Abstract
This study examined the effects of continuous endurance training on motor unit (MU) mean firing rates (MFR), percent myosin heavy chain (%MHC) isoforms, and muscle cross-sectional area (mCSA) of the vastus lateralis (VL). Twelve females completed 5-weeks of continuous cycling-training (CYC), while 8 females were controls (CON). Participants performed maximal voluntary contractions (MVCs) and 40% MVCs of the knee extensors before (PRE) and after the 5-week treatment period at the same absolute pre-treatment submaximal torque (POSTABS) and relative to post-treatment MVCs (POSTREL). Surface electromyographic (EMG) signals were decomposed with the Precision Decomposition III algorithm. MU firing times and waveforms were validated with reconstruct-and-test and spike trigger average procedures. MFRs at steady torque, recruitment thresholds (RT), and normalized EMG amplitude (N-EMGRMS) were analyzed. Y-intercepts and slopes were calculated for the MFR vs. RT relationships. MHC isoforms and mCSA were determined with muscle biopsies and ultrasonography. CYC decreased MVCs and type IIX %MHC isoform without changes in mCSA. The slopes for the MFR vs. RT relationships decreased for CYC during POSTREL and POSTABS while N-EMGRMS increased for POSTABS with no differences between PRE and POSTREL. Type I %MHC isoform was correlated with the slope for the MFR vs. RT relationship during POSTABS and POSTREL for CYC. This study provides evidence that decreases in the MFRs of higher threshold MUs post-CYC is likely a function of changes in input excitation (POSTABS) and the firing frequency–excitation relationships (POSTREL). Evidence is provided that MHC isoforms influence the firing rate scheme of the muscle following short-term training.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is interest in motor unit (MU) firing rate adaptations as a function of continuous endurance training (De Luca et al. 1982a, b; Herda et al. 2015; Dimmick et al. 2018). The mechanical output of a MU is proportional to its force twitch and firing rate (De Luca and Hostage 2010). Thus, adaptations in firing rate behavior following endurance training may have implications on MU force production. However, questions remain as only a few longitudinal studies have been conducted and they reported mixed results. Short-term continuous endurance training programs have resulted in decreases (Vila-Cha et al. 2010) or no change in MU firing rates (Martinez-Valdes et al. 2017). The discrepancies are likely related to differences in training programs and analyses of MU firing rates. For example, continuous endurance training programs have been performed primarily at moderate intensities (Vila-Cha et al. 2010) and training was for a short-duration (Martinez-Valdes et al. 2017). In addition, some investigations recorded few MUs per contraction that did not allow the firing rate scheme to be interpreted on a subject-by-subject basis (Vila-Cha et al. 2010). Furthermore, Vila-Cha et al. (2010) did not account for recruitment thresholds which are correlated with firing rates during voluntary submaximal isometric efforts (Milner-Brown et al. 1973; Trevino et al. 2016). Thus, examining the effects of continuous endurance training on firing rates relative to recruitment thresholds can allow comparisons on a subject-by-subject basis, and can provide more insight on MU behavior than comparing the average firing rates among subjects (Dimmick et al. 2018).
It is hypothesized that differences in MU firing rates between individuals with various exercise training backgrounds or changes following exercise training are attributed to contractile properties of the muscle (De Luca et al. 1982a). Myosin heavy chain isoform composition is a valid measure of absolute peak power and shortening velocity (contractile properties) of single skeletal muscle fibers (Trappe et al. 2003) and the electrophoretically determined % myosin heavy chain isoform composition correlates with the histochemical derived percentage of fiber type area (Fry et al. 1994; Staron et al. 2000). The muscle fiber type (I, IIA, IIX) with the largest fiber diameters yields the greatest % myosin heavy chain isoform composition (Staron et al. 2000). Thus, % myosin heavy chain isoform composition can provide insight on muscle fiber cross-sectional areas, as individuals with greater amounts of type II myosin heavy chain composition have exhibited larger type II fibers for higher-threshold MUs (Trevino et al. 2018; Herda et al. 2019). However, there are no differences in peak power of single skeletal muscle fibers when normalized to fiber diameter in young individuals (Trappe et al. 2003) and myosin heavy chain isoforms do not always reflect the contractile properties of the whole skeletal muscle, particularly in untrained subjects (Harridge et al. 1996; Herda et al. 2019). Significant correlations have previously been reported between type I % myosin heavy chain isoform with MU firing rates at moderate- (Trevino et al. 2016) and high-intensity (Colquhoun et al. 2018) targeted torques for chronically trained individuals of the vastus lateralis (VL). Continuous endurance training results in shifts in fiber type area (Howald et al. 1985) and could provide evidence that changes in muscle contractile properties influence alterations in MU firing rates. For example, myosin heavy chain isoform shifts to type I suggests peak power of the muscle fibers is becoming weaker and, thus, greater input excitation is required to maintain the same contraction level post-intervention. Therefore, the higher firing rates of individuals with greater type I myosin heavy chain isoform may indicate greater excitation is needed to maintain the same relative task due to reduced force generation capabilities of recruited MUs during moderate intensity contractions (Trevino et al. 2016). Of note, the correlation between myosin heavy chain isoforms and MU firing rates has not been examined in sedentary individuals. Furthermore, despite sex-related differences in % myosin heavy chain for the VL (Staron et al. 2000; Trevino et al. 2019), all the aforementioned studies only investigated males. Thus, it is plausible females may experience different MU firing rate adaptations due to greater amounts of type I % myosin heavy chain for the VL. However, this has yet to be investigated and examination may provide insight regarding neuromuscular adaptations in response to endurance training of females.
All previous continuous endurance cycling training investigations reported no change in maximal voluntary contraction (MVC) of the knee extensors and thus, MU firing rates were analyzed relative to post-training MVC (Vila-Cha et al. 2010; Martinez-Valdes et al. 2017). Continuous endurance training is reported to increase type I % myosin heavy chain isoform composition which could reduce MU twitch forces (Garnett et al. 1979). Therefore, it is plausible that a continuous cycling program performed with a greater intensity and total training time than previous investigations would require the recruitment of a greater percentage of MUs and negatively affect the force generating capabilities of the MU pool to a greater extent, decreasing MVC and requiring an increase in overall excitation to match pre-training targeted torques. Subsequently, an investigation examining the effects of endurance training on MU behavior should include contractions at the same pre- and post-training absolute torque levels and relative to the post-training MVC to provide a better understanding of possible underlying mechanisms for alterations in MU firing rates. For example, alterations in firing rates may be a function of changing the mean level of input excitation (operating point) (Contessa and De Luca 2013), the firing frequency-excitation relationship, or both.
Therefore, the primary purposes of this investigation were to examine the adaptations to a short-term (5 weeks) continuous endurance cycling protocol performed at a vigorous intensity (Norton et al. 2010) on MU firing rates recorded during a submaximal isometric trapezoidal contraction of the knee extensors performed at 40% MVC pre- and post-endurance training and the % myosin heavy chain isoform composition content of the VL in sedentary females. In addition, participants completed a 40% MVC at the same relative torque as pre-training. Normalized electromyographic amplitude (EMGRMS) was measured to examine possible differences for input excitation to the motoneuron pool at a targeted torque following training. Ultrasound images were analyzed to examine potential continuous endurance training-related changes in muscle cross-sectional area and subcutaneous fat of the VL that may confound interpretations of MU analyses. Furthermore, correlations examined the association of type I % myosin heavy chain isoform of the VL and MU firing rates at pre- and post-endurance training.
Experimental procedures
Subjects
Twenty healthy females completed this investigation. Twelve subjects completed the continuous endurance cycling training (CYC) program (age: 22.2 ± 5.7 years; height: 164.2 ± 5.5 cm, body mass: 63.6 ± 13.7 kg), whereas eight controls (CON) (age: 19.4 ± 2.5 years; height: 169.5 ± 6.2 cm, body mass: 63.9 ± 11.7 kg) only completed the experimental procedures. Changes in MU activity following exercise interventions have been reported with as few as ten participants (Vila-Cha et al. 2010; Pope et al. 2016). Thus, a quasi-random allocation was utilized where the first fifteen subjects were assigned to the CYC group to ensure ten completed the training, whereas the next 10 subjects were assigned to CON to ensure changes in MU parameters were not a function of type I error. Three CYC and two CON subjects voluntarily withdrew from the study. No subject reported any form of structured exercise for the previous 3 years or any history of current or ongoing neuromuscular diseases or musculoskeletal injuries specific to the ankle, knee, or hip joints. This study was approved by the university’s institutional review board for human subjects research and each subject signed a written informed consent. Subjects were provided monetary compensation for their participation.
Experimental approach
CYC and CON attended three laboratory sessions before (visits 1–3) and after (visits 4–6) five weeks of CYC or CON. Visits one and four consisted of ultrasound imaging of the VL to determine muscle cross-sectional area and subcutaneous fat followed by a maximal aerobic capacity test. Visits two and five were familiarization visits, where subjects practiced isometric MVCs and submaximal isometric trapezoid muscle actions of the knee extensors at 40% MVC. During test visits three and six, the muscle actions were recorded. The MVCs were used to determine maximal strength and the torque level for the submaximal contractions at pre- (PRE) and post-treatment. For post-treatment, subjects completed submaximal muscles actions at the same absolute torque as pre- (POSTABS) and relative to the post-treatment MVC (POSTREL). Surface EMG signals were collected from the VL to measure peak EMGRMS during the MVC and calculate normalized EMGRMS during the submaximal muscle actions. In addition, surface EMG signals recorded during the submaximal muscle actions were decomposed to extract recruitment threshold, mean firing rate at the targeted torque, and action potential amplitude for each observed MU. Subjects concluded visits 3 and 6 with a muscle biopsy of the VL. To avoid fatigue, there was 48 h of rest prior to testing visits.
Continuous cycling training
All CYC subjects completed 5 weeks of continuous endurance cycling on Life Fitness Upright Bikes (Model CLSC, Rosemont, IL, USA) at a frequency of 4 sessions per week for 20 total cycling sessions. Exercise intensity was prescribed on the upper limits of heart rate reserve. Target heart rates were calculated with the Karvonen method [(maximal heart rate – resting heart rate) × %intensity + resting heart rate)] (Karvonen et al. 1957). Percent heart rate reserve is recommended for prescribing exercise intensity in cycling activities (Lounana et al. 2007) for low fitness level populations (Swain 2000).
Weeks 1–3 consisted of 30 min of continuous cycling at 70% of the heart rate reserve, whereas, weeks 4–5 were 40 min at 75% of heart rate reserve. Heart rates were monitored with a Polar FT7 Heart Rate Monitor (Polar Electro, INC, Lake Success, NY, USA). All cycling sessions were supervised and subject heart rates were recorded every 3 min to ensure exercise intensity compliance.
Ultrasound imaging
A portable brightness mode ultrasound image device with a multi-frequency linear array probe (12 L-RS; 5–13 MHz; 38.4-mm field of view) in conjunction with GE logiq e Logic View software was used to generate panoramic real-time images of the VL. ImageJ software (National Institutes of Health, Bethesda, MD) was used to quantify anatomical muscle cross-sectional area and subcutaneous fat of the VL. For detailed information regarding subject positioning, ultrasound device settings, and image analyses, refer to Dimmick et al. (2018). Data from cadaver (Cartwright et al. 2013) and magnetic resonance imaging studies (Scott et al. 2012) have supported ultrasound to be a valid and reliable tool for assessing muscle cross-sectional area.
Maximal aerobic capacity testing
A graded exercise test was completed on an electronically braked cycle ergometer (Lode, Groningen, Netherlands) to determine maximal aerobic capacity (VO2MAX) and respiratory gases were collected with a two-way rebreathing valve (Hans Rudolph Inc., Shawnee, Kansas, USA) and monitored with a metabolic cart and respective software (Parvo Medics TrueOne® 2400 Metabolic Measurement System, Sandy, Utah, USA). Values were reported as ventilated oxygen and carbon dioxide and calculated relative VO2MAX. Prior to testing, participant seat height was measured and recorded for consistency across trials. Tests were considered valid if participants met three of the five American College of Sports Medicine Guidelines indicators (Amstrong et al. 2005). During testing, heart rates were recorded with a Polar FT7 Heart Rate Monitor (Polar Electro, INC, Lake Success, NY, USA) and the maximal heart rate was used to calculate the target heart rate for the cycling intervention. For detailed information regarding metabolic cart calibration, equipment settings, and test protocol refer to Walter et al. (2009).
Isometric strength testing
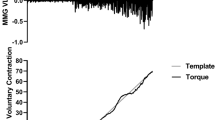
All isometric knee extensor strength assessments were performed on a Biodex System 3 isokinetic dynamometer (Biodex Medical Systems, Shirley, NY) in accordance to Trevino et al. (2016). All Biodex settings were recorded for consistency among trials. For pre-treatment testing, subjects performed an isometric trapezoidal muscle action at 40% of MVC (PRE). For post-treatment testing, subjects performed a muscle action at 40% of pre- and 40% of post-treatment MVC to investigate mean firing rates at POSTABS and POSTREL torques, respectively. For all isometric trapezoidal muscle actions, the torque increased at 10% MVC/s to the targeted torque level, where it was held for 10 s and then decreased to baseline at a rate of 10% MVC/s (Fig. 1). Five minutes of rest was given between each muscle action. Subjects were given a second attempt if unable to maintain the targeted torque during the initial trial.
Top graph: an example of the firing instances of the 26 detected motor units (MUs) recorded from the five-pin surface array sensor for the vastus lateralis during an isometric trapezoidal contraction at 40% maximal voluntary contraction (MVC) for one participant. The black line shows the force signal as it appeared to the participant during the trial. Each vertical bar represents the firing time of an action potential with MUs 5 and 26 indicated in bold. Middle graphs: the MU action potential (MUAP) waveforms from channels (CH) 1–4 for MU 5 and 26, respectively. Bottom graph: the mean firing rate (pps) versus recruitment threshold (expressed as percentage of maximal voluntary contraction [%MVC]) relationship is presented along with the calculated linear regression equations for the relationship
Electromyographic recording and decomposition
During the trapezoid muscle actions, surface EMG signals were recorded from the VL using a 5-pin surface array sensor (Delsys, Boston, MA). The pins have a diameter of 0.5 mm and are positioned at the corners of a 5 × 5 mm square, with the fifth pin in the center. Prior to sensor placement, skin was prepared by shaving, removing superficial dead skin with surgical tape, and sterilized with an alcohol swab. The sensor was placed over the VL muscle at 50% of the distance between the greater trochanter and the lateral condyle of the femur with adhesive tape. A permanent marker was used to mark the location of the electrode on the skin and participants were instructed to remark the location when necessary. The position of the electrodes was also measured using anatomical landmarks as a reference to confirm similar electrode placement between experimental sessions. The reference electrode was placed over the left patella. Signals were sampled at 20 kHz and stored on a computer for off-line analysis.
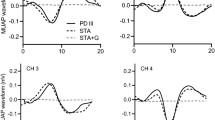
For detailed information regarding the signal processing of the EMG signals, refer to De Luca et al. (2006) and Nawab et al. (2010). Action potentials were extracted into firing events of single MUs from the four separate EMG signals via the Precision Decomposition III algorithm (version 4.1.1.0) as described by De Luca et al. (2006). This algorithm is designed for decomposing EMG signals into their constituent MU action potential trains and provides a unique waveform for each EMG channel. The accuracy of the decomposed firing instances was initially tested with the reconstruct-and-test procedure (Nawab et al. 2010) and only MUs with > 90% accuracies were used for further analysis. It is suggested a 90% accuracy criterion is appropriate for investigating MU firing rate characteristics as utilizing more stringent accuracy thresholds (92% and 95%) does not statistically change regression coefficients, yet may result in a reduction of subjects (~ 40%) due to a lack of usable MUs (Trevino et al. 2016). Additionally, a secondary spike trigger average procedure was performed to validate the firing times and action potential waveforms created via the Precision Decomposition III algorithm. The derived firing times from the Precision Decomposition III algorithm were used to spike trigger average the 4 raw EMG signals (Hu et al. 2013; Herda et al. 2020). MUs with high correlations (r > 0.7) across the 4 channels between the Precision Decomposition III algorithm and the spike trigger average derived action potential waveforms and a coefficient of variation for the spike trigger average derived peak to peak amplitudes < 0.3 across time (Hu et al. 2013) were selected for further analysis. Seemingly valid MU action potential waveforms may be constructed from trigger events that don’t correspond with MU discharge times (Farina et al. 2014). Thus, Gaussian noise set at 1% of the standard deviation for the interspike interval was added to the discharge times for each MU (Hu et al. 2013; Thompson et al. 2018). Correlations were performed on the MU action potential waveforms created from the raw EMG signals with the small amount of noise (< 2 ms shift) added to the firing times with the action potential waveforms that were triggered with the Precision Decomposition III algorithm derived firing times. Adding a small amount of noise to the firing times should decrease the correlation between action potential waveforms derived from the Precision Decomposition III algorithm and the spike trigger average procedures. Furthermore, peak-to-peak amplitudes of the spike trigger averaged action potential waveforms were compared to the action potential waveforms derived from the firing times with the addition of Gaussian noise. The peak-to-peak amplitudes derived from the firing times with Gaussian noise should decrease if no true action potential waveforms is consistently present (Fig. 2). For each MU, 2 parameters were extracted from the firing rate data: (1) recruitment threshold (expressed as %MVC) and (2) mean firing rates at the targeted torque level (pulses per second [pps]). The recruitment threshold was calculated as the average torque from a 0.10 ms epoch of force that began at the first discharge of the MU (Trevino et al. 2016). The mean firing rates were calculated as the inverse of the average interspike intervals (time between MU action potentials) during an 8 s epoch of the steady torque segment of the isometric trapezoidal template. Great care was taken to ensure the analysis was as close to the beginning of the steady torque segment as possible, but did not include any toque overshoot during the transition from the linearly increasing segment to steady torque. The start times for the analysis among all three contractions were not different (p > 0.05).
The four unique motor unit action potential waveforms for a single motor unit from the spike triggered averaging procedure (STA), the Precision Decomposition III algorithm (PD III), and the STA procedure with the addition of Gaussian noise to the firing times (STA + G). The average peak-to-peak amplitude for the waveforms calculated from the STA procedure (0.08899 mV) was substantially greater compared to STA + G (0.00127 mv). The large decrease in amplitude values for STA + G suggests minimal error in the firing times derived from the PD III algorithm
EMG amplitude
Channel 1 of the 4 bipolar EMG channels from the 5-pin surface array sensor was selected for the time-domain (amplitude) analyses (Dimmick et al. 2018). EMG signals were bandpass filtered (fourth-order Butterworth) at 10–500 Hz. Peak EMG amplitude was calculated as the average root mean square (RMS) value that corresponded to the highest 0.25 s peak torque epoch during the pre- and post-treatment MVCs. In addition, the average EMGRMS calculated during the 8 s epoch analyzed during the steady torque segment of the PRE, POSTABS, and POSTREL isometric trapezoidal muscle actions was normalized to peak EMGRMS for the respective visit.
Muscle biopsy
Thirty minutes after test visits three and six, muscle biopsies were taken from the VL (similar positon of EMG electrode) utilizing the percutaneous needle biopsy methods (Bergstrom 1962) and the double chop and suction method (Evans et al. 1981; Staron et al. 1990). For detailed information regarding subject preparation and follow-up care, biopsy storage, and SDS-PAGE methods used to investigate myosin heavy chain isoforms, refer to Trevino et al. (2016). The myosin heavy chain isoforms (types I, IIA, and IIX [fast fatigable]) were identified according to their molecular masses (Staron and Johnson 1993). The percentage of type I myosin heavy chain isoform content (type I % myosin heavy chain) was used for the correlations with the MU data. Fiber type distribution of the whole VL muscle in humans is not different along the axis of the muscle (Lexell et al. 1983) and differences in variation with single-fiber measurements from muscle biopsies account for < 2% of a change in fiber type proportion (Williamson et al. 2001).
Statistical analysis
For each contraction, linear regressions were applied to the mean firing rate vs. recruitment threshold relationships (Colquhoun et al. 2018). Slopes and y-intercepts were calculated for each subject and used for statistical analysis.
Potential changes in MVC, maximal aerobic capacity, muscle cross-sectional area, subcutaneous fat, and body mass were examined via separate two-way mixed factorial repeated measure analysis of variance (ANOVA) [Group (CYC vs. CON) × Time (PRE vs. POST)]. In addition, three separate two-way mixed factorial repeated measure ANOVAs [Group (CYC vs. CON) × Contraction (PRE vs. POSTABS vs. POSTREL)] were used to analyze possible differences in normalized EMGRMS and the slopes and y-intercepts for the mean firing rate vs. recruitment threshold relationships. Furthermore, a three-way mixed factorial repeated measures ANOVA [Group (CYC vs. CON) × Time (PRE vs. POST) × Isoform (Type I vs. Type IIX)] was used to analyze possible differences in % myosin heavy chain for the VL. Type IIA % myosin heavy chain isoform was not included in the ANOVA model as it is not statistically independent of the other two myosin heavy chain isoforms. When appropriate, follow-up analyses included one-way repeated measures ANOVAs, and paired and independent samples t tests with Bonferroni corrections. For pre-treatment, Pearson’s product moment correlation coefficients were calculated comparing the type I% myosin heavy chain isoform of the VL among the slopes and y-intercepts for the mean firing rate vs. recruitment threshold relationships separately for each group and all subjects (CYC + CON) together. For post-treatment, Pearson’s product moment correlation coefficients were calculated separately for CYC and CON comparing the type I% myosin heavy chain isoform of the VL among the slopes and y-intercepts for the mean firing rate vs. recruitment threshold relationships for POSTABS and POSTREL. Slopes and y-intercepts were calculated using Microsoft Excel version 2016 (Microsoft, Redmond, WA).
To investigate the validity of the MU action potential waveforms detected from the additional spike trigger average procedures, a Fisher’s r to z transformation (Fisher 1921) was performed for each subject on the correlation coefficient values for the MU action potential waveforms triggered with the Precision Decomposition III algorithm and the waveforms created from the raw EMG signals with Gaussian noise added to the firing times for the pre-treatment contraction. Potential differences in the z scores were investigated with a paired samples t test. Additionally, paired samples t tests were used to investigate potential differences between the peak-to-peak amplitudes of the spike trigger average action potential waveforms and the action potential waveforms derived from the firing times with the addition of Gaussian noise. The level of significance was set at p ≤ 0.05. Effect sizes were estimated using Hedges’s g and effect size estimates were classified as minimal (0–0.2), small (0.2–0.5), medium (0.5–0.8) or large (> 0.8). All statistical analyses were performed with SPSS 24 (IBM Corp, Armonk, NY).
Results
Ultrasound and body mass data
There were no significant two-way interactions for muscle cross-sectional area (p = 0.784), subcutaneous fat (p = 0.649), or body mass (p = 0.404). In addition, there were no main effects for time (p = 0.428–0.872) or group (p = 0.259–0.908) for muscle cross-sectional area, subcutaneous fat, or body mass. The means (SD) for muscle cross-sectional area, body mass, and subcutaneous fat for each treatment and time point are presented in Table 1. Thus, potential changes in MU parameters, or lack, therefore, were not a function of changes in subcutaneous fat.
Maximal aerobic capacity
There was a significant two-way interaction (p = 0.006). For CYC, there was a large, significant increase in maximal aerobic capacity (p < 0.001; g = 0.55) from pre- (32.52 ± 5.08 mL kg−1 min−1) to post-treatment (35.16 ± 4.42 mL kg−1 min−1), whereas, there was a minimal, non-significant (p = 0.295; g = 0.16) change from pre- (31.15 ± 4.00 mL kg−1 min−1) to post-treatment for CON (31.74 ± 3.35 mL kg−1 min−1). There were minimal and large non-significant differences between groups for pre- (p = 0.529; g = 0.29) and post-treatment (p = 0.080; g = 0.85), respectively.
Maximal strength
There was a significant two-way interaction (p = 0.033). For CYC, a small, significant maximal strength decrease was observed from pre- (131.03 ± 32.01 Nm) to post-treatment (122.45 ± 36.64 Nm; p = 0.044; g = 0.25), whereas, there was a minimal, non-significant (p = 0.275; g = 0.11) change from pre- (127.72 ± 32.57 Nm) to post-treatment (131.08 ± 29.23 Nm) for CON. There were minimal and small, non-significant differences between the groups at pre- (p = 0.824; g = 0.10) and post-treatment (p = 0.584; g = 0.26), respectively.
Normalized EMGRMS
Normalized EMGRMS could not be calculated for 3 subjects due to removal of the EMG sensor for additional skin preparation between MVC testing and the submaximal contractions; thus, statistical analyses were performed on the remaining 17 (11 CYC and 6 CON) subjects. There was a significant two-way interaction (p = 0.016). The follow-up one-way ANOVA for contraction was significant for CYC (p = 0.006), but not for CON (p = 0.461). For CYC, there was a large, significant increase for POSTABS compared to PRE (p = 0.026; g = 0.85), and a medium, significant difference compared to POSTREL (p = 0.042; g = 0.52). However, there was a small, non-significant difference for CYC between PRE and POSTREL (p = 0.640; g = 0.42). There were medium, non-significant differences between groups at PRE (p = 0.192; g = 0.70) and POSTABS (p = 0.247; g = 0.61) and a small, non-significant difference at POSTREL (p = 0.530; g = 0.24) (Fig. 3). Table 2 contains the mean ± SD for CYC and CON.
Plotted individual values (black markers) and means (bars) ± SD for normalized electromyographic amplitude (N-EMGRMS) for 11 cycling and 6 control subjects from pre- (PRE) and post-treatment absolute (POSTABS) and relative (POSTREL) torque levels. † indicates POSTABS is significantly greater than PRE (p = 0.026) and POSTREL (p = 0.042) for the cycling group
MU data
The spike trigger average procedure resulted in the rejection of 144 MUs (PRE = 32, POSTABS = 41, POSTREL = 71; 2.40 ± 1.80 MUs per contraction). Therefore, a total of 1324 MUs were analyzed (PRE = 478, POSTABS = 483, POSTREL = 404; 22.07 ± 3.88 per contraction). The mean (SD), MU counts, recruitment threshold ranges, and interspike intervals for each treatment and contraction are presented in Table 3. The mean firing rate (r = − 0.864 ± 0.082) vs. recruitment threshold relationships were significant (p ≤ 0.05) for each subject contraction and, thus, support the inverse relationship (onion skin control scheme) between MU firing rates and recruitment thresholds previously reported (Person and Kudina 1972; Tanji and Kato 1973; De Luca and Erim 1994). Adding a small amount of Gaussian noise to the Precision Decomposition III derived firing times deteriorated the action potential waveforms, such as, there was a significant decrease in the z-scores calculated from the correlation coefficients (p < 0.001; 1.370 ± 0.845 to 1.057 ± 0.100; g = 0.52) and peak-to-peak amplitudes (p < 0.001; 0.023 ± 0.010 to 0.002 ± 0.001 mV; g = 2.96) (Fig. 2) with minor shifts in the firing times (Thompson et al. 2018). Therefore, if the Precision Decomposition III derived firing times were primarily erroneous, the introduction of additional error would have little impact on the correlation and action potential amplitudes.
For the mean firing rate vs. recruitment threshold relationships, there was a significant interaction (p = 0.030) for the slopes. The follow-up one-way ANOVA for contraction was significant for CYC (p < 0.001), but not for CON (p = 0.921). For CYC, there were large, significant decreases in the slopes for POSTABS (p = 0.006; g = 1.44) and POSTREL (p = 0.009; g = 1.33) compared to PRE, however, there were no significant differences between POSTREL and POSTABS (p > 0.999). There was a large, significant difference between CYC and CON for PRE (p = 0.016; g = 1.03), but a minimal, non-significant difference for POSTABS (p = 0.687; g = 0.19) and POSTREL (p = 0.612; g = 0.24) (Fig. 4). For the y-intercepts from the mean firing rate vs. recruitment threshold relationships, there was no two-way interaction (p = 0.461) or significant main effect for group (p = 0.538). There was a significant main effect for contraction (p = 0.021); however, there were no significant differences among PRE, POSTABS, and POSTREL when collapsed across groups with Bonferroni corrections applied (p = 0.090–0.999) (Fig. 4). Table 2 contains the mean ± SD values for slopes and y-intercepts from the mean firing rate vs. recruitment threshold relationships for CYC and CON. Figure 5 illustrates the plotted predicted mean firing rate vs. recruitment threshold relationships for a CYC subject with y-intercept and slope values similar to the group means for PRE, POSTABS, and POSTREL.
Plotted individual values (black markers) and means (bars) ± SD for the slopes (top graphs) and y-intercepts (bottom graphs) of the mean firing rate (pulses per second [pps]) vs. recruitment threshold (% maximal voluntary contraction [MVC]) relationship for the cycling and control subjects from the pre- (PRE) and post-treatment absolute (POSTABS) and relative (POSTREL) torque levels. † indicates PRE is significantly different than POSTABS (p = 0.006) and POSTREL (p = 0.009) for the cycling group. * indicates significant differences between the cycling and control groups (p = 0.016) for the PRE torque level contraction
Plotted predicted mean firing rate (pulses per second [PPS]) vs. recruitment threshold (expressed as percentage of maximal voluntary contraction [MVC]) relationships calculated from the linear regressions for one subject with slope and y-intercept values that were similar to the group means for the pre- (PRE), post-cycling absolute (POSTABS), and post- relative (POSTREL) contractions
Myosin heavy chain
One of the muscle biopsy samples could not be analyzed due to complications in tissue preparation for storage and, therefore, statistical analyses were performed on the remaining 19 (11 CYC and 8 CON) subjects. There was a significant three-way interaction (p = 0.043). For CYC, there was a large, significant decrease in type IIX % myosin heavy chain isoform pre- to post-treatment (p = 0.006; g = 1.26), whereas, there was a minimal, non-significant change from pre- to post-treatment for CON (p = 0.457; g = 0.22). There were small and minimal, non-significant changes in type I % myosin heavy chain isoform for CYC (p = 0.083; g = 0.35) and CON (p = 0.634: g = 0.12). For CYC and CON, type I % myosin heavy chain isoform was significantly greater than type IIX % myosin heavy chain isoform at pre- and post-treatment (p ≤ 0.001–0.004). There were small and large, non-significant differences between CYC and CON for type I and IIX % myosin heavy chain isoform at pre- (p = 0.358; g = 0.40) or post-treatment (p = 0.056; g = 0.95), respectively. Type IIA % myosin heavy chain was not included in the ANOVA model (Table 4) as it is not statistically independent of the other myosin heavy chain isoforms (Fig. 6). Table 4 contains the mean ± SD values for myosin heavy chain isoforms percentages for CYC and CON.
Top: Electrophoretic separation of myosin heavy chain (MHC) isoforms for subject 9 from the PRE- (left) and post-cycling (POST CYC) treatment (right). Bottom: Mean ± SD for the percent type I, type IIA, and type IIX MHC isoform for 11 CYC and 9 control (CON) subjects for PRE- and post-treatment (POST). * indicates type I %MHC isoform was significantly greater than type IIX %MHC isoform at PRE and POST (p ≤ 0.001–0.004) within each treatment group. † indicates a significant decrease in type IIX %MHC isoform for CYC from PRE to POST (p = 0.006)
Correlations
For pre-treatment, there were no significant correlations (p = 0.320–0.648; r = − 0.192–0.331) for type I % myosin heavy chain isoform and the slopes for the mean firing rate vs. recruitment threshold relationships when including all or only CYC or CON. However, for post-treatment, there were significant correlations for CYC among type I % myosin heavy chain isoform and the slopes from the mean firing rate vs. recruitment threshold relationships for POSTABS (p = 0.024; r = 0.671) and POSTREL (p = 0.049; r = 0.603), but not for CON (p = 0.351–0.676; r = 0.176–0.382) for POSTABS or POSTREL (Fig. 7). The correlations for CYC from the POSTABS and POSTREL indicated that individuals with greater percent type I myosin heavy chain isoform had less negative slopes from the mean firing rate vs. recruitment threshold relationships. For the y-intercepts from the mean firing rate vs. recruitment threshold relationships, there were no significant correlations (p = 0.200–0.894, r = − 0.388 to − 0.077) at pre- or post-treatment when including all subjects or when separated by group.
Plotted relationships between type I % myosin heavy chain (%MHC) area and the slopes for the mean firing rate (pulses per second [pps]) vs. recruitment threshold (% maximal voluntary contraction [%MVC]) relationships from the pre- (PRE, left column) and post-treatment absolute- (POSTABS, middle column) and relative (POSTREL, right column) torque levels for 11 cycling (black markers) and 9 control (grey markers) subjects
Discussion
In agreement with previous studies, maximal aerobic capacity significantly increased following 5 weeks (20 sessions) of continuous cycling training (Howald et al. 1985; Martinez-Valdes et al. 2017). Significant findings following 5 weeks of training include correlations between type I % myosin heavy chain isoform composition of the VL and mean firing rates in relation to recruitment threshold, and knee extensor maximal strength decreased without changes in muscle cross-sectional area. Additionally, subjects exhibited alterations in mean firing rates at steady torque in relation to recruitment threshold at the same absolute and relative torque level post-cycling training despite normalized EMGRMS only increasing for the contraction performed at the same absolute torque, and cycling training decreased type IIX % myosin heavy chain isoform of the VL.
In the present study, knee extensor maximal strength significantly decreased following 5 weeks of CYC. Previously, it was reported that normalized twitch forces of gastrocnemius fibers following chronic endurance running training were substantially less compared to untrained individuals (Harber et al. 2004). In contrast, Vila-Cha et al. (2010) and Martinez-Valdes et al. (2017) reported no differences in maximal strength following 6 and 2 weeks of moderate-intensity continuous endurance cycling. The differences in findings may be due to training protocols and subject populations. The cycling training program used by Vila-Cha et al. (2010) was primarily a moderate intensity (50–70% heart rate reserve) compared to the vigorous intensity (Norton et al. 2010) utilized for the current study, while Martinez-Valdes et al. (2017) used a constant intensity (65% VO2PEAK) and only increased training duration over 6 cycling sessions (90–120 min). Thus, subjects for the current study trained at greater exercise intensities (70–75% heart rate reserve) and overall amount of time (680 min) compared to Vila-Cha et al. (2010) and Martinez-Valdes et al. (2017) (630 min). Consequently, the higher exercise intensities likely led to the recruitment of additional MUs (Saltin and Gollnick 1983) and negatively affected the force generation capabilities for a greater percentage of the motoneuron pool. Myosin heavy chain isoform composition shifts are linked to changes in muscle fiber twitch forces and it is unclear if the subjects for Vila-Cha et al. (2010) and Martinez-Valdes et al. (2017) altered myosin heavy chain isoform composition. Furthermore, Vila-Cha et al. (2010) and Martinez-Valdes et al. (2017) investigated sedentary- and recreationally trained college-aged males respectively, whereas the subjects for the current study were sedentary, college-aged females.
Following 5 weeks of continuous endurance cycling training, there were alterations in mean firing rates relative to recruitment threshold for the POSTREL torque level, such as, a decreased slope for the mean firing rate vs. recruitment threshold relationship. However, there was no change in normalized EMGRMS for POSTREL. Although POSTREL was performed at a lower steady torque, the required input excitation was not different between contractions at the same relative intensity (40% MVC). The lack of difference in normalized EMGRMS between PRE and POSTREL may indirectly indicate the contractions were performed at similar operating points (Contessa and De Luca 2013).
Linear regressions were used to calculate predicted mean firing rates for each subject at the targeted torque for MUs with recruitment thresholds of 5%, 10%, 15%, and 20% MVC. The slopes provide a relative measure of the difference in mean firing rates among MUs at the targeted torque as a function of recruitment thresholds and, thus, change scores were calculated for all CYC subjects and contractions. The mean firing rates for the lowest-threshold MUs (y-intercepts of the mean firing rate vs. recruitment threshold relationships) at the targeted torque were not different between PRE and POSTREL. However, the change scores for PRE indicated that the predicted mean firing rates at the targeted torque decreased 1.58 pps for every 5% increase in recruitment thresholds whereas for POSTREL, subjects exhibited a significantly greater (p = 0.009) rate of change (− 2.16 pps). Thus, participants exhibited significantly lower mean firing rates in comparison to PRE following CYC and the differences between contractions became progressively larger with increments in recruitment thresholds (i.e. 10% [− 0.58 pps), 15% [− 1.16 pps] and 20% MVC [− 1.74 pps]). Therefore, the similar levels of normalized EMGRMS between PRE and POSTREL may provide evidence that differences in mean firing rates post-cycling could be due to changes in the firing frequency-excitation relationship rather than input excitation to the motoneuron pool. These findings contrast Martinez-Valdes et al. (2017) that reported no change in EMGRMS or mean firing rates for the VL during a 30% MVC following 2 weeks of long duration endurance cycling.
The decrease in mean firing rates exhibited POSTREL may be due to alterations in motoneuron membrane potentials (Beaumont and Gardiner 2003) that decrease the firing frequency-excitation relationship (Gardiner 2006). This is tentatively supported with the decreases in mean firing rates post-training despite similar levels of input excitation (normalized EMGRMS) between the PRE and POSTREL. In addition, greater afterhyperpolarization amplitudes, which would decrease MU firing rates, have been reported for motoneurons innervating slow-twitch muscle fibers (Beaumont and Gardiner 2003). Thus, the effect of CYC may be more pronounced on higher- as lower threshold MUs would primarily express type I myosin heavy chain isoforms at PRE. This would be supported by the mean firing rate vs. recruitment threshold relationships as the mean firing rates were unchanged for the lower threshold MUs (y-intercepts), whereas, mean firing rates at the targeted torque progressively decreased with increments in MU recruitment thresholds (more negative slopes for POSTREL). Another possible explanation for the changes in mean firing rates is alterations in twitch relaxation rates. Five weeks of endurance cycling has resulted in downregulation of sarco(endo)plasmic reticulum calcium ATPase isoform (SERCA) pumps for the VL in humans (Majerczak et al. 2008), which may prolong muscle relaxation rates and allow MU tetanus at lower MU firing rates. This would allow MU twitch forces to summate (Grottel et al. 1993) and may explain how individuals in the current study maintained the same relative torque despite no change in normalized EMGRMS and overall decreases in mean firing rates.
Following 5 weeks of continuous endurance cycling training, there were also alterations in mean firing rates relative to recruitment threshold and likely recruitment patterns at the POSTABS torque level. There was a significant decrease in the slope for the mean firing rate vs. recruitment threshold relationship with greater normalized EMGRMS in comparison to PRE and POSTREL. Again, the secondary analysis indicated there were significant differences (p = 0.006) in the changes scores of the predicted mean firing rates for POSTABS compared to PRE. For POSTABS, mean firing rates at the targeted torque decreased 2.21 pps for every 5% increase in recruitment thresholds compared to PRE (− 1.58 pps). Therefore, the predicted mean firing rates at the targeted torque for POSTABS were becoming progressively lower compared to PRE with increments in MU recruitment thresholds (i.e. 10% [− 0.63 pps), 15% [− 1.27 pps] and 20% MVC [− 1.90 pps]). In theory, the operating point of excitation to the MU pool is altered to compensate for changes in MU twitch forces. In the present study, maximal torque production was significantly lower (− 6.6%) following 5 weeks of CYC. Therefore, the significant increase in normalized EMGRMS may indicate a rightward shift in the operating point (greater excitation) and the recruitment of a greater percentage and larger MUs (De Luca and Contessa 2012; Contessa and De Luca 2013; Martinez Valdes et al. 2018) to achieve the same submaximal torque level (POSTABS). The greater recruitment of larger MUs to sustain the same task post-CYC may be the result of weaker lower-threshold MUs (Farina et al. 2009; Contessa et al. 2016) coupled with increases in higher-threshold MUs twitch durations. For example, increased twitch durations would result in tetanus at lower frequencies (Bellemare et al. 1983). Thus, a greater reliance would be placed on the recruitment of additional higher-threshold MUs, which possess lower firing rates, instead of increasing the firing rates of active higher-threshold MUs when trying to match the POSTABS torque level and, consequently, would make the slopes from the mean firing rate vs. recruitment threshold relationships more negative (Fig. 5).
The mean firing rate and normalized EMGRMS data from the POSTABS contraction provides support for Vila-Cha et al. (2010) that examined the influence of continuous endurance cycling on firing rates recorded via intramuscular electrodes. Although Vila-Cha et al. (2010) reported no change in knee extensor MVC following 6 weeks of moderate intensity endurance cycling in sedentary males, the authors reported increased surface EMG amplitude, likely suggesting that larger (Martinez Valdes et al. 2018) and a greater total percentage of MUs were active during a 30% MVC that coincided with a significant decrease (10.6%) in pooled mean firing rates. Similarly, normalized EMGRMS increased and the mean firing rates of MUs recruited at 20% MVC for the current study decreased 11.4% at the targeted torque following training. Therefore, the decrease in mean firing rates with an increase in EMG amplitude reported by Vila-Cha et al. (2010) and for the current study likely suggests more MUs were recruited post-endurance cycling training to match the same relative and absolute submaximal force/torque level, respectively.
Only one study has longitudinally investigated the effects of continuous endurance cycling on the three myosin heavy chain isoforms in healthy, college-aged sedentary individuals (Howald et al. 1985). For the current study, there was a significant decrease in type IIX % myosin heavy chain isoforms (18.31– 9.45%) for the VL. Similarly, Howald et al. (1985) reported a decrease from 12.4 to 9.6% for type IIX % myosin heavy chain isoforms of the VL as measured with no changes in the diameters of the muscle fibers. The gel electrophoresis technique for the present study cannot quantify muscle fiber cross-sectional areas. However, there was no change in overall muscle cross-sectional area for the VL in the present study that would align with the findings of Howald et al. (1985). Therefore, it is proposed that continuous endurance cycling does not alter muscle fiber diameters despite changes in overall myosin heavy chain isoform composition.
Although muscle fibers for a given MU can coexpress two of the myosin heavy chain isoforms (Carroll et al. 2005; Rowan et al. 2012), it is generally accepted muscle fibers are distributed along a continuous spectrum (Enoka and Duchateau 2017) with fibers exhibiting primarily type IIX characteristics comprising higher- compared to lower-threshold MUs (Garnett et al. 1979). A decrease in hybrid fiber expression (Grosicki et al. 2016) and the conversions to type I and/or type IIA myosin heavy chain isoforms will occur if the MUs that possess these fiber types are continuously activated. Therefore, it is speculated that the loss of type IIX % myosin heavy chain isoform in the present study would support the notion that these fibers exist in smaller to moderate size MUs as the magnitude of input excitation would not recruit the highest threshold MUs during a contraction at 40% MVC (Miller et al. 2020) or during the cycling training in this study (Saltin and Gollnick 1983).
Interestingly, there were divergent findings for the correlations among type I % myosin heavy chain isoform and the slopes for the mean firing rate vs. recruitment threshold relationships at pre- and post-CYC training. Previously, Trevino et al. (2016) reported a strong positive correlation (r = 0.853) between type I % myosin heavy chain isoform and the slopes for the mean firing rate vs. recruitment threshold relationships during a 40% MVC. Individuals exhibiting greater amounts of type I % myosin heavy chain isoform displayed less negative slopes. Therefore, it was hypothesized the slopes would be correlated with type I% myosin heavy chain isoform, which was unfounded at PRE. A possible explanation for the lack of significant relationships is the influence of exercise training status. The subjects for Trevino et al. (2016) averaged 8 h/week exercise (unpublished data), whereas, the subjects in the current study reported no structured exercise program for the previous 3 years. However, following 5 weeks of cycling training (2.25 h/week), there were significant relationships among type I % myosin heavy chain isoform and the slopes for the mean firing rate vs. recruitment threshold relationships at POSTABS (r = 0.671) and POSTREL (r = 0.603). Subsequently, this is the first study to longitudinally examine sedentary individuals and the findings highlight the importance of training status on relationships between myosin heavy chain isoform and MU firing rate behavior.
A likely explanation for the change in the strength of the relationships from pre- to post-cycling training was the reduction of type IIX % myosin heavy chain isoform. It has been suggested that hybrid fiber expression increases with lack of physical activity (Grosicki et al. 2016). In addition, indirect evidence has previously been reported that MUs recruited during a 40% MVC possess type II fibers (Trevino et al. 2019). Thus, it is likely the percentage of type IIX myosin heavy chain isoform at baseline (Schilling et al. 2005; Fry et al. 2017) resulted in lower-threshold MUs with some muscle fibers that would express type IIX characteristics and may have masked the correlations between the slopes from the mean firing rate vs. recruitment threshold relationships and type I % myosin heavy chain isoforms. Previous research has reported similar non-correlations with myosin heavy chain isoforms with various muscular performance measures in untrained subjects (Schilling et al. 2005; Herda et al. 2019). In addition, the weaker correlations post-cycling training compared to Trevino et al. (2016) could be the result of remaining differences in exercise training status. Therefore, it is likely the cycling protocol reduced the amount of fibers that expressed type IIX myosin heavy chain isoform in the frequently activated MUs and, consequently, increased the strength of the relationships between type I % myosin heavy chain isoform and the slopes for the mean firing rate vs. recruitment threshold relationships for the POSTABS and POSTREL torque levels due to a decrease in myosin heavy chain coexpression of the MUs recruited post-training (Grosicki et al. 2016).
Similar to Trevino et al. (2016), individuals with greater amounts of type I % myosin heavy chain isoform had less negative slopes for the mean firing rate vs. recruitment threshold relationships, indicating a smaller decline in mean firing rates with increments in recruitment thresholds at the targeted POSTABS and POSTREL torque levels. It is well understood that MUs comprised of a greater percentage of type I % myosin heavy chain isoform are smaller (Trevino et al. 2018) and produce lower twitches forces (Garnett et al. 1979) which, consequently, influences adjustments in the amplitude of the neural drive from the central nervous system to the MU pool when sustaining a targeted torque (De Luca et al. 1996). Thus, Trevino et al. (2016) hypothesized the greater mean firing rates of higher-threshold MUs during a moderate-intensity contraction for individuals with more type I myosin heavy chain isoforms might be due to smaller force generation capabilities of the MU that led to greater neural drive to sustain the targeted torque, causing greater mean firing rates. It should be noted that the slopes for the mean firing rate vs. recruitment threshold relationships were more negative for CON in comparison to CYC at PRE. The differences in slopes may be due to the non-significant differences in type IIA % myosin heavy chain isoform between CYC (36.17 ± 12.65%) and CON (47.19 ± 9.79%), which has previously been reported (Trevino et al. 2016).
This study provides compelling evidence, via ultrasound images, muscle biopsies, and calculated relationships among MU mean firing rates and recruitment thresholds, that previously reported changes in firing rates at steady torque following continuous endurance cycling (Vila-Cha et al. 2010) resulted from changes in input excitation and the firing frequency-excitation relationships. In addition, this is the first study to provide evidence that short-term endurance training can result in relationships between MU mean firing rates and recruitment threshold at steady torque and myosin heavy chain isoform composition for previously sedentary individuals.
Data availability
Data in this study are available upon request. Please contact the corresponding author for access.
Abbreviations
- CON:
-
Control treatment group
- CYC:
-
Cycling treatment group
- MU:
-
Motor unit
- MVC:
-
Maximal voluntary contraction
- EMGRMS :
-
Electromyographic amplitude
- POSTABS :
-
Post-treatment absolute torque level
- POSTREL :
-
Post-treatment relative torque level
- PRE:
-
Baseline testing
- VL:
-
Vastus lateralis
References
Amstrong L, Whaley M, Brubaker P, Otto R (2005) ACSM’Guidelines for Exercise Testing and Prescription. In: Lippincott Williams & Wilkins, American College of Sport Medicine, Guidelines Skin Fold M easurement, Philadelphia PA
Beaumont E, Gardiner PF (2003) Endurance training alters the biophysical properties of hindlimb motoneurons in rats. Muscle Nerve 27:228–236
Bellemare F, Woods J, Johansson R, Bigland-Ritchie B (1983) Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol 50:1380–1392
Bergstrom J (1962) Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens. Scand J Clin Lab Investig (England) 14(Suppl 68)
Carroll CC, Gallagher PM, Seidle ME, Trappe SW (2005) Skeletal muscle characteristics of people with multiple sclerosis. Arch Phys Med Rehabil 86:224–229
Cartwright MS, Demar S, Griffin LP, Balakrishnan N, Harris JM, Walker FO (2013) Validity and reliability of nerve and muscle ultrasound. Muscle Nerve 47:515–521
Colquhoun RJ, Magrini MA, Haun CT et al (2018) Muscle phenotype is related to motor unit behavior of the vastus lateralis during maximal isometric contractions. Physiol Rep 6:e13636
Contessa P, De Luca CJ (2013) Neural control of muscle force: indications from a simulation model. J Neurophysiol 109:1548–1570
Contessa P, De Luca CJ, Kline JC (2016) The compensatory interaction between motor unit firing behavior and muscle force during fatigue. J Neurophysiol 116:1579–1585
De Luca CJ, Contessa P (2012) Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107:178–195
De Luca CJ, Erim Z (1994) Common drive of motor units in regulation of muscle force. Trends Neurosci 17:299–305
De Luca CJ, Hostage EC (2010) Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol 104:1034–1046
De Luca C, LeFever R, McCue M, Xenakis A (1982a) Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329:113–128
De Luca C, LeFever R, McCue M, Xenakis A (1982b) Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329:129–142
De Luca CJ, Foley PJ, Erim Z (1996) Motor unit control properties in constant-force isometric contractions. J Neurophysiol 76:1503–1516
De Luca CJ, Adam A, Wotiz R, Gilmore LD, Nawab SH (2006) Decomposition of surface EMG signals. J Neurophysiol 96:1646–1657
Dimmick HL, Miller JD, Sterczala AJ, Trevino MA, Herda TJ (2018) Vastus lateralis muscle tissue composition and motor unit properties in chronically endurance-trained vs. sedentary women. Eur J Appl Physiol 118(9):1789–1800
Enoka RM, Duchateau J (2015) Inappropriate interpretation of surface EMG signals and muscle fiber characteristics impedes progress on understanding the control of neuromuscular function. J Appl Physiol 119:1516–1518
Evans W, Phinney S, Young V (1981) Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14:101–102
Farina D, Holobar A, Gazzoni M, Zazula D, Merletti R, Enoka RM (2009) Adjustments differ among low-threshold motor units during intermittent, isometric contractions. J Neurophysiol 101:350–359
Farina D, Merletti R, Enoka RM (2014) The extraction of neural strategies from the surface EMG: an update. J Appl Physiol 117:1215–1230
Fisher RA (1921) On the “probable error” of a coefficient of correlation deduced from a small sample. Metron 1:1–32
Fry AC, Allemeier CA, Staron RS (1994) Correlation between percentage fiber type area and myosin heavy chain content in human skeletal muscle. Eur J Appl Physiol Occup Physiol 68:246–251
Fry AC, Housh TJ, Cramer JB et al (2017) Noninvasive assessment of skeletal muscle myosin heavy chain expression in trained and untrained men. J Strength Condition Res 31:2355–2362
Gardiner PF (2006) Changes in α-motoneuron properties with altered physical activity levels. Exerc Sport Sci Rev 34:54–58
Garnett R, O’donovan M, Stephens J, Taylor A (1979) Motor unit organization of human medial gastrocnemius. J Physiol 287:33–43
Grosicki GJ, Standley RA, Murach KA et al (2016) Improved single muscle fiber quality in the oldest-old. J Appl Physiol 121:878–884
Grottel K, Celichowski J, Anissimova N (1993) The relationship between the stimulation frequency and the tension of rat medial gastrocnemius motor units. Roczniki Naukowe AWF (poznan) 42:73–80
Harber MP, Gallagher PM, Creer AR, Minchev KM, Trappe SW (2004) Single muscle fiber contractile properties during a competitive season in male runners. Am J Physiol Regul Integr Compar Physiol 287:R1124–R1131
Harridge S, Bottinelli R, Canepari M, Pellegrino M, Reggiani C, Esbjörnsson M, Saltin B (1996) Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch 432:913–920
Herda TJ, Siedlik J, Trevino MA, Cooper MA, Weir JP (2015) Motor unit control strategies of endurance versus resistance trained individuals. Muscle Nerve 52(5):832–843
Herda TJ, Trevino MA, Sterczala AJ et al (2019) Muscular strength and power are correlated with motor unit action potential amplitudes, but not myosin heavy chain isoforms in sedentary males and females. J Biomech 86:251–255
Herda TJ, Parra ME, Miller JD, Sterczala AJ, Kelly MR (2020) Measuring the accuracies of motor unit firing times and action potential waveforms derived from surface electromyographic decomposition. J Electromyogr Kinesiol 52:102421
Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R (1985) Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch 403:369–376
Hu X, Rymer WZ, Suresh NL (2013) Motor unit pool organization examined via spike-triggered averaging of the surface electromyogram. J Neurophysiol 110:1205–1220
Karvonen MJ, Kentala E, Mustala O (1957) The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn 35:307
Lexell J, Henriksson-Larsen K, Sjöström M (1983) Distribution of different fibre types in human skeletal muscles 2. A study of cross-sections of whole m. vastus lateralis. Acta Physiol Scand 117:115–122
Lounana J, Campion F, Noakes TD, Medelli J (2007) Relationship between% HRmax,% HR reserve,% VO2max, and% VO2 reserve in elite cyclists. Med Sci Sports Exerc 39:350–357
Majerczak J, Karasinski I, Zoladz A (2008) Training induced decrease in oxygen cost of cycling is accompanied by down regulation of SERCA expression in human vastus lateralis muscle. Acta Physiol Pol 59:589
Martinez Valdes E, Negro F, Falla D, De Nunzio AM, Farina D (2018) Surface EMG amplitude does not identify differences in neural drive to synergistic muscles. J Appl Physiol (1985) 124(4):1071–1079
Martinez-Valdes E, Falla D, Negro F, Mayer F, Farina D (2017) Differential motor unit changes after endurance or high-intensity interval training. Med Sci Sports Exerc 49(6):1126–1136
Miller JD, Lippman JD, Trevino MA, Herda TJ (2020) Neural drive is greater for a high-intensity contraction than for moderate-intensity contractions performed to fatigue. J Strength Condition Res 34:3013–3021
Milner-Brown HS, Stein RB, Yemm R (1973) Changes in firing rate of human motor units during linearly changing voluntary contractions. J Physiol 230:371–390
Nawab SH, Chang SS, De Luca CJ (2010) High-yield decomposition of surface EMG signals. Clin Neurophysiol 121:1602–1615
Norton K, Norton L, Sadgrove D (2010) Position statement on physical activity and exercise intensity terminology. J Sci Med Sport 13:496–502
Person RS, Kudina LP (1972) Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32:471–483
Pope ZK, Hester GM, Benik FM, DeFreitas JM (2016) Action potential amplitude as a non-invasive indicator of motor unit specific hypertrophy. J Neurophysiol 00039:02016
Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT (2012) Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS ONE 7:e29082
Saltin B, Gollnick PD (1983) Skeletal muscle adaptability: significance for metabolism and performance. In: Handbook of Physiology. Skeletal Muscle pp 555–631
Schilling BK, Fry AC, Weiss LW, Chiu LZ (2005) Myosin heavy chain isoform expression: influence on isoinertial and isometric performance. Res Sports Med 13:301–315
Scott JM, Martin DS, Ploutz-Snyder R et al (2012) Reliability and validity of panoramic ultrasound for muscle quantification. Ultrasound Med Biol 38:1656–1661
Staron RS, Johnson P (1993) Myosin polymorphism and differential expression in adult human skeletal muscle. Compar Biochem Physiol Part B: Compar Biochem 106:463–475
Staron R, Malicky E, Leonardi M, Falkel J, Hagerman F, Dudley G (1990) Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. Eur J Appl Physiol 60:71–79
Staron RS, Hagerman FC, Hikida RS et al (2000) Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48:623–629
Swain DP (2000) Energy cost calculations for exercise prescription. Sports Med 30:17–22
Tanji J, Kato M (1973) Firing rate of individual motor units in voluntary contraction of abductor digiti minimi muscle in man. Exp Neurol 40:771–783
Thompson CK, Negro F, Johnson MD et al (2018) Robust and accurate decoding of motoneuron behaviour and prediction of the resulting force output. J Physiol 596:2643–2659
Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T (2003) Single muscle fibre contractile properties in young and old men and women. J Physiol 552:47–58
Trevino MA, Herda TJ, Fry AC, Gallagher PM, Vardiman JP, Mosier EM, Miller JD (2016) Influence of the contractile properties of muscle on motor unit firing rates during a moderate-intensity contraction in vivo. J Neurophysiol 116:552–562
Trevino MA, Sterczala AJ, Miller JD et al (2018) Sex-related differences in muscle size explained by amplitudes of higher-threshold motor unit action potentials and muscle fiber typing. Acta Physiol 225:e13151
Trevino M, Sterczala A, Miller J et al (2019) Sex-related differences in muscle size explained by amplitudes of higher-threshold motor unit action potentials and muscle fibre typing. Acta Physiol 225:e13151
Vila-Cha C, Falla D (2010) Farina D (2010) Motor unit behavior during submaximal contractions following six weeks of either endurance or strength training. J Appl Physiol (1985) 109:1455–1466
Walter AA, Herda TJ, Ryan ED et al (2009) Acute effects of a thermogenic nutritional supplement on cycling time to exhaustion and muscular strength in college-aged men. J Int Soc Sports Nutr 6:15
Williamson D, Gallagher P, Carroll C, Raue U, Trappe S (2001) Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol 91:1955–1961
Acknowledgements
We would like to thank P.R. Maier and J.D. Lippman who aided in data collection and analysis, as well as each subject for their selfless participation.
Funding
This publication was made possible by a National Strength and Conditioning Association Foundation (NSCAF) Graduate Research Doctoral Grant FND0074499 and a University of Kansas Doctoral Student Research Fund (DSRF) grant.
Author information
Authors and Affiliations
Contributions
MAT and TJH designed the experiments, MAT, AJS, JDM, MEW, HLD, JAD, JPW, ACF, and PMG conducted the experiments, MAT, AJS, JDM, MEW, HLD, JAD, JPW, ACF, PMG, and TJH analyzed the data. MAT wrote the manuscript. MAT, TJH, ACF, and PMG edited and revised the manuscript. All authors approved the final version of the manuscript submitted for publication and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Ethics approval
University of Kansas-Lawrence Institutional Review Board (# STUDY00002953).
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trevino, M.A., Dimmick, H.L., Parra, M.E. et al. Effects of continuous cycling training on motor unit firing rates, input excitation, and myosin heavy chain of the vastus lateralis in sedentary females. Exp Brain Res 240, 825–839 (2022). https://doi.org/10.1007/s00221-021-06278-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06278-3