Abstract
Purpose
The effects on muscle swelling were compared between low-load resistance exercise to exhaustion with (BFR) and without blood flow restriction (NBFR).
Methods
Ten young men [aged 27 (SD 5) years, standing height 1.74 (SD 0.05) m, body mass 70.3 (SD 4.3) kg] performed 20 % of one repetition maximal dumbbell curl exercise to exhaustion (four sets, rest intervals were 30 s for BFR and/or 3 min for NBFR, respectively). One arm was randomly chosen for BFR exercise and the other arm performed NBFR exercise. During the BFR exercise session, an elastic cuff was worn proximally on the testing arm at 160 mmHg. Electromyography (EMG) signals were recorded from surface electrodes placed on the biceps brachii muscle and analyzed for integrated EMG (iEMG). Biceps brachii muscle thickness (MTH) was measured using B-mode ultrasound.
Results
The total number of exercise repetitions was greater (p < 0.01) in NBFR (221 ± 67 reps) than in BFR (111 ± 36 reps). During the exercise session, iEMG for biceps brachii muscles increased (p < 0.01) during BFR and NBFR (3.94 and 4.45 times of baseline value). Immediately after the exercise, MTH sharply increased (p < 0.01) with BFR and NBFR (1.21 and 1.20 times of baseline value). These results demonstrate that both BFR and NBFR exercises lead to pronounced muscle activation and muscle swelling.
Conclusion
Low-load resistance exercise to exhaustion is an effective method for promoting muscle swelling regardless of BFR. Furthermore, our data indicate that the increase in muscle swelling for both NBFR and BFR is maintained even 60 min after the exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past decade, numerous studies have reported that low-intensity resistance training [20–30 % one-repetition maximum (1-RM)] combined with blood flow restriction (BFR) elicits muscle fatigue and hypertrophy similar to that induced by traditional high-load resistance training (HL, >70 % 1-RM), regardless of age (Abe et al. 2005; Karabulut et al. 2010; Loenneke et al. 2012d; Takarada et al. 2000; Yasuda et al. 2009, 2010a, 2014). BFR training was originally developed in Japan and it is better known as KAATSU training (Sato 2005). This technique may serve as an alternative training method to improve muscle size and strength in elderly patients who are capable of tolerating only low-load resistance training (i.e., multiple sclerosis patients, arthritis patients, etc.).
Recently, low-load (30 % 1-RM) resistance exercise to volitional fatigue was reported to stimulate muscle protein synthesis for a longer duration than HL (90 % 1-RM) or work-matched resistance exercise (Burd et al. 2010). In addition, Ogasawara et al. (2013) revealed that low-load (30 % 1-RM) bench press training to volitional fatigue results in muscle hypertrophy similar to HL (75 % 1-RM) bench press training. Therefore, there is a high possibility that low-load resistance training to volitional fatigue is an effective method for the promotion of muscle protein synthesis and hypertrophy, regardless of whether or not BFR is applied.
Acute cell swelling has been shown to stimulate protein synthesis and suppress proteolysis (Berneis et al. 1999; Häussinger et al. 1993). A previous study showed that increased leg circumference, an index of muscle swelling, was more pronounced in BFR than in non-BFR immediately after low-intensity knee extension exercise (Fry et al. 2010). Interestingly, following a single bout of low-intensity BFR bench press exercise, acute changes in muscle size were observed in both the blood flow restricted triceps muscle as well as the blood flow non-restricted chest muscle. The muscle cross-sectional area of both the triceps and chest muscles increased following BFR bench press training (Yasuda et al. 2010b). Therefore, it appears that BFR training-induced muscle cell swelling may contribute significantly to the anabolic benefits of BFR (Loenneke et al. 2012a; Yasuda et al. 2012). However, previous BFR or NBFR studies (Fahs et al. 2014; Martín-Hernández et al. 2013; Yasuda et al. 2012) have only evaluated muscle swelling at the beginning and end of the resistance exercise, giving no inference as to the time-course of change. We hypothesized that low-load resistance training to volitional fatigue is an effective method for the promotion of muscle swelling regardless of BFR, and the time-course of the increase in muscle swelling will be similar for both conditions. Thus, the purpose of the present study was to investigate the effect of low-load resistance exercise to volitional fatigue with and without BFR on muscle swelling.
Methods
Subjects
Ten healthy young men volunteered for the study. A priori sample size estimation indicated that eight subjects would be needed to detect a group by time interaction for measurements of muscle size with an effect size of 1.5–1.8, alpha level of 0.05, and a power of 0.80. Subjects were excluded if they were hypertensive (blood pressure > 140/90 mmHg) or obese (BMI > 30 kg/m2). The subjects were classified as “recreationally active”; three of ten participated in regular aerobic type exercises (jogging, or cycling; 2–3 times/week for approximately 30 min). Three other subjects had light to moderate resistance training experience and performed upper body, but they were not professionally trained. Each subject was informed of the risks associated with the exercise, measurements to be taken and the purpose of the study, which conformed to the Declaration of Helsinki and was approved by the Ethics Committee for Human Experiments, University of Tokyo. Written informed consent was obtained from each subject prior to participation.
Protocol
One week prior to experiments, all subjects completed an orientation session which included measurement of resting blood pressure and familiarization with arm curl exercise and BFR. During the orientation session, subjects sat in a chair with the testing arm placed on a table at heart level, and blood pressure was measured after 3 min rest. Then, 1-RM for each arm was determined. Subjects performed 5–6 unilateral arm curls with a low load (approximately 30–40 % predicted 1-RM) as a warm-up and to familiarize subjects with the arm curl exercise. After the warm-up period, the intensity was set at about 80 % of predicted 1-RM. Following each successful lift, the intensity was increased by about 5 % until the subject could not complete the lift through the entire range of motion. A test was considered valid only when the subject used the proper form and completed the entire lift in a controlled manner without assistance. On average, five trials were necessary to complete a 1-RM test (2–3 min rest between each attempt) (Yasuda et al. 2008, 2009, 2010a, 2012).
In the experiments, the subjects randomly performed unilateral arm curl exercises with two different exercise conditions that were scheduled 1 week apart (Visit 1 and Visit 2). The order of exercise conditions was randomized. Subjects were instructed to refrain from drinking alcohol and caffeine for 24 h before the sessions and from performing any strenuous exercise for 48 h before the sessions.
Exercise
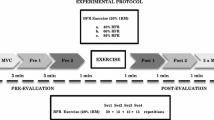
The subjects performed four sets of arm curl exercise without BFR (NBFR, with 3 min rest period between sets) and with BFR (with 30 s rest period between sets). In each set of exercise, the subjects repeated the movements until exhaustion, which was defined as failure to keep up with the metronome. This protocol is typical of previously reported BFR and NBFR studies (Kubo et al. 2006; Ogasawara et al. 2013). Exercise intensity was 20 % of the predetermined 1-RM. During each exercise condition, subjects sat on the arm curl bench, with their arm positioned in front of their body such that the shoulder was supported at 45° flexion. The range of elbow joint motion during exercise was completed from 0° to 150° (0° being full extension). Contraction duration was 2.4 s with a 1.2:1.2 s concentric-eccentric contraction duty cycle controlled by a metronome (50 beats per min) (Yasuda et al. 2008, 2009). One arm was randomly chosen to perform BFR exercise, while the other arm performed NBFR exercise, but the use of dominant or non-dominant arm was randomized between subjects. The protocols are summarized in Fig. 1.
Blood flow restriction
Before the experiment, all subjects were familiarized with the specialized pressure cuff (30 mm width, Kaatsu–Master, KAATSU Japan Co., Ltd, Tokyo, Japan). The pressure was set to 160 mmHg, according to previous studies (Yasuda et al. 2008, 2009) for the upper arm which reported that 160 mmHg is the optimal level of BFR for altering blood flow, energy supply, and increasing muscle activation when the predetermined exercise protocol is the same among all BFR conditions. The restriction pressure intensity was applied to the upper arms as previously described (Yasuda et al. 2009, 2010a, 2011). During acclimatization, no sign of discomfort or pain was observed in the subjects. In the BFR session experiment, subjects were seated on a chair and the cuff was tightened around the arm to a “belt pressure” (i.e., the pressure the cuff is applying to the arm prior to inflation) of 30 mmHg on the most proximal region of the testing arm. The cuff was then inflated to 160 mmHg (Yasuda et al. 2009, 2010a, 2011). In the BFR session, the cuff was inflated for the entire exercise session including the rest periods between sets and contractions.
Ultrasound-measured muscle thickness
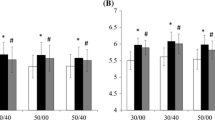
The muscle thickness of the elbow flexors was measured using B-mode ultrasound (Acuson Sequoia 512, Siemens, Tokyo, Japan) at the biceps brachii muscle (at 60 % distal between the lateral epicondyle of the humerus and the acromial process of the scapula). Briefly, the measurements were carried out while the subjects stood with their elbows extended and relaxed. A 10.0 MHz scanning head (5.5 cm length probe) was placed on the skin perpendicular to the tissue interface. The scanning head was coated with a water-soluble transmission gel to facilitate acoustic contact without depressing the dermal surface. The subcutaneous adipose tissue-muscle interface and the muscle-bone interface were identified from the ultrasonic image. The perpendicular distance from the adipose tissue-muscle interface to the muscle–bone interface was taken as muscle thickness (MTH). Ink markers on the elbow flexors were used to ensure similar positioning over repeated MTH measurements (Yasuda et al. 2012). Multiple images were recorded by the same investigator (TY) each time and printed for analysis. Then, each image analysis was performed by the same investigator who was blinded to the both condition and time assignments of the subjects. Test–retest reliability of MTH measurements using intraclass correlation coefficient (ICC3,1) was 0.97. The MTH was recorded before the resistance exercise (pre), during rest periods between sets (1st, 2nd and 3rd set), immediately after the resistance exercise (post), and at 15, 30 and 60 min after the resistance exercise (Fig. 1).
Electromyography (EMG)
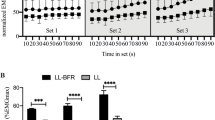
The skin was shaved, abraded with a skin preparation gel (Skinpure, Nihon Kohden, Japan), and cleaned with alcohol wipes. During the experiment, skin impedance was less than 2 kΩ. The ground electrode was positioned on the lateral epicondyle. Bipolar (2-cm center-to-center) surface EMG (sEMG) electrodes (Ag/AgCl; Vitrode F; Nihon Kohden; Tokyo, Japan) were placed over the muscle belly (mid-portion) along the longitudinal axis of the biceps brachii of the testing upper arm (Yasuda et al. 2008, 2009). The electrode placement on the biceps brachii was at 60 % of the upper arm limb length. EMG signals were recorded and collected on a personal computer (T7300 Macintosh, Apple, Japan) for subsequent analysis. All EMG signals were digitized at a sampling rate of 1,024 Hz with a bandwidth of 0 Hz to 500 kHz (AB 6216; Nihon Kohden; Tokyo, Japan). The load cell signal was low-pass filtered with a 10 Hz cutoff. Raw EMG signals were digitized and stored on hard disk in a computer using the Chart software program (LabChart 7 software, ADInstruments, Japan). To determine integrated EMG (iEMG), signals were fully rectified and automatically integrated (“Integral” function in LabChart 7 software) on the same computer. During the experimental session, sEMG was recorded continuously and each iEMG value was divided into groups of five successive repetitions (12 s). When the last iEMG group of each set was less than five repetitions, the iEMG value was divided according to the number of repetitions. The highest of iEMG group over each entire set was represented as a single data point for statistical analysis. iEMG during each set was normalized to pre, which was iEMG of the same exercise without BFR (three to five repetitions) before the first set of each visit day. The coefficient of variation (CV) for this measurement from test to retest was 5.7 %.
Blood sampling and biochemical analyses
Venous blood samples (2 mL) were obtained from the antecubital vein and measured for hematocrit, lactate concentration (n = 10), and creatine kinase (CK, n = 3). Hematocrit and lactate concentration were obtained before (pre), immediately after [post (without cuff)], and at 15, 30 and 60 min after the resistance exercise (Fig. 1). CK was obtained at pre, post, and daily for 4 days after the resistance exercise. Lactate concentration was analyzed with a rapid lactate analyzer (Lactate Pro, Arkray, Tokyo, Japan) and hematocrit and CK were measured at a commercial laboratory (SRL Inc., Tokyo, Japan).
Heart rate
During two exercise sessions, heart rate was measured during each set (Model 9560, Onyx II, Nonin Medical Inc., Plymouth, MN, USA).
Ratings of perceived exertion (RPE)
RPE was measured using the Borg scale (6–20) every 10 repetitions and immediately after each set (Borg 1973).
Visual analog scale (VAS)
VAS, drawn as a 100-mm line with 0 mm indicating “no pain” and 100 mm indicating “extremely sore”, was used to quantify soreness levels. The subject was instructed to mark a point on the line describing their soreness after they palpated their upper arm with their fingers (palpation of ~5 mm skin indention), while the investigator passively extended and flexed the subject’s forearm. The highest value of soreness from these three methods for each subject was used for muscle soreness analysis. VAS was measured before, immediately after (without cuff), and daily for 4 days after the exercise bout of each arm (Thiebaud et al. 2013). VAS of each subject was measured at the same time of day throughout the investigation.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). Two-way ANOVA with repeated measures (condition × time) was used to evaluate the training effects for all dependent variables. When significant main effects and/or interaction were observed, post hoc testing was performed using the Tukey technique. Statistical significance was set at p < 0.05. Effect sizes (ESs, Cohen’s d) in MTH and lactate concentration were calculated with the following formula: [(α mean − pre mean)/pre SD, α = 1st set, 2nd set, 3rd set, post, 15, 30, or 60 min; d = 0.2–0.5 is a small effect, d = 0.5–0.8 is a moderate effect, and d > 0.8 is a large effect] (Cohen 1988).
Results
The mean age, height, body mass and BMI for the subjects were 27 ± 5 years, 173.6 ± 4.9 cm, 70.3 ± 4.3 kg and 23.3 ± 1.6, respectively. On each visit day, there were no differences (p > 0.05) between BFR and NBFR for 1-RM (13.6 ± 2.5 and 13.7 ± 2.1 kg), heart rate (67.4 ± 8.0 and 64.7 ± 11.0 BPM), systolic (124 ± 7 and 120 ± 8 mmHg) and diastolic blood pressures (75 ± 9 and 71 ± 13 mmHg).
Exercise repetitions decreased progressively (p < 0.01) in the two conditions for arm curl exercise from the 1st to 4th set and NBFR was greater (p < 0.01) than BFR at the 1st and 2nd sets. The total number of (4 sets) exercise repetitions was greater (p < 0.01) in NBFR (221 ± 67 reps) than in BFR (111 ± 36 reps) (Fig. 2). MTH for biceps brachii increased (p < 0.01) with BFR and NBFR (1.21 and 1.20-fold the baseline value), which was similar from the 1st set to 60 min (Fig. 3). All ESs for the differences in MTH were large for BFR (0.88–1.51 from 2nd set to 60 min) and NBFR (0.84–1.81 from 1st set to 60 min) except for 1st set for BFR (0.71). During the exercise session, iEMG for biceps brachii muscles increased (p < 0.01) progressively in BFR and NBFR (3.94 and 4.45 times baseline value); the iEMG increases for BFR and NBFR were similar from the 1st to 4th sets (Fig. 4).
Immediately after the exercise session, the lactate concentration was similarly increased with both conditions (Fig. 5). Immediately after the exercise session, hematocrit was increased with both BFR (pre: 46.4 ± 2.5 %, post: 48.4 ± 2.7 %) and NBFR (pre: 46.6 ± 1.7 %, post: 48.2 ± 2.0 %) to similar levels. During the exercise session, the heart rate increased progressively similarly in both BFR and NBFR from the 1st (96 ± 10 and 95 ± 13 BPM), 2nd (96 ± 10 and 97 ± 15 BPM), 3rd (93 ± 8 and 99 ± 12 BPM), and 4th set (93 ± 7 and 99 ± 17 BPM). All ESs for the differences in lactate concentration were large for BFR (0.8–10.4 from post to 60 min) and NBFR (1.6–10.9 from 1st set to 60 min) except for 60 min for NBFR (0.64).
In summation of four sets, high RPE score (more than 15) showed a longer duration (p < 0.01) in NBFR (130 ± 56 reps) than in BFR (90 ± 39 reps). In both BFR and NBFR, VAS significantly increased: 39 ± 27 and 47 ± 23 mm at 24 h, 54 ± 26 and 69 ± 23 mm at 48 h, 50 ± 22 and 58 ± 17 mm at 72 h, and 28 ± 21 and 32 ± 17 mm at 96 h. There was no significant difference in VAS between the two conditions in any set.
In both BFR and NBFR, CK gradually increased: 165 ± 73 and 160 ± 74 U/L at pre, 169 ± 72 and 162 ± 78 U/L at post, 420 ± 429 and 583 ± 559 U/L at 24 h, 3,668 ± 5,442 and 3,825 ± 3,767 U/L at 48 h, 9,102 ± 7,267 and 8,788 ± 7,570 U/L at 72 h, and 13,415 ± 7,267 and 11,305 ± 8,712 U/L at 96 h.
Discussion
The main findings of the present study were: (1) increased muscle swelling following exercise and increased muscle activation during resistance exercise did not differ between BFR and NBFR, and, (2) time-course of the increase in muscle swelling was similar between two conditions, (3) low-load resistance exercise to volitional fatigue induced an increase in muscle swelling 15 min after exercise, which was mainly dependent on muscle damage or inflammation of the muscle tissues regardless of BFR.
It is known that acute cell swelling due to osmotic water shifting into the cell stimulates anabolic processes, both through an increase in protein synthesis and a decrease in proteolysis (Berneis et al. 1999; Häussinger et al. 1993). Recent BFR studies (Martín-Hernández et al. 2013; Loenneke et al. 2012a; Yasuda et al. 2012) suggest that BFR training leads to pronounced acute changes in muscle size, an index of muscle swelling, the response to which may be an important factor for promoting muscle hypertrophy. However, recent studies (Mitchell et al. 2012; Ogasawara et al. 2013) have reported that NBFR training to volitional fatigue results in muscle hypertrophy similar to high-load training, but their studies were unclear regarding the relationship between NBFR and muscle swelling. In the present study, we revealed the effect size for muscle swelling was similar in both BFR and NBFR exercises [1.5 (large) and 1.8 (large) for post, respectively]. Therefore, it would appear that low-load resistance exercise to volitional fatigue could stimulate muscle protein metabolism induced by muscle swelling. On the other hand, Fahs et al. (2014) demonstrated that BFR training induced a greater increase in muscle size compared with non-BFR training although similar muscle swelling was observed following low-load resistance training to volitional fatigue with and without BFR. In addition, Loenneke et al. (2012b) observed that the induced increase in muscle size after a brief application of BFR (in the absence of exercise) may have been mediated through an acute fluid shift. Taken together, these findings suggest that acute muscle swelling may be a requisite factor for muscle hypertrophy but by itself is not enough.
In previous BFR studies, greater muscle activation during low-load BFR resistance exercise was hypothesized to occur as a compensation for a deficit in force development, secondary to changes in energy supply; these changes resulted from the decreased oxygen available to the muscle and an accumulation of metabolites (Bigland-Ritchie et al. 1986; Moritani et al. 1986; Yasuda et al. 2010a). In this study, muscle activation during the exercise session increased progressively in both BFR and NBFR conditions, although the number of exercise repetitions was approximately two-fold greater in NBFR than in BFR. Additionally, the magnitude of the increased blood lactate concentration and hematocrit following exercise was similar between the two conditions. Therefore, it can be speculated that increased muscle activation during NBFR was also caused by a mismatch in energy demand/energy supply, although the metabolic product per unit exercise repetition with NBFR was approximately one-half that with BFR. Taken together, muscle swelling and muscle activation during low-load resistance exercise to volitional fatigue could reach a plateau with NBFR as well as with BFR, even though the number of exercise repetitions was approximately two-fold greater in NBFR than in BFR.
Previous studies (Loenneke et al. 2012a, c; Yasuda et al. 2012) reported that an increase in muscle swelling with BFR was due to a fluid shift from the plasma into the muscle under BFR. In the present study, however, the increased muscle swelling with both NBFR and BFR remained at least 60 min post-exercise whereas plasma volume and blood lactate concentration returned to baseline at 15 min post-exercise. On the other hand, increased muscle soreness scores (assessed by VAS) with NBFR and BFR (54 and 69 mm, respectively) were very high score compared with a previously reported BFR study (20 mm; Thiebaud et al. 2013). In addition, muscle damage (CK, n = 3) gradually increased to 13,415 U/L for BFR and 11,305 U/L for NBFR at 96 h post-exercise. These low-intensity exercise results suggest that muscle soreness and muscle damage with BFR and NBFR were comparable with that reported with a high-intensity exercise study (Nosaka and Newton 2002), which performed maximal eccentric exercise of unilateral elbow flexors (3 sets of 10 reps). In contrast, previous BFR studies of low-load BFR exercise not to volitional fatigue reported that there are no changes in markers of muscle damage between before and after an acute bout of exercise (Abe et al. 2006; Fujita et al. 2008). Taken together, it is speculated that low-load resistance exercise to volitional fatigue induced an increase in muscle swelling 15 min after exercise, which was mainly dependent on muscle damage or inflammation of the muscle tissues regardless of BFR.
Some limitations of this study should be discussed. First, the rest period between sets was different between NBFR (3 min rest period between sets) and BFR (30 s rest period between sets). Based on previous studies (Stull and Kearney 1978; Salles et al. 2009), it can be speculated that NBFR with 30 s rest periods between sets recovered maximal strength and exercise repetitions were approximately twice as much as 3 min rest periods between sets for the upper bodies. Therefore, it is likely that increased MTH in NBFR was a little suppressed after the 2nd set when NBFR with 30 s rest periods between sets was applied. In this study, the highest effect size in MTH was 1.5 for BFR and 1.8 for NBFR (at the 3rd set and post, respectively), but MTH at the 1st set of NBFR already reached a large effect size (1.3). Taken together, there is a high possibility that increased muscle swelling between the two conditions with 30 s rest periods between sets are also similar. Second, because the number of repetitions at the 1st set (67 reps) were higher compared with a previous BFR study (25 reps) (Kubo et al. 2006), it appears that the optimal level of BFR was higher than 160 mmHg in this study. Notably, this study used an arbitrary pressure. Previous studies (Loenneke et al. 2012b, 2013; Yasuda et al. 2008) reported that differences in limb size do result in differences in BFR, thus it is always necessary to pay attention to the relationship between the level of BFR and the exercise and/or training effect. Third, NBFR exercise was limited to highly motivated individuals who are capable of tolerating the perceptual response of the exercise session because the high RPE score (more than 15) was approximately a 1.5-fold longer period in NBFR than in BFR. Fourth, our results were acute and may not necessarily be translated to chronic adaptation. Hence, more studies are necessary to understand the relationship between NBFR exercise and safety and/or arterial function.
In conclusion, low-load resistance exercise to volitional fatigue is an effective method to promote muscle swelling and muscle activation regardless of BFR, and the time-course of the increase in muscle swelling was similar for both conditions. Furthermore, our data indicate that the increase in muscle swelling for both NBFR and BFR is maintained even 15–60 min after the exercise, which may be mainly dependent on muscle damage or inflammation of the muscle tissues.
Abbreviations
- 1-RM:
-
One repetition maximum
- BFR:
-
Blood flow restriction
- HL:
-
High-load resistance training
- iEMG:
-
Integrated electromyography
- MTH:
-
Muscle thickness
- NBFR:
-
No blood flow restriction
References
Abe T, Yasuda T, Midorikawa T, Sato Y, Kearns CF, Inoue K, Koizumi K, Ishii N (2005) Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int J Kaatsu Train Res 1:6–12
Abe T, Kearns CF, Sato Y (2006) Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol 100:1460–1466
Berneis K, Ninnis R, Haussinger D, Keller U (1999) Effects of hyper- and hypoosmolality on whole body protein and glucose kinetics in humans. Am J Physiol 276:E188–E195
Bigland-Ritchie B, Cafarelli E, Vollestad NK (1986) Fatigue of submaximal static contractions. Acta Physiol Scand Suppl 556:137–148
Borg GA (1973) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM (2010) Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One 9:e12033
Cohen J (1988) Statistical power analysis for the behavior sciences, 2nd edn. Lawrence Erlbaum, Hillsdale, pp 273–406
de Salles BF, Simão R, Miranda F, Novaes Jda S, Lemos A, Willardson JM (2009) Rest interval between sets in strength training. Sports Med 39:765–777
Fahs CA, Loenneke JP, Thiebaud RS, Rossow LM, Kim D, Abe T, Beck TW, Feeback DL, Bemben DA, Bemben MG (2014) Muscular adaptations to fatiguing exercise with and without blood flow restriction. Clin Physiol Funct Imaging [Epub ahead of print]
Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB (2010) Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol 108:1199–1209
Fujita T, Brechue WF, Kurita K, Sato Y, Abe T (2008) Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int J Kaatsu Train Res 4:1–8
Häussinger D, Roth E, Lang F, Gerok W (1993) Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet 22:1330–1332
Karabulut M, Abe T, Sato Y, Bemben MG (2010) The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur J Appl Physiol 108:147–155
Kubo K, Komuro T, Ishiguro N, Tsunoda N, Sato Y, Ishii N, Kanehisa H, Fukunaga T (2006) Effects of low-load resistance training with vascular occlusion on the mechanical properties of muscle and tendon. J Appl Biomech 22:112–119
Loenneke JP, Fahs CA, Rossow LM, Abe T, Bemben MG (2012a) The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses 78:151–154
Loenneke JP, Fahs CA, Rossow LM, Sherk VD, Thiebaud RS, Abe T, Bemben DA, Bemben MG (2012b) Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol 112:2903–2912
Loenneke JP, Fahs CA, Thiebaud RS, Rossow LM, Abe T, Ye X, Kim D, Bemben MG (2012c) The acute muscle swelling effects of blood flow restriction. Acta Physiol Hung 99:400–410
Loenneke JP, Wilson JM, Marin PJ, Zourdos MC, Bemben MG (2012d) Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol 112:1849–1859
Loenneke JP, Fahs CA, Rossow LM, Thiebaud RS, Mattocks KT, Abe T, Bemben MG (2013) Blood flow restriction pressure recommendations: a tale of two cuffs. Front Physiol 10:249
Martín-Hernández J, Marín PJ, Menéndez H, Loenneke JP, Coelho-e-Silva MJ, García-López D, Herrero AJ (2013) Changes in muscle architecture induced by low load blood flow restricted training. Acta Physiol Hung 100:411–418
Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM (2012) Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol 113:71–77
Moritani T, Muro M, Nagata A (1986) Intramuscular and surface electromyogram changes during muscle fatigue. J Appl Physiol 60:1179–1185
Nosaka K, Newton M (2002) Difference in the magnitude of muscle damage between maximal and submaximal eccentric loading. J Strength Cond Res 16:202–208
Ogasawara R, Loenneke JP, Thiebaud RS, Abe T (2013) Low-load bench press training to fatigue results in muscle hypertrophy similar to high-load bench press training. Int J Clin Med 4:114–121
Sato Y (2005) The history and future of kaatsu training. Int J Kaatsu Train Res 1:1–5
Stull GA, Kearney JT (1978) Recovery of muscular endurance following submaximal, isometric exercise. Med Sci Sports 10:109–112
Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N (2000) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88:2097–2106
Thiebaud RS, Yasuda T, Loenneke JP, Abe T (2013) Effects of low-intensity concentric and eccentric exercise combined with blood flow restriction on indices of exercise-induced muscle damage. Interv Med Appl Sci 5:53–59
Yasuda T, Brechue WF, Fujita T, Sato Y, Abe T (2008) Muscle activation during low-intensity muscle contractions with varying levels of external limb compression. J Sports Sci Med 7:467–474
Yasuda T, Brechue WF, Fujita T, Shirakawa J, Sato Y, Abe T (2009) Muscle activation during low-intensity muscle contractions with restricted blood flow. J Sports Sci 27:479–489
Yasuda T, Abe T, Brechue WF, Iida H, Takano H, Meguro K, Kurano M, Fujita S, Sato Y, Nakajima T (2010a) Venous blood gas and metabolite response to low-intensity muscle contractions with external limb compression. Metabolism 59:1510–1519
Yasuda T, Fujita S, Ogasawara R, Sato Y, Abe T (2010b) Effects of low-intensity bench press training with restricted arm muscle blood flow on chest muscle hypertrophy: a pilot study. Clin Physiol Funct Imaging 30:338–343
Yasuda T, Ogasawara R, Sakamaki M, Ozaki H, Sato Y, Abe T (2011) Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur J Appl Physiol 111:2525–2533
Yasuda T, Loenneke JP, Thiebaud RS, Abe T (2012) Effects of low-intensity blood flow restricted concentric or eccentric training on muscle size and strength. PLoS One 12:e52843
Yasuda T, Fukumura K, Fukuda T, Uchida Y, Iida H, Meguro M, Sato Y, Yamasoba T, Nakajima T (2014) Muscle size and arterial stiffness after blood flow-restricted low-intensity resistance training in older adults. Scand J Med Sci Sports 24:799–806
Acknowledgments
The authors thank the participants who participated in this study. We also thank Yusuke Uchida, Seiya Oosumi, Kenji Kinpara, and Shinpei Sugimoto (Okamoto-Ishii Hospital, Shizuoka, Japan). This study was supported, in part, by Grant-in-aid (#25750288 and #20549604 to TY) from the Japan Ministry of Education, Culture, Sports, Science, and Technology, and the Descente Foundation. Our department is funded by KAATSU Japan Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Yasuda, T., Fukumura, K., Iida, H. et al. Effect of low-load resistance exercise with and without blood flow restriction to volitional fatigue on muscle swelling. Eur J Appl Physiol 115, 919–926 (2015). https://doi.org/10.1007/s00421-014-3073-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-3073-9