Abstract
Glutamate receptors and transporters, including T1R1 and T1R3 (taste receptor 1, subtypes 1 and 3), mGluRs (metabotropic glutamate receptors), EAAC-1 (excitatory amino acid carrier-1), GLAST-1 (glutamate-aspartate transporter-1), and GLT-1 (glutamate transporter-1), are expressed in the gastrointestinal tract. This study determined effects of oral administration of monosodium glutamate [MSG; 0, 0.06, 0.5, or 1 g/kg body weight (BW)/day] for 21 days on expression of glutamate receptors and transporters in the stomach and jejunum of sow-reared piglets. Both mRNA and protein levels for gastric T1R1, T1R3, mGluR1, mGluR4, EAAT1, EAAT2, EAAT3, and EAAT4 and mRNA levels for jejunal T1R1, T1R3, EAAT1, EAAT2, EAAT3 and EAAT4 were increased (P < 0.05) by MSG supplementation. Among all groups, mRNA levels for gastric EAAT1, EAAT2, EAAT3, and EAAT4 were highest (P < 0.05) in piglets receiving 1 g MSG/kg BW/day. EAAT1 and EAAT2 mRNA levels in the stomach and jejunum of piglets receiving 0.5 g MSG/kg BW/day, as well as jejunal EAAT3 and EAAT4 mRNA levels in piglets receiving 1 g MSG/kg BW/day, were higher (P < 0.05) than those in the control and in piglets receiving 0.06 g MSG/kg BW/day. Furthermore, protein levels for jejunal T1R1 and EAAT3 were higher (P < 0.05) in piglets receiving 1 g MSG/kg BW/day than those in the control and in piglets receiving 0.06 g MSG/kg BW/day. Collectively, these findings indicate that dietary MSG may beneficially stimulate glutamate signaling and sensing in the stomach and jejunum of young pigs, as well as their gastrointestinal function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutamate, a major amino acid in animal and plant proteins (Li et al. 2011) as well as in the milk (Haynes et al. 2009; Wu and Knabe 1994), is extensively degraded in the small intestine of mammals, including piglets (Wu 2009, 2010a; Wu et al. 2007, 2013a). Emerging evidence also shows that a small amount of dietary glutamate is metabolized in the stomach (Torii et al. 2013). T1R1 and T1R3 (taste receptor 1, subtypes 1 and 3) are expressed in epithelial cells of the gastrointestinal tract (Bezenc et al. 2007; Kawai et al. 2012). In addition, mGluR-1 (metabotropic glutamate receptor-1) is highly expressed in the rat gastric fundus (San Gabriel et al. 2007). Glutamate transporters, including EAAC-1 (excitatory amino acid carrier 1), GLAST-1 (glutamate-aspartate transporter-1), GLT-1 (glutamate transporter 1), VGLUT1 (vesicular transporter-1), and VGLUT2 (vesicular transporter-2) are present in the stomach (Aoyama and Nakaki 2013; Falalyeyeva and Beregova 2007). Glutamate transport by the enterocyte’s apical membrane takes place mainly via the high-affinity \({\text{X}}_{\text{AG}}^{ - }\) system and to a lesser extent by the low-affinity B0 system, with the \({\text{X}}_{\text{AG}}^{ - }\) system transporting both glutamate and aspartate (Had-Aissouni 2012; Gras et al. 2012; Lewerenz et al. 2012; Wu 2013b).
The molecular identities of the four proteins possessing an \({\text{X}}_{\text{AG}}^{ - }\) system activity have been described in various tissues, including GLAST-1, GLT-1, EAAC-1, EAAC-4, and EAAC-5 (Torii et al. 2013). Studies with pigs and rodent species show that EAAC-1 is the most abundant glutamate transporter in the small intestine and is expressed on the apical, brush border membrane throughout the gut (Fan et al. 2004). At present, little is known about effects of monosodium glutamate (MSG) on expression of glutamate receptors and transports in the gastrointestinal tract. The main goal of this study was to determine mRNA and protein levels for T1R1, T1R3, mGluR1, mGluR4, EAAT1, EAAT2, EAAT3, and EAAT4 in the stomach and jejunum of sow-reared piglets receiving oral administration of 0, 0.06, 0.5, or 1 g MSG/kg body weight/day.
Materials and methods
MSG was purchased from Henan Lianhua Gourmet Powder Co., Ltd (Henan, China). Its purity was 99.9 %. Unless indicated, all chemicals were obtained from Sigma (St. Louis, MO, USA).
Animals and diets
Twenty-four Duroc × Large White × Landrace newborn piglets with an average body weight of 1.55 ± 0.20 kg were assigned randomly to one of four treatments. There was one sow with her offpsring per pen in an environmentally controlled facility. Lactating sows had free access to a corn- and soybean meal-based diet (Wu et al. 2011a) and drinking water. The piglets were nursed by their mothers and received oral administration of 0 (control), 0.06 (low dose), 0.5 (intermediate dose), or 1 g (high dose) MSG/kg body weight/day twice daily after birth until 21 days of age. There were six piglets per treatment group. On each day, the first and second MSG administration were given between 08:00 and 09:00 and between 18:00 and 19:00, respectively, after piglets were nursed by their mothers. The MSG was dissolved in 2 ml water per kg of body weight before gavage. Piglets in the 0, 0.06, and 0.5 g MSG/kg body weight/day groups received the same amount of sodium in the form of NaCl as piglets in the 1 g MSG/kg body weight/day group, as previously described (Rezaei et al. 2013). The study was conducted in accordance with the Chinese guidelines for animal welfare and approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, The Chinese Academy of Sciences (Yin et al. 2010b).
Tissue collection
At 21 days of age, 24 piglets were sacrificed for tissue collection. Approximately 5 g each of the jejunum and the stomach was collected, their contents were removed, and their mucosal surface was cleaned with sapline. Thereafter, the tissues were immediately frozen in liquid nitrogen, and stored at −80 °C until mRNA analysis (Liu et al. 2012).
RNA extraction and cDNA synthesis
Total RNA was extracted from the jejunal and stomach tissues using Trizol Reagents according to the instructions from Invitrogen Corporation (He et al. 2013). The quantity of the RNA obtained was checked by measuring optical density at 260 and 280 nm (Geng et al. 2011). Before reverse transcription (RT), all RNA samples were treated with Dnase I enzyme (amplification grade) to remove any residual DNA according to the manufacturer’s instructions (Invitrogen). Each RNA (1 μg) sample was combined with 1 μl of 10× reaction buffer, 1 μL of DNase I (1 U/μL), and diethyl pyrocarbonate-treated H2O up to 10 μL, and incubated at 37 °C for 30 min. Next, 1 μl of 50 mM EDTA was added to stop the reaction by incubation at 65 °C for 10 min. Subsequently, the DNase-treated RNA samples were reverse-transcribed to cDNA in accordance with the manufacturer’s instructions (TakaRa Biotechnology, Dalian Co., Ltd, China). The reaction mixture, which included 5× PrimeScript® Buffer (4 μl), PrimeScript® RT Enzyme Mix 1 (1 μl), RT Primer Mix (1 μl), and diethyl pyrocarbonate-treated H2O up to 20 μL, was incubated at 37 °C for 15 min and at 85 °C for 5 s.
Quantification of mRNA levels for AA and peptide transporters
Components of a 10 μL real-time PCR mixture included 5 μl SYBRR Premix Ex TaqTM (2×), 0.4 μL PCR Forward Primer (10 μM), 0.4 μL PCR Reverse Primer (10 μM), 0.2 μL ROX Reference Dye (50×), 1 μL template DNA, and nuclease-free water up to 10 μl. The PCR conditions used for amplification and quantification included an initial denaturing stage (95 °C for 30 s), followed by the PCR (95 °C for 5 s, 60 °C for 34 s), fusion (95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s), and continuous fluorescence measurements. The primers are shown in Table 1. The amplification of GAPDH was used for each sample to normalize the expression of selected genes. The relative expression ratios of mRNA were calculated as previously described (Bustin et al. 2009; Liu et al. 2012; Yin et al. 2010a).
Quantification of protein amounts of AA and peptide transporters
The frozen samples were powdered under liquid nitrogen, and lysed in an assay buffer (150 mM NaCl, 1 % Triton X-100, 0.5 % sodium deoxycholate, 0.1 % SDS, 50 mM Tris-HCl at pH 7.4, and a protease inhibitor cocktail) purchased from Roche (Shanghai, China). After centrifugation at 10,000×g and 4 °C for 10 min, protein concentration in the supernatant fluid was determined using the Bicinchoninic Acid assay (Beyotime Biotechnology, China). All samples were adjusted to an equal protein concentration and then diluted with the 2× loading buffer [0.63 ml of 0.5 M Tris-HCl (pH 6.8), 0.42 ml 75 % glycerol, 0.125 g sodium dodecyl sulfate (SDS), 0.25 ml β-mercaptoethanol, 0.2 ml 0.05 % solution of bromphenol blue, and 1 ml water] to a final volume of 2.5 ml and heated in boiling water for 5 min. The solution was cooled on ice before use for Western blot analysis as previously described (Fu et al. 2013).
The denatured proteins were separated using SDS-PAGE (10 % gradient gel), transferred to PVDF membranes (Millipore, Billerica, MA, USA) overnight at 12 V using the Bio-Rad Transblot apparatus (Hercules, CA, USA). The membranes were blocked in 5 % fat-free milk in Tris-Tween buffered saline (TTBS: 20 mM Tris/150 mM NaCl, pH 7.5, and 0.1 % Tween-20) for 3 h and then incubated with an antibody for T1R1, mGluR1, EAAT3 or β-actin (Table 2) at 4 °C overnight with gentle rocking. After washing three times with TTBS, the membranes were incubated at room temperature for 2 h with horseradish peroxidase-linked secondary antibodies. Finally, the membranes were washed with TTBS, followed by development using Supersignal West Dura Extended Duration Substrate according to the manufacturer’s instructions (Pierce, Rockford, IL, USA). The images were detected on chemiluminescence (Applygen Technologies Inc., Beijing, China). Multiple exposures of each Western blot were performed to ensure linearity of chemiluminescence signals. Western blots were quantified by measuring the intensity of correctly sized bands using AlphaImager 2200 (Alpha Innotech Corporation, CA, USA) software (Yang et al. 2013).
Statistics
Data are expressed as mean ± standard deviation (SD). Data were subjected to one-way analysis of variance using the General Linear Model procedures of SAS (SAS 9.1, SAS Institute, Cary, USA). Log transformation of variables was performed when variance of data was not homogenous among treatment groups, as assessed using the Levene’s test (Wei et al. 2012). Differences among treatment means were determined by the Tukey multiple comparison test (Fu et al. 2010). Differences were considered significant at P < 0.05.
Results
Overall observations
The body weights of piglets at 0, 7, 14, and 21 days of age are shown in Table 3. Compared with the control group, dietary supplementation with 0.5 and 1 g MSG/kg body weight/day did not affect (P > 0.05) the weight gains of piglets during the 21-day period. Oral administration of 0.5 g MSG/kg body weight/day over a 3-week period increased (P < 0.05) the average day weight gain of piglets than that for the 1 g MSG/kg body weight/day group. No adverse effects of oral MSG administration on any piglet were observed during the entire experimental period.
mRNA levels for AA transporters in the stomach
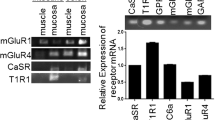
The mRNA levels for T1R1, T1R3, mGluR1, mGluR4, EAAT1, EAAT2, EAAT3 and EAAT4 in the stomach were increased (P < 0.05) by dietary MSG supplementation (Fig. 1). Gastric T1R1 mRNA levels in the High-MSG group and intermediate-MSG group were higher (P < 0.05) than those in the other groups. Compared with the control group, mRNA levels for gastric T1R3, mGluR1, mGluR4, EAAT1, EAAT2, EAAT3 and EAAT4 in the Low-MSG, intermediate-MSG, and High-MSG groups were elevated (P < 0.05). The mRNA levels for EAAT1, EAAT2, EAAT3, and EAAT4 in the stomach of piglets in the High-MSG group were higher (P < 0.05) than those in the other groups. Gastric EAAT1 and EAAT2 mRNA levels in the intermediate-MSG group were higher (P < 0.05) than those in the control and the low-MSG group.
Effects of dietary supplementation with MSG on mRNA levels for AA and peptide transporters in the stomach of sow-reared piglets. mRNA abundances of T1R1, T1R3, mGluR1, mGluR4, EAAT1, EAAT2, EAAT3, EAAT4 were normalized using GAPDH as an internal control. Data are expressed as mean ± SEM, n = 6. a–c Within a variable, values with different superscripts differ (P < 0.05). CN control; LMG = 0.06 g MSG/kg BW/day; MMG = 0.5 g MSG/kg BW/day; HMG = 1 g MSG/kg BW/day
mRNA levels for AA transporters in the jejunum
The T1R1 and T1R3 mRNA levels in the jejunum were increased (P < 0.05) by dietary MSG supplementation (Fig. 2). Supplementation with MSG had no effect on intestinal mGluR1 mRNA abundance. Jejunal EAAT1, EAAT2, EAAT3 and EAAT4 mRNA levels in the low-MSG, intermediate-MSG and high-MSG groups were higher (P < 0.05) than those in the control group. Abundances of EAAT1 and EAAT2 mRNA in the jejunum of piglets in the intermediate-MSG and high-MSG groups were higher (P < 0.05) than those in the control and low-MSG group. Similar results were obtained for jejunal EAAT3 and EAAT4 abundance.
Effects of dietary supplementation with MSG on mRNA levels for AA and peptide transporters in the jejunum of sow-reared piglets. The mRNA expression abundances of T1R1, T1R3, mGluR1, mGluR4, EAAT1, EAAT2, EAAT3, EAAT4 were normalized using GAPDH as an internal control. Data are expressed as mean ± SEM, n = 6. a–c Within a variable, values with different superscripts differ (P < 0.05). CN control; LMG = 0.06 g MSG/kg BW/day; MMG = 0.5 g MSG/kg BW/day; HMG = 1 g MSG/kg BW/day
Protein levels for AA transporters in the stomach
Abundances of proteins for T1R1, mGluR1 and EAAT3 in the stomach were higher (P < 0.05) in the MSG-supplemented piglets compared with the control group (Fig. 3). The values were the highest (P < 0.05) in the high-MSG group among all piglets.
Effects of dietary supplementation with MSG on the protein abundance of AA and peptide transporters in the stomach of sow-reared piglets. The protein abundances of T1R1, mGluR1 and EAAT3 were normalized using β-actin as an internal control. Data are expressed as mean ± SEM, n = 6. a–c Within a variable, values with different superscripts differ (P < 0.05). CN control; LMG = 0.06 g MSG/kg BW/day; MMG = 0.5 g MSG/kg BW/day; HMG = 1 g MSG/kg BW/day
Protein levels for AA transporters in the jejunum
The amounts of jejunal T1R1 and EAAT3 proteins were higher (P < 0.05) in the High-MSG group compared with the control and the low-MSG group (Fig. 4). Dietary supplementation with MSG had no effect (P < 0.05) on the abundance of mGluR1 protein in the jejunum.
Effects of dietary supplementation with MSG on the protein abundance of AA and peptide transporters in the jejunum of sow-reared piglets. The protein abundances of T1R1, mGluR1 and EAAT3 were normalized using β-actin as an internal control. Data are expressed as mean ± SEM, n = 6. a–c Within a variable, values with different superscripts differ (P < 0.05). CN control; LMG = 0.06 g MSG/kg BW/day; MMG = 0.5 g MSG/kg BW/day; HMG = 1 g MSG/kg BW/day
Discussion
While glutamate was traditionally considered as a nutritionally nonessential amino acid for humans and other animals (see Wu 2010b for review), this amino acid plays an important role in physiology (Brosnan and Brosnan 2012; Parpura and Verkhratsky 2013; Yao et al. 2012), nutrient metabolism (Wu et al. 2011a, b), anti-oxidative responses (Aoyama et al. 2012; Had-Aissouni 2012), and immunity (Gupta et al. 2013). In addition, glutamate is a major energy substrate for the small intestine and an excitatory neurotransmitter (Burrin and Stoll 2009; Wu 1998). This AA activates taste receptors in the digestive tract (Kawai et al. 2012; San Gabriel and Uneyama 2012), enhances diet-induced thermogenesis in brown adipose tissue of young adult rats (Smriga et al. 2000), and regulates the release of certain hormones [e.g., norepinephrine (Smriga and Torii 2000) and glucagon-like peptide-1 (Iwatsuki and Torii 2012)] in animals. Interestingly, glutamate reduces white-fat deposition in adult rats (Kondoh and Torii 2008) and growing pigs (Rezaei et al. 2013). Thus, there is growing interest in glutamate nutrition in swine (Tan et al. 2012; Wu 2010a), humans (Shi et al. 2012), and other mammals (Boutry et al. 2012; Brosnan and Brosnan 2012; Wu 2013a, b).
The stomach has the capacity for the transport of glutamate into the blood circulation and also is a site for glutamate sensing and glutamate-mediated regulation of digestive function (Burrin and Stoll 2009). Humans have an ability to detect at least five basic taste qualities: sweet, umami, bitter, salty, and sour (Iwatsuki and Torii 2012). Receptors for umami taste and sweet taste are closely related to each other. The three subunits of the T1R family form two heteromeric receptors: umami (T1R1/T1R3) (Li et al. 2002; Nelson et al. 2002) and sweet (T1R2/T1R3) (Li et al. 2002; Nelson et al. 2001). Currently, two l-glutamate receptors have been identified in the cells lining the gut: metabotropic l-glutamate receptor type 1 is located in the chief cells (pepsinogen-secreting cells) of the stomach (San Gabriel et al. 2007), and a heterodimer l-glutamate taste receptor, T1R1 + T1R3 (taste receptor 1, subtypes 1 and 3), is found in epithelial cells of the stomach, small intestine, and colon (Bezenc et al. 2007).
Results of our study indicate that expression of T1R1 and T1R3 as well as mGluR1 and mGluR4 in the stomach and the jejunum was increased by dietary MSG supplementation. Thus, it is possible that glutamate receptors in the gastrointestinal tract may detect ingested glutamate, transmitting this information to adjacent cells and neurons. Some receptors, such as N-methyl-d-aspartate (NMDA) and mGluR1 to mGluR8, are present in the stomach and regulate gastrointestinal function (San Gabriel et al. 2007; Torii et al. 2013). In support of this view, there is evidence that dietary supplementation with 1 % l-glutamate increased pepsinogen C expression in the stomach mucosa and gastric secretions (Khropycheva et al. 2011; Zolotarev et al. 2009). Furthermore, Nakamura et al. (2010) reported that mGluR1 expression in gastric chief cells was much lower than that in parietal cells and the entire gastric mucosa. In contrast, the endocrine cells, possibly D cells, specifically expressed multiple metabotropic glutamate receptors such as mGluR2, 3, 4, and 7, although the relative level of expression was low (Nakamura et al. 2010). Taken together, these data suggest that mGluR1 is involved in the gastric phase control of protein digestion.
Glutamate transporters (EAAC-1, GLAST and GLT-1) and vesicular transporters (VGLUT1 and VGLUT2) are expressed in the stomach (Falalyeyeva and Beregova 2007). Burrin and Stoll (2009) compared the metabolic fate of dietary [13C]-glutamate in young pigs when administered the same control diet and supplemental glutamate intakes by the intragastric and intraduodenal feeding route. They reported that the fractional rate of gastrointestinal glutamate absorption in pigs when given intragastrically was higher than that for intraduodenal feeding. This suggests glutamate transport by the stomach mucosal cells. Consistent with these data, we found that dietary supplementation with MSG increased the expression of EAAT1, EAAT2, EAAT3 and EAAT4 in the piglet stomach. At present, the metabolic fate of glutamate in the gastric mucosa is unknown. However, we surmise that dietary glutamate upregulates expression of key genes for glutamate transport and sensing in the stomach.
The first step for the utilization of glutamate by the small intestine is its transport from the lumen into enterocytes, which involves the Na+-dependent high-affinity \({\text{X}}_{\text{AG}}^{ - }\) system and/or the low-affinity B0 system (Conrad and Sato 2012; Wu et al. 2013b). The \({\text{X}}_{\text{AG}}^{ - }\) system transports both glutamate and aspartate. The molecular identities of the four proteins in the \({\text{X}}_{\text{AG}}^{ - }\) system have been described in various tissues, including GLAST-1, GLT-1, EAAC-1, and excitatory amino acid transporters 4 and 5 (Beart and O’Shea 2007; Fan et al. 2004; Iwanaga et al. 2005; Kanai and Hediger 2003). Studies with pigs, rats and mice have demonstrated that EAAC-1 is the most abundant glutamate transporter in the intestine and is expressed on the apical, brush border membrane throughout the small intestine (Fan et al. 2004). In our work, we found that the expression of EAAT1, EAAT2, EAAT3 and EAAT4 in the jejunum was enhanced by dietary MSG supplementation. Whether glutamate directly or indirectly regulates gene expression in intestinal cells is unknown. Nonetheless, increased abundance of glutamate transporters in enterocytes aid in maximizing the absorption of dietary glutamate for utilization, thereby reducing its entry into the large intestine.
In conclusion, dietary supplementation with MSG increases expression of glutamate signaling receptors and glutamate transporters, including T1R1, T1R3, mGluR1, EAAT1, EAAT2, EAAT3, and EAAT4, in the stomach and small intestine of neonatal pigs. Taken together from previous studies, glutamate is a functional amino acid to beneficially enhance nutrient sensing and transport in the gastrointestinal tract. MSG is safe and may serve as a low-cost ingredient to improve the intestinal absorptive capacity in sucking piglets.
Abbreviations
- AA:
-
Amino acid
- EAAC-1:
-
Excitatory amino acid carrier 1
- GLAST:
-
Glutamate-aspartate transporter
- GLT-1:
-
Glutamate transporter 1
- mGluRs:
-
Metabotropic glutamate receptors
- MSG:
-
Monosodium glutamate
- RT-PCR:
-
Real-time polymerase chain reaction
- SDS:
-
Sodium dodecyl sulfate
- T1R1:
-
Taste receptor 1, subtype 1
- T1R3:
-
Taste receptor 1, subtype 3
- TTBS:
-
Tris-Tween buffered saline
References
Aoyama K, Nakaki T (2013) Neuroprotective properties of the excitatory amino acid carrier 1 (EAAC1). Amino Acids 45:133–142
Aoyama K, Watabe M, Nakaki T (2012) Modulation of neuronal glutathione synthesis by EAAC1 and its interacting protein GTRAP3-18. Amino Acids 42:163–169
Beart PM, O’Shea RD (2007) Transporters for l-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol 150:5–17
Bezenc C, le Coutre J, Damak S (2007) Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32:41–49
Boutry C, Matsumoto H, Bos C et al (2012) Decreased glutamate, glutamine and citrulline concentrations in plasma and muscle in endotoxemia cannot be reversed by glutamate or glutamine supplementation: a primary intestinal defect? Amino Acids 43:1485–1498
Brosnan JT, Brosnan ME (2012) Glutamate: a truly functional amino acid. Amino Acids. doi:10.1007/s00726-012-1280-4
Burrin DG, Stoll B (2009) Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr 90(suppl):1S–7S
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Conrad M, Sato H (2012) The oxidative stress-inducible cystine/glutamate antiporter, system x(c)(-): cystine supplier and beyond. Amino Acids 42:231–246
Falalyeyeva T, Beregova T (2007) Role of peripheral glutamate receptors in regulation of gastric secretion and motor function of stomach. J Pre-Clin Clin Res 1:107–111
Fan MZ, Matthews JC, Etienne NM et al (2004) Expression of apical membrane l-glutamate transporters in neonatal porcine epithelial cells along the small intestinal crypt-villus axis. Am J Physiol Gastrointest Liver Physiol 287:G385–G398
Fu WJ, Stromberg AJ, Viele K et al (2010) Statistics and bioinformatics in nutritional sciences: analysis of complex data in the era of systems biology. J Nutr Biochem 21:561–572
Fu D, Yang H, Kong X et al (2013) Molecular cloning and expression profiling of excitatory amino acid carrier 1 in suckling Huanjiang mini-piglets with large or small body weight at birth. Mol Biol Rep 40:3341–3350
Geng MM, Li TJ, Kong XF et al (2011) Reduced expression of intestinal N-acetylglutamate synthase in suckling piglets: a novel molecular mechanism for arginine as a nutritionally essential amino acid for neonates. Amino Acids 40:1513–1522
Gras G, Samah B, Hubert A et al (2012) EAAT expression by macrophages and microglia: still more questions than answers. Amino Acids 42:221–229
Gupta R, Palchaudhuri S, Chattopadhyay D (2013) Glutamate induces neutrophil cell migration by activating class I metabotropic glutamate receptors. Amino Acids 44:757–767
Had-Aissouni L (2012) Maintenance of antioxidant defenses of brain cells: plasma membrane glutamate transporters and beyond. Amino Acids 42:159–161
Haynes TE, Li P, Li XL et al (2009) l-Glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37:131–142
He LQ, Yang HS, Li TJ et al (2013) Effects of dietary l-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids 45:383–391
Iwanaga T, Goto M, Watanabe M (2005) Cellular distribution of glutamate transporters in the gastrointestinal tract of mice: an immunohistochemical and in situ hybridization approach. Biomed Res 26:271–278
Iwatsuki K, Torii K (2012) Peripheral chemosensing system for tastants and nutrients. Curr Opin Endocrinol Diabetes Obes 19:19–25
Kanai Y, Hediger MA (2003) The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol 479:237–247
Kawai M, Sekine-Hayakawa Y, Okiyama A et al (2012) Gustatory sensation of l- and d-amino acids in humans. Amino Acids 43:2349–2358
Khropycheva R, Andreeva J, Uneyama H et al (2011) Dietary glutamate signal evokes gastric juice excretion in dogs. Digestion 83(Suppl 1):7–12
Kondoh T, Torii K (2008) MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male Sprague–Dawley rats. Physiol Behav 95:135–144
Lewerenz J, Maher P, Methner A (2012) Regulation of xCT expression and system xc(-) function in neuronal cells. Amino Acids 42:171–179
Li X, Staszewski L, Xu H et al (2002) Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99:4692–4696
Li XL, Rezaei R, Li P et al (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Liu XD, Wu X, Yin YL, Liu YQ, Geng MM, Yang HS, Blachier F, Wu GY (2012) Effects of dietary l-arginine or N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids 42:2111–2119
Liu XD, Wu X, Yin YL et al (2012) Effects of dietary l-arginineor N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids 42:2111–2119
Nakamura E, Hasumura M, San Gabriel Ana (2010) New frontiers in gut nutrient sensor research: luminal glutamate-sensing cells in rat gastric mucosa. J Pharmacol Sci 112:13–18
Nelson G, Hoon MA, Chandrashekar J et al (2001) Mammalian sweet taste receptors. Cell 106:381–390
Nelson G, Chandrashekar J, Hoon MA et al (2002) An amino-acid taste receptor. Nature 416:199–202
Parpura V, Verkhratsky A (2013) Astroglial amino acid-based transmitter receptors. Amino Acids 44:1151–1158
Rezaei R, Knabe DA, Tekwe CD et al (2013) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
San Gabriel A, Uneyama H (2012) Amino acid sensing in the gastrointestinal tract. Amino Acids. doi:10.1007/s00726-012-1371-2
San Gabriel AM, Maekawa T, Uneyama H, Yoshie S, Torii K (2007) mGluR1 in the fundic glands of rat stomach. FEBS Lett 581:1119–1123
Shi Z, Yuan B, Taylor AW et al (2012) Monosodium glutamate intake increases hemoglobin level over 5 years among Chinese adults. Amino Acids 43:1389–1397
Smriga M, Torii K (2000) Release of hypothalamic norepinephrine during MSG intake in rats fed normal and nonprotein diet. Physiol Behav 70:413–415
Smriga M, Murakami H, Mori M et al (2000) Use of thermal photography to explore the age-dependent effect of monosodium glutamate, NaCl and glucose on brown adipose tissue thermogenesis. Physiol Behav 71:403–407
Tan BE, Li XG, Wu G et al (2012) Dynamic changes in blood flow and oxygen consumption in the portal-drained viscera of growing pigs receiving acute administration of l-arginine. Amino Acids 43:2481–2489
Torii K, Uneyama H, Nakamura E (2013) Physiological roles of dietary glutamate signaling via gut-brain axis due to efficient digestion and absorption. J Gastroenterol 48:442–451
Wei JW, Carroll RJ, Harden KK et al (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2010a) Recent advances in swine amino acid nutrition. J Anim Sci Biotech 1:49–61
Wu G (2010b) Functional amino acids in growth, reproduction and health. Adv Nutr 1:31–37
Wu G (2013a) Amino Acids: Biochemistry and Nutrition. CRC Press, Boca Raton
Wu G (2013b) Functional amino acids in nutrition and health. Amino Acids. doi:10.1007/s00726-013-1500-6
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr 124:415–424
Wu GY, Bazer FW, Davis TA, Johnson GA, Kim SW, Knabe DA, Spencer TE, Yin YL (2007) Important roles for arginine-family amino acids in swine nutrition and production. Livest Sci 122:8–22
Wu G, Bazer FW, Johnson GA et al (2011a) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Bazer FW, Burghardt RC et al (2011b) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu G, Bazer FW, Satterfield MC et al (2013a) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Wu G, Wu ZL, Dai ZL et al (2013b) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Yang HS, Fu DZ, Kong XF et al (2013) Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets. J Anim Sci 91:1–8
Yao K, Yin YL, Li XL et al (2012) Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids 42:2491–2500
Yin FG, Zhang ZZ, Huang J, Yin YL (2010a) Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. Br J Nutr 103:1404–1412
Yin YL, Yao K, Liu ZJ, Gong M, Ruan Z, Deng D, Tan BE, Liu ZQ, Wu GY (2010b) Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486
Zolotarev V, Khropycheva R, Uneyama H et al (2009) Effect of free dietary glutamate on gastric secretion in dogs. Ann N Y Acad Sci 1170:87–90
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 31110103909, 31272217, 31272450, and 31272451), National Science and Technology Support Program Funding (2012BAD39B03), Nanjing Branch Academy of Chinese Academy of Science and Jiangxi Province Cooperation Project, National Basic Research Program of China (2012CB124704), and Texas A&M AgriLife Research (H-8200).
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, J., Yin, Y., Shu, X.G. et al. Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids 45, 1169–1177 (2013). https://doi.org/10.1007/s00726-013-1573-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1573-2