Abstract
Purpose
To investigate the effects of blood flow restriction (BFR) on electromyographic amplitude (EMGRMS)–force relationships of the biceps brachii (BB) during a single high-load muscle action.
Methods
Twelve recreationally active males and eleven recreationally active females performed maximal voluntary contractions (MVCs), followed by an isometric trapezoidal muscle action of the elbow flexors at 70% MVC. Surface EMG was recorded from the BB during BFR and control (CON) visits. For BFR, cuff pressure was 60% of the pressure required to completely occlude blood at rest. Individual b (slope) and a terms (gain) were calculated from the log-transformed EMGRMS–force relationships during the linearly increasing and decreasing segments of the trapezoid. EMGRMS during the steady force segment was normalized to MVC EMGRMS.
Results
For BFR, the b terms were greater during the linearly increasing segment than the linearly decreasing segment (p < 0.001), and compared to the linearly increasing segment for CON (p < 0.001). The a terms for BFR were greater during the linearly decreasing than linearly increasing segment (p = 0.028). Steady force N-EMGRMS was greater for BFR than CON collapsed across sex (p = 0.041).
Conclusion
BFR likely elicited additional recruitment of higher threshold motor units during the linearly increasing- and steady force-segment. The differences between activation and deactivation strategies were only observed with BFR, such as the b terms decreased and the a terms increased for the linearly decreasing segment in comparison to the increasing segment. However, EMGRMS–force relationships during the linearly increasing- and decreasing-segments were not different between sexes during BFR and CON.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood flow restriction (BFR) is used during exercise to partially restrict arterial inflow and completely occlude venous outflow, via a tourniquet or cuff applied over the proximal portion of the training limb (Scott et al. 2015; Patterson et al. 2019). Previous studies have reported that when matched for the same relative low-load, training with BFR set at 120–270 mmHg resulted in greater muscle hypertrophy (Yasuda et al. 2015; Takarada et al. 2002) and strength (Takarada et al. 2002; Yasuda et al. 2015) compared to non-occluded exercise. Additionally, a recent meta-analysis indicated that electromyographic amplitude (EMGRMS) was increased to a greater degree during low-load exercise when BFR was applied compared to similar non-occluded relative load exercise (Centner and Lauber 2020). Greater increases for EMGRMS may reflect the additional recruitment of higher threshold motor units (MUs) (Martinez-Valdes et al. 2018), which could partially explain why utilizing BFR during low-load training can result in greater muscle hypertrophy than without BFR as increases in EMG activity have been associated with single fiber and whole muscle growth (Häkkinen et al. 2001; Seynnes et al. 2007). Despite research indicating benefits of utilizing BFR with low-load exercise, less is known regarding the influence of BFR during high-load exercise. For example, Behringer et al. (2018) reported four sets of unilateral eccentric knee extensions at 75% of one repetition maximum to volitional failure with femoral BFR set at 20 mmHG below individual occlusion pressure resulted in metabolic stress, hormonal responses, muscle damage markers, and muscle swelling that were similar to non-BFR despite ~40% less repetitions (85.6 ± 15.4 vs. 142.3 ± 44.1). However, the authors did not record any EMG measures. Therefore, information regarding the effects off BFR on neuromuscular behavior at higher loads is still lacking.
We are aware of only one study that has examined the effects of BFR on neuromuscular behavior during acute high-load isometric exercise (Cayot et al. 2016). Cayot et al. (2016) reported BFR at 130% systemic blood pressure did not alter EMGRMS of the vasti muscles (i.e., quadriceps) during one set of four, five second isometric step contractions at randomly ordered submaximal loads (20, 40, 60, and 80% maximal voluntary contraction [MVC]) that were separated by 30 s of rest. Considering BFR commonly increases EMGRMS during low-load exercise performed with slower rates of force development (Yasuda et al. 2008, 2013; Moritani et al. 1992), it is possible that the rate of force development influences EMGRMS responses for BFR and non-BFR conditions during high-load exercise (Cayot et al. 2016). A slower rate of force development may be necessary for differences in EMGRMS to manifest with BFR; however, this has yet to be investigated. Therefore, more information is needed regarding potential benefits of BFR with high-load exercise.
Surface EMGRMS is commonly used to assess the level of muscle excitation, and is influenced by MU recruitment and/or firing rates of active MUs (Farina et al. 2004, 2014). However, the surface EMGRMS signal can be influenced by other factors, such as the quantity, length, and diameter of the muscle fibers that comprise the signal, distance from the electrode to the muscle fibers, and the amount of subcutaneous fat (sFAT), among others (Farina et al. 2010). Therefore, it has been suggested that EMGRMS–force relationships should be examined on a subject-by-subject basis (Farina et al. 2004). Previous studies have demonstrated that log-transforming the EMGRMS–force relationships during linearly increasing and decreasing muscle actions and applying simple linear regression provides a quantitative and reliable method for describing the individual patterns, and allows for subject-by-subject comparisons (Hill et al. 2023; Jeon et al. 2023; Trevino and Herda 2015; Herda et al. 2010). For instance, the log-transformation procedure produces the equation Y = a ∙ Xb, where Y = EMGRMS, X = force, a = gain coefficient, and b = exponential coefficient. The a term (anti-log of the y-intercept) can be viewed as a “gain factor” since the exponential model forces the a term through the zero, and it reflects a downward or upward shift in EMGRMS without altering the linearity of the relationship. Conversely, the b term (slope) reflects the rate of change of the Y variable (EMGRMS) in relation to the X variable (force). Previously, cross-sectional studies examining the a and/or b terms from the log-transformed EMGRMS–force/torque relationships during isometric trapezoidal muscle actions have been sensitive to MU activation and muscle action-related differences among chronic training statuses (i.e., aerobically trained, resistance-trained, sedentary) during single (Trevino and Herda 2015) and repeated contractions to fatigue (Jeon et al. 2023), and during prolonged contractions following short-term (5 weeks) endurance cycling training (Olmos et al. 2023a). Furthermore, Herda et al. (2009) reported a good day-to-day reliability for the a and b terms from the log-transformed EMGRMS–torque relationships across multiple days, during numerous contractions loads. Therefore, the aforementioned studies suggest that this analytical method is sensitive to acute and longitudinal changes in neuromuscular behavior. However, no study has examined the influence of BFR on EMGRMS–force relationships during a high-load isometric trapezoidal muscle action. Furthermore, utilizing an isometric trapezoidal muscle action to examine the effects of BFR may provide additional information regarding neuromuscular adjustments, as it has been reported that motor control strategies differ during MU activation and deactivation, and at steady force (Trevino and Herda 2015; Olmos et al. 2023a; Orizio et al. 2010). For example, differences in MU recruitment and derecruitment thresholds (Herda et al. 2015; Jesunathadas et al. 2010; De Luca et al. 1982a), as well as MU firing rates during increasing, steady, and decreasing muscle actions at the same effort level have been reported (Herda et al. 2015; Trevino et al. 2016; Del Vecchio et al. 2019).
Although equivocal findings have been reported regarding the level of required muscle excitation between males and females across various fatiguing intensities for the biceps brachii (BB) (Hill et al. 2016; Kalra et al. 2012; Yoon et al. 2007; Kavanagh et al. 2020), there are only a few studies that have examined sex-related differences in EMGRMS during a single high-load muscle action not taken to failure (Bilodeau et al. 1992; Pradhan et al. 2020). However, these studies did not account for the normalization of EMGRMS. Normalizing EMGRMS reduces variability in the measurement (De Luca 1997; Farina et al. 2010) and may provide further insight regarding sex-related differences in neuromuscular behavior at a targeted force (Diong et al. 2022). Therefore, the purpose of this study was to examine the influence of BFR on EMGRMS–force relationships in males and females during a 70% MVC isometric trapezoidal muscle action of the BB. Although high-load exercise to volitional failure with BFR has induced metabolic stress, hormonal responses, muscle damage markers, and muscle swelling similar to non-BFR (Behringer et al. 2018), it remains unknown if utilizing BFR during a single higher load muscle action can alter EMG responses without volitional failure (Kolind et al. 2023; Wernbom et al. 2009). Thus, we examined a load that is commonly used in applied settings (Maestroni et al. 2020; Bandy and Hanten 1993) is similar to the methods of Behringer et al. (2018), and recruits the majority of the MU pool (96%) for the BB (De Luca and Kline 2011). We hypothesized that BFR would elicit divergent MU control strategies, such as greater muscle excitation at steady force and alterations in the linearity (b terms) and gain (a terms) of the EMGRMS patterns compared to a non-BFR treatment. In addition, we hypothesized that females would require a greater level of muscle excitation at the targeted force for both treatments to compensate for smaller muscle fibers and MU twitch forces compared to the males, due to smaller muscle cross-sectional areas (mCSA) previously reported for the BB (Miller et al. 1993; Nonaka et al. 2006). It has been reported that sFAT can low-pass filter EMG signals (Bartuzi et al. 2010; Farina et al. 2004). Therefore, sFAT of BB was examined to provide confidence that any treatment- and/or sex-related differences for EMGRMS parameters were not the result of low-pass filtering.
Methods
Subjects
Twelve healthy males (mean ± SD; age: 25 ± 4 years, height: 177.59 ± 6.72 cm, body mass: 90.64 ± 8.90 kg) and eleven healthy females (age: 22 ± 5 years, height: 165.74 ± 5.43 cm, body mass: 61.54 ± 11.72 kg) participated in this study. All participants were currently resistance training (6.41 ± 2.89 h/wk) and had more than 1 year of resistance training experience (6.57 ± 4.14 years). Additionally, participants had reported no ongoing neuromuscular diseases, or musculoskeletal injuries specific to the shoulder, elbow, or wrist. The sample size was based on a recent investigation that reported sex-related differences for N-EMGRMS during a 70% MVC isometric trapezoidal contraction (Olmos et al. 2023b). This study was approved by the University’s institutional review board for human subject’s research. Written consent was obtained from all participants before their participation.
Experimental design
This study used a randomized cross-over design. Participants reported to the laboratory on three occasions separated by a minimum of 2 days, but no more than 7 days. All experimental visits were scheduled at the same time of day (±1 h) from their original testing visit. Visit one consisted of ultrasonography for the BB to determine sFAT, and familiarization of MVCs and the isometric trapezoidal muscle actions for the elbow flexors with blood flow restriction (BFR) and without (control [CON]). Visits two and three were randomized for either the CON or BFR treatments, where participants performed MVC followed by a submaximal isometric trapezoidal muscle action of the elbow flexors at 70% MVC. EMG was recorded from the BB during the BFR and CON visits. Participants were instructed to refrain from any alcohol and caffeine consumption, and physical activity for 24 and 48 h, respectively, before each visit.
Ultrasonography
Muscle cross-sectional area (mCSA) of the BB and sFAT overlying the BB was evaluated via ultrasonography using a portable brightness-mode (B-mode) Logiq® S8 ultrasound device (LOGIQ S8 GE Ultrasound System; GE Healthcare, Milwaukee, WI, USA) with a 4–15 MHz multi-frequency linear array probe (ML6-15-D; 50 mm field of view; GE Healthcare system). Scan depth was set to 5 cm, gain was 58 dB, and transducer frequency was 17 MHz to optimize image quality, and was held constant across all subjects. During testing, participants were examined on a padded table in the supine position with their arm abducted, relaxed, and supported on a wooden table, and their forearm extended at the elbow. After 10 min of rest to allow fluid shifts to settle (Berg et al. 1993), a generous amount of water soluble transmission gel was applied to the skin to reduce possible near field artifacts and enhance acoustic coupling, and three panoramic images were captured at half the distance from medial acromion to the fossa cubit (similar placement as the EMG sensor). Great care was taken to limit the compression of the muscle with the probe. ImageJ software (National Institutes of Health, Bethesda, MD) was used to analyze all ultrasound images. Each image was scaled from pixels to cm using the straight line function. For mCSA, the muscle was outlined using the polygon function, with care taken to exclude the surrounding fascia. Furthermore, using the straight line function, sFAT was quantified as the distance between the skin and the superficial aponeurosis of the BB.
Isometric strength testing
Isometric strength assessments of the dominant elbow flexors were performed on an isometric ergometer (MUC1; OT Bioelettronica SRL, Torino, Italy) fitted with a load cell (CCT Transducer, linear, full scale 100 kg) and the signal recorded with the force transducer was amplified (500×). During testing, participants were seated in an upright position with their shoulder and elbow joint flexed at 90° and the upper forearm strapped to the ergometer.
For each experimental visit, participants first performed two, 3–4 s held submaximal isometric warm-up contractions at 50 and 70% of perceived maximal effort, followed by two-to-three MVCs, each separated by 2 min of rest. During the MVCs, participants were instructed to “pull as hard and as fast as possible” and sustain the maximal contraction for 3–4 s. Additionally, verbal encouragement was provided during each attempt. The MVC with the highest force (N) averaged over a 0.25 s epoch determined maximal strength and the force level for the subsequent submaximal contractions. Following 2 min of rest, participants performed an isometric trapezoidal muscle action at 70% MVC with BFR or without (CON). For the isometric trapezoidal muscle action trajectory, baseline consisted of 5 s, followed by an increase in force at a rate of 10% MVC/s to the desired force level, a 12 s steady force segment, followed by a decrease of 10% MVC/s to baseline. Therefore, the duration of the 70% contraction lasted 26 s (Fig. 1). During each isometric trapezoidal muscle action, participants were instructed to maintain their force output as accurately as possible to the target force presented digitally in real time on a computer monitor. For the BFR visit, the occlusion cuff was inflated immediately prior to performing the trapezoidal muscle action and remained inflated during the entirety of the contraction. The occlusion cuff was immediately deflated following the completion of the muscle action. A second attempt was given after 90 s if a participant was unable to sustain their target force output on the first trial.
The electromyographic (EMG) signal from the Biceps Brachii (BB) during the 70% isometric trapezoidal contraction from one participant (top). The force signal (bottom) is overlaid onto the trapezoidal template as it appeared for the participant during the trial. The vertical lines represent the (A) linear force increasing, (B) steady force, and (C) linear force decreasing segments of the 70% isometric trapezoidal contraction. The EMG signals that corresponds with the contraction segments (A–C) were selected for analysis
Blood flow restriction
During the BFR condition, participants performed the isometric trapezoidal muscle action with a (5 cm) pneumatic nylon cuff (model: SC5D™, Hokanson®, Bellevue, WA, USA) applied around the most proximal portion of the contraction arm. Before starting the session, arterial occlusion pressure (AOP) was determined in the supine position for each participant. The cuff pressure was steadily increased at a rate of 5 mmHg per sec until resting brachial arterial pulse was no longer detected (100% arterial occlusion), and then reduced slowly at a rate of 1–2 mmHg per second until reappearance of pulse velocity. This process was complete within 30 s. AOP was assessed by Doppler ultrasound system (LOGIQ S8; GE Medical systems, Milwaukee, WI) equipped with a multi-frequency linear array transducer operating at 10 MHz. During the submaximal muscle action, the cuff pressure was inflated to 60% of each individual’s AOP, which is in agreement with the previous studies that have examined the acute effects of BFR on neuromuscular behavior (Hill et al. 2022, 2023).
Electromyography
During the trapezoidal muscle actions, surface EMG signals were recorded from the BB using a 5-pin array sensor (Delsys, Boston, MA, USA). Each pin has a diameter of 0.5 mm and is positioned at the corners of a 5 × 5 mm square, with the fifth pin in the center. Prior to sensor placement, the surface of the skin was prepared by shaving, removing superficial dead skin with adhesive tape, and sterilized with alcohol. The sensor was placed over the muscle belly of the BB (Ye et al. 2015; Petersen et al. 2002; Kidgell et al. 2010). The reference electrode was placed over the seventh cervical vertebrae at the neck. The signals recorded from the four pairs of the sensor electrode were differentially amplified and filtered with a bandwidth of 20 Hz–9.5 kHz. Channel 1 of the four differential EMG signals was selected for the time-domain (amplitude) analyses (Dimmick et al. 2018; Trevino et al. 2022; Olmos et al. 2023a) and used for all subsequent analyses and statistical comparisons.
Signal processing
The EMG (μV) and force (N) signals were simultaneously sampled at 2 kHZ with a National Instrument compact data acquisition system (NI cDAQ-9174) during each contraction. All subsequent signals were stored and processed off-line with custom-written software (LabVIEW version 18; National Instruments, Austin, TX). EMG signals were band-pass-filtered (fourth-order Butterworth) at 10–500 Hz. During the isometric trapezoidal muscle action, consecutive, non-overlapping 0.25-s epochs were analyzed for the force and EMG signals. Root mean square (RMS) was used to calculate the amplitude of the EMG signals.
Statistical analysis
For the linearly increasing and decreasing segments of the isometric trapezoid, simple linear regression models were fit to the natural log-transformed EMGRMS–force relationships (Trevino and Herda 2015; Jeon et al. 2023). The equations were represented as
where ln[Y] = the natural log of the EMGRMS values, ln[X] = the natural log of the force values, b = slope, and ln[a] = the natural log of the y-intercept. This can also be expressed as an exponential equation after the anti-log transformation of both sides of the equation
where Y = predicted EMGRMS values, X = force, b = slope of Eq. (1), and a = the anti-log of the y-intercept from Eq. (1). Individual slopes and the y-intercepts were calculated using Microsoft Excel® version 2016 (Microsoft, Inc., Redmond, WA, USA) to allow subject-by-subject analysis. For the steady force segment of the trapezoid, EMGRMS was calculated by averaging the values from each 0.25-s epoch during the steady targeted contraction force, and normalized (N-EMGRMS) to the EMGRMS value that corresponded to the highest 0.25 s peak force during the MVC for the respective visit.
The test–retest reliability for MVC force was assessed between the BFR and CON visits with a model 2,1 to calculate intraclass correlation coefficients (ICC), standard error of measurement (SEM), and minimal difference (MD). Two independent samples t tests were performed to examine potential sex-related differences in mCSA and sFAT. A two-way mixed factorial repeated-measures analysis of variances (ANOVA) (sex [male vs. female] × visit [CON vs. BFR]) were used to analyze possible differences in MVC between sexes and experimental visits. Additionally, a separate two-way mixed factorial repeated-measures analysis of variances (ANOVAs) (sex [male vs. female] × condition [CON vs. BFR]) were used to analyze possible differences in N-EMGRMS between sexes and conditions. Separate, three-way mixed factorial repeated-measures ANOVAs (sex [males vs. females] × condition [CON vs. BFR] × segment [increase vs. decrease]) were performed to examine differences in the b and a terms from the log-transformed EMGRMS–force relationships during the linearly increasing and decreasing segment of the 70% MVC isometric trapezoidal muscle action. When appropriate, follow-up analyses included independent and paired samples t tests with Bonferroni corrections. Lastly, to assess if sFAT may have biased EMG parameters, ten Pearson’s product moment correlation coefficients were calculated comparing sFAT with N-EMGRMS during the steady force segment, and the b terms and a terms from the log-transformed EMGRMS–force relationships during the linear increasing and decreasing segments of the isometric trapezoidal muscle actions for CON and BFR, respectively. The level of significance was set at p ≤ 0.05. Effect sizes for interactions were estimated using partial eta squared and were classified as small (0.01–0.06), medium (0.06–0.14), or large (>0.14). Additionally, effect sizes for between or within comparisons were estimated using Hedges’s g and were classified as minimal (0–0.2), small (0.2–0.5), medium (0.5–0.8), or large (>0.8). All statistical analyses were performed using SPSS 20 (IBM Corporation, Armonk, New York, USA).
Results
Reliability
For the MVCs during the BFR and CON visits, the ICC (0.989) indicated excellent reliability (Koo and Li 2016). In addition, the SEM was MD were 8.39 and 23.26 N, respectively. Only 1 subject out of 23 (4%) displayed a difference in MVCs between trials that exceeded the MD.
Maximal strength
There was no two-way interaction (sex × visit; F[1,21] = 4.139; p = 0.055; ηp2 = 0.165) or main effect for visit (F[1,21] = 3.655; p = 0.070; ηp2 = 0.148) for MVC. However, there was a main effect for sex (F[1,21] = 34.900; p < 0.001; ηp2 = 0.624). Males had a greater MVC (328.70 ± 53.00 N) than females (200.05 ± 49.64; g = 2.50) when collapsed across condition.
Linear increasing and decreasing EMGRMS–force relationships
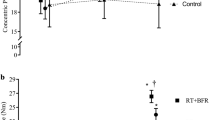
All 92 log-transformed EMGRMS–force relationships were significant during the linearly increasing (p < 0.05; r range = 0.912–0.995) and decreasing segments (p < 0.05; r range = 0.914–0.993) of the isometric trapezoidal muscle actions for BFR and CON. The lower limits of the 95% CIs constructed around the b terms from the natural log-transformed EMGRMS–force relationships during the linearly increasing and decreasing muscle actions were >1. Therefore, the EMGRMS–force relationships were non-linear with an upward acceleration in EMGRMS across the force spectrum for both muscle actions during BFR and CON. For a clearer interpretation of the EMGRMS–force patterns for each treatment and segment, the predicted N-EMGRMS values were calculated for each subject at the targeted force using the exponential equation (Eq. 2 of the methods) from 10 to 70% MVC (Fig. 2).
Plotted means and standard error of the mean for the blood flow restriction (BFR; closed triangle) and control (CON; open triangle) treatments during the (A) linearly increasing and (B) decreasing segments of the predicted normalized electromyographic (N-EMGRMS)–force relationships. *Indicates predicted N-EMGRMS during the linearly increasing segment was greater for BFR in comparison to CON at 60% (p = 0.034) and 70% MVC (p = 0.019)
For the b terms, there was no significant three-way interaction (sex × condition × segment; F[1,21] = 0.006; p = 0.939; ηp2 < 0.001). Additionally, there were no two-way interactions for segment and sex (F[1,21] = 0.973; p = 0.335; ηp2 = 0.044), or condition and sex (F[1,21] = 2.223; p = 0.151; ηp2 = 0.096). However, there was a significant two-way interaction for condition and segment (F[1,21] = 9.109; p = 0.007; ηp2 = 0.303). During the linear increase, b terms were greater for BFR (1.57 ± 0.38) than CON (1.30 ± 0.29; p < 0.001; g = 0.78). For BFR, the b terms were greater during the linear increase (1.57 ± 0.38) than the linear decrease (1.27 ± 0.28; p < 0.001; g = 0.88) (Fig. 3). For CON, b terms were similar between the linearly increasing and decreasing segments (1.22 ± 0.22; p = 0.148; g = 0.31). In addition, there were no differences in the linear decrease segments between CON and BFR (p = 0.366; g = 0.19).
Plotted individual values for the b and a terms from the electromyographic amplitude vs. force relationships for males (closed circles) and females (open circles) from the linearly increasing and decreasing segments of the isometric trapezoidal contraction for the blood flow restriction (BFR) and control (CON) treatments. Bars represent the means, with the 95% confidence intervals and standard deviations displayed for the b and a terms, respectively. *Indicates that the b terms during the linearly increasing segment were greater for BFR in comparison to CON (p < 0.001) and the linearly decreasing segment for BFR (p < 0.001). †Indicates greater a terms for BFR during the linearly decreasing segment in comparison the linearly increasing segment (p = 0.045)
For the a terms, there was no significant three-way interaction (sex × condition × segment; F[1,21] = 0.782; p = 0.387; ηp2 = 0.036). In addition, there were no two-way interactions for segment and sex (F[1,21] = 2.513; p = 0.128; ηp2 = 0.107), or condition and sex (F[1,21] = 1.075; p = 0.312; ηp2 = 0.049). However, there was a significant two-way interaction for condition and segment (F[1,21] = 5.7324; p = 0.026; ηp2 = 0.214). For BFR, the a terms were greater during the linearly decreasing (0.43 ± 0.80) than the linearly increasing segment (0.15 ± 0.29; p = 0.028; g = 0.46) (Fig. 3). There were no differences in a terms between segments for CON (p = 0.799; g = 0.06), nor were there differences between the a terms for CON and BFR (p = 0.115–0.532; g = 0.13–0.46).
Steady force segment
For N-EMGRMS, there was no significant two-way interaction (sex × condition; F[1,21] = 1.872; p = 0.186; ηp2 = 0.082). However, there were main effects for condition (F[1,21] = 4.739; p = 0.041; ηp2 = 0.184) and sex (F[1,21] = 5.598; p = 0.028; ηp2 = 0.210). N-EMGRMS was significantly greater for BFR (82.50 ± 22.92%) than CON (72.27 ± 17.28%; g = 0.50) when collapsed across sex. In addition, N-EMGRMS was greater for females (81.72 ± 23.44%) than males (68.55 ± 18.01%; g = 0.63) when collapsed across conditions (Fig. 4).
Plotted individual values for normalized electromyographic amplitude (N-EMGRMS) from the control (CON) and blood flow restriction (BFR) treatments for the males (closed circle) and females (open circle) during the steady force segments of the isometric trapezoidal contraction. Bars represent the means and standard deviations. †Indicates greater N-EMGRMS for BFR than CON (p = 0.041) when collapsed across sex. *Indicates greater normalized EMGRMS for the females than males (p = 0.028) when collapsed across conditions
Ultrasound measurements
mCSA was significantly greater for males (12.71 ± 2.11 cm2) than females (6.38 ± 1.90 cm2; t[21] = 7.545; p < 0.001; g = 3.14). sFAT was not significant different between males (0.17 ± 0.08 cm) and females (0.22 ± 0.12 cm; t[21] = −1.438; p = 0.176; g = 0.49).
Correlations
Only one of the ten Pearson’s product moment correlations (10%) for sFAT among EMGRMS parameters were significant. For CON, sFAT was not correlated with the b or a terms for the linearly increasing and decreasing segments (p = 0.227–0.719; r = −0.079 to 0.262). For BFR, sFAT was not correlated with b and a terms during the linearly increasing (p = 0.078–0.171; r = −0.375 to 0.296) and a terms during the linearly decreasing segment (p = 0.127; r = 0.328). However, sFAT was correlated with the b terms during the linearly decreasing segment of the EMGRMS-force relationships for BFR (p = 0.026; r = −0.464). In addition, sFAT was not correlated with N-EMGRMS for the CON (p = 0.248; r = 0.251) or BFR (p = 0.257; r = 0.246) treatments.
Discussion
Previous studies have only examined the influence of BFR on electromyographic parameters at failure during a series of repetitive low-load contractions, rapid high-load step contractions, or after an acute bout of exercise (Moritani et al. 1992; Hill et al. 2022, 2023; Cayot et al. 2016; Yasuda et al. 2013). Therefore, this study examined MU control strategies during activation, constant force, and deactivation via EMGRMS–force relationships during a single high-load (70% MVC) isometric trapezoidal muscle action with and without BFR for males and females. Significant and novel findings as a result of the BFR treatment include: (1) an increase in the slope (greater b terms) for the EMGRMS–force relationship during the linearly increasing segment of the trapezoidal muscle action, (2) greater N-EMGRMS during the targeted steady force segment, and (3) a decrease in the slope (reduced b terms) coupled with an increase in the gain (greater a terms) for the EMGRMS-force relationships during the linearly decreasing muscle action compared to the linearly increasing-muscle action of the isometric trapezoidal muscle action. Another interesting finding was that females displayed greater N-EMGRMS at steady force compared to the males for both treatments (CON and BFR). The findings of this study suggest that BFR may elicit changes in motor control strategies within seconds of starting a controlled high-load contraction, as muscle excitatory input (EMGRMS) significantly increased during the first segment of isometric trapezoidal muscle action, and remained elevated at the targeted force. In addition, the sex-related difference for N-EMGRMS at the targeted force suggests that females require greater muscle excitation when completing a high-load contraction at the same relative intensity. The similar sFAT between sexes coupled with the lack of correlations between sFAT and EMGRMS parameters provides confidence the findings were not the result of sFAT filtering of the EMG signal (Jeon et al. 2023; Trevino and Herda 2015; Herda et al. 2010).
This is the first study to report that BFR alters electromyographic behavior during a single muscle action. During the linearly increasing segment, the b terms were greater for the BFR treatment, indicating EMGRMS increased at a greater rate relative to force compared to the CON treatment. It is well understood that during submaximal isometric muscle actions, increases in muscle excitation to the motor unit pool when augmenting force induce the orderly recruitment of MUs by order of size (Henneman et al. 1965; Goldberg and Derfler 1977; Milner-Brown et al. 1973; Trevino et al. 2019), and a simultaneous increase in the firing rates of active MUs (Monster and Chan 1977; Farina et al. 2009; De Luca and Hostage 2010). However, surface EMGRMS may be more representative of the size of the action potentials from active MUs, rather than a reflection of firing rate changes (Martinez-Valdes et al. 2018). For example, Martinez-Valdez et al. (2018) examined surface EMGRMS, the change in MU firing rates from recruitment to the targeted torque (neural drive), and MU action potential sizes of the vastus lateralis and vastus medialis during four contraction intensities ranging from 10 to 70% MVC. Although both muscles received similar neural drive, differences in surface EMGRMS between muscles were mainly explained by the size of MU action potentials. Therefore, the larger b terms (greater increase in EMGRMS relative to force) during the linearly increasing segment of the isometric trapezoidal muscle action with BFR may likely suggest the accelerated recruitment of higher threshold MUs, which possess larger action potentials, was necessary to match the targeted force trajectory. In addition, it is speculated that increased accumulation of metabolic byproduct, mechanosensitivity, and nociception from venous occlusion during BFR exercise may increase feedback input of group III/IV afferent neurons to the central nervous system (Yasuda et al. 2010; Moritani et al. 1992; Kolind et al. 2023). Initial input of these afferent neurons during muscle contraction can excite the flexor MU pool at the presynaptic level of group Ia afferents, and/or directly synapse with α-motoneurons (Laurin et al. 2015; Kniffki et al. 1981; Martin et al. 2008), which may result in the recruitment of additional MUs (Martin et al. 2008; Moritani et al. 1992). Therefore, during a single non-fatiguing muscle action, it is possible that group III/IV afferent neurons may be eliciting a potential feedback response that initially warrants greater muscle excitation (EMGRMS) to the motor neuron pool to recruit additional MUs when matching the desired force levels (Fatela et al. 2019; Moritani et al. 1992; Tucker et al. 2009). Consequently, the accelerated increase for EMGRMS with BFR may provide support that the additional recruitment of higher threshold MUs occurred when producing the targeted force during the linearly increasing segment.
Previous studies have reported differences for EMGRMS between BFR and CON conditions during the later stages of intermittent low-load acute exercise (Yasuda et al. 2009, 2013; Moritani et al. 1992; Fatela et al. 2016). This is likely the result of their prolonged exercise duration coupled with BFR exacerbating the metabolic environment and, consequently, increasing the feedback response of group III/IV afferents to augment muscle excitation. Interestingly, the current study indicated differences for the b terms between treatments during the linearly increasing segment (first 7 s). Considering, non-occluded isometric contractions have been shown to alter oxygenation upon the onset of higher force production (Muthalib et al. 2010), it can be speculated the BFR quickly evoked the afferent response due to a combination of mechanical and metabolic stimuli. It should be noted that differences in predicted N-EMGRMS for the current study appeared to become larger as the target force increased during the linearly increasing segment (Fig. 2). In support, a secondary analysis comparing predicted N-EMGRMS at 10% MVC increments between treatments and segments indicated BFR elicited greater values than CON at 60% and 70% MVC during the linearly increasing segment (p < 0.05; Fig. 2). Therefore, using BFR during slowly increasing high-load efforts altered motor control strategies during a single contraction. Future research should investigate if BFR can alter EMGRMS–force relationships during a single isometric trapezoidal muscle action at lower intensities than the one utilized for the current study.
In agreement with Moritani et al., (1992), but in contrast to Cayoet et al. (2016), N-EMGRMS during the steady force segment was greater for the BFR (~83%) compared to the CON (~72%) treatment. Cayot et al., (2016) reported no differences for EMGRMS of the vasti muscles during BFR when performing one set of four, 5 s isometric step contractions at various submaximal target intensities (20, 40, 60, and 80% MVC) separated by a 30 s rest period with occlusion set at 130% systemic blood pressure. The authors stated the testing protocol was designed to reduce the influence of fatigue and/or metabolic stress on neuromuscular function. Consequently, their 30 s rest period between contractions may not have been sufficient to elicit divergent electromyographic responses between the BFR and non-BFR conditions. However, Moritani et al. (1992) reported greater EMGRMS during 2 s repeated contractions at 20% MVC followed by 2 s of rest for 4 min when 200 mmHg of occlusion pressure was applied between the 1st and 2nd minute. Therefore, it appears low-load intermittent isometric exercise may require multiple sets and/or numerous repetitions to elicit divergent EMGRMS responses between BFR and non-BFR conditions, which may be the result of increased difficulty clearing metabolites and partial pressure CO2 during longer applications (Yasuda et al. 2010). Conversely, our testing protocol included a gradual linearly increasing segment (10% MVC/s [7 s]) to 70% MVC, where it was maintained for an additional 12 s. It has been reported that slower rates of force production require a greater recruitment of higher threshold motor units than faster rates to produce to same relative load (Miller et al. 2019). Thus, our slower rate of increase preceding the targeted steady force segment likely resulted in greater recruitment than Cayot et al. (2016), and was further accentuated with the BFR treatment. Subsequently, BFR appears to increase the recruitment of higher threshold MUs, which are comprised of fibers that primarily express type II characteristics (Yoshida and Watari 1997; Suga et al. 2009, 2010). The greater N-EMGRMS values with BFR for the steady force segment may suggest a greater percentage of the MU pool was likely active during our BFR treatment. Furthermore, these findings may highlight the importance of utilizing slower rates of force development with BFR for training and clinical applications during a single high-load contraction.
During the linearly decreasing segment, there were muscle action-related differences for only the BFR treatment (Fig. 3), specifically, decreases and increases for the b and a terms in comparison to the linearly increasing segment, respectively. Therefore, the decreased b term indicated a smaller rate of change for EMGRMS in relation to force during the linearly decreasing segment, whereas the greater a term indicated an upward gain for the EMGRMS–force relationship across the force spectrum. Orizio et al. (2010) suggested that initial decreases for EMGRMS during the onset of a linearly decreasing isometric task reflect the selective derecruitment and/or a decrease in the firing rates of larger MUs, resulting in a greater reliance on smaller MUs with longer action potential durations to match force levels, which may be due to the size-related sensitivity of MUs to inhibition (De Luca et al. 1982b). For example, the density of inhibitory inputs is suggested to be similar across the MU pool (Clamann et al. 1974); therefore, afferent impulses may have more difficulty reaching the terminal branching structures of the nerve fibers for the larger compared to the smaller MUs (De Luca et al. 1982b). Subsequently, a greater percentage of the synaptic endings on the smaller MUs are activated, resulting in larger excitatory postsynaptic potentials and higher excitability (Lüscher et al. 1979). In regards to an isometric trapezoidal contraction during BFR, initial input of the group III/IV afferent neurons may be excitatory, resulting in greater recruitment of higher threshold MUs during the linearly increasing segment (Kniffki et al. 1979, 1981). However, higher threshold MUs are more fatigable (Burke 1978; Fuglevand et al. 1999; Burke et al. 1973), and as fatigue increases, activated group III/IV afferents can decrease central motor output (Taylor et al. 2006; Laurin et al. 2015; Gandevia 2001) and result in greater MU derecruitment (Moritani et al. 1992; Leonard et al. 1994; Garland 1991). Therefore, visually anticipating the force decrease, coupled with greater fatigue of higher threshold MUs, may have increased inhibitory input to the MU pool, which is also competing with the excitatory input to match the targeted force. The reduction of excitatory input leads to the orderly derecruitment and decrease in firing rates of active larger, higher threshold MUs. Consequently, the size of the largest activated MUs at the onset of the linearly decreasing segment may have been smaller compared to the same relative force (%MVC) during the linearly increasing segment. The decrease in large MUs at the onset of the linearly decreasing segment may explain the smaller rate of change for EMGRMS in relation to force. It is also possible that discomfort during BFR stimulated inhibitory inputs from spinal/supraspinal and/or presynaptic levels (Ia and group III/IV afferents). For example, previous studies have reported greater levels of discomfort with AOP ≥ 40% during acute exercise (Kolind et al. 2023; Bell et al. 2018; Wernbom et al. 2006). Therefore, increasing discomfort during the steady force segment may have also allowed greater derecruitment of higher threshold MUs at the onset of the linearly decreasing segment (Taylor et al. 2006; De Luca et al. 1982b; Kennedy et al. 2014). The upward gain for the EMGRMS–force relationship for the BFR treatment may be due to hysteresis of the active lower threshold MUs (Powers and Heckman 2015), such that firing rates are greater for the lower threshold MUs during the linearly decreasing- in comparison to linearly increasing-segment, and the MUs are derecruited at lower levels of excitation than their recruitment threshold (De Luca et al. 1982a; De Luca and Contessa 2012). Considering N-EMGRMS values for BFR are likely representative of a higher percentage and larger MUs that were active prior to the linearly decreasing segment, we speculate that their accelerated derecruitment during the onset of the linearly decreasing segment resulted in a greater contribution of the lower threshold MU to match the targeted force (Fig. 2).
Another interesting finding was that regardless of treatment (CON or BFR), females (~82%) exhibited higher N-EMGRMS values than males (~69%) during the steady segment of the isometric trapezoidal muscle action (Fig. 4), suggesting that greater muscle excitation was necessary to match the targeted force. Muscle excitation to the MU pool will adjust in response to MU force twitches when producing a desired muscle force output (Contessa and Luca 2013; De Luca and Contessa 2015; Trevino et al. 2022). In addition, previous studies have indicated that larger MUs produce greater force twitches (Milner-Brown et al. 1973; Goldberg and Derfler 1977; De Luca and Hostage 2010; Olson et al. 1968), and that the BB for males have greater muscle cross-sectional areas and larger mean muscle fiber sizes (Miller et al. 1993; Nonaka et al. 2006). In the current study, the mCSA of the BB for the males was roughly double that of the females. Therefore, it is plausible that our sex-related discrepancies for N-EMGRMS could be due to smaller MUs and muscle fibers for the females (Trevino et al. 2019; Miller et al. 1993; Simoneau and Bouchard 1989; Olmos et al. 2023b), resulting in greater recruitment of additional higher threshold MUs and greater firing rates of active MUs to compensate for smaller MU force twitches when modulating force. Future research should examine single MU firing rate and recruitment patterns of the BB between males and females at high-load targeted forces.
A limitation of the current study is the lack of muscle architectural imaging during the muscle actions. EMGRMS–force relationships can be influenced by force–fascicle length relationships (de Brito Fontana and Herzog 2016); therefore, it is possible that cuff inflation may have affected fascicle shortening and lengthening during the increasing and decreasing segments of the isometric trapezoidal template, respectively. In addition, physical compression of the muscle belly from the inflated cuff may have reduced the force generating capabilities of the elbow flexors (de Brito Fontana et al. 2018), requiring greater effort to match the relative load during the BFR treatment.
Conclusions
In summary, this is the first study to demonstrate BFR influences motor control strategies during a single, high-load isometric muscle action. The greater b terms (i.e., slope) during the linearly increasing segment and N-EMGRMS at steady force with the BFR treatment may suggest the additional recruitment of higher threshold MUs to match the targeted force. In addition, BFR resulted in muscle action-related differences during the isometric trapezoidal muscle action, such as decreases and increases for the slope and gain of the EMGRMS–force relationships during the linearly decreasing- in comparison to the linearly increasing segment, respectively. The findings suggest that it may be beneficial to utilize BFR during a high-load muscle action(s) with a controlled tempo during resistance training or clinical settings to potentially recruit additional higher threshold MUs compared to non-BFR exercise. It is reported that higher threshold MUs experience greater atrophy and denervation with aging/disuse compared to lower threshold MUs (Inns et al. 2022; Sterczala et al. 2018; Lexell et al. 1988). Therefore, using high isometric load exercise with BFR in rehabilitation settings may be more practical than traditional exercise, as isometric training is a common method for the rehabilitation process when mobility is limited (Maestroni et al. 2020; De Ruiter et al. 2005; Bandy and Hanten 1993). Additionally, there were sex-related differences for N-EMGRMS at steady force, regardless of treatment, suggesting that females recruited a greater percentage of the MU pool to complete the same relative load. Future research should investigate if a resistance training program for females can reduce the amount of necessary muscle excitation (N-EMGRMS) during high-load efforts, as this may be beneficial for activities of daily living/occupational performance.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- AOP:
-
Arterial occlusion pressure
- BB:
-
Biceps brachii
- BFR:
-
Blood flow restriction
- BABF:
-
Brachial artery blood flow
- CON:
-
Control
- EMGRMS :
-
Electromyography amplitude
- MVC:
-
Maximal voluntary contraction
- MU:
-
Motor unit
- mCSA:
-
Muscle cross-sectional area
- N-EMGRMS :
-
Normalized electromyographic amplitude
- sFAT:
-
Subcutaneous fat
References
Bandy WD, Hanten WP (1993) Changes in torque and electromyographic activity of the quadriceps femoris muscles following isometric training. Phys Ther 73(7):455–465. https://doi.org/10.1093/ptj/73.7.455
Bartuzi P, Tokarski T, Roman-Liu D (2010) The effect of the fatty tissue on EMG signal in young women. Acta Bioeng Biomech 12(2):87–92
Behringer M, Heinke L, Leyendecker J, Mester J (2018) Effects of blood flow restriction during moderate-intensity eccentric knee extensions. J Physiol Sci 68(5):589–599. https://doi.org/10.1007/s12576-017-0568-2
Bell ZW, Buckner SL, Jessee MB, Mouser JG, Mattocks KT, Dankel SJ, Abe T, Loenneke JP (2018) Moderately heavy exercise produces lower cardiovascular, RPE, and discomfort compared to lower load exercise with and without blood flow restriction. Eur J Appl Physiol 118:1473–1480. https://doi.org/10.1007/s00421-018-3877-0
Berg H, Tedner B, Tesch P (1993) Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 148(4):379–385. https://doi.org/10.1111/j.1748-1716.1993.tb09573.x
Bilodeau M, Arsenault AB, Gravel D, Bourbonnais D (1992) Influence of gender on the EMG power spectrum during an increasing force level. J Electromyogr Kinesiol 2(3):121–129. https://doi.org/10.1016/1050-6411(92)90009-8
Burke R (1978) Motor units: physiological/histochemical profiles, neural connectivity and functional specializations. Am Zool 18(1):127–134
Burke R, Levine D, Tsairis P, Zajac Iii F (1973) Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234(3):723–748. https://doi.org/10.1113/jphysiol.1973.sp010369
Cayot TE, Lauver JD, Silette CR, Scheuermann BW (2016) Effects of blood flow restriction duration on muscle activation and microvascular oxygenation during low-volume isometric exercise. Clin Physiol Funct Imaging 36(4):298–305. https://doi.org/10.1111/cpf.12228
Centner C, Lauber B (2020) A systematic review and meta-analysis on neural adaptations following blood flow restriction training: what we know and what we don’t know. Front Physiol 11:887. https://doi.org/10.3389/fphys.2020.00887
Clamann HP, Gillies JD, Henneman E (1974) Effects of inhibitory inputs on critical firing level and rank order of motoneurons. J Neurophysiol 37(6):1350–1360. https://doi.org/10.1152/jn.1974.37.6.1350
Contessa P, Luca CJD (2013) Neural control of muscle force: indications from a simulation model. J Neurophysiol 109(6):1548–1570. https://doi.org/10.1152/jn.00237.2012
de Brito Fontana H, Herzog W (2016) Vastus lateralis maximum force-generating potential occurs at optimal fascicle length regardless of activation level. Eur J Appl Physiol 116:1267–1277. https://doi.org/10.1007/s00421-016-3381-3
de Brito Fontana H, Han S-W, Sawatsky A, Herzog W (2018) The mechanics of agonistic muscles. J Biomech 79:15–20. https://doi.org/10.1016/j.jbiomech.2018.07.007
De Luca CJ (1997) The use of surface electromyography in biomechanics. J Appl Biomech 13(2):135–163. https://doi.org/10.1123/jab.13.2.135
De Luca CJ, Contessa P (2012) Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107(1):178–195. https://doi.org/10.1152/jn.00961.2010
De Luca CJ, Contessa P (2015) Biomechanical benefits of the onion-skin motor unit control scheme. J Biomech 48(2):195–203. https://doi.org/10.1016/j.jbiomech.2014.12.003
De Luca CJ, Hostage EC (2010) Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol 104(2):1034–1046. https://doi.org/10.1152/jn.01018.2009
De Luca C, Kline J (2011) Influence of proprioceptive feedback on the firing rate and recruitment of motoneurons. J Neural Eng 9(1):016007. https://doi.org/10.1088/1741-2560/9/1/016007
De Luca C, LeFever R, McCue M, Xenakis A (1982a) Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329(1):113–128. https://doi.org/10.1113/jphysiol.1982.sp014293
De Luca C, LeFever R, McCue M, Xenakis A (1982b) Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329(1):129–142. https://doi.org/10.1113/jphysiol.1982.sp014294
De Ruiter C, De Boer M, Spanjaard M, De Haan A (2005) Knee angle-dependent oxygen consumption during isometric contractions of the knee extensors determined with near-infrared spectroscopy. J Appl Physiol 99(2):579–586. https://doi.org/10.1152/japplphysiol.01420.2004
Del Vecchio A, Casolo A, Negro F, Scorcelletti M, Bazzucchi I, Enoka R, Felici F, Farina D (2019) The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J Physiol 597(7):1873–1887. https://doi.org/10.1113/JP277250
Dimmick HL, Miller JD, Sterczala AJ, Trevino MA, Herda TJ (2018) Vastus lateralis muscle tissue composition and motor unit properties in chronically endurance-trained vs sedentary women. Eur J Appl Physiol 118(9):1789–1800. https://doi.org/10.1007/s00421-018-3909-9
Diong J, Kishimoto KC, Butler JE, Héroux ME (2022) Muscle electromyographic activity normalized to maximal muscle activity, not to Mmax, better represents voluntary activation. PLoS ONE 17(11):e0277947. https://doi.org/10.1371/journal.pone.0277947
Farina D, Merletti R, Enoka RM (2004) The extraction of neural strategies from the surface EMG. J Appl Physiol 96(4):1486–1495. https://doi.org/10.1152/japplphysiol.01070.2003
Farina D, Holobar A, Gazzoni M, Zazula D, Merletti R, Enoka RM (2009) Adjustments differ among low-threshold motor units during intermittent, isometric contractions. J Neurophysiol 101(1):350–359. https://doi.org/10.1152/jn.90968.2008
Farina D, Holobar A, Merletti R, Enoka RM (2010) Decoding the neural drive to muscles from the surface electromyogram. Clin Neurophysiol 121(10):1616–1623. https://doi.org/10.1016/j.clinph.2009.10.040
Farina D, Merletti R, Enoka RM (2014) The extraction of neural strategies from the surface EMG: an update. J Appl Physiol 117(11):1215–1230. https://doi.org/10.1152/japplphysiol.00162.2014
Fatela P, Reis JF, Mendonca GV, Avela J, Mil-Homens P (2016) Acute effects of exercise under different levels of blood-flow restriction on muscle activation and fatigue. Eur J Appl Physiol 116:985–995. https://doi.org/10.1007/s00421-016-3359-1
Fatela P, Mendonca GV, Veloso AP, Avela J, Mil-Homens P (2019) Blood flow restriction alters motor unit behavior during resistance exercise. Int J Sports Med 40(09):555–562. https://doi.org/10.1055/a-0888-8816
Fuglevand AJ, Macefield VG, Bigland-Ritchie B (1999) Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. J Neurophysiol 81(4):1718–1729. https://doi.org/10.1152/jn.1999.81.4.1718
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. https://doi.org/10.1152/physrev.2001.81.4.1725
Garland SJ (1991) Role of small diameter afferents in reflex inhibition during human muscle fatigue. J Physiol 435(1):547–558. https://doi.org/10.1113/jphysiol.1991.sp018524
Goldberg LJ, Derfler B (1977) Relationship among recruitment order, spike amplitude, and twitch tension of single motor units in human masseter muscle. J Neurophysiol 40(4):879–890. https://doi.org/10.1152/jn.1977.40.4.879
Häkkinen K, Pakarinen A, Kraemer WJ, Häkkinen A, Valkeinen H, Alen M (2001) Selective muscle hypertrophy, changes in EMG and force, and serum hormones during strength training in older women. J Appl Physiol 91(2):569–580. https://doi.org/10.1152/jappl.2001.91.2.569
Henneman E, Somjen G, Carpenter DO (1965) Excitability and inhibitibility of motoneurons of different sizes. J Neurophysiol 28(3):599–620. https://doi.org/10.1152/jn.1965.28.3.599
Herda TJ, Weir JP, Ryan ED, Walter AA, Costa PB, Hoge KM, Beck TW, Stout JR, Cramer JT (2009) Reliability of absolute versus log-transformed regression models for examining the torque-related patterns of response for mechanomyographic amplitude. J Neurosci Methods 179(2):240–246. https://doi.org/10.1016/j.jneumeth.2009.01.028
Herda TJ, Housh TJ, Fry AC, Weir JP, Schilling BK, Ryan ED, Cramer JT (2010) A noninvasive, log-transform method for fiber type discrimination using mechanomyography. J Electromyogr Kinesiol 20(5):787–794. https://doi.org/10.1016/j.jelekin.2010.01.004
Herda TJ, Siedlik JA, Trevino MA, Cooper MA, Weir JP (2015) Motor unit control strategies of endurance-versus resistance-trained individuals. Muscle Nerve 52(5):832–843. https://doi.org/10.1002/mus.24597
Hill EC, Housh TJ, Smith C, Cochrane KC, Jenkins N, Cramer JT, Schmidt RJ, Johnson G (2016) Effect of sex on torque, recovery, EMG, and MMG responses to fatigue. J Musculoskelet Neuronal Interact 16(4):310
Hill EC, Rivera PM, Proppe CE, Gonzalez Rojas DH, Wizenberg AM, Keller JL (2022) Greater neuromuscular fatigue following low-load blood flow restriction than non-blood flow restriction resistance exercise among recreationally active men. J Neurophysiol 128(1):73–85. https://doi.org/10.1152/jn.00028.2022
Hill EC, Rivera PM, Proppe CE, Rojas DHG, Lawson JE (2023) Acute effects of low load blood flow restricted and non restricted exercise on muscle excitation, neuromuscular efficiency, and average torque. J Musculoskelet Neuronal Interact 23(2):165
Inns TB, Bass JJ, Hardy EJ, Wilkinson DJ, Stashuk DW, Atherton PJ, Phillips BE, Piasecki M (2022) Motor unit dysregulation following 15 days of unilateral lower limb immobilisation. J Physiol 600(21):4753–4769. https://doi.org/10.1113/JP283425
Jeon S, Sontag SA, Herda TJ, Trevino MA (2023) Chronic training status affects muscle excitation of the vastus lateralis during repeated contractions. Sports Medicine and Health Science. https://doi.org/10.1016/j.smhs.2022.12.005
Jesunathadas M, Marmon AR, Gibb JM, Enoka RM (2010) Recruitment and derecruitment characteristics of motor units in a hand muscle of young and old adults. J Appl Physiol 108(6):1659–1667. https://doi.org/10.1152/japplphysiol.00807.2009
Kalra C, Kumar DK, Arjunan S (2012) Age and gender related differences in surface electromyograms for various force levels of contraction. J Med Biol Eng 32:405–410. https://doi.org/10.1109/IEMBS.2011.6090957
Kavanagh JJ, Smith KA, Minahan CL (2020) Sex differences in muscle activity emerge during sustained low-intensity contractions but not during intermittent low-intensity contractions. Physiol Rep 8(7):e14398. https://doi.org/10.14814/phy2.14398
Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL (2014) Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol 116(4):385–394. https://doi.org/10.1152/japplphysiol.01166.2013
Kidgell DJ, Stokes MA, Castricum TJ, Pearce AJ (2010) Neurophysiological responses after short-term strength training of the biceps brachii muscle. J Strength Cond Res 24(11):3123–3132. https://doi.org/10.1519/JSC.0b013e3181f56794
Kniffki K-D, Schomburg E, Steffens H (1979) Synaptic responses of lumbar α-motoneurones to chemical algesic stimulation of skeletal muscle in spinal cats. Brain Res 160(3):549–552. https://doi.org/10.1016/0006-8993(79)91085-0
Kniffki K-D, Schomburg E, Steffens H (1981) Synaptic effects from chemically activated fine muscle afferents upon α-motoneurones in decerebrate and spinal cats. Brain Res 206(2):361–370. https://doi.org/10.1016/0006-8993(81)90537-0
Kolind MI, Gam S, Phillip JG, Pareja-Blanco F, Olsen HB, Gao Y, Søgaard K, Nielsen JL (2023) Effects of low load exercise with and without blood-flow restriction on microvascular oxygenation, muscle excitability and perceived pain. Eur J Sport Sci 23(4):542–551. https://doi.org/10.1080/17461391.2022.2039781
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Laurin J, Pertici V, Dousset E, Marqueste T, Decherchi P (2015) Group III and IV muscle afferents: role on central motor drive and clinical implications. Neuroscience 290:543–551. https://doi.org/10.1016/j.neuroscience.2015.01.065
Leonard CT, Kane J, Perdaems J, Frank C, Graetzer DG, Moritani T (1994) Neural modulation of muscle contractile properties during fatigue: afferent feedback dependence. Electroencephalogr Clin Neurophysiol 93(3):209–217. https://doi.org/10.1016/0168-5597(94)90042-6
Lexell J, Taylor CC, Sjöström M (1988) What is the cause of the ageing atrophy?: Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-to 83-year-old men. J Neurol Sci 84(2–3):275–294. https://doi.org/10.1016/0022-510x(88)90132-3
Lüscher H-R, Ruenzel P, Henneman E (1979) How the size of motoneurones determines their susceptibility to discharge. Nature 282(5741):859–861. https://doi.org/10.1038/282859a0
Maestroni L, Read P, Bishop C, Turner A (2020) Strength and power training in rehabilitation: underpinning principles and practical strategies to return athletes to high performance. Sports Med 50(2):239–252. https://doi.org/10.1007/s40279-019-01195-6
Martin PG, Weerakkody N, Gandevia S, Taylor J (2008) Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586(5):1277–1289. https://doi.org/10.1113/jphysiol.2007.140426
Martinez-Valdes E, Negro F, Falla D, De Nunzio AM, Farina D (2018) Surface electromyographic amplitude does not identify differences in neural drive to synergistic muscles. J Appl Physiol 124(4):1071–1079. https://doi.org/10.1152/japplphysiol.01115.2017
Miller AEJ, MacDougall J, Tarnopolsky M, Sale D (1993) Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol 66(3):254–262. https://doi.org/10.1007/BF00235103
Miller JD, Lund C, Gingrich MD, Schtul KL, Wray ME, Herda TJ (2019) The effect of rate of torque development on motor unit recruitment and firing rates during isometric voluntary trapezoidal contractions. Exp Brain Res 237:2653–2664. https://doi.org/10.1007/s00221-019-05612-0
Milner-Brown H, Stein R, Yemm R (1973) The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol 230(2):359. https://doi.org/10.1113/jphysiol.1973.sp010192
Monster AW, Chan H (1977) Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol 40(6):1432–1443. https://doi.org/10.1152/jn.1977.40.6.1432
Moritani T, Sherman WM, Shibata M, Matsumoto T, Shinohara M (1992) Oxygen availability and motor unit activity in humans. Eur J Appl Physiol 64:552–556. https://doi.org/10.1007/BF00843767
Muthalib M, Millet GY, Quaresima V, Nosaka K (2010) Reliability of near-infrared spectroscopy for measuring biceps brachii oxygenation during sustained and repeated isometric contractions. J Biomed Opt 15(1):017008. https://doi.org/10.1117/1.3309746
Nonaka H, Mita K, Akataki K, Watakabe M, Itoh Y (2006) Sex differences in mechanomyographic responses to voluntary isometric contractions. Med Sci Sports Exerc 38(7):1311–1316. https://doi.org/10.1249/01.mss.0000227317.31470.16
Olmos AA, Sontag SA, Sterczala AJ, Parra ME, Dimmick HL, Miller JD, Deckert JA, Herda TJ, Trevino MA (2023a) High-intensity cycling training necessitates increased neuromuscular demand of the vastus lateralis during a fatiguing contraction. Res Q Exerc Sport:1–12. https://doi.org/10.1080/02701367.2023.2201311
Olmos AA, Sterczala AJ, Parra ME, Dimmick HL, Miller JD, Deckert JA, Sontag SA, Gallagher PM, Fry AC, Herda TJ (2023b) Sex‐related differences in motor unit behavior are influenced by myosin heavy chain during high‐but not moderate‐intensity contractions. Acta Physiol:e14024. DOI: https://doi.org/10.1111/apha.14024
Olson CB, Carpenter DO, Henneman E (1968) Orderly recruitment of muscle action potentials: motor unit threshold and EMG amplitude. Arch Neurol 19(6):591–597. https://doi.org/10.1001/archneur.1968.00480060061008
Orizio C, Baruzzi E, Gaffurini P, Diemont B, Gobbo M (2010) Electromyogram and force fluctuation during different linearly varying isometric motor tasks. J Electromyogr Kinesiol 20(4):732–741. https://doi.org/10.1016/j.jelekin.2010.03.005
Patterson SD, Hughes L, Warmington S, Burr J, Scott BR, Owens J, Abe T, Nielsen JL, Libardi CA, Laurentino G (2019) Corrigendum: blood flow restriction exercise: Considerations of methodology, application, and safety. Front Physiol 10:1332. https://doi.org/10.3389/fphys.2019.01332
Petersen NT, Taylor JL, Gandevia SC (2002) The effect of electrical stimulation of the corticospinal tract on motor units of the human biceps brachii. J Physiol 544(1):277–284. https://doi.org/10.1113/jphysiol.2002.024539
Powers RK, Heckman CJ (2015) Contribution of intrinsic motoneuron properties to discharge hysteresis and its estimation based on paired motor unit recordings: a simulation study. J Neurophysiol 114(1):184–198. https://doi.org/10.1152/jn.00019.2015
Pradhan A, Malagon G, Lagacy R, Chester V, Kuruganti U (2020) Effect of age and sex on strength and spatial electromyography during knee extension. J Physiol Anthropol 39(1):1–11. https://doi.org/10.1186/s40101-020-00219-9
Scott BR, Loenneke JP, Slattery KM, Dascombe BJ (2015) Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med 45:313–325. https://doi.org/10.1007/s40279-014-0288-1
Seynnes OR, de Boer M, Narici MV (2007) Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol 102(1):368–373. https://doi.org/10.1152/japplphysiol.00789.2006
Simoneau J-A, Bouchard C (1989) Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab 257(4):E567–E572. https://doi.org/10.1152/ajpendo.1989.257.4.E567
Sterczala AJ, Herda TJ, Miller JD, Ciccone AB, Trevino MA (2018) Age-related differences in the motor unit action potential size in relation to recruitment threshold. Clin Physiol Funct Imaging 38(4):610–616. https://doi.org/10.1111/cpf.12453
Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, Takada S, Takahashi T, Omokawa M, Kinugawa S (2009) Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. J Appl Physiol 106(4):1119–1124. https://doi.org/10.1152/japplphysiol.90368.2008
Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, Takada S, Omokawa M, Kinugawa S, Tsutsui H (2010) Dose effect on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. J Appl Physiol 108(6):1563–1567. https://doi.org/10.1152/japplphysiol.00504.2009
Takarada Y, Sato Y, Ishii N (2002) Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 86:308–314. https://doi.org/10.1007/s00421-001-0561-5
Taylor JL, Todd G, Gandevia SC (2006) Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol 33(4):400–405. https://doi.org/10.1111/j.1440-1681.2006.04363.x
Trevino MA, Herda TJ, Fry AC, Gallagher PM, Vardiman JP, Mosier EM, Miller JD (2016) Influence of the contractile properties of muscle on motor unit firing rates during a moderate-intensity contraction in vivo. J Neurophysiol 116(2):552–562. https://doi.org/10.1152/jn.01021.2015
Trevino M, Sterczala A, Miller J, Wray M, Dimmick H, Ciccone A, Weir J, Gallagher P, Fry A, Herda T (2019) Sex-related differences in muscle size explained by amplitudes of higher-threshold motor unit action potentials and muscle fibre typing. Acta Physiol 225(4):e13151. https://doi.org/10.1111/apha.13151
Trevino MA, Dimmick HL, Parra ME, Sterczala AJ, Miller JD, Deckert JA, Gallagher PM, Fry AC, Weir JP, Herda TJ (2022) Effects of continuous cycling training on motor unit firing rates, input excitation, and myosin heavy chain of the vastus lateralis in sedentary females. Exp Brain Res 240(3):825–839. https://doi.org/10.1007/s00221-021-06278-3
Trevino MA, Herda TJ (2015) The effects of training status and muscle action on muscle activation of the vastus lateralis. Acta Bioeng Biomech 17(4)
Tucker K, Butler J, Graven-Nielsen T, Riek S, Hodges P (2009) Motor unit recruitment strategies are altered during deep-tissue pain. J Neurosci 29(35):10820–10826. https://doi.org/10.1523/JNEUROSCI.5211-08.2009
Wernbom M, Augustsson J, Thomeé R (2006) Effects of vascular occlusion on muscular endurance in dynamic knee extension exercise at different submaximal loads. J Strength Cond Res 20(2):372–377. https://doi.org/10.1519/R-16884.1
Wernbom M, Järrebring R, Andreasson MA, Augustsson J (2009) Acute effects of blood flow restriction on muscle activity and endurance during fatiguing dynamic knee extensions at low load. J Strength Cond Res 23(8):2389–2395. https://doi.org/10.1519/JSC.0b013e3181bc1c2a
Yasuda T, Brechue WF, Fujita T, Sato Y, Abe T (2008) Muscle activation during low-intensity muscle contractions with varying levels of external limb compression. J Sports Sci Med 7(4):467
Yasuda T, Brechue WF, Fujita T, Shirakawa J, Sato Y, Abe T (2009) Muscle activation during low-intensity muscle contractions with restricted blood flow. J Sports Sci 27(5):479–489. https://doi.org/10.1080/02640410802626567
Yasuda T, Abe T, Brechue WF, Iida H, Takano H, Meguro K, Kurano M, Fujita S, Nakajima T (2010) Venous blood gas and metabolite response to low-intensity muscle contractions with external limb compression. Metabolism 59(10):1510–1519. https://doi.org/10.1016/j.metabol.2010.01.016
Yasuda T, Loenneke J, Ogasawara R, Abe T (2013) Influence of continuous or intermittent blood flow restriction on muscle activation during low-intensity multiple sets of resistance exercise. Acta Physiol Hung 100(4):419–426. https://doi.org/10.1556/APhysiol.100.2013.4.6
Yasuda T, Fukumura K, Uchida Y, Koshi H, Iida H, Masamune K, Yamasoba T, Sato Y, Nakajima T (2015) Effects of low-load, elastic band resistance training combined with blood flow restriction on muscle size and arterial stiffness in older adults. J Gerontol Ser A 70(8):950–958. https://doi.org/10.1093/gerona/glu084
Ye X, Beck TW, DeFreitas JM, Wages NP (2015) Acute effects of dynamic exercises on the relationship between the motor unit firing rate and the recruitment threshold. Hum Mov Sci 40:24–37. https://doi.org/10.1016/j.humov.2014.11.011
Yoon T, Schlinder Delap B, Griffith EE, Hunter SK (2007) Mechanisms of fatigue differ after low-and high-force fatiguing contractions in men and women. Muscle Nerve 36(4):515–524. https://doi.org/10.1002/mus.20844
Yoshida T, Watari H (1997) Effect of circulatory occlusion on human muscle metabolism during exercise and recovery. Eur J Appl Physiol 75:200–205. https://doi.org/10.1007/s004210050148
Acknowledgements
The authors would like to thank each subject for their selfless participation.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
AAO, TKD, SMH, ECH, HCB, and MAT designed the experiments. AAO, TRM, KNS, TKD, SMH, HCB, ECH, PJS, JL, and MAT conducted the experiments. AAO, TRM, KNS, TKD, SMH, HCB, ECH, PJS, JL, and MAT analyzed the data. AAO and MAT wrote the manuscript. AAO and MAT edited and revised the manuscript. All authors approved the final version of the manuscript submitted for publication and agreed to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest, financial, or otherwise. No funding was received for conducting this study.
Additional information
Communicated by Toshio Moritani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olmos, A.A., Montgomery, T.R., Sears, K.N. et al. Blood flow restriction increases necessary muscle excitation of the elbow flexors during a single high-load contraction. Eur J Appl Physiol 124, 1807–1820 (2024). https://doi.org/10.1007/s00421-023-05405-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05405-y