Abstract
Key message
We developed transgenic sweet potato with Spomin (sucrose-inducible minimal promoter)-GUS gene-fused constructs. Induced GUS activities by Spomin were higher than those by CaMV 35S promoter.
Abstract
We developed transgenic sweet potato (Ipomoea batatas L. Lam. cv. Kokei no. 14) plants with Spomin (sucrose-inducible minimal promoter)-GUS gene-fused constructs with signal peptides for sorting to cytosol, apoplast and ER, and we analyzed the GUS expression pattern of cut tissue after sucrose treatment. Induced GUS activities by Spomin were several hundred times higher than those by the CaMV 35S promoter. Also, GUS activities in storage roots induced with a Spomin–cytosol-GUS construct were higher than those with either Spomin–apoplast or –ER-GUS constructs. The induced GUS activities by Spomin were higher in storage roots without sucrose treatment than those with sucrose treatment. Chilling (4 °C) storage roots with Spomin constructs for 4 weeks produced higher GUS activities than in storage roots stored at 25 °C for 4 weeks. The calculated maximum GUS content in the storage roots was up to about 224.2 μg/g fresh weight. The chilling treatment increased the free sucrose content in the storage roots, and this increase in endogenous sugar levels induced increased GUS activities in the storage roots. Therefore, Spomin appears to be a useful promoter to develop protein production systems using sweet potato variety Kokei no. 14 storage roots by postharvest treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various promoters are used in plant engineering. Constitutive promoters can be able to induce heterologous gene expression in whole plant tissues constitutively. For example, cauliflower mosaic virus 35S RNA promoter (35S) is used for many plant species (Guan et al. 2013). Actin promoter or ubiquitin promoter is also used for plants (Guan et al. 2013). However, constitutive heterologous gene expression in plant can be harmful to plant growth (Guan et al. 2013). Growth inhibition or gene silencing of heterologous gene was reported in transgenic plants with 35S construct (Elkind et al. 1990; Chen et al. 2006). To overcome this problem, there are many promoter studies using tissue-specific promoters or inducible promoters. Tissue-specific promoters induce expressions of heterologous genes in specific organs or specific tissues, for example, endosperm, fruit, or root. Inducible promoters induce expressions of heterologous genes in specific conditions, for example, light, osmotic stress, mechanical shock, hormone, or chemical compound treatment.
The accumulation level of cholera toxin B (CTB) subunit was about 4.4 μg/g tomato fruits with 35S (Jani et al. 2002); however, CTB accumulation was about 3.37 mg/g in rice seeds under the control of the rice endosperm-specific storage protein glutelin GluB-1 promoter (Kajiura et al. 2013). In cassava storage roots, 35S was not suitable for inducing high expression of genes; however, cassava storage root-specific promoters induced very high expression in storage roots (Zhang et al. 2003; Koehorst-van Putten et al. 2012). Sweet potato ADP-glucose pyrophosphorylase gene (ibAGP1) transcript was detected in sweet potato leaf, stem and storage root and ibAGP1 transcript was strongly induced by sucrose treatment (Noh et al. 2004). ibAGP1 promoter, which was constitutive and sucrose-inducible promoter, was isolated and ibAGP1 promoter showed high-level heterologous gene expression by sucrose treatment in sweet potato storage roots and carrot taproots (Kwak et al. 2006). Heterologous gene expression system with sucrose-inducible promoter will be suitable for inducing high levels of gene expression in storage roots.
Sweet potato is one of the most widely cultivated crops in the world. The yield in 2017 was about 12.2 ton/ha (FAO 2017) which is about three times higher than that of rice paddy (FAO 2017). The biomass production is unlimited because of its vegetative reproduction by storage roots. It is cultivated throughout the world (Kim et al. 2011; Muramoto et al. 2012). It is a multipurpose crop, suitable as a staple or supplementary food, animal feed, and raw brewing materials for industrial production (Muramoto et al. 2012). Storage roots are eating raw in some cultures.
Sporamin is the most abundant storage protein in storage roots of sweet potato (Maeshima et al. 1985). Although sporamin gene expression is normally specific to storage roots (Maeshima et al. 1985), its expression has also been induced in sweet potato leaves, petioles, stems and roots by exogenous sucrose, glucose or fructose treatment (Hattori et al. 1990, 1991). A study by Nakamura et al. (1991) reported high sporamin accumulation levels in leaves and petioles 7–14 days after treatment with 6% sucrose, but accumulation gradually decreased from 14 to 21 days. With 3% sucrose treatment, accumulation levels were lower than those with 6% treatment for 21 days, but then increased. However, in the case of 0 or 1% sucrose treatment, sporamin accumulation levels were very low. These reports showed that heterologous gene expression by sporamin promoter can be induced in whole sweet potato plants (leaves, stems, roots and storage roots) from 7 to 14 days under 6% concentration of sucrose, glucose or fructose; however, heterologous gene expression will be induced under 3% sucrose condition for more than 21 days. In transgenic tobacco with sporamin promoter–anthocyanin biosynthetic gene-fused constructs, high-level expression of anthocyanin biosynthetic genes and high-level accumulation of anthocyanin pigment were induced (An et al. 2015). The levels of gene expression and anthocyanin pigment accumulation were higher than those in transgenic tobacco with 35S–anthocyanin biosynthetic gene-fused constructs (An et al. 2015). Morikami et al. (2005) reported cis-acting regulatory sequences in the promoter region of the sporamin gene (gSpo-A1) and 204-bp fused promoter fragment in gSpo-A1 (− 282 to − 165 and − 86 to − 1) was sucrose-inducible minimal promoter region (Spomin). Induced GUS activities by Spomin were higher than those by gSpo-A1 promoter full region (Ohta et al. 1991; Morikami et al. 2005). We developed a transgenic tobacco plant system which produces a heterologous GUS protein with Spomin derived from sweet potato (Honma and Yamakawa 2015; Honma and Yamakawa 2019). In the tobacco leaves, maximum GUS activity levels were induced by 6% sucrose solution treatment and were higher than those expressed by 35S (Honma and Yamakawa 2015).
The sweet potato variety Kokei no. 14 is a variety for table use in Japan. Accumulated sugar levels (mg sugar/g fresh weight (FW) of storage roots) are about 1.7% sucrose, 0.70% glucose and 0.62% fructose (3% sugar in total) (Takahata et al. 1992). It takes about 3 months for root tuberization. Sporamin promoter will induce heterologous gene expression in storage roots with root tuberization. Sucrose concentration in Kokei no. 14 storage roots has been shown to increase when the roots were stored at 4 °C for 30 days (Sakamoto et al. 2014).
From those reports, we hypothesize that exogenous sucrose treatment and endogenous sucrose during root tuberization, and suitable postharvest storage could induce heterologous gene expression with Spomin enabling accumulation of heterologous proteins in storage roots. Some transgenic sweet potatoes have been shown to accumulate some secondary metabolites or starch (Tanaka et al. 2009; Pan et al. 2012; Park et al. 2015). In this paper, we show the potential development of heterologous protein accumulation systems in transgenic sweet potato storage roots with Spomin-GUS constructs.
Materials and methods
Plant materials
Sweet potato variety Kokei no. 14, a variety for table use in Japan, was kindly provided by The National Agriculture and Food Research Organization (NARO), Kyushu Okinawa Agricultural Research Center. The sweet potatoes were grown in pots with soil (Nippi YOSAIBAIDO SP200, JA, Japan) at 25 °C in a 16-h light/8-h dark cycle. Growing shoot tips were sterilized with 2% sodium hypochlorite solution, then rinsed three times in sterilized water and transferred to Linsmaier and Skoog (LS) solid medium containing 1.5% sucrose and 0.32% Gelrite, where they were incubated at 25 °C in a 16-h light/8-h dark cycle (Linsmaier and Skoog 1965). About 5 cm lengths with 2 or 3 nodes were cut from top of the shoots and transferred to fresh LS solid medium. The sweet potato plants grown in these bottles were used to produce transgenic sweet potato, from which storage roots were harvested after 3–4-month cultivation in pots with soil.
Induction of transformants
We have prepared Agrobacterium tumefaciens EHA105 harboring each construct with Spomin to sort the GUS protein to cytosol (SU), apoplast (SA) and ER (SE) (Fig. 1; Honma and Yamakawa 2015). In addition, we also prepared A. tumefaciens harboring each construct with 35S to sort the GUS protein to cytosol (3U), apoplast (3A) and ER (3E) by the constitutive expression with 35S (Fig. 1; Honma and Yamakawa 2015). We made transformants using A. tumefaciens EHA105 with 6 GUS constructs, based on previous methods (Otani et al. 1998; Anwar et al. 2010). Transgenic sweet potato plants were transplanted to pots with soil to induce storage roots. The storage roots were obtained 4–6 months after transplantation. We named six transgenic lines with each SU, SA, SE, 3U, 3A, and 3E constructs as SU, SA, SE, 3U, 3A, and 3E, respectively.

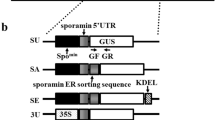
(reproduced and modified from Fig. 1 in Honma and Yamakawa (2015) Plant Biotechnol. Vol. 32, p48.)
Constructs of pTFPBIT vector for GUS introduction with Spomin or 35S and signal sequences. a The pTFPBIT vector was constructed from a pBI121 vector by adding HPT selection marker, and replacing THSP18.2 from the NOS terminator following GUS gene. THSP18.2 was the terminator of A. thaliana heat-shock protein 18.2. IS was insertion site of Spomin or 35S + signal sequences + GUS. b 6 GUS constructs are shown. GF: 5′-AAATCAAAAAACTCGACGGCCTGTG-3′ and GR: 5′-TATAAAGACTTCGCGCTGATA-3′ were GUS gene-specific primers. GUS gene (uidA): white boxes, Spomin: black boxes (Morikami et al. 2005), 35S (dot boxes), sporamin 5′UTR: vertical stripe boxes (accession number X13509), sporamin ER sorting sequences: horizontal stripe boxes (accession number X13509), ER retention signal KDEL: broken line boxes, sporamin vacuole sorting sequences: gray box (accession number X13509) and transit peptide of sweet potato ADP-glucose pyrophosphorylase (TP1) (Kwak et al. 2008): slash box (accession number AY544766). Signal sequences were collected from National Center for Biotechnology Information
Treatment with sucrose solution
Excised leaves and stems were treated with deionized water containing 0, 6 or 10% sucrose for 7 days at 25 °C in a 16-h light/8-h dark cycle. Excised white roots, red roots, and excised and sliced storage roots were incubated in deionized water containing 0, 6 or 10% sucrose for 7 days at 25 °C in the dark. The sucrose-treated tissues were used for the GUS assay. Non-sucrose-treated tissues were excised and sliced just before the GUS assay. The non-sliced storage roots were treated just like the sliced red and white roots, with 0, 6 or 10% sucrose solution for 7 days at 25 °C in the dark. Non-sucrose-treated non-sliced storage roots were also prepared without sucrose solution treatment. These non-sucrose-treated non-sliced storage roots were excised and sliced just before the GUS assay.
Expression analysis of the introduced GUS gene (uidA) in transgenic sweet potato leaves
Total RNAs were extracted from leaves grown in culture bottles, and were treated with 0 or 6% sucrose for 7 days at 25 °C in a 16-h light/8-h dark cycle (Honma and Yamakawa 2015). Semi-qRT-PCRs were performed with uidA-specific primers (GF: 5′-AAATCAAAAAACTCGACGGCCTGTG-3′ and GR: 5′-TATAAAGACTTCGCGCTGATA-3′) to confirm uidA expression. actin (FM244697) primers (actinF: 5′-CTGTTCTCTTGACTGAAGCAC-3′ and actinR 5′-TAACCTTCATAGATAGGGACGGT-3′) were used as an internal standard. PCR products were analyzed by agar gel electrophoresis. Signal intensities of the electrophoresed bands were determined using Image J software.
Chilling treatment
Non-excised storage roots were stored at 4 °C or 25 °C for 4 weeks in the dark before being used for the GUS assay. The sucrose concentration of storage root after chilling treatment was detected by tissue staining.
Protein extraction and fluorometric assay of GUS activity
Protein extraction and fluorometric assays of GUS activity were done on excised plant tissue, using the method reported by Jefferson et al. (Jefferson et al. 1987). The detailed methods were described in a previous paper (Honma and Yamakawa 2015). The GUS activity was expressed as pmol of 4-methylumbelliferone (MU) produced per minute per milligram of proteins in transgenic tissues.
Statistical analysis
A standard Student’s t test or Mann–Whitney U test was performed to determine the differences.
Calculation of theoretical production of GUS protein from GUS activities
Theoretical production of GUS protein was calculated based on the relationship between enzyme activities and on the amount of purified GUS protein from Escherichia coli (Kim et al. 1995).
Detection of free sucrose in storage root
Sucrose detection was carried by color development with anisaldehyde reagent. Storage root quarter cross sections were put on a silica gel TLC glass plate to transfer the tissue fluid for 3 min after chilling treatment. Anisaldehyde regent was sprayed on to the TLC plate and the plate was heated at 80 °C for 10 min. The anisaldehyde regent was prepared by mixing 99.5% ethanol 70 ml, acetic acid 1.2 ml, sulfuric acid 2.5 ml and anisaldehyde 2 ml. Standard sucrose solutions (0, 10−2, 10−1, 100 and 10%) were prepared and used for semi-quantitative determination by color development after spotting on to TLC plates.
Results
Development of transgenic lines
At least four transgenic lines (T) were confirmed in every introduced construct by genomic PCR (data not shown). There were no differences between any transgenic line and wild-type (WT) line by FWs. uidA transcription levels were examined by semi-qRT-PCR in just expanded leaves after 0% or 6% sucrose treatment for 7 days. In transgenic lines with Spomin constructs, uidA transcription levels in the leaves after 7 days 6% sucrose treatment were higher than those after 7 days 0% sucrose treatment and the levels were also higher than those of 35S constructs after 0 or 6% sucrose treatment (Fig. 2).
Relative expression (RE) levels of uidA in transgenic I. batatas grown in pots after 0 or 6% sucrose treatment. a RE levels of uidA in transgenic I. batatas leaves by semi-qRT-PCR. Upper bands show uidA and actin transcripts after sucrose treatment at 0 days. Lower bands show uidA and actin transcripts after sucrose treatment at 7 days. (%), sucrose concentration: WT, wild type: P, SU construct. b The graph shows RE of uidA actin−1. White bars show uidA actin−1 levels after 7 days. Black bars show uidA actin−1 levels after 0 days
GUS activities in excised and sliced organs with sucrose treatment
In SU, SA and SE storage roots, GUS activities were from 30 times to thousands of times higher than those induced in 3U, 3A and 3E storage roots (Fig. 3b, d, f). The highest expression level of GUS was detected in SU storage roots with 6% sucrose solution treatment. High GUS activities were also detected in other SU organs (Fig. 3a). There was no correlation between GUS activity and the concentration of sucrose solution in SA and SE excised organs, except for storage roots (Fig. 3c, e), unlike the earlier results reported for transgenic tobacco with an introduced Spomin–GUS construct (Honma and Yamakawa 2015).
GUS activities in excised and sliced leaves, stems, white roots, red roots and storage roots of transgenic I. batatas after sucrose treatment. GUS activities of SU and 3U (a, b), SA and 3A (c, d), SE and 3E (e, f) leaves (L), stems (S), white roots (WR), red roots (RR) and storage roots (TR) after 0, 6 or 10% sucrose treatment. White bars show SU, SA and SE. Black bars show 3U, 3A and 3E. Plants were cultured in pots for 2–3 months after planting. Vertical axes show GUS activities (pmol MU/min/mg protein). Error bars show standard error, N. *, significant differences between Spomin and 35S (*p < 0.05)
GUS activities in excised and sliced organs without sucrose treatment
GUS activities were very high in Spomin excised and sliced organs with 0% sucrose treatment (Fig. 3b–f). We investigated GUS activities before sucrose treatment (Fig. 4), and found that very high GUS activities were already induced in SU, SA and SE storage roots, and that these GUS activities were higher than in the same organs with sucrose treatment (Figs. 3b, d, f, 4b, d, f). GUS activities of SU storage roots were about 1.2–1.7 higher than those of SA and SE (Fig. 4b, d, f). High GUS activities were also detected in other SU organs except storage roots, and these activities were higher than those in 3U organs except storage roots (Fig. 4a). However, apart from in storage roots, the detected GUS activities in SA and SE organs were indistinguishable from those of 3a and 3E (Fig. 4c, e).
GUS activities in excised and sliced leaves, stems, white roots, red roots and storage roots of transgenic I. batatas without sucrose treatment. GUS activities of SU and 3U (a, b), SA and 3A (c, d), SE and 3E (e, f) leaves (L), stems (S), white roots (WR), red roots (RR) and storage roots (TR) without sucrose treatment. White bars show SU, SA and SE. Black bars show 3U, 3A and 3E. Plants were cultured in pots for 2–3 months after planting. Vertical axes show GUS activities (pmol MU/min/mg protein). Error bars show standard error, N. *, significant differences between Spomin and 35S (*p < 0.05)
GUS activities in non-sliced storage roots with sucrose solution treatment
GUS activities in excised and sliced storage roots with sucrose treatment were lower than in those without sucrose treatment (Figs. 3, 4). Microscopic observation (data not shown) suggested that some materials including sucrose or GUS had leaked from the storage root section into the sucrose solution. We studied GUS activities in non-sliced storage roots with exogenous sucrose treatment to increase GUS accumulation levels in storage roots more. We found that GUS activities decreased in transgenic storage roots with the rooting of adventitious fine roots (Fig. 5).
GUS activities in non-sliced transgenic storage roots (SE) with sucrose treatment. GUS activities (pmol MU/min/mg protein) of non-excised transgenic storage roots after 7 days with 0, 6 or 10% sucrose treatment. Error bars show standard error, N = 3. 0, 6 and 10, sucrose %. n, without sucrose treatment
GUS activities after chilling treatment
In our study, GUS activities of storage roots with Spomin constructs stored at 4 °C were higher than those stored at 25 °C treatment (Fig. 6a). We confirmed the increase of free sucrose in the storage roots of wild-type Kokei no. 14 without weight loss after the storage at 4 °C for 4 weeks (Fig. 6b).
GUS activities and sucrose detection after chilling treatment. a GUS activities of SU, SA and SE transgenic storage roots after 4 °C or 25 °C treatment. Scale bar = 1 cm. Graph shows GUS activities (pmol MU/min/mg protein) of all 12 transgenic lines (SU = 5, SA = 3, SE = 4) after chilling treatment. White bars show 4 °C treatment. Black bars show 25 °C treatment. b Sucrose detection using staining solution after 4 °C or 25 °C treatment. M shows the marker spots of sucrose standard solutions (0, 10−2, 10−1, 100, 10%)
It had previously been reported that black rot symptoms were induced under 8 °C and that the suitable storage temperature for sweet potato was about 13 °C (Ohashi and Uritani 1972). However, in our studies, soft rot symptoms were not observed in the storage roots after 4 °C treatment (Fig. 6a).
Discussion
Some researchers have shown that fusing sporamin promoter to a heterologous gene induced the expression of the gene in some plants (Morikami et al. 2005; Hong et al. 2008; Kim et al. 2010; Fukutomi et al. 2013; An et al. 2015). However, the current study is the first time that heterologous gene expression has been induced in sweet potato using Spomin. The qRT-PCR results showed that high uidA transcript levels were induced by Spomin under high sucrose concentrations and that high GUS accumulation could be induced in transgenic storage roots (Fig. 2). Transgenic sweet potato that was obtained by introducing the full-length cDNA in the sense orientation showed co-suppression of introduced cDNA gene (Kimura et al. 2001). In our studies, we confirmed that co-suppression was not observed in sweet potato and that it would be possible to develop heterologous protein production systems with sweet potato and Spomin. This possibility of development is supported by the fact that although sporamin is normally only detected in storage roots of sweet potato grown under normal field conditions, previous studies showed that sporamin was produced in sweet potato leaves, petioles and stems in either water or MS medium with high sucrose concentration (Hattori et al. 1990; Nakamura et al. 1991).
Generally, heterologous proteins in cytosol are degraded (Benchabane et al. 2008). To overcome this problem, many studies showed some solutions to transport heterologous proteins to organelles. However, in storage roots, cytosol (SU) GUS activities were about 100 times higher than those of apoplast (SA) and ER (SE) (Figs. 3b, d, f, 4b, d, f). SA and SE GUS proteins were glycosylated with N-glycosylation through Golgi apparatus because sporamin sorting sequences were introduced in SA and SE constructs (Iturriaga et al. 1989; Matsuoka and Nakamura 1991). The decrease of GUS activities with N-glycosylation through the Golgi apparatus has been discussed in previous studies (Iturriaga et al. 1989; Firek et al. 1994; Honma and Yamakawa 2015). Sporamin proteins are sorted to vacuoles and are accumulated in vacuole (Matsuoka and Nakamura 1991; Shewry 2003; Yang et al. 2005). Unlike in some cereals, the vacuole may be a more suitable organelle than ER to accumulate proteins in sweet potato storage roots (Kawakatsu and Takaiwa 2010; Kim et al. 2010; Ibl and Stoger 2012). We hypothesize that Spomin and vacuole sorting construct will be useful tools to develop heterologous protein production systems with sweet potato storage roots.
In storage roots, SU, Sa and SE GUS activities by Spomin in storage roots were higher than 3U, 3a, and 3E GUS activities by 35S (Fig. 3b, d, f). Heterologous gene expression levels by sucrose-inducible promoter were higher than those by constitutive promoter in storage roots. These results are supported by previous cassava and carrot reports (Zhang et al. 2003; Arango et al. 2010; Koehorst-van Putten et al. 2012).
Anthocyanin pigments were accumulated in red roots such as storage roots (Nishiyama and Yamakawa 2004) and in the early developmental stage of storage roots, the accumulation of starch granules and anthocyanin pigments was induced (Wang et al. 2016). However, the GUS activity in red roots was not high (Figs. 3a, c, e, 4a, c, e). We hypothesize that accumulation systems of anthocyanin pigments or starch granules can be different from accumulation systems of proteins in the developmental stage of storage roots. Storage roots are more useful than red roots to develop heterologous protein production systems.
Previous reports showed that sporamin accumulation in sweet potato tissues was decreased from 14 to 21 days after sucrose treatment (Nakamura et al. 1991). In our study, we showed GUS activities by Spomin with sucrose treatment were almost same at 7 days and 14 days (data not shown), and it may be possible that some materials including sucrose or GUS leaked from the storage root sections (data not shown). When we studied non-sliced storage roots, we found that GUS activities after rooting were lower in roots with sucrose treatment than in roots without sucrose treatment (Fig. 5). Protein amounts in storage roots were decreased from 0.53 to 0.16% during sprouting (Maeshima et al. 1985). Therefore, we hypothesize that GUS activities in storage roots with sucrose treatment would decrease because of the consumption of proteins containing GUS during rooting. Consequently, exogenous sucrose treatment of storage roots may not be suitable for increasing GUS accumulation. We have to improve sucrose-inducible method for sweet potato storage roots to develop heterologous protein production systems with sweet potato storage roots.
High GUS activities by Spomin were detected without sucrose treatment (Fig. 4). Sporamin promoter has been shown to induce gene expression in plantlets under suitable conditions (Hattori et al. 1990, 1991). Previous reports have also shown that (1) sporamin promoter induced gene expression with glucose, fructose and sucrose (Hattori et al. 1990; Nakamura et al. 1991), (2) sucrose, glucose and fructose levels in Kokei no. 14 storage roots (mg sugar/g FW of storage roots) were about 3% (Takahata et al. 1992), (3) that sporamin protein accumulation was induced under 3% sucrose condition (Nakamura et al. 1991) and (4) ibAGP1 promoter was up-regulated by increasing endogenous sucrose contents in sweet potato storage roots without exogenous sucrose treatment (Kwak et al. 2006). Thus, we hypothesize that GUS accumulation by Spomin has already been induced by endogenous sucrose derived from photosynthesis before exogenous sucrose treatment.
Previous studies have shown that it is possible to increase sucrose content in sweet potato storage roots. For example, sweet potato storage roots which were stored at 0–7 °C showed weight loss and an increased sucrose content (Hasselbring and Hawkins 1915a, 1915b). Also, the sucrose concentration of Kokei no. 14 storage roots increased up to three times (6 g/100 g FW) when the roots were stored at under 3–5 °C for 30 days (Sakamoto et al. 2014). In our study, increases in GUS activities and the amount of endogenous sucrose were induced without weight loss when storage roots were stored at 4 °C instead of 25 °C (Fig. 6). It should be possible to increase endogenous sucrose levels in storage roots under chilling storage conditions. However, sweet potato storage roots are susceptible to chilling injury (Picha 1984). Low-temperature conditioning (LTC) treatment at 10 °C for 5 days before chilling treatment is useful to alleviate chilling injury in sweet potato storage roots (Li et al. 2018). Moreover, LTC pretreatment before chilling treatment increased sucrose contents (mg/g FW of storage roots) in storage roots, compared with chilling treatment without LTC pretreatment (Li et al. 2018). Sucrose contents (g 100/g FW of storage roots) in sweet potato storage roots were also increased by curing pretreatment (30 °C for 7 days and relative humidity 96–100%) (Miyazaki 1990). Curing pretreatments of storage roots are reported to prevent diseases or physiological decay in some crops (Miyagawa et al. 2003; Tortoe et al. 2014; Zainuddin et al. 2018). In sweet potato, curing pretreatment of storage roots before storage is used to prevent postharvest infection or decay of storage roots during storage term (Chakraborty et al. 2017). Curing pretreatment (40 °C for 1.5 days) of Kokei no. 14 storage roots prevented sweet potato diseases; however, sucrose contents (%) in Kokei no. 14 storage roots were not affected (Tanoue et al. 1989). Curing pretreatment, LTC pretreatment, and chilling treatment can be useful methods to develop heterologous protein production systems with Kokei no. 14 storage roots while preventing chilling injury and postharvest disease. We hypothesize that it should be possible to increase endogenous sucrose levels in storage roots under suitable storage conditions. In addition, the GUS accumulation levels with Spomin constructs should also increase without exogenous sucrose treatment. It will be useful to produce heterologous proteins at low cost.
GUS accumulation levels in each organ except storage roots were low, compared with transgenic Nicotiana plumbaginifolia (Honma and Yamakawa 2015). In leaves, stems, white roots and red roots, there was not always a consistent sucrose concentration-dependent increase in GUS activity (Fig. 3c, e), as was found in an earlier study on transgenic N. plumbaginifolia (Honma and Yamakawa 2015). The decrease of GUS activities of Sa and SE in leaves, stems, white roots, and red roots of after sucrose treatment is probably due to the N-glycosylation of GUS according to the previous reports (Iturriaga et al. 1989; Matsuoka and Nakamura 1991). However, GUS activities in leaves, stems, white roots, and red roots of SU, Sa, and SE were increased by sucrose treatment, compared with no sucrose treatment (Figs. 3a, c, e, 4a, c, e). These results were different from GUS activities in storage roots of SU, Sa and SE. Sucrose might have been consumed to accumulate more sporamin protein than GUS protein expressed by a heterologous gene in sweet potato leaves, petioles and stems. As the sporamin gene is a multigene family with 49 sporamin full-length cDNAs (Hattori and Nakamura 1988; Hattori et al. 1989), a heterologous gene has not been able to accumulate GUS protein in short term. Further studies will be needed to accumulate heterologous protein in leaves, stems, white roots, and red roots of sweet potato with Spomin derived from storage roots.
Conclusion
We were able to develop transgenic sweet potato variety Kokei no. 14 storage roots which express introduced uidA using the Spomin expression system. Maximum GUS yield (μg) in transgenic storage roots has been calculated to be up to 224.2 μgGUS/g FW (Kim et al. 1995), which is much higher than yields in transgenic N. plumbaginifolia leaves or transgenic storage roots with 35S (Table 1). Moreover, transgenic sweet potato storage root protein production systems with Spomin can induce high levels of protein accumulation at low cost because exogenous sucrose treatment is unnecessary, unlike with transgenic N. plumbaginifolia with Spomin systems. Therefore, the sweet potato storage roots and Spomin system will be useful tools to develop protein production systems, for example, for use as edible or drinkable vaccines.
Author contribution statement
YH and TY conceived and designed research. YH and TY conducted experiments and analyzed data. YH and TY wrote the manuscript. All authors read and approved the manuscript.
Abbreviations
- GUS:
-
β-Glucuronidase
- Spomin :
-
Sucrose-inducible minimal promoter
- ER:
-
Endoplasmic reticulum
- UTR:
-
Untranslated region
- Semi-qRT-PCR:
-
Semi-quantitative reverse transcription polymerase chain reaction
- HPT:
-
Hygromycin phosphotransferase
References
An CH, Lee KW, Lee SH, Jeong YJ, Woo SG, Chun H, Park YI, Kwak SS, Kim CY (2015) Heterologous expression of IbMYB1a by different promoters exhibits different patterns of anthocyanin accumulation in tobacco. Plant Physiol Biochem 89:1–10
Anwar N, Kikuchi A, Watanabe KN (2010) Assessment of somaclonal variation for salinity tolerance in sweet potato regenerated plants. Afr J Biotechnol 9:7256–7265
Arango J, Salazar B, Welsch R, Sarmiento F, Beyer P, Al-Babili S (2010) Putative storage root specific promoters from cassava and yam: cloning and evaluation in transgenic carrots as a model system. Plant Cell Rep 6:651–659. https://doi.org/10.1007/s00299-010-0851-7
Benchabane M, Goulet C, Rivard D, Faye L, Gomord V, Michaud D (2008) Preventing unintended proteolysis in plant protein biofactories. Plant Biotechnol J 6:633–648. https://doi.org/10.1111/j.1467-7652.2008.00344.x
Chakraborty C, Roychowdhury R, Chakraborty S, Chakravorty P, Ghosh D (2017) A review on post-harvest profile of sweet potato. Int J Curr Microbiol Appl Sci 6:1894–1903
Chen HJ, Wang SJ, Chen CC, Yeh KW (2006) New gene construction strategy in T-DNA vector to enhance expression level of sweet potato sporamin and insect resistance in transgenic Brassica oleracea. Plant Sci 171:367–374
Elkind Y, Edwards R, Mavandad M, Hedrick SA, Ribak O, Dixon RA, Lamb CJ (1990) Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc Natl Acad Sci USA 87:9057–9061
Firek S, Whitelam GC, Draper J (1994) Endoplasmic reticulum targeting of active modified beta-glucuronidase (GUS) in transgenic tobacco. Transgenic Res 3:326–331. https://doi.org/10.1007/BF01973593
FAO (2017) FAOSTAT DATA 2017
Fukutomi D, Yoshinaka K, Kawamoto S, Mitsunari T, Kajita S, Kawai S (2013) High-level fructooligosaccharide production in transgenic tobacco plants. Plant Biotechnol 30:77–81
Guan ZJ, Guo B, Huo YL, Guan ZP, Dai JK, Wei YH (2013) Recent advances and safety issues of transgenic plant-derived vaccines. Appl Microbiol Biotechnol 97:2817–2840. https://doi.org/10.1007/s00253-012-4566-2
Hasselbring H, Hawkins LA (1915a) Respiration experiments with sweet potatoes. J Agric Res 5:509–517
Hasselbring H, Hawkins LA (1915b) Carbohydrate transformations in sweet potatoes. J Agric Res 5:543–560
Hattori T, Nakamura K (1988) Genes coding for the major tuberous root protein of sweet potato: identification of putative regulatory sequence in the 5′ upstream region. Plant Mol Biol 11:417–426. https://doi.org/10.1007/BF00039022
Hattori T, Yoshida N, Nakamura K (1989) Structural relationship among the members of a multigene family coding for the sweet potato tuberous root storage protein. Plant Mol Biol 5:563–572. https://doi.org/10.1007/BF00027316
Hattori T, Nakagawa S, Nakamura K (1990) High-level expression of tuberous root storage protein genes of sweet potato in stems of plantlets grown in vitro on sucrose medium. Plant Mol Biol 14:595–604. https://doi.org/10.1007/BF00027505
Hattori T, Fukumoto H, Nakagawa S, Nakamura K (1991) Sucrose-induced expression of genes coding for tuberous root storage protein, sporamin, of sweet potato in leaves and petioles. Plant Cell Physiol 32:79–86. https://doi.org/10.1093/oxfordjournals.pcp.a078055
Hong YF, Liu CY, Cheng KJ, Hour AL, Chan MT, Tseng TH, Chen KY, Shaw JF, Yu SM (2008) The sweet potato sporamin promoter confers high-level phytase expression and improves organic phosphorus acquisition and tuber yield of transgenic potato. Plant Mol Biol 67:347–361. https://doi.org/10.1007/s11103-008-9324-6
Honma Y, Yamakawa T (2015) High-level expression of sucrose inducible sweet potato sporamin gene promoter: β-glucuronidase fusion gene in transgenic Nicotiana plumbaginifolia. Plant Biotechnol 32:47–53
Honma Y, Yamakawa T (2019) High-level expression of sucrose inducible sweet potato sporamin gene promoter: β-glucuronidase fusion gene in transgenic Nicotiana plumbaginifolia hairy roots. (submitted)
Ibl V, Stoger E (2012) The formation, function and fate of protein storage compartments in seeds. Protoplasma 249:379–392. https://doi.org/10.1007/s00709-011-0288-z
Iturriaga G, Jefferson RA, Bevan MW (1989) Endoplasmic reticulum targeting and glycosylation of hybrid proteins in transgenic tobacco. Plant Cell 1:381–390
Jani D, Meena LS, Rizwan-ul-Haq QM, Singh Y, Sharma AK, Tyagi AK (2002) Expression of cholera toxin B subunit in transgenic tomato plants. Transgenic Res 11:447–454. https://doi.org/10.1023/A:1020336332392
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907. https://doi.org/10.1002/j.1460-2075.1987.tb02730.x
Kajiura H, Wasai M, Kasahara S, Takaiwa F, Fujiyama K (2013) N-glycosylation and N-glycan moieties of CTB expressed in rice seeds. Mol Biotechnol 54:784–794. https://doi.org/10.1007/s12033-012-9626-4
Kawakatsu T, Takaiwa F (2010) Cereal seed storage protein synthesis: fundamental processes for recombinant protein production in cereal grains. Plant Biotechnol J 8:939–953. https://doi.org/10.1111/j.1467-7652.2010.00559.x
Kim DH, Jin YH, Jung EA, Han MJ, Kobashi K (1995) Purification and characterization of beta-glucuronidase from Escherichia coli HGU-3, a human intestinal bacterium. Biol Pharm Bull 18:1184–1188
Kim CY, Ahn YO, Kim SH, Kim YH, Lee HS, Catanach AS, Jacobs JM, Conner AJ, Kwak SS (2010) The sweet potato IbMYB1 gene as a potential visible marker for sweet potato intragenic vector system. Physiol Plant 139:229–240. https://doi.org/10.1111/j.1399-3054.2010.01365.x
Kim YH, Kim MD, Park SC, Yang KS, Jeong JC, Lee HS, Kwak SS (2011) SCOF-1-expressing transgenic sweetpotato plants show enhanced tolerance to low-temperature stress. Plant Physiol Biochem 49:1436–1441
Kimura T, Otani M, Noda T, Ideta O, Shimada T, Saito A (2001) Absence of amylose in sweet potato (Ipomoea batatas (L.) Lam.) following the introduction of granule-bound starch synthase I cDNA. Plant Cell Rep 20:663–666. https://doi.org/10.1007/s002990100376
Koehorst-van Putten HJ, Wolters AM, Pereira-Bertram IM, van den Berg HH, van der Krol AR, Visser RG (2012) Cloning and characterization of a tuberous root-specific promoter from cassava (Manihot esculenta Crantz). Planta 236:1955–1965. https://doi.org/10.1007/s00425-012-1796-6
Kwak MS, Noh SA, Oh MJ, Huh GH, Kim KN, Lee SW, Shin JS, Bae JM (2006) Two sweetpotato ADP-glucose pyrophosphorylase isoforms are regulated antagonistically in response to sucrose content in storage roots. Gene 366:87–96
Kwak MS, Oh MJ, Paek KH, Shin JS, Bae JM (2008) Dissected effect of a transit peptide of the ADP-glucose pyrophosphorylase gene from sweetpotato (ibAGP2) in increasing foreign protein accumulation. Plant Cell Rep 27:1359–1367. https://doi.org/10.1007/s00299-008-0563-4
Li X, Yang HQ, Lu GQ (2018) Low-temperature conditioning combined with cold storage inducing rapid sweetening of sweetpotato tuberous roots (Ipomoea batatas (L.) Lam) while inhibiting chilling injury. Postharvest Biol Technol 142:1–9
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue culture. Physiol Plant 18:100–127. https://doi.org/10.1111/j.1399-3054.1965.tb06874.x
Maeshima M, Sasaki T, Asahi T (1985) Characterization of major proteins in sweet potato tuberous roots. Phytochemistry 24:1899–1902
Matsuoka K, Nakamura K (1991) Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci USA 88:834–838
Miyagawa I, Fujiwara T, Kosakai K, Shiga Y (2003) Studies on the environmental control for curing storage of Konjak ubers. J Agric Meteorol 59:237–244
Miyazaki T (1990) Effects of curing, storage conditions, and cooking on the composition of sweet potatoes. J Jpn Soc Hortic Sci 3:649–656
Morikami A, Matsunaga R, Tanaka Y, Suzuki S, Mano S, Nakamura K (2005) Two cis-regulatory elements are involved in the sucrose-inducible expression of the sporamin gene promoter from sweet potato in transgenic tobacco. Mol Genet Genom 272:690–699. https://doi.org/10.1007/s00438-004-1100-y
Muramoto N, Tanaka T, Shimamura T, Mitsukawa N, Hori E, Koda K, Otani M, Hirai M, Nakamura K, Imaeda T (2012) Transgenic sweet potato expressing thionin from barley gives resistance to black rot disease caused by Ceratocystis fimbriata in leaves and storage roots. Plant Cell Rep 6:987–997. https://doi.org/10.1007/s00299-011-1217-5
Nakamura K, Ohto M, Yoshida N, Nakamura K (1991) Sucrose-induced accumulation of β-amylase occurs concomitant with the accumulation of starch and sporamin in leaf-petiole cuttings of the sweet potato. Plant Physiol 96:902–909
Nishiyama Y, Yamakawa T (2004) Effect of medium composition on the production of anthocyanins by hairy root cultures of Ipomoea batatas. Plant Biotechnol 21:411–414
Noh SA, Kwak MS, Lee HS, Huh GH, Liu JR, Shin JS, Bae JM (2004) Genomic organizations of two small subunit ADP-glucose pyrophosphorylase genes from sweetpotato. Gene 339:173–180
Ohashi H, Uritani I (1972) The mechanism of chilling injury in sweet potato IX. The relation of chilling to changes in mitochondrial respiratory activities. Plant Cell Physiol 13:1065–1073
Ohta S, Hattori T, Morikami A, Nakamura K (1991) High-level expression of a sweet potato sporamin gene promoter: β-glucuronidase (GUS) fusion gene in the stems of transgenic tobacco plants is conferred by multiple cell type-specific regulatory elements. Mol Gen Genet 225:369–378. https://doi.org/10.1007/BF00261676
Otani M, Shimada T, Kimura T, Saito A (1998) Transgenic plant production from embryogenic callus of sweet potato (Ipomoea batatas (L.) Lam.) using Agrobacterium tumefaciens. Plant Biotechnol 15:11–16
Pan LP, Yu SL, Chen CJ, Li H, Wu YL, Li HH (2012) Cloning a peanut resveratrol synthase gene and its expression in purple sweet potato. Plant Cell Rep 1:121–131. https://doi.org/10.1007/s00299-011-1145-4
Park SC, Kim SH, Park S, Lee HU, Lee JS, Park WS, Ahn MJ, Kim YH, Jeong JC, Lee HS, Kwak SS (2015) Enhanced accumulation of carotenoids in sweetpotato plants overexpressing IbOr-Ins gene in purple-fleshed sweetpotato cultivar. Plant Physiol Biochem 86:82–90
Picha DH (1984) Chilling injury and low temperature sugar changes in sweet potato roots. HortScience 3:592
Sakamoto T, Masuda D, Nishimura K, Ikeshita Y (2014) Relationship between invertase gene expression and sucrose concentration in the tuberous roots of sweet potato (Ipomoea batatas L. Lam.) during cold storage. J Hortic Sci Biotechnol 89:229–235. https://doi.org/10.1080/14620316.2014.11513073
Shewry PR (2003) Tuber storage proteins. Ann Bot 91:755–769
Takahata Y, Noda T, Nagata T (1992) Varietal diversity of free sugar composition in storage root of sweet potato. Jpn J Breed 42:515–521
Tanaka M, Takahata Y, Nakayama H, Nakatani M, Tahara M (2009) Altered carbohydrate metabolism in the storage roots of sweet potato plants overexpressing the SRF1 gene, which encodes a Dof zinc finger transcription factor. Planta 230:737–746. https://doi.org/10.1007/s00425-009-0979-2
Tanoue H, Shimozono K, Maeya Y (1989) Studies on the heated moist air treatment for reducing decay of sweet potato during storage. Bull Kagoshima Agric Exp Stn 17:59–69
Tortoe C, Dowuona S, Dziedzoave N, Rees D (2014) Effect of curing treatments on seven key farmers’ yams (Dioscorea spp.) in Ghana. Agric Sci 5:1119–1128
Wang H, Yang J, Zhang M, Fan W, Firon N, Pattanaik S, Yuan L, Zhang P (2016) Altered phenylpropanoid metabolism in the maize Lc-expressed sweet potato (Ipomoea batatas) affects storage root development. Sci Rep 6:18645
Yang J, Barr LA, Fahnestock SR, Liu ZB (2005) High yield recombinant silk-like protein production in transgenic plants through protein targeting. Transgenic Res 14:313–324. https://doi.org/10.1007/s11248-005-0272-5
Zainuddin IM, Fathoni A, Sudarmonowati E, Beeching JR, Gruissem W, Vanderschuren H (2018) Cassava post-harvest physiological deterioration: from triggers to symptoms. Postharvest Biol Technol 142:115–123
Zhang P, Bohl-Zenger S, Puonti-Kaerlas J, Potrykus I, Gruissem W (2003) Two cassava promoters related to vascular expression and storage root formation. Planta 218:192–203. https://doi.org/10.1007/s00425-003-1098-0
Acknowledgements
We would like to express our thanks to Dr. Elizabeth Hood of ProdiGene Inc. (Present address: Arkansas State University) for providing A. tumefaciens strain EHA105.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sang-Soo Kwak.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Honma, Y., Yamakawa, T. High expression of GUS activities in sweet potato storage roots by sucrose-inducible minimal promoter. Plant Cell Rep 38, 1417–1426 (2019). https://doi.org/10.1007/s00299-019-02453-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-019-02453-7