Abstract
Black rot of sweet potato caused by pathogenic fungus Ceratocystis fimbriata severely deteriorates both growth of plants and post-harvest storage. Antimicrobial peptides from various organisms have broad range activities of killing bacteria, mycobacteria, and fungi. Plant thionin peptide exhibited anti-fungal activity against C. fimbriata. A gene for barley α-hordothionin (αHT) was placed downstream of a strong constitutive promoter of E12Ω or the promoter of a sweet potato gene for β-amylase of storage roots, and introduced into sweet potato commercial cultivar Kokei No. 14. Transgenic E12Ω:αHT plants showed high-level expression of αHT mRNA in both leaves and storage roots. Transgenic β-Amy:αHT plants showed sucrose-inducible expression of αHT mRNA in leaves, in addition to expression in storage roots. Leaves of E12Ω:αHT plants exhibited reduced yellowing upon infection by C. fimbriata compared to leaves of non-transgenic Kokei No. 14, although the level of resistance was weaker than resistance cultivar Tamayutaka. Storage roots of both E12Ω:αHT and β-Amy:αHT plants exhibited reduced lesion areas around the site inoculated with C. fimbriata spores compared to Kokei No. 14, and some of the transgenic lines showed resistance level similar to Tamayutaka. Growth of plants and production of storage roots of these transgenic plants were not significantly different from non-transgenic plants. These results highlight the usefulness of transgenic sweet potato expressing antimicrobial peptide to reduce damages of sweet potato from the black rot disease and to reduce the use of agricultural chemicals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant crops are susceptible to various diseases caused by a variety of pathogens such as bacteria, fungi, insects and viruses. Losses due to these diseases have been estimated to amount to 12% of the potential crop production (James et al. 1990). Disease control utilizing transgenic plants has already put into practical use for diseases caused by insects and viruses through the expression of an insecticidal protein from Bacillus thuringiensis and the virus coat protein, respectively (Kahl and Winter 1995; Estruch et al. 1997). However, to control diseases caused by pathogenic bacteria and fungi, which actually accounts for the largest part of plant diseases, antibiotics such as streptomycin and fungicides such as copper-containing agricultural chemicals are still being used in the field, causing various environmental problems (Kummerer 2004; Komarek et al. 2010).
Sweet potato [Ipomoea batatas (L.) Lam.] is one of the most widely cultivated tuber crops in the world, in particular in the tropical and subtropical zones of Asia, Oceania, Latin America and Africa. Sweet potato can be used for a variety of purposes, such as for staple and subsidiary food, brewing, animal feed, and industrial feedstock for materials such as bioplastics. Although sweet potato can be produced in harsh environment conditions, it is particularly weak in its storage stability. In addition to insect damages by sweet potato weevil, the black rot caused by pathogenic fungus Ceratosistis fimbriata is one of the most serious diseases of sweet potato that causes significant losses during storage (Uritani and Stahmann 1961; Akazawa and Uritani 1962; Clark and La Bonte 1992). Since sweet potato has hexaploid genome and usually propagated by vegetative propagation, the conventional breeding strategy by sexual hybridization has not been very effective. Recently, Agrobacterium-mediated transformation and regeneration methods have been developed to establish transgenic sweet potato plants (Moran et al. 1998; Otani et al. 1998). Application of transgenic technology is expected to open a new way to add black rot resistance to sweet potato to the widely used sweet potato cultivars without changing their basic characters.
A large number of natural antimicrobial peptides have been identified among various living organisms (Zasloff 2002). Antimicrobial peptides are generally composed of 12 to 50 amino acids, and exhibit a broad range of antimicrobial activities against gram-negative and -positive bacteria, mycobacteria and fungi. The mode of action of the antimicrobial peptides is bacteriocidal, or killing microbes, instead of bacteriostatic, or inhibiting their growth. Although the exact mechanism is not precisely known, antimicrobial peptides may kill microbes by forming trans-membrane pores or by penetrating into the cell to bind intracellular molecules crucial for survival (Matsuzaki 1999; Shai 1999; Yang et al. 2000). Small size, characteristic amino acid composition, cationic charge and amphipathic nature are suggested to allow them to attach and insert into membrane (Matsuzaki 1999). Antimicrobial peptides are regarded as an excellent candidate to develop therapeutic agents for humans, because they have a broad host range activity, do not appear to induce resistance, and require only a short contact time to induce killing (Zasloff 2002; Peters et al. 2010).
Transgenic plants that express antimicrobial peptides have already shown to give a resistance to infection by bacteria (Allefs et al. 1996; Carmona et al. 1993; Iwai et al. 2002), fungi (Epple et al. 1997; Gao et al. 2000) or both (Osusky et al. 2000; Ponti et al. 2003; Ovard and Enright 2006) to various plants. As a first step to develop transgenic sweet potato resistant to C. fimbriata through the expression of antimicrobial peptide, we examined toxicity of plant thionins against C. fimbriata. Thionins are cysteine-rich peptides of about 50 amino acids found only in seeds and other tissues of plants (Florack and Stiekema 1994; Stec 2006), and they show a broad cellular toxicity against wide range of bacteria and fungi. Transgenic tobacco (Carmona et al. 1993), Arabidopsis (Epple et al. 1997), rice (Iwai et al. 2002), and tomato (Chan et al. 2005) that express thionin have previously shown to exhibit resistance to bacterial and fungal pathogens.
Since we observed that purothionin from wheat endosperm shows strong toxicity to C. fimbriata in vitro, and we then tried to develop transgenic sweet potato that expresses α-hordothionin (αHT) from barley endosperm with structure similar to purothionin (Florack et al. 1994). Sweet potato cultivar Kokei No. 14, which is one of the most widely cultivated cultivars in Japan and highly susceptible to infection by C. fimbriata, was used as a host. We developed transgenic sweet potato Kokei No. 14 lines that express αHT under a chimeric El2Ω promoter (Mitsuhara et al. 1996) and a promoter of the endogenous β-Amy gene encoding the major β-amylase of storage roots (Nakamura et al. 1991; Takeda et al. 1994; Maeo et al. 2001). Expression of αHT in leaves and storage roots of these transgenic plants conferred resistance to inoculation with C. fimbriata spores, suggesting that transgenic sweet potato expressing antimicrobial peptide is a promising way to reduce losses of sweet potato due to black rot and to reduce the use of agricultural chemicals.
Materials and methods
Anti-fungal activity of peptide against C. fimbriata
Corystein™ (Takara Bio Inc, Shiga, Japan) purified from wheat endosperm was used as purothionin peptide. C. fimbriata IFO 30501 was grown on agar plate with potato dextrose medium at 26°C for 14 days, and conidiospores were collected. The spores were suspended in 0.01% Tween20, and filtrated by sterile gauze. In the wells of a 96-well microtitre plate, 60 μl of spore suspension (1 × 105 spores/mL in 80% potato dextrose broth) was mixed with 10 μl of 200 mM MES buffer (pH 6.0), 30 μl of peptide solution diluted to different concentrations, and cultured at 26°C. At 30 min and 48 h after mixing, OD at 415 nm was measured using a micro-plate reader. Growth inhibition was calculated as follows;
Growth inhibition (%) = [ΔC − ΔT]/ΔC × 100, where ΔC = (OD415 at 48 h − OD415 at 30 min) without peptide, and ΔT = (OD415 at 48 h − OD415 at 30 min) with peptide.
Plant materials
Sweet potato (Ipomoea batatas (L.) Lam.) cv. Kokei No. 14 was used as the wild type control plant material, and cv. Tamayutaka was used as a control cultivar resistant to inoculation with C. fimbriata. Sweet potato plants were grown in 80 mm diameter plastic pot filled with Kreha culture soil (Kureha Co., Tokyo, Japan) in growth chamber at 26°C under 16 h photoperiod for examination of susceptibility of leaves to inoculation with spores of C. fimbriata. Alternatively, they were grown in 450 mm diameter plastic pot filled with Kureha culture soil in glasshouse at 26°C under natural light for examination of susceptibility of storage roots to inoculation with spores of C. fimbriata.
Expression vectors for αHT gene
A 384 bp-long coding sequence for αHT was obtained by chemical DNA synthesis, and placed downstream of a chimeric El2Ω promoter for high-level constitutive expression (Mitsuhara et al. 1996). In addition to this El2Ω:αHT fusion gene, the β-Amy:αHT fusion gene, in which the αHT gene was placed downstream of a 2 kb-long promoter and 5′-UTR regions of β-Amy gene of sweet potato encoding the major β-amylase of sweet potato storage root (Yoshida et al. 1992; Maeo et al. 2001), was constructed. These fusion genes were placed between kanamycin-resistance and hygromycin-resistance marker genes on a binary vector derived from pIG121-Hm (Ohta et al. 1990; Mita et al. 1995), and introduced into A. tumefascience EHA101 by electroporation.
Transformation and regeneration of sweet potato
Transformation of sweet potato by A. tumefacience was performed essentially as described by Otani et al. (1998) with some modifications. A. tumefaciens EHA101 harboring the binary vector plasmid was grown in liquid LS medium (Linsmaier and Skoog 1965) supplemented with 3% sucrose, 10 mg/L acetosyringone, and 1 mg/L 4-fluorophenoxyacetic acid (4FA) at 30°C. The embryogenic calli induced from shoot apical meristems of sweet potato were soaked in the bacterial suspension for 2 min, and transferred onto LS gellan gum (3.2 g/L) plate containing 3% sucrose, 10 mg/L acetosyringone, and 1 mg/L 4FA (pH 5.8). After 3 days of co-cultivation at 26°C in the dark, the calli were transferred to recovery medium containing LS gellan gum plate containing 3% sucrose, 1 mg/L 4FA, and 6 mg/L Meropenem (Dainippon Sumitomo Pharma, Osaka, Japan). After 7 days, calli were transferred onto LS gellan gum plate containing 5 mg/L hygromycin (Wako Pure Chemical Industries, Osaka, Japan) for selection. After 60 days with a change of medium every 2 weeks, calli with light yellow color were transferred to LS gellan gum plate containing 3% sucrose, 4 mg/L abscisic acid, 1 mg/L gibberellic acid (GA3), 5 mg/L hygromycin, and 6 mg/L Meropenem, and incubated at 26°C under 16 h photoperiod for regeneration. After 3 weeks, somatic embryos formed from hygromycin-resistant calli were transferred to regeneration medium of LS gellan gum plate containing 3% sucrose, 5 mg/L hygromycin, and 6 mg/L Meropenem. After 4 weeks, regenerated plants were transferred onto the fresh medium, and further grow for 8 weeks before transferred to plastic pot filled with Kureha culture soil. Plants were naturalized under high humidity condition for 3 days.
Quantitative RT-PCR
Total RNA was isolated from sweet potato using an RNeasy plant mini kit (Qiagen, Valencia, CA, USA), and levels of mRNA were determined by quantitative RT-PCR (Holland et al. 1991) with TaqMan Reverse Transcription Reagents and TaqMan Universal PCR Master Mix (Applied biosystems, Foster City, CA, USA) using an ABI PRISM 7000. The primer set for αHT mRNA was 5′-CCCAAATTGGCCCTTGTG-3′, 5′-GGGGGAGCTCTTAGGCAGTAAGGGATGTGAGTC-3′ and 5′-CCAACTCAGATGAACCAGACACCGTCAA-3′ as TaqMan probe. The primer set for Act2 mRNA was 5′-TGGAACCGGAATGGTTAAGG-3′, 5′-ACAGTGTGGCTCACACCATCAC-3′ and 5′-TCCTAGGGCAGTCTTCCCGAGTATAGTTGG-3′ as TaqMan probe. The level of αHT mRNA was normalized by the level of Act2 mRNA.
Resistance of sweet potato against inoculation with C. fimbriata
Leaves of 5 to 6 weeks sweet potato cut at the petioles were dipped in a suspension of C. fimbriata spores (1 × 105 spores/mL) for 30 s, and incubated at 26°C under moist conditions. After 7 or 8 days, the whole leaf surface area and the leaf area that turned yellow were measured by image analysis, and the ratio of yellow leaf area was calculated as a measure of disease susceptibility.
Surface of storage roots of 15 to 16 weeks sweet potato plant was scratched with a needle, and a suspension of C. fimbriata spores (1 × 105 spores/mL) was inoculated to scars with needle. After incubation for 20 days at 26°C under moist condition in the dark black lesion areas developed around the site of inoculation were measures by image analysis.
Results
Anti-fungal activity against C. fimbriata of purothionin from wheat
Generally, anti-microbial peptides from plants such as thionin exhibit high anti-fungal activity. We assayed for anti-fungal activity of purothionin from wheat endosperm towards C. fimbriata in vitro. The growth of C. fimbriata as measured by the increase in OD at 415 nm was almost completely inhibited by purothionin at concentrations above 10 μg/mL (Fig. 1a). Both germination of spore and hyphal elongation of C. fimbriata observed under the microscope were inhibited by 30 μg/mL of purothionin (Fig. 1b).
Inhibition of growth of C. fimbriata by purothionin. a Effect of purothionin on growth of C. fimbriata spores. Suspension of spores was mixed with various concentrations of purothionin and cultured at 26°C. OD at 415 nm was measured at 30 min and 48 h later, and growth inhibition was calculated as described in “Materials and methods”. b Hyphal growth anomalies in the presence of purothionin. Photographs were taken 48 h after incubation of C. fimbriata without purothionin (control, left), or with 30 μg/mL purothionin (right). Bars 100 μm
Construction of vectors for expression of α-hordothionin and transformation of sweet potato
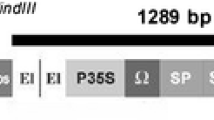
We selected αHT from barley, which is 45 amino acid-long thionin, for the expression in sweet potato to develop black rot resistance. Since αHT has high sequence identity and similar toxicity for plant pathogenic bacteria with purothionin, these two peptides are considered to be functional orthologs to each other (Bohlmann and Apel 1991; Florack et al. 1993). A DNA fragment containing the coding sequence of αHT was placed downstream of a strong constitutive E12Ω promoter (Mitsuhara et al. 1996), with tandem repeat of the enhancer sequence from the cauliflower mosaic virus (CaMV) 35S promoter and the omega sequence from tobacco mosaic virus (TMV) to develop E12Ω:HT fusion gene and placed in a binary Ti-plasmid vector pIG121-Hm (Mita et al. 1995) (Fig. 2). We also prepared β-Amy:αHT fusion gene, in which the αHT coding sequence was placed downstream of a 2 kb-long promoter and 5′-UTR regions of a sweet potato gene for the major β-amylase of storage root (β-Amy; Yoshida et al. 1992) and placed in pIG121-Hm vector (Fig. 2). The 2.0 kb-long promoter fragment of β-Amy confers sugar-inducible expression of the β-glucuronidase (GUS) reporter gene in the leaves of tobacco and Arabidopsis (Takeda et al. 1994; Maeo et al. 2001). The β-Amy:GUS also shows sugar-inducible expression in leaves and constitutive expression in tubers of transgenic potato.
Binary Ti plasmid vectors used for expression of αHT in transformed sweet potato plants. The El2Ω:αHT or β-Amy:αHT fusion gene was inserted between the nos-NPTII and 35S-HPT genes for kanamycin resistance and hygromycin resistance, respectively, in pIG121-Hm binary vector. El2-35S duplicated enhancer and the promoter of CaMV 35S gene, 5′UTR 5′-untranslated region of β-Amy, Ω omega sequence of TMV, RB and LB the right and left border, Pnos and Tnos promoter and terminator of the nopaline synthase gene, p35S CaMV 35S promoter
Agrobacterium tumefaciens EHA101 cells harboring Ti plasmid with the E12Ω:αHT or β-Amy:αHT fusion gene were used to transform sweet potato commercial cultivar Kokei No. 14 by infection to embryonic calli (Otani et al. 1998). We obtained many hygromycin-resistant calli for both constructs, and these calli were used for plant regeneration. Regenerated plants were examined for the integration of αHT sequence into genomic DNA, and we obtained three lines transformed with El2Ω:αHT and six lines transformed with β-Amy:αHT. All of these transgenic plants grew normally and developed mature leaves and storage roots that were similar to those of the non-transgenic Kokei No. 14.
Expression of αHT in leaves and storage roots of transgenic sweet potato
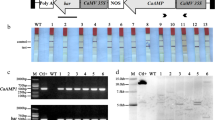
Total RNAs were prepared from mature leaves and storage roots, and the amount of αHT mRNA was determined by quantitative real-time PCR. The amount of Act2 mRNA was also determined and served as an internal control. High-level expression of αHT mRNA was detected in mature leaves of all of the three El2Ω:αHT lines (Fig. 3a). The levels of αHT mRNA relative to Act2 mRNA in mature leaves of six β-Amy:αHT lines were more than 100-fold lower than those detected in El2Ω:αHT plants or αHT mRNAs could not be detected. Since the expression of β-Amy in leaves of sweet potato (Nakamura et al. 1991) and the expression of β-Amy:GUS in leaves of transgenic tobacco plants (Maeo et al. 2001) are strongly inducible by sucrose, we examined whether the expression of β-Amy:αHT in leaves of transgenic sweet potato is also inducible by sucrose. Leaf petiole cuttings of β-Amy:αHT plants were treated with a 6% solution of sucrose or water for 24 h before the isolation of total RNAs. As shown in Fig. 3b, marked increases in αHT mRNA occurred by treatment with 6% sucrose in all of the six transgenic lines. The level of Act2 mRNA did not change significantly by the treatment of leaves with sucrose.
Expression of αHT gene in transgenic sweet potato plants. a RT-PCR analysis of the levels of αHT mRNA in leaves of non-transgenic Kokei No. 14, three transgenic lines with El2Ω:αHT, and six transgenic lines with β-Amy:αHT. The amount of Act2 mRNA served as an internal control, and the levels of αHT mRNA relative to Act2 mRNA are shown on vertical axis in logarithmic scale. b The levels of αHT mRNA relative to Act2 mRNA in leaves of transgenic sweet potato with β-Amy:αHT. Leaf-petiole cuttings of β-Amy:αHT plants were treated with a 6% solution of sucrose or water for 24 h before the isolation of total RNAs. c The levels of αHT mRNA relative to Act2 mRNA in storage roots of Kokei No. 14, Tamayutaka, El2Ω:αHT transgenic line No. 1, and six transgenic lines with β-Amy:αHT
The αHT mRNA was detected in storage roots of all of the transgenic lines with El2Ω:αHT and β-Amy:αHT, but not in non-transgenic plants (Fig. 3c). The levels of αHT mRNA relative to Act2 mRNA in storage roots appeared to be much smaller than those in leaves. This was due to nearly 100-fold higher amount of Act2 mRNA in storage roots compared to leaves. The amounts of αHT mRNAs in storage roots of β-Amy:αHT plants were similar to those detected in sucrose-treated leaves. Unlike leaves, the levels of αHT mRNA in storage roots were not significantly different between the El2Ω:αHT and β-Amy:αHT plants.
Resistance against black rot fungus in leaves of transgenic sweet potato with El2Ω:αHT
To evaluate the resistance of transgenic sweet potato plants against C. fimbriata, leaves of non-transgenic Kokei No. 14 and three transgenic lines with El2Ω:αHT were challenged by inoculation with C. fimbriata spores. Sweet potato cultivar Tamayutaka, a natural variety resistant to C. fimbriata, was also used as a positive control. Leaves cut from seedlings at the petiole were dipped in a suspension of C. fimbriata spores for 30 s, and incubated at 26°C under moist condition. Leaves of non-transgenic Kokei No. 14 plants inoculated with spores progressively turned into yellow from the cut surface to the periphery of leaf vein, and from the leaf edges to the whole area of leaves, resulting in browning of leaves after 7 to 8 days of inoculation (Fig. 4a). Since leaves without inoculation with spores showed only slight yellowing in the peripheral region of veins (data not shown), this yellowing of leaves is probably caused by spreading of C. fimbriata infection to the entire leaf. In contrast to the leaves of Kokei No. 14, leaves of Tamayutaka, which has darker green color than Kokei No. 14, stayed green with little yellowing even 8 days after inoculation with spores. Leaves of transgenic lines with El2Ω:αHT showed yellowing after inoculation with C. fimbriata spores. However, area of leaf surface that turned yellow was smaller compared to leaves of non-transgenic Kokei No. 14 after inoculation. In particular, leaves of transgenic plants did not show yellowing of edges. Transgenic line No. 2 was particularly resistant, and it showed severe yellowing only along the side of leaf vein even after 8 days post-inoculation (Fig. 4a).
Resistance of leaves of transgenic sweet potato with El2Ω:αHT to C. fimbriata. a Morphological characteristics of leaves of Kokei No. 14 and Tamayutaka control plants, and three lines of the El2Ω:αHT transgenic plants after 7 and 8 days of inoculation with C. fimbriata spores. b Ratio of yellow leaf area to the whole leaf area was evaluated by image analysis. Bars means Standard errors, and asterisk indicates a statistical significance by Dunnett’s multiple comparisons test; n = 13 for Kokei No. 14 and Tamayutaka, and n = 4–7 in transgenic lines; ***P < 0.001; **P < 0.01
We measured the whole leaf surface area and the area of leaf surface which turned into yellow, and calculated the ratio of leaf area that turned yellow 7 days after inoculation as a measure of disease susceptibility. Mean of the ratio of yellow leaf area for 13 leaves of untransformed Kokei No. 14 plant and Tamayutaka was 62.6 and 7 %, respectively. On the other hand, the mean value of the ratio of yellow leaf area in 4 to 7 leaves of No. 1, No. 2 and No. 3 transgenic lines expressing El2Ω:αHT was 48.0, 20.9, and 34.3%, respectively (Fig. 4b). Among these, low ratio of the yellow leaf area of No. 2 transgenic line was statistically significant.
Resistance against black rot fungus in storage roots of transgenic sweet potato with El2Ω:αHT and β-Amy:αHT
We also examined resistance of storage roots of transgenic sweet potato after inoculation with C. fimbriata spores. Storage roots were scratched with a needle on their surface, and a suspension of C. fimbriata spores was inoculated to these wound scars. Infected storage roots were incubated at 26°C under moist condition for 20 days in the dark. Storage roots of non-transgenic Kokei No. 14, a transgenic line with El2Ω:αHT and six transgenic lines with β-Amy:αHT were challenged by inoculation with C. fimbriata spores. Storage roots of Tamayutaka were also used as a positive control. Storage roots of non-transgenic Kokei No. 14 infected with spores developed black circles around the inoculation sites that were considered to be lesions of black rot (Fig. 5a). Storage roots without inoculation showed no changes around scars (data not shown). Storage roots of Tamayutaka after inoculation with spores developed black circles around inoculation sites that were smaller than those developed on storage roots of Kokei No. 14. Storage roots of transgenic lines developed black lesion areas around the inoculation sites smaller than those developed on storage roots of Kokei No. 14. Among them, the El2Ω:αHT No. 1 and β-Amy:αHT No. 060201 transgenic lines developed particularly small size of black lesions around the inoculation sites (Fig. 5a).
Resistance of storage roots of transgenic sweet potato with El2Ω:αHT and β-Amy:αHT to C. fimbriata. a Lesion development of storage roots of control Kokei No. 14, transgenic El2Ω:αHT line No. 1, and transgenic β-Amy:αHT line No. 06020 after 20 days inoculated with C. fimbriata spores. White circles indicate black lesion areas developed around the sites inoculated with spores. b Size of lesion area was evaluated by measuring the area turned black around the site of inoculation on storage root by image analysis. Bars means standard errors, and asterisk indicates a statistical significance by Dunnett’s multiple comparisons test; n = 17 for Kokei No. 14, 12 for Tamayutaka, 15 for transgenic El2Ω:αHT line No. 1, and 7–13 for transgenic β-Amy:αHT lines; ***P < 0.001; **P < 0.01
We measured the area of lesions turned into black around the inoculation sites as a measure of disease susceptibility. The mean of black area of lesions around inoculation sites for 17 lesions of untransformed Kokei No. 14 and for 12 lesions of Tamayutaka was 283 and 84 mm2, respectively (Fig. 5b). On the other hand, the mean value of black area of lesions around inoculation sites for 15 lesions of El2Ω:αHT No. 1 line was 119 mm2, which is significantly lower than that of Kokei No. 14. The mean values of black area of lesions around inoculation sites for six transgenic lines with β-Amy:αHT were between 111 and 201 mm2. Among them, small sizes of the black lesion areas on No. 060101, No. 060201, No. 060901, No. 061201 transgenic lines compared to those on Kokei No. 14 were statistically significant.
Discussion
Antimicrobial peptides from plants are known to show anti-fungal activities. Indeed, we could first demonstrate that purothionin peptide from wheat endosperm shows growth inhibitory activity against black rot fungus C. fimbriata. It inhibited both germination of spores and the growth of fungal hyphae (Fig. 1). Several other antimicrobial peptides also inhibited growth of C. fimbriata with different inhibitory activities (Muramoto et al. unpublished results). These results prompted us to examine whether transgenic sweet potato plants expressing thionin, or other antimicrobial peptide, acquire enhanced resistance to black rot disease. To develop transgenic sweet potato expressing thionin, we used a gene for barley thionin αHT (Florack et al. 1994). The entire coding sequence for the precursor form of αHT was chemically synthesized and placed downstream of the strong constitutive promoter of E12Ω (Mitsuhara et al. 1996; E12Ω:αHT) and the promoter of a sweet potato gene for β-amylase of storage roots (Maeo et al. 2001; β-Amy:αHT) (Fig. 2). The modified method of Agrobacterium-mediated transformation of calli (Otani et al. 1998) was used to deliver these fusion genes into the chromosome of sweet potato.
All of the hygromycin-resistant regenerated plants with the αHT gene sequence integrated into the genome showed expression of αHT mRNA. High levels of αHT mRNA were detected in leaves of three transgenic lines with E12Ω:αHT. Levels of αHT mRNA in leaves of six transgenic lines with β-Amy:αHT were quite low or not detected in some lines (Fig. 3a). However, similar to endogenous β-Amy mRNA in sweet potato (Nakamura et al. 1991), αHT mRNA was strongly induced after treatment of leaves with sucrose in all of these β-Amy:αHT transgenic lines (Fig. 3b). The levels of αHT mRNA in leaves after treatment with sucrose were not in parallel with the levels of αHT mRNA in non-treated leaves or leaves treated with water among these transgenic lines, and they were not as high as levels in leaves of transgenic E12Ω:αHT plants. In addition to transgenic line with E12Ω:αHT, all of the six transgenic lines with β-Amy:αHT showed expression of αHT mRNA in storage roots (Fig. 3c). The amounts of αHT mRNAs in storage roots of the β-Amy:αHT plants were similar to those in the E12Ω:αHT plants. These results suggest that the β-Amy promoter is useful for selective expression in storage roots.
Transgenic lines with El2Ω:αHT showed reduced leaf surface area that turned into yellow after inoculation with spores of C. fimbriata compared to leaves of non-transgenic Kokei No. 14 (Fig. 4a, b). In addition, unlike leaves of Kokei No. 14 which showed severe yellowing at the edges of leaves after inoculation, leaves of transgenic lines did not show yellowing of leaf edges even at 8 days after inoculation (Fig. 4a). In addition to leaves, storage roots of the El2Ω:αHT transgenic line showed reduced development of black lesion area after inoculation with C. fimbriata spores to wounded sites on the surface (Fig. 5a). Similar reduction in black lesion area around the infected wound sites were also observed in storage roots of transgenic lines with β-Amy:αHT. Significant levels of reduction in lesion area compared to the non-transgenic Kokei No. 14 occurred in many of the transgenic lines, and some of them showed resistance levels similar to storage roots of Tamayutaka. Altogether, these results suggest that sweet potato can acquire increased level of resistance against black rot disease caused by C. fimbriata, in particular in storage roots, by the overexpression of thionin peptide. It is assumed that increased resistance is due to inhibition of fungal growth by thionin at the site of inoculation.
Previously, transgenic tobacco overexpressing αHT has been shown to exhibit enhanced resistance to attack by bacterial pathogen Pseudomonas syringae (Carmona et al. 1993), and overexpression of oat thionin in rice also gives resistance to pathogenic bacteria (Iwai et al. 2002). Expression of an Arabidopsis gene for thionin, Thi2.1, is inducible by infection of phytopathogenic fungi, and overexpression of Thi2.1 enhanced resistance against infection by Fusarium oxysporum (Epple et al. 1997). Overexpression of Thi2.1 in transgenic tomato has also been shown to give enhanced resistance not only to bacterial wilt but also to fungal wilt (Chan et al. 2005). Results of these previous studies also support that expression of thionin peptide is a promising approach to enhance black rot resistance to commercial cultivars of sweet potato, and it may also gives even the enhanced resistance to bacterial pathogens.
The level of resistance of transgenic sweet potato with El2Ω:αHT or β-Amy:αHT to inoculation with C. fimbriata is much weaker than cultivar Tamayutaka, in particular in leaves (Figs. 4, 5). However, we might be able to increase the level of resistance by various modifications of the transgene to be expressed in sweet potato. In the present study, we used the entire coding sequence for αHT for expression in sweet potato. In the barley endosperm, αHT is first synthesized as a larger precursor protein with an amino-terminal signal peptide and the carboxy-terminal pro-peptide (Florack et al. 1994), yet αHT is stored in the cell probably associated externally with the protein body or endoplasmic reticulum (Ponz et al. 1983). Antimicrobial peptides such as thionin are likely to kill microbes directly by acting to their membranes, and infection of C. fimbriata to storage roots of sweet potato generally occurs through scars on the surface. By modifying the structure of protein to be expressed in sweet potato, we may be able to accumulate antimicrobial peptide in the extracellular apoplast. Modification of the structure of the peptide itself might also be useful to improve the growth inhibitory activity of the peptide against C. fimbriata and to enhance the stability of the peptide in planta. By changing the promoter for expression, we might be able to enhance the level of expression and select the site of expression in sweet potato, such as the wound scars on the surface.
In the future, the level and nature of αHT protein expressed in leaves and storage roots of transgenic sweet potato need to be determined. The sugar-inducible expression of αHT in leaves might offer a good system to evaluate the relationship between the expression of αHT and the level of resistance to C. fimbriata.
References
Akazawa T, Uritani I (1962) Pattern of carbohydrate breakdown in sweet potato roots infected with Ceratocystis fimbriata. Plant Physiol 37:662–670
Allefs SJHM, De Jong ER, Florack DEA, Hoogendoorn C, Stiekema WJ (1996) Erwinia soft rot resistance of potato cultivars expressing antimicrobial peptide tachyplesin I. Mol Breed 2:97–105
Bohlmann H, Apel K (1991) Thionins. Annu Rev Plant Physiol Plant Mol Biol 42:227–240
Carmona MJ, Molina A, Fernandez JA, Lopez-Fando JJ, Garcia-Olmedo F (1993) Expression of the alpha-thionin gene from barley in tobacco confers enhanced resistance to bacterial pathogens. Plant J 3:457–462
Chan YL, Prasad V, Sanjaya, Chen KH, Liu PC, Chan MT, Cheng CP (2005) Transgenic tomato plants expressing an Arabidopsis thionin (Thi2.1) driven by fruit-inactive promoter battle against phytopathogenic attack. Planta 221:386–393
Clark CA, La Bonte DR (1992) Disease factors in breeding and biotechnology for sweet potato. In: Hill WA, Bonsi CK, Loretan PA (eds) Sweet potato technology for the 21st century. Tuskegee University Press, Tuskegee, pp 484–494
Epple P, Apel K, Bohlmann H (1997) Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum. Plant Cell 9:509–520
Estruch JJ, Estruch JJ, Carozzi NB, Desai N, Duck NB, Warren GW, Koziel MG (1997) Transgenic plants: an emerging approach to pest control. Nat Biotechnol 15:137–141
Florack DE, Stiekema WJ (1994) Thionins: properties, possible biological roles and mechanisms of action. Plant Mol Biol 26:25–37
Florack DE, Visser B, Vries PMD, Van Vuurde JWL, Stiekema WJ (1993) Analysis of the toxicity of purothionins and hordothionins for plant pathogenic bacteria. Neth J P1ant Path 99:259–268
Florack DE, Dirkse WG, Visser B, Heidekamp F, Stiekema WJ (1994) Expression of biologically active hordothionins in tobacco. Effects of pre- and pro-sequences at the amino and carboxyl termini of the hordothionin precursor on mature protein expression and sorting. Plant Mol Biol 24:83–96
Gao AG, Hakimi SM, Mittanck CA, Wu Y, Woerner BM, Stark DM, Shah DM, Liang J, Rommens CM (2000) Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat Biotechnol 18:1307–1310
Holland PM, Abramson RD, Watson R, Gelfand DH (1991) Detection of specific polymerase chain-reaction product by utilizing the 5′3′ exonuclease activity of thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA 88:7276–7280
Iwai T, Kaku H, Honkura R, Nakamura S, Ochiai H, Sasaki T, Ohashi Y (2002) Enhanced resistance to seed-transmitted bacterial diseases in transgenic rice plants overproducing an oat cell-wall-bound thionin. Mol Plant Microbe Interact 15:515–521
James WC, Teng PS, Nutter FW (1990) Estimated losses of crops from pathogens. In: Pimentel D (ed) Handbook of pest management in agriculture, vol I, 2nd edn. CRC Press, Boca Raton, pp 15–52
Kahl G, Winter P (1995) Plant genetic engineering for crop improvement. World J Microbiol Biotechnol 11:449–460
Komarek M, Cadkova E, Chrastny V, Bordas F, Bollinger JC (2010) Contamination of vineyard soils with fungicides: a review of environmental and toxicological aspects. Environ Int 36:138–151
Kummerer K (2004) Resistance in the environment. J Antimicrob Chemother 54:311–320
Linsmaier EM, Skoog F (1965) Organic growth factor requirement of tobacco tissue culture. Physiol Plant 18:100–127
Maeo K, Tomiya T, Hayashi K, Akaike M, Morikami A, Ishiguro S, Nakamura K (2001) Sugar-responsible elements in the promoter of a gene for β-amylase of sweet potato. Plant Mol Biol 46:627–637
Matsuzaki K (1999) Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta 1462:1–10
Mita S, Suzuki-Fujii K, Nakamura K (1995) Sugar-inducible expression of a gene for β-amylase in Arabidopsis thaliana. Plant Physiol 107:895–904
Mitsuhara I, Ugaki M, Hirochika H, Ohshima M, Murakami T, Gotoh Y, Katayose Y, Nakamura S, Honkura R, Nishimiya S, Ueno K, Mochizuki A, Tanimoto H, Tsugawa H, Otsuki Y, Ohashi Y (1996) Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol 37:49–59
Moran R, Garcia R, Lopez A, Zaldua Z, Mena J, Garcia M, Armas R, Somonte D, Rodriguez J, Gomez M, Pimentel E (1998) Transgenic sweet potato plants carrying the delta-endotoxin gene from Bacillus thuringiensis var. tenebrionis. Plant Sci 139:175–184
Nakamura K, Ohto MA, Yoshida N (1991) Sucrose-induced accumulation of β-amylase occurs concomitant with the accumulation of starch and sporamin in leaf-petiole cuttings of sweet potato. Plant Physiol 96:902–909
Ohta S, Mita S, Hattori T, Nakamura K (1990) Construction and expression in tobacco of a β-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31:805–813
Osusky M, Zhou G, Osuska L, Hancock RE, Kay WW, Misra S (2000) Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nat Biotechnol 18:1162–1166
Otani M, Shimada T, Kimura T, Saito A (1998) Transgenic plant production from embryogenic callus of sweet potato (Ipomoea batatas (L.) Lam.) using Agrobacterium tumefaciens. Plant Biotechnol 15:11–16
Ovard SV, Enright FM (2006) Expression of the antimicrobial peptides in plants to control phytopathogenic bacteria and fungi. Plant Cell Rep 25:561–572
Peters BM, Shirtliff ME, Jabra-Rizk MA (2010) Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog 6:e1001067
Ponti D, Mangoni ML, Mignogna G, Simmaco M, Barra D (2003) An amphibian antimicrobial peptide variant expressed in Nicotiana tabacum confers resistance to phytopathogens. Biochem J 370:121–127
Ponz F, Paz-Ares J, Hernandez-Lucas C, Carbonero P, Garcia-Olmedo F (1983) Synthesis and processing of thionin precursors in developing endosperm from barley (Hordeum vulgare L.). EMBO J 2:1035–1040
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462:55–70
Stec B (2006) Plant thionins; the structural perspective. Cell Mol Life Sci 63:1370–1385
Takeda S, Mano S, Ohto M, Nakamura K (1994) Inhibitors of protein phosphatases 1 and 2A block the sugar-inducible gene expression in plants. Plant Physiol 106:567–574
Uritani I, Stahmann MA (1961) Changes in nitrogen metabolism in sweet potato with black rot. Plant Physiol 36:770–782
Yang L, Weiss TM, Lehrer RI, Huang HW (2000) Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys J 79:2002–2009
Yoshida N, Hayashi K, Nakamura K (1992) A nuclear gene encoding β-amylase of sweet potato. Gene 120:255–259
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Acknowledgments
This work was financially supported in part by a grant from the New Energy and Industrial Technology Development Organization, Japan (NEDO), and the Ministry of Economy, Trade and Industry, Japan (METI).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Sato.
This paper is dedicated to Professor Emeritus Ikuzo Uritani of Nagoya University, who passed away on 25th September 2010 at the age of 91.
Rights and permissions
About this article
Cite this article
Muramoto, N., Tanaka, T., Shimamura, T. et al. Transgenic sweet potato expressing thionin from barley gives resistance to black rot disease caused by Ceratocystis fimbriata in leaves and storage roots. Plant Cell Rep 31, 987–997 (2012). https://doi.org/10.1007/s00299-011-1217-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1217-5