and general defense responses in mice immunized with inactivated Pasteurella multocida vaccine. Amino Acids 46, 2365–2375 (2014). https://doi.org/10.1007/s00726-014-1789-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1789-9