Abstract
Purpose
There is some evidence that muscular activation during exercise is enhanced by higher levels of blood flow restriction (BFR). However, the impact of different relative levels of BFR on the acute neuromuscular response to resistance exercise is not yet fully understood. We examined the acute effects of low-intensity knee extensions [20 % of 1-repetition maximum (1RM)] with BFR on muscle activation, neuromuscular fatigue and torque in the rectus femoris (RF) and vastus medialis (VM) muscles.
Methods
Fourteen men (24.8 ± 5.4 years) exercised at 20 % 1RM combined with 40, 60 and 80 % BFR. Restrictive pressures were calculated based on direct blood-flow measurements taken at rest on each participant. Torque was determined during pre- and post-exercise maximal voluntary contractions. Surface electromyographic activity [root mean square (RMS)] was obtained during dynamic and sustained isometric contractions before and after exercise. The median frequency (MF) of the electromyographic power spectrum was computed for isometric contractions.
Results
Torque only decreased in the 80 % BFR condition (−5.2 %; p < 0.01). Except for the VM in the 40 % BFR, MF decreased in both muscles post-exercise in all conditions (p < 0.01). MF decrements were of greater magnitude post-exercise at higher levels of BFR. RMS increased within all sets in both muscles (p < 0.01) and attained higher values in the 80 % BFR condition; except for set 1 in the RF muscle (p < 0.01).
Conclusion
Muscular activation, as well as neuromuscular fatigue, varies as a function of relative BFR intensity. Therefore, the individual determination of vascular restriction levels is crucial before engaging in BFR exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Training at reduced intensities [20–50 % of maximal voluntary activation (MVC)] combined with blood flow restriction (BFR) has challenged many principles of resistance training (Moritani et al. 1992; Wernbom et al. 2008). Numerous studies have reported that low-intensity resistance training with BFR elicits increases both in muscle size and strength (Shinohara et al. 1997; Takarada et al. 2000a, b, 2002; Abe et al. 2006) to a similar, or even greater, extent than resistance training performed at intensities ≥65 % of 1 repetition maximum (1RM) (American College of Sports Medicine 2009). Despite an extensive effort to understand the physiological basis of muscle hypertrophy post-training with BFR, the exact mechanisms underlying such response remain largely unknown (Moritani et al. 1992; Takarada et al. 2000a, b; Moore et al. 2004; Abe et al. 2006, 2010; Fujita et al. 2007; Renzi et al. 2010). Past reports, examining the interaction between BFR and neuromuscular adaptations, have shown that this form of exercise implicates greater muscular activation to maintain the same total work output (Moritani et al. 1992; Shinohara et al. 1997; Takarada et al. 2000b; 2002, Yasuda et al. 2008; Wernbom et al. 2009; Cook et al. 2013; Fahs et al. 2015). It has also been shown that BFR induces heightened neural activation and enhanced recruitment of type II motor units (MU) during exercise (Moritani et al. 1992; Takarada et al. 2000b; Moore et al. 2004). Taken together, these data indicate that muscle hypertrophy might be partially driven by neuromuscular responses accompanying BFR exercise (Loenneke et al. 2011). In agreement with this hypothesis, similar levels of neuromuscular activity (measured by means of surface electromyography–EMG) have been previously reported between low-intensity BFR exercise and non-BFR high-intensity resistance training (Shinohara et al. 1997; Takarada et al. 2000b; 2002). This suggests that both training options may be equally effective for the purpose of enhancing the recruitment of type II MU (Moritani et al. 1992; Takarada et al. 2000b; Moore et al. 2004). Nevertheless, whether the neuromuscular component represents a pivotal role in the genesis of muscle adaptations to BFR training is not known.
Several methodological inconsistencies have generated conflicting findings between studies; thus limiting our understanding of the physiological phenomena priming muscle hypertrophy post-BFR resistance training. For example, the vast majority of studies on this topic have implemented BFR training protocols prescribing an arbitrary restrictive pressure for all participants (Moore et al. 2004; Pierce et al. 2005; Yasuda et al. 2009, 2010; Wernbom et al. 2009; Cook et al. 2013). In contrast, other experimental designs have based the restrictive pressure on resting systolic blood pressure values (Abe et al. 2006, 2010; Yasuda et al. 2006; Cook et al. 2007). Recent findings, however, indicate that neither one of these previously used approaches represents an effective strategy for controlling the magnitude of BFR in the lower body (Loenneke et al. 2012a, b).
It is presently believed that restrictive pressure should be individualized to each participant (Scott et al. 2014). Laurentino et al. (2008) were the first to use a Doppler probe for determining the pressure required for complete vascular occlusion in the upper thigh at resting conditions. In that study, the authors found that combining low-intensity resistance training with BFR, set at 80 % of resting arterial occlusive pressure, was as effective as high-load training for inducing muscle hypertrophy.
To our knowledge, only two studies have specifically explored changes in EMG amplitude across different BFR pressures combined with resistance exercise (Yasuda et al. 2008; Loenneke et al. 2015). While one of these experimental designs used different arbitrary BFR pressures within the context of upper-limb exercise (Yasuda et al. 2008), the other involved lower-limb exercise and estimated BFR relative pressure based on limb circumference (Loenneke et al. 2015). Unfortunately, none of these studies actually quantified pre-exercise resting vascular occlusion pressure for adjusting BFR relative values on an individual basis; thus limiting subsequent interpretations of the data. For this reason, we aimed to gain a better understanding of how torque, muscular activation and neuromuscular fatigue are affected by different levels of BFR (determined directly at rest on an individual basis) combined with low-intensity resistance exercise (20 % 1RM). We hypothesized that, for a given volume and intensity of exercise, muscle activation, together with neuromuscular fatigue, would vary as a function of BFR relative pressure.
Methods
Participants
Fourteen healthy young men (age: 24.8 ± 5.4 years; height: 175.2 ± 4.4 cm; body mass: 71.1 ± 6.9 kg, and body mass index: 23.1 ± 1.8 kg/m2), with minor experience in exercise training (not participating in any form of resistance or endurance training for the last 6 months), were recruited to participate in this study. The experimental risks involved in the present investigation were thoroughly explained to each participant. Subsequently, participants were asked to sign an informed consent document before being enrolled in the first testing session. This study was approved by the Faculty’s Ethics Committee (CEFMH 17/2014) and complied with the principles set forth in the Declaration of Helsinki. At study entry, participants were also required to fulfill a questionnaire focused on medical and training history. Participants were all non-smokers and free from any known cardiovascular and metabolic diseases, as assessed by medical history. Finally, the participants were instructed to maintain the same level of physical activity throughout the course of the study.
Study design
After being familiarized with all testing procedures, the participants were required to visit the laboratory within a week for testing. All participants were tested on three different conditions over a period of ~3 weeks. Familiarization consisted of a single session that served the purpose of simulating a real testing protocol (including BFR exercise) and determining the cuff pressure required for eliciting absolute vascular occlusion in right lower limb (at rest). Then, on a different day, all participants performed a baseline session, where (1) the vascular occlusion pressure for the right lower limb was reassessed, (2) 1RM was determined for the right knee extension and (3) testing (including metronomic pacing) was again reproduced.
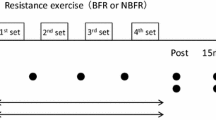
Each participant was tested on 3 different conditions of unilateral knee extension, using a Biodex System 3 isokinetic dynamometer (Biodex Medical Systems, Shirley, NY). Participants exercised with various BFR levels at 20 % 1RM throughout all testing sessions. This training intensity was selected because of its effectiveness in inducing fatigue when combined with BFR (Cook et al. 2007). In each condition, the level of vascular restriction was set according to the following relative BFR pressures (normalized to the pressure values compatible with absolute vascular occlusion obtained at rest): (1) 40 % BFR (2) 60 % BFR and (3) 80 % BFR (Fig. 1). Participants were tested in the seated position and were fixed with chest and abdominal straps. Vascular restriction was elicited using a 13 × 124 cm pneumatic cuff (SC12L Tourniquet Cuffs, D. E. Hokanson, Inc. Bellevue, WA) applied to the most proximal portion of the right thigh. Before inflating the pneumatic cuff to the target pressure, an adaptive cycle of cuff inflation/deflation was performed, with inflations of 30 s and deflations of 10 s executed gradually at 50, 75, and 100 % of each target point. The cuff was inflated to the target inflation pressure prior to the first set of exercise and then deflated and removed immediately after the final post-exercise measurement, for a total of ~10 min restriction time.
Experimental protocol. All participants performed 4 sets of leg extension (30 + 15 + 15 + 15 repetitions) at 20 % 1 repetition maximum (1RM), with three different blood flow restriction (BFR) conditions (40, 60, and 80 %). All measurements were taken at 3 times, with 1 min rest for the maximal voluntary contractions (MVC’s), and 30 s rest for pre 1, pre 2, post 1, post 2. MVC was defined as the highest value obtained during testing. During submaximal isometric contractions (20 % MVC sustained contractions lasting 5 s) a mean value was determined for each measurement point (pre 1, pre 2, post 1, post 2)
The following warm-up protocol was performed in each visit to the laboratory before testing: (1) 6 min of unloaded cycling on a cycle ergometer (60–70 rpm); (2) 3 sets of 4 progressive isokinetic knee extensions at 90° s−1, with a maximal contraction in the last execution of each set. Following the warm-up period, the participants completed three different pre-evaluations time points before exercise: (1) MVC, (2) pre 1–20 % MVC without BFR and (3) pre 2–20 % MVC with BFR (1 min after cuff inflation). These exact same three evaluation time points were repeated in an inverse order post-exercise: (1) post 1–20 % MVC with BFR, (2) post 2–20 % MVC without BFR (1 min after cuff deflation) and (3) MVC. All BFR conditions integrated 4 sets of knee extension at 20 % 1-RM (30 + 15 + 15 + 15 repetitions), with 30 s of passive rest between sets. This training volume has been used consistently in most studies examining the physiological responses to BFR resistance training (Loenneke et al. 2012b; Yasuda et al. 2009, 2010). A metronome was used to control the concentric–concentric mode, with 1 s for knee extension (20 % 1-RM) and another for knee flexion (unloaded). Verbal encouragement was provided to ensure that each participant completed the full exercise protocol. For safety reasons, a pulse oximeter (Onyx® II 9560, Nonin Medical Inc., Plymouth, MN) was employed to the right hallux immediately after each set to ensure that blood flow was not completely halted by tissue edema. There were at least 5 days between testing sessions and the order of conditions was randomized after baseline measurements. All participants were asked to avoid the intake of caffeine, alcohol and performing any form of exercise for at least 48 h before testing. Additionally, to minimize the circadian effect, they were all tested at approximately the same time of day (±2 h).
1RM testing
1RM was determined for the right leg extension, using the isotonic mode of the isokinetic device. The participants were asked to complete 1 repetition through a full range of motion (90°). A strong verbal encouragement was provided in each trial and, at least, 2 min of recovery were allowed between attempts. 1RM was defined as the maximal load lifted in a full range of motion for a total of one single repetition. For all participants, 1RM was determined within five attempts (mean values 221.8 ± 32.3 N m).
Maximal voluntary contraction
MVC was determined for the right leg extension. During baseline measurements, a torque–angle curve was constructed on an individual basis to determine the optimal knee joint angle to be used in all subsequent measurements (pre- and post-exercise). MVC determination implicated 3 isometric contraction trials, with at least 3 s of duration, each separated by 1 min of recovery. Participants were instructed to exert their maximum force as fast as possible and peak torque was defined as the pre- and post-exercise MVC values.
Submaximal voluntary contraction measurements
Submaximal voluntary contractions were performed with and without BFR, in an isometric fashion, at a pre-determined force level (20 % MVC). As mentioned above, the knee joint angle was individualized to the optimal torque–angle value. In each trial, participants performed three sustained isometric contractions with a force plateau of at least 5 s, each separated by 30 s of recovery. They were instructed to attain the target value smoothly and to sustain the contraction throughout the predefined period of time; using the visual feedback of a computer monitor. The mean value of the three sustained isometric contractions obtained during each trial was used for further analyses.
Determination of vascular occlusion pressure at rest
The vascular occlusion pressure was determined at rest, using a vascular Doppler probe (PD1 + Combi, Ultrasound Technologies Ltd, Caldicot, UK) that was placed over the tibial artery. A 13 × 124 cm pneumatic cuff was placed on the most proximal end of the right thigh (inguinal fold region) and inflated up to the point when the auscultatory pulse of the tibial artery was interrupted (Laurentino et al. 2008). Determination of vascular occlusion pressure was conducted during the familiarization session and again at baseline (mean values 139.4 ± 7.8 mmHg). The between-session differences were <5 % in all participants; thus indicating that the pressure of absolute vascular occlusion was highly reproducible. To guarantee similar cuff placement between testing sessions, a photographic record was made for each participant. Vascular restriction used in each condition was then set as a relative measure of absolute vascular occlusion (40, 60, and 80 % BFR).
EMG and force signal acquisition
Electrodes were placed following SENIAM recommendations and recorded continuously from the vastus medialis (VM) and rectus femoris (RF) of the right leg during all protocols. The recording sites were shaved, abrased and cleaned with alcohol wipes. EMG signals were collected using the Trigno™ wireless EMG system (Delsys Inc, USA). The signals had a baseline noise <4.5 μV pk–pk and a bandwidth filter between 20 and 450 Hz ±10 %. Digitizing was performed at a resolution of 16 bits and at a sampling frequency of 2 kHz (MP150, Biopac Systems, Inc.). Data collection was synchronized with the force signal collected from the Biodex System. EMG amplitude (root mean square–RMS) was measured from EMG signals: (1) during MVC measurements–RMS was calculated based on a 500 ms time window centered on the highest force value, (2) during the submaximal voluntary contractions at 20 % MVC–RMS was calculated in a 5000 ms plateau of each isometric contraction using the mean value of the 3 trials in each measurement point and (3) during the dynamic exercise protocols–RMS was calculated for each repetition based on a 500 ms time window centered on the highest force level. All RMS measurements were normalized to pre-exercise MVC. Median frequency (MF) was calculated from EMG raw signals: (1) MVC (pre- and post-exercise) for a time window of 500 ms, centered on the highest force level and (2) 20 % MVC measurements for 5000 ms, using the mean value of the three trials in each measurement point.
Statistical analysis
All data are reported as mean ± SD, unless otherwise specified. The Mauchly’s test was used to test the assumption of sphericity. The Greenhouse-Geisser correction was implemented to adjust the degrees of freedom for the averaged tests of significance when the assumption of sphericity was not met. Paired t tests were used for exploring possible differences in MVC derived parameters (torque, MF, RMS) from pre- to post-exercise time points at each condition (40, 60, and 80 % BFR). A two-way repeated measures ANOVA [3 conditions (40 vs. 60 vs. 80 % BFR) ×4 times (pre 1 vs. pre 2 vs. post 1 vs. post 2)] was conducted to determine the effects pre- to post-exercise, of relative BFR intensity on neuromuscular fatigue (MF) and muscular activation (RMS). An additional two-way repeated measures ANOVA [3 conditions (40 vs. 60 vs. 80 % BFR) ×5 times (start set 1 vs. end set 1 vs. end set 2 vs. end set 3 vs. end set 4) was used for RMS analyses within each exercise protocol. For this analysis, sets were divided into thirds and, in each time point, mean value ± SD was compared. When a significant effect was detected at a significance level of p < 0.05, t tests were used for post hoc comparisons. Adjustment for multiple comparisons was made with Bonferroni’s correction. The intraclass correlation coefficients (ICC) were additionally calculated for all pre-exercise data (all conditions) with the purpose of exploring the between-sessions reliability of all parameters (torque, MF and RMS). The ICC values varied from 0.71 to 0.94 (95 % confidence interval) which represents good reliability (Cicchetti and Sparrow 1981). All statistical calculations were computed using the SPSS 22.0 statistical software package (SPSS Inc., Chicago, IL) and a significance level of p < 0.05 was used.
Results
Pre- and post-exercise measurements were primarily focused on the acute impact of each condition on the following variables: (1) mechanical output (MVC’s torque), (2) neuromuscular function and fatigue (MF of the EMG power spectrum and (3) muscular activation (RMS of the EMG signal). Data obtained before and after exercise are shown in Table 1.
Maximal voluntary contraction
Torque decreased by 5.2 % post-exercise; however, this was limited to the 80 % BFR condition (p < 0.01). No significant differences from pre- to post-exercise measurements were observed on RMS or MF values for either muscle.
Submaximal voluntary contraction
Median frequency. The pre-exercise MF and RMS values were compared between all conditions and no differences were detected for either the VM or the RF muscle. However, in the RF muscle, we obtained a condition by time interaction for MF (F = 4.0, p < 0.05). Post hoc analyses indicated that, from pre 1 to pre 2, MF decreased in the 80 % BFR condition (−7.8 %, p < 0.01), but not in the 40 or the 60 % BFR conditions (Fig. 2). In contrast, from pre 2 to post 1, MF decreased in all conditions (40 % BFR: −8.4, 60 % BFR: −15.2, 80 % BFR: −21.2 %; p < 0.01). This represented a reduction of 9.5, 19.9, and 30.6 % (at 40, 60, and 80 % BFR, respectively) in MF values from pre 1 to post 1 (p < 0.01). There was a significant increase in MF at exactly 1 min post-cuff release in all conditions (from post 1 to post 2) (40 % BFR: 9.3, 60 % BFR: 10.4, 80 % BFR: 14.5 %; p < 0.01). Despite this, in the 80 % BFR condition, MF values remained lower than those obtained at pre 1 (−11.8 %, p < 0.01). Conversely, this was not the case for the 40 or 60 % BFR conditions.
Median frequency of the electromyographic power spectrum of the Rectus Femoris and Vastus Medialis obtained pre and post-exercise with different levels of blood flow restriction (BFR): 40 % BFR; 60 % BFR; 80 % BFR. Pre-exercise without BFR (pre 1); pre-exercise with BFR (pre 2); post-exercise with BFR (post 1); Post-exercise without BFR (post 2). Asterisk significant differences from pre 1 to pre 2; pre 2 to post 1; post 1 to post 2 (p < 0.05)
There was a time main effect for MF in the VM (F = 17.5, p < 0.05). Follow up t tests indicated that MF was not affected by cuff inflation (pre 1 to pre 2) in any condition (Fig. 2). Nevertheless, from pre 2 to post 1, MF decreased significantly in both the 60 and 80 % BFR conditions (−3.2 and −7.5 %, respectively) (p < 0.01). In contrast, no differences were seen between pre 1 and post 1, time points. Finally, at 1 min post-cuff release (from post 1 to post 2), there was a significant increase in MF and this occurred similarly in all conditions (40 % BFR: 5.3, 60 % BFR: 6.3, 80 % BFR: 8.8 %; p < 0.01). Importantly, in the 40 % BFR condition, such increase implicated that MF values recorded at post 2 attained higher values than those obtained at post 1 (3.9 %, p < 0.01).
Root mean square. There was a time (F = 4.1, p < 0.05) and condition main effect (F = 3.9, p < 0.05) for RMS in the RF. After cuff inflation (from pre 1 to pre 2), the RMS of the RF decreased significantly in the 40 % BFR condition (−13.9 %, p < 0.01) (Fig. 3). On the other hand, the 80 % BFR condition produced a significant increase in RMS (28.1 %, p < 0.01) after exercise (from pre 2 to post 1). Figure 3 also depicts that cuff deflation (from post 1 to post 2) did not affect the RMS of the RF in any condition.
Root mean square of the electromyographic signal of the Rectus Femoris and Vastus Medialis obtained pre and post-exercise with different levels of blood flow restriction (BFR): 40 % BFR; 60 % BFR; 80 % BFR. Pre-exercise without BFR (pre 1); pre-exercise with BFR (pre 2); post-exercise with BFR (post 1); Post-exercise without BFR (post 2). Asterisk Significant differences from pre 1 to pre 2; pre 2 to post 1; post 1 to post 2 (p < 0.05)
In the VM, there was a condition by time interaction for RMS (F = 10.3, p < 0.05). Cuff inflation increased the RMS in the VM, but only in the 80 % BFR condition (9.8 %, p < 0.01) (Fig. 3). RMS was also enhanced after exercise (from pre 2 to post 1) in the 60 % BFR (15.5 %, p < 0.01) and 80 % BFR (32.8 %, p < 0.01) conditions. Cuff deflation (from post 1 to post 2) only induced significant decreases in RMS values for the 80 % BFR condition (−25.7 %, p < 0.01). Despite this, in the 80 % BFR condition, RMS remained persistently higher compared to that seen at pre 1 (23.8 %, p < 0.01).
Exercise protocol
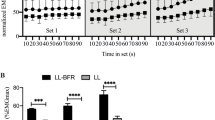
Root mean square: There was a time by condition interaction for the RMS values of both the RF (F = 13.8, p < 0.05) and the VM muscle (F = 8.0, p < 0.05). RMS increased significantly within each set of exercise in all conditions (p < 0.05). Importantly, this pattern of response was sustained from the first to the last set of exercise. Despite this, RMS values were similar between sets in the 40 and 60 % BFR condition. In contrast, the 80 % BFR condition evoked a significant increase in RMS between the end of set 1 and the end of set 2 (p < 0.05). From then on, RMS remained unchanged until exercise termination.
We found no significant differences in the RMS of either muscle during the 1/3 of set 1 (start) when comparing its values between conditions throughout the sequence of sets (Fig. 4). Additionally, while the RMS of the RF was similar between conditions by the end of the set 1, this was not the case for the VM. Our analyses revealed that, in this muscle, there were overall differences between conditions at the end of set 1 (p < 0.05). Furthermore, at the end of sets 2, 3, and 4, the RMS of the RF was different between 40 and 80 % BFR (p < 0.01) and also between 60 and 80 % BFR (p < 0.01). No differences were detected between 40 and 60 % BFR. In the VM, by the end of set 2, there were significant differences between 40 and 80 % BFR (p < 0.01) and between 60 and 80 % BFR (p < 0.01). Finally, by the end of sets 3 and 4, there were significant differences in the RMS of the VM between all conditions (p < 0.01).
Discussion
To our knowledge, this is the first study exploring the acute neuromuscular changes resulting from different levels of lower limb relative BFR, following a direct measurement of absolute vascular occlusion obtained at rest. We aimed to resolve some methodological limitations previously reported in the existent literature (Loenneke et al. 2015), and to extent the findings of past research on the neuromuscular changes secondary to resistance exercise performed at different levels of BFR pressure (Loenneke et al. 2015; Yasuda et al. 2008). Accordingly, we used a more direct and individualized approach for adjusting BFR; thus ensuring similar level of relative vascular restriction between participants at pre-exercise conditions. More importantly, we demonstrated that muscle fatigue is dependent on the relative level of vascular restriction and this is novel. Specifically, by analyzing the MF of the EMG power spectrum, our data suggest that neuromuscular fatigue is of greater magnitude and duration when using a cuff pressure equivalent to 80 % BFR during resistance exercise. Given that we also obtained significant increases in RMS throughout the different BFR protocols, the same holds true for muscular activation; a finding that is line with that reported in past research (Moritani et al. 1992; Moore et al. 2004; Yasuda et al. 2008, 2009; Wernbom et al. 2009; Cook et al. 2013; Fahs et al. 2015; Loenneke et al. 2015). Finally, by showing that the VM and RF responded differently to the same restrictive stimulus, we also present preliminary evidence that not all thigh muscles are similarly affected by acute BFR. This suggests that cuff placement, size and/or proximity to the muscle belly likely model the interaction between BFR, muscular activation and neuromuscular fatigue. Alternatively, such different levels of activation within the thigh muscles may simply reflect a pattern of neural compensation triggered by neuromuscular fatigue.
Mechanical output
Mechanical output was only affected by exercise combined with 80 % BFR. There is general agreement that BFR resistance exercise decreases torque (Cook et al. 2007, 2013; Karabulut et al. 2010; Wernbom et al. 2012; Loenneke et al. 2012b, 2015) and that this is secondary to neuromuscular fatigue, not muscle damage (Loenneke et al. 2014). Even though the magnitude of torque reduction is not a direct surrogate of neuromuscular fatigue or less blow flow (Cook et al. 2007), as torque was not affected by lower restrictive pressures (i.e. 40 and 60 % BFR), our findings do not entirely corroborate those of past research. Importantly, these seemingly discrepant findings between studies might be explained by several factors inherent to our experimental design. First, post 1 and post 2 measurements required at least 5 min to be completed. For this reason, post-exercise MVC was not obtained immediately after exercise cessation and this likely affected the results. Second, in contrast to that prescribed in most previous studies, BFR exercise was not performed until mechanical failure. Third, since MVC’s were taken without BFR, flow mediated dilation might have induced faster post-exercise recovery by means of muscle reperfusion secondary to reactive hyperemia.
Median frequency
With the exception of that obtained for the 80 % BFR condition in the RF, MF was not affected by cuff inflation before exercise. This suggests that, when using high values of restrictive pressure, BFR is an effective strategy for eliciting significant changes in neuromuscular function. The exact mechanisms underlying such response are presently unknown. From a hypothetical standpoint, this might be secondary to an increased synchronization of the MU firing frequency, a decrease in active MU and/or changes in intrinsic muscle properties (Bigland-Ritchie et al. 1986). Most studies indicate that MF reduction is more strongly associated with a decreased muscle fiber conduction velocity (Eberstein and Beattie 1985). Moreover, there is compelling evidence supporting a crucial role of intramuscular acidity, together with a reduced sarcoplasmic reticulum Ca2+ uptake, in the impairment of muscle contractile function (Place et al. 2009).
We also found that higher levels of BFR elicited greater decrements in MF values post-exercise. Importantly, this occurred in the context of similar exercise volume and BFR duration between all conditions. Despite some methodological differences in cuff characteristics (narrower cuff) and BFR pressure values (~280 mmHg), one previous report had already shown that BFR knee extension exercise elicits a progressive reduction in MF values for both the vastus lateralis (VL) and RF muscles (Pierce et al. 2005). More recently, the MF of the VM and VL was also documented to decrease (by 18.5 and 18.2 %, respectively) after one set of high intensity (80 % 1-RM) squats (until failure), using approximately 60 % BFR (measured in the femoral artery) (Neto et al. 2014). Taken together, these data corroborate the notion that the magnitude of neuromuscular fatigue varies as a function of relative vascular restriction when using this form of exercise. Moreover, we found that, in the 80 % BFR condition, MF values remained lower at post 2 (after cuff release) compared to that seen at pre 1. Thus, we provide evidence that higher levels of vascular restriction also determine a slower recovery from neuromuscular fatigue (for a given volume of exercise and BFR duration). This finding may have two important practical applications. First, since the recovery from neuromuscular fatigue is slower at high levels of BFR, we could speculate that the interval period between training sessions should be manipulated (i.e., longer duration) to optimize the physiological effects of BFR resistance exercise. Nevertheless, full conclusions can only be addressed after a follow up study on the time required for full recovery from neuromuscular fatigue. Second, our data show that, when adjusted to individual blood flow determinations made at resting conditions, relative BFR affects the acute neuromuscular response of training. Therefore, it should be viewed as a decisive factor when adjusting the overall characteristics of a BFR resistance training prescription (particularly in terms of training frequency). From a different perspective, as exercise at 80 % BFR was the most effective stimulus for prolonging neuromuscular fatigue, high levels of BFR can be relevant for muscle adaptation. This concept is corroborated by past findings showing that BFRE-induced fatigue (secondary to heightened lactate accumulation) may further stimulate muscle protein synthesis via the Akt/mTOR signaling pathway (Loenneke et al. 2010). Furthermore, since it has been showed that MF decrements are sensitive to biochemical changes in type II fibers (Gerdle and Fugl-Meyer 1992), we can speculate that BFR exercise with high levels of relative restriction is an important factor whenever this type of neuromuscular stimulation is the primary focus of the BFR training program.
Finally, BFR exercise had different effects in the MF of the VM and RF; particularly when comparing pre 1 to pre 2 and pre 1 to post 2. These findings are similar to those reported in the existent literature (Pierce et al. 2005). Since the restrictive cuff was positioned exactly over the RF muscle, it should determine a more pronounced degree tissue compression compared to that seen in the VM. Ultimately, as reported in one previous study, direct cuff compression on the proximal portion of the thigh likely predisposes the RF muscle to exercise-induced neuromuscular fatigue (Kacin and Strazar 2011).
Root mean square
There is general agreement that BFR increases EMG amplitude during an acute stimulus of resistance exercise (Takarada et al. 2000a, b, 2002; Yasuda et al. 2008, 2009; Wernbom et al. 2009; Cook et al. 2013; Fahs et al. 2015; Loenneke et al. 2015). In line with these studies, we also observed an increment for the RMS values in response to multiple sets of low-intensity BFR exercise. As there were no between-set differences for external load or muscle force production, we believe that this response may be representative of physiological compensation to maintain force against conduction failure (Moritani et al. 1992). Hypothetically, as previously postulated, these increments in EMG amplitude might reflect an enhanced recruitment of type II MU (Moritani et al. 1992; Takarada et al. 2000a; Moore et al. 2004) due to the local accumulation of metabolites (Loenneke et al. 2015). According to this premise, BFR should have induced RMS increments on both muscles (VM and RF) at pre- and post-exercise time-points. However, it is interesting to note that this was not the case as the VM displayed a different pattern of response in RMS compared to that seen in the RF muscle. Specifically, while the VM responded with a significant increase in RMS from pre 2 (pre-exercise) to post 1 (post-exercise) both in the 60 and 80 % BFR conditions, this did not occur in the RF muscle. In the RF, RMS was only increased post-exercise in the 80 % BFR condition. Mechanistically, we believe that these differences reflect a strategy to compensate impaired muscle contraction of the RF in the fatigued state. Nevertheless, future research is warranted to confirm these possible explanations. Irrespectively of this, we found that, 80 % BFR was the only condition leading to significant increases in the RMS values of both the VM and RF muscles. Thus, taking this into consideration, our data indicate that this represents the minimal level of relative BFR required to evoke significant changes in MU activity during exercise, not performed to volitional fatigue. These findings do not corroborate those of past research indicating for the purpose of eliciting significant changes in MU activity during exercise (iEMG), it is not necessary to go beyond a restrictive pressure of 60 % BFR (Loenneke et al. 2015). Nevertheless, the methodological differences between studies likely underlie these discrepant results.
The analyses resulting from dynamic exercise protocols are in consonance with that seen during isometric contractions. Accordingly, the RMS values obtained in both muscles were consistently greater in the 80 % BFR condition throughout all sets of exercise. As for the set-by-set comparisons, we obtained no differences in RMS between conditions for the RF at the end of the 1st set of exercise. Conversely, in the 80 % BFR condition, the VM reported a significant increase in RMS values at this specific time point. This means that the threshold for enhancing muscle activation with vascular restriction was attained at the end of 30 repetitions performed with 80 % BFR for the VM, but not for the RF muscle (independently of BFR relative intensity). To our knowledge, only one previous study has investigated changes in EMG activity across different BFR pressures using the same external load (Yasuda et al. 2008). While focusing on the upper-limb response to BFR exercise, the authors found that muscle EMG activity was more strongly potentiated by high levels of external compression (147 mmHg). Importantly, in another study using low-intensity BFR exercise, Yasuda et al. (2006) reported that vascular restriction enhanced the EMG integrated activity of the triceps brachii muscle; however this was only seen after the end of the 2nd set. Taken together, these data substantiate that relative BFR pressure represents a proven way to fine-tune the acute impact of BFR resistance training on acute neuromuscular activation.
Conclusion
Based on our findings, we conclude that, for a given volume and intensity of exercise, the magnitude of neuromuscular activation, as well as fatigue, varies as a function of BFR relative pressure. Accordingly, within the context of resistance training, BFR should not be prescribed using standardized values of absolute pressure. Ultimately, as the relationship between absolute and relative values of BFR pressure is not the same among different persons, this approach might shift the dose–response towards the domain of pure unpredictability. For this reason, we believe that engagement in BFR training programs should invariably be preceded by the quantification of resting vascular occlusion pressure.
Limitations
Women were not included in the present study and this represents an important limitation to our findings. Future research, using a mixed-sex population, is needed to overcome this limitation and strengthen the interpretation of these results. Nevertheless, it is important to note that adipose tissue distribution follows a sexually dimorphic pattern and that this may induce significant decreases in surface EMG signal amplitude; thus affecting data analyses (Kuiken et al. 2003).
Abbreviations
- BFR:
-
Blood flow restriction
- 1RM:
-
1-Repetition maximum
- RF:
-
Rectus femoris
- VM:
-
Vastus medialis
- RMS:
-
Root mean square
- MF:
-
Median frequency
- MU:
-
Motor units
- EMG:
-
Electromyography
- MVC:
-
Maximal voluntary contraction
- SD:
-
Standard deviation
- ANOVA:
-
Analysis of variance
- ICC:
-
Intraclass correlation coefficients
- Iemg:
-
Integrated electromyographic activity
References
Abe T, Kearns CF, Sato Y (2006) Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, KAATSU-walk training. J Appl Physiol 100(5):1460–1466
Abe T, Fujita S, Nakajima T, Sakamaki M, Ozaki H, Ogasawara R et al (2010) Effects of low-intensity cycle training with restricted leg blood flow on thigh muscle volume and VO. J Sport Sci Med 9:452–458
American College of Sports Medicine (2009) Position stands: progression models in resistance training for healthy adults. Med Sci Sport Exer 41(3):687–708
Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC (1986) Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol 379:451–459
Cicchetti DV, Sparrow SA (1981) Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 86(2):127–137
Cook SB, Clark BC, Ploutz-Snyder LL (2007) Effects of exercise load and blood-flow restriction on skeletal muscle function. Med Sci Sport Exer 39(10):1708–1713
Cook SB, Murphy BG, LaBarbera KE (2013) Neuromuscular function after a bout of low-load blood flow–restricted exercise. Med Sci Sport Exer 45(1):67–74
Eberstein A, Beattie B (1985) Simultaneous measurement of muscle conduction velocity and EMG power spectrum changes during fatigue. Muscle Nerve, p. 768–773
Fahs CA, Loenneke JP, Thiebaud RS, Rossow L, Kim D, Feeback DL, Bembem DA, Bembem MG (2015) Muscular adaptations to fatiguing exercise with and without blood flow restriction. Clin Physiol Funct I 35(3):167–176
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y et al (2007) Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 103(3):903–910
Gerdle B, Fugl-Meyer A (1992) Is the mean power frequency shift of the EMG a selective indicator of fatigue of the fast twitch motor units? Acta Physiol Scand 145(2):129–138
Kacin A, Strazar K (2011) Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Spor 21(6):231–241
Karabulut M, Cramer JT, Abe T, Sato Y, Bemben MG (2010) Neuromuscular fatigue following low-intensity dynamic exercise with externally applied vascular restriction. J Electromyogr Kines 20(3):440–447
Kuiken T, Lowery M, Stoykov N (2003) The effect of subcutaneous fat on the myoelectric signal and cross talk. Prosthet Orthot Int 27(1):48–54
Laurentino G, Ugrinowitsch C, Aihara A, Fernandes A, Parcell A, Ricard M, Tricoli V (2008) Effects of strength training and vascular occlusion. Int J Sports M 29(08):664–667
Loenneke JP, Wilson G, Wilson J (2010) A mechanistic approach to blood flow occlusion. Int J Sports Med 31:1–4
Loenneke JP, Fahs CA, Wilson JM, Bemben MG (2011) Blood-flow restriction: the metabolite/volume threshold theory. Med Hypotheses 77(5):748–752
Loenneke JP, Fahs CA, Rossow LM, Sherk VD, Thiebaud RS, Abe T et al (2012a) Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol 112(8):2903–2912
Loenneke JP, Thiebaud RS, Fahs CA, Rossow LM, Abe T, Bemben MG (2012b) Blood flow restriction does not result in prolonged decrements in torque. Eur J Appl Physiol 113(4):923–931
Loenneke JP, Thiebaud RS, Abe T (2014) Does blood flow restriction result in skeletal muscle damage? A critical review of available evidence. Scand J Med Sci Sports 24(6):415–422
Loenneke JP, Kim D, Fahs CA, Thiebaud RS, Abe T, Larson RD et al (2015) Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve 51(5):713–721
Moore D, Burgomaster K, Schofield L, Gibala M, Sale D, Phillips S (2004) Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur J Appl Physiol 92(4–5):399–406
Moritani T, Sherman WM, Shibata M, Matsumoto T, Shinohara M (1992) Oxygen availability and motor unit activity in humans. Eur J Appl Physiol 64:552–556
Neto GR, Santos HH, Sousa JB, Júnior AT, Araújo JP, Aniceto RR, Sousa MS (2014) Effects of high-intensity blood flow restriction exercise on muscle fatigue. J Hum Kinet 41:163–172
Pierce JR, Clark BC, Ploutz-Snyder LL, Kanaley JA (2005) Growth hormone and muscle function responses to skeletal muscle ischemia. J Appl Physiol 101(6):1588–1595
Place N, Bruton JD, Westerblad H (2009) Mechanisms of fatigue induced by isometric contractions in exercising humans and in mouse isolated single muscle fibres. Clin Exp Pharmacol Physiol 36(3):334–339
Renzi CP, Tanaka H, Sugawara J (2010) Effects of leg blood flow restriction during walking on cardiovascular function. Med Sci Sport Exer 42(4):726–732
Scott BR, Loenneke JP, Slattery KM, Dascombe BJ (2014) Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med 45(3):313–325
Shinohara M, Kouzaki M, Yoshihisa T, Fukunaga T (1997) Efficacy of tourniquet ischemia for strength training with low resistance. Eur J Appl Physiol O 77(1–2):189–191
Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N (2000a) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88(6):2097–2106 (Bethesda, Md: 1985)
Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N (2000b) Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol, 61–65
Takarada Y, Sato Y, Ishii N (2002) Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 86(4):308–314
Wernbom M, Augustsson J, Raastad T (2008) Ischemic strength training: a low-load alternative to heavy resistance exercise? Scand J Med Sci Sports 18(4):401–416
Wernbom M, Järrebring R, Andreasson MA, Augustsson J (2009) Acute effects of blood flow restriction on muscle activity and endurance during fatiguing dynamic knee extensions at low load. J Strength Cond Res 23(8):2389–2395
Wernbom M, Paulsen G, Nilsen TS, Hisdal J, Raastad T (2012) Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur J Appl Physiol 112(6):2051–2063
Yasuda T, Fujita T, Miyagi Y, Kubota Y, Sato Y, Nakajima T et al (2006) Electromyographic responses of arm and chest muscle during bench press exercise with and without KAATSU. Int J KAATSU Train Res 2(1):15–18
Yasuda T, Brechue WF, Fujita T, Sato Y, Abe T (2008) Muscle activation during low-intensity muscle contractions with varying levels of external limb compression. J Sport Sci Med 7(4):467–474
Yasuda T, Brechue WF, Fujita T, Shirakawa J, Sato Y, Abe T (2009) Muscle activation during low-intensity muscle contractions with restricted blood flow. J Sport Sci 27(5):479–489
Yasuda T, Abe T, Brechue WF, Iida H, Takano H, Meguro K et al (2010) Venous blood gas and metabolite response to low-intensity muscle contractions with external limb compression. Metabolism 59(10):1510–1519
Acknowledgments
This work was supported by CIPER–Foundation for Science and Technology (FCT), Portugal (PEst-OE/SAU/UI447/2014).
J.F. Reis would also like to acknowledge FCT, Portugal for her Postdoctoral Grant (SFRH/BPD/84315/2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatela, P., Reis, J.F., Mendonca, G.V. et al. Acute effects of exercise under different levels of blood-flow restriction on muscle activation and fatigue. Eur J Appl Physiol 116, 985–995 (2016). https://doi.org/10.1007/s00421-016-3359-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3359-1