Abstract
Imazethapyr is an herbicide that is used in a variety of crops worldwide, including soybean and corn. The aim of the present study was to evaluate the biomarkers responses of adult Leptodactylus latinasus exposed to the formulation Pivot® H (10.59% imazethapyr) in the laboratory at concentrations and under conditions that simulate two potential field exposure scenarios: an immersion in field runoff (Scenario 1: 10 mg/L) and a direct exposure to the droplets emitted by spray noozles (Scenario 2: 1000 mg/L). In both scenarios, the experimental procedure involved completely immersing the frogs over a period of 15 s. Different endpoints were evaluated at several ecotoxicological levels 48 and 96 h after the herbicide exposure. These included individual (biometric indices and behavior alterations), histological (liver pigments and lesions), biochemical (catalase, glutathione system and cholinesterase activities) and genotoxic effects (micronuclei induction and nuclear abnormalities). Forty-eight hours after imazethapyr exposure, frogs submitted to Scenario 1 presented an inhibition of liver glutathione-S-transferase activity, whereas histological alterations and increased hepatic cholinesterase levels were observed in frogs exposed under Scenario 2. Ninety-six hours after exposure to the imazethapyr formulation, frogs from the Scenario 1 treatment presented a decrease in liver melanin and hemosiderin, increased hepatic catalase activity and micronuclei induction. For their part, frogs exposed to Scenario 2 presented a decrease in the hepatosomatic index, an increase in liver alterations, melanin reduction and micronuclei induction. The multivariate analysis enables correlations to be made between biomarkers of different organizational level in exposed anurans. Our result indicates that real exposure to imazethapyr formulations under field conditions may pose a risk to Leptodactylus latinasus populations living in the agroecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticide applications are considered critical in order to maintain high agricultural output. However, they can also cause adverse effects in nontarget organisms, either through the same mechanisms they activate in pests, or through completely different modes of action (Howarth 2000; Köhler and Triebskorn 2013). The imidazolinones are widely employed broad-spectrum herbicides that are used for selective pre- or post-emergence weed control in a variety of crops. The mode of action for these herbicides in controlling weeds is through an inhibition of the acetolactate synthase (ALS), an enzyme involved in the synthesis of branched-chain amino acids (Tan et al. 2005; Kawai et al. 2007; Lin et al. 2007).
Imazethapyr or IMZT (Cas No. 81335-77-5) is an imidazolinone herbicide that is widely used in genetically modified soybean and corn crops. The recommended application rate of IMZT ranges between 0.8 and 1L/ha for both aerial and terrestrial applications, respectively (CASAFE 2013). IMZT is classified as a slightly toxic (Class III) by the USEPA (1989), and as a dangerous compound for the environment by the European Union (EU) (Kegley et al. 2020). Furthermore, they affirm that IMZT is an unsafe pesticide for the environment and is associated with to adverse effect in humans such as irritant eye irritations, skin lessons lesions and respiratory tract difficulties (Kegley et al. 2020).
Relatively little is known regarding IMZT toxicity to nontarget organisms. Low acute toxicity has been reported for the green alga Raphidocelis subcapitata (Sphaeropleales) and the aquatic invertebrate Daphnia magna (Cladocera) (Kegley et al. 2020), whereas high acute toxicity was reported in the gibbous duckweed Lemna gibba (Alismatales) (Magdaleno et al. 2015; Reimche et al. 2015). The sensitivity of terrestrial invertebrates to IMZT is variable being low in earthworms but high in honey bees (Kegley et al. 2020). Previous reports show that IMZT is not acutely toxic to fish such as the channel catfish Ictalurus punctatus (Siluriformes), the rainbow trout Oncorhynchus mykiss (Salmoniformes) and the bluegill Lepomis macrochirus (Perciformes) (Kegley et al. 2020). An alteration of oxidative stress-related hepatic enzymes activity was reported in the common carp Cyprinus carpio (Cypriniformes) after an exposure to 0.0148 mg/L IMZT formulations (Moraes et al., 2011) In addition, acetylcholinesterase (AChE) was inhibited after exposure to a commercial formulation of IMZT in the Mozambique tilapia Oreochromis mossambicus (Cichliformes), as reported by Pasha and Singh (2005) and Pasha (2013). Recently, our group demonstrated toxicological effects at the genetic, biochemical and individual level in two local amphibian species exposed to IMZT formulation in their larval phase: Boana pulchella and Leptodactylus latinasus (Pérez-Iglesias et al. 2015, 2017, 2018, 2020). More specifically, several biomarkers were altered such as primary DNA lesions and micronuclei (MNs) frequency and glutathione-S-transferase (GST) activity. Morphological abnormalities and an alteration of swimming performance were also observed. These studies demonstrate that IMZT formulation is a stress factor in larval amphibians since the herbicide alters the tadpole homeostasis and creates a state of oxidative and physiological stress (Pérez-Iglesias et al. 2015, 2017, 2018, 2020).
Amphibians have been widely used as bioindicators of environmental quality due to their shell-less eggs, exposed embryogenesis, free-living aquatic larvae, permeable skin, narrow home range, key position in trophic webs and their dependence upon both the aquatic and terrestrial environment (Blaustein and Wake 1990; Stebbins and Cohen 1995; Blaustein et al. 2011). On the other hand, increased pesticide use has been claimed as one of the main causes responsible for the recent declines in amphibians populations reported worldwide (Blaustein and Wake 1990; Houlahan et al. 2000; Kiesecker et al. 2001; Beebee and Griffiths 2005; Mann et al. 2009). Several studies have demonstrated that amphibians are negatively affected at the physiological, histological, biochemical and cytogenetic levels when living in agroecosystems where pesticides are applied (Mann et al. 2009; Shutler and Marcogliese 2011; Hegde and Krishnamurthy 2014). Particularly in Argentina, several authors have reported evidences of alterations in anurans inhabiting the agricultural Pampa region, where large amounts of pesticides were applied (Cabagna et al. 2006; Attademo et al. 2007, 2011, 2014; Peltzer et al. 2008; Brodeur et al. 2011, 2012; Agostini et al. 2013; Sanchez et al. 2013; Babini et al. 2015; Josende et al. 2015; Guerra and Aráoz 2016). In this sense, amphibians are an important group of nontarget organisms in Argentinean agricultural landscapes that can be exposed directly or residually to a variety of pesticides through either plant material, soil, water or contaminated prey items (Mann et al. 2009; Brodeur et al. 2011, 2012; Van Meter et al. 2014, 2019; Suárez et al. 2016). Dermal uptake of pesticides is a significant exposure pathway in terrestrial amphibians (Brühl et al. 2013; Van Meter et al. 2014, 2015, 2018, 2019), and dermal absorption of environmental pollutants has been shown to be faster in amphibians than in mammals (Quaranta et al. 2009).
Adult amphibians are known to move across terrestrial landscapes in search of breeding ponds and/or overwintering habitats (Brühl et al. 2013; Van Meter et al. 2018, 2019). In agricultural areas, this situation means that terrestrial amphibians are at risk of dermal exposure to pesticides by contact with contaminated soil, water or vegetation (Van Meter et al. 2018, 2019). Nevertheless, few studies have previously evaluated the effects of dermal exposure to pesticides in adult anurans (Brühl et al. 2013; Van Meter et al. 2014, 2015, 2018, 2019).
In this context, the objective of the present study was to evaluate biomarker responses at various organizational levels in adult anurans exposed in laboratory conditions to an IMZT-based herbicide formulation under two scenarios illustrative of real-life field exposures. The terrestrial, fossorial L. latinasus was selected as the test organism because it is easy to collect and breed in captivity (Pérez-Iglesias et al. 2016) and because its populations are not at risk as the species is classified as least concern (Vaira et al. 2012; IUCN 2020). In addition, L. latinasus has previously been recommended for biomonitoring pesticide contamination in the Pampa region (Brodeur and Vera Candioti 2017), being the most frequent species in Neotropical agroecosystems and in undisturbed areas (Agostini et al. 2016; Medina et al. 2016; Suárez et al. 2016), and has been previously employed in a number of ecotoxicological studies both in the field and in the laboratory (Brodeur et al. 2011; Guerra and Aráoz 2016; Pérez-Iglesias et al. 2016, 2020). However, to our knowledge, there are no studies reported in the literature on the effects of active ingredient or formulation of IMZT in adult frogs evaluated using multiple biomarkers.
Materials and Methods
Chemical Reagents and Determinations
All chemicals, reactive and solvents were of analytical grade and were purchased from Sigma Chemical Co. (St. Louis, MO). Pivot® H (10.59% w/v of IMZT, CAS 081335-77-5) was obtained from BASF Argentina S.A. All test solutions were prepared immediately before use. According to the concentration of the active ingredient of IMZT in the Pivot® H safety sheet (10.59 g in 100 mL), the corresponding calculations were made to obtain a stable stock solution of 1 g of IMZT per liter of dechlorinated tap water (USEPA 1975). Then, from stable stock solution, the corresponding dilutions were made in dechlorinated tap water to obtain the concentrations of the exposure scenarios.
IMZT concentrations in bioassay solutions were analyzed by equipment in the CIM Institute (National University of La Plata, La Plata, Argentina) according to the procedures described in Report 01–4134 of the US Geological Survey (Furlong 2001). Briefly, IMZT active ingredient was identified and quantified by instrumental analysis with a Waters Acquity Ultra Performance Liquid Chromatography (UPLC) system coupled to a Quattro Premier XE tandem quadrupole mass spectrometer (MS/MS), with an electrospray ionization (ESI) source. For the chromatographic separation of the herbicide, a C18 column (1.7 μm, 100 × 2.1 mm) was used, at a flow of 0.3 mL/min with acetonitrile/methanol-nanopure water gradient (previously conditioned with formic acid). The identification and quantification of IMZT were performed by injection a volume of 2 μL of the standard solutions without additional extraction system. The software MassLynx v4.1 and the TargetLynx package were used for data analysis. Quantification was done by means of an external calibration curve and through a measurement of the area under the chromatographic peaks (using as a reference a 1 ppm solution of technical grade of IMZT to 95%), after considering the dilution effected with each matrices and the recovery of the compound. The detection limit of IMZT was 0.5 μg/L.
Test Organisms and Specimens Recollection
Leptodactylus latinasus (Jimenez de la Espada, 1875) (Anura, Leptodactylidae) is a small sized terrestrial frog species (38–40 mm) that hides under rocks or debris. Specimens feed on the insects encountered on the soil among the vegetation (Cei 1980). Males of this species build small caves in the mud where they call females and eggs are laid in a foam nest where tadpoles begin their development and emerge when inundated by rain or overflowing brooks (Cei 1980; Brodeur et al. 2011). This species was chosen due to its particular characteristic of reproducing and foraging within soybean, wheat and maize crops, where it builds caves between the plants, and in small depressions where puddles form after rainfalls.
Individuals of L. latinasus (n = 60) were obtained at night during the breeding season from a typical unpolluted field from the Pampa region where no large-scale agricultural activities occurred (La Plata, Buenos Aires, Argentina 35° 00′ 02″ S, 57° 52′ 15″ W). All frog collections were approved by the Flora and Fauna Directorate from the Buenos Aires Province (Permit # 22500–22339/13). Adult males of L. latinasus were transported to the laboratory within a few hours from capture. They were then weighed (average, 3.52 ± 0.65 g) and measured (snout-vent length average, 31.27 ± 2.60 mm). Afterward, specimens were acclimated during 7 days in 2800 cm3 glass containers containing water at the bottom. During this period, photoperiod was maintained stable at 16:8 h light/dark and temperature at 25.0 ± 1 °C. No food was supplied.
Experimental Design and Exposure Protocol
The experimental design is illustrated in Fig. 1. Briefly, before the start of bioassay, frogs were placed in a clean glass 20 L aquarium for a 24-h period of dehydration. This dehydration period was intended to facilitate the movement of water and xenobiotics throughout the anuran dermis upon exposure, because rehydration starts in the bioassay once exposure begins, according to the suggestions of Van Meter et al. (2018). Experiments were carried out according to the exposure protocol previously adopted by Wang and Jia (2009) and Van Meter et al. (2014, 2015, 2018, 2019) following two plausible real-life scenarios: (1) an immersion in field runoff (S1: 10 mg/L) and (2) a direct exposure to the droplets emitted by spray nozzles (S2: 1000 mg/L). For each scenario, ten adult frogs per treatment were exposed to an acute pulse of Pivot® H, by immersing the entire organisms in the each test solution (S1: 10 mg/L and S2: 1000 mg/L) during 15 s. Test solutions of IMZT were prepared according to procedures proposed by USEPA (1975). Also, a negative control group consisted of 10 frogs immersed in dechlorinated tap water that were run in parallel to herbicide-exposed specimens (Van Meter et al. 2015; 2018). Immediately after the acute pulse, each frog was placed separately and individually in a 3-L glass flasks containing 200 mL base of humidified fertile soil. Frogs were not fed throughout the experiment and, after the exposure, they were rehydrated by spraying dechlorinated tap water every 24 h to avoid frog death due to drying (Van Meter et al. 2018). Evaluation of the proposed endpoints was performed 48 and 96 h after the acute pulse of exposure based on previous studies by our group which highlight the importance of analyzing these two time points (Franco-Belussi et al. 2013; Pérez-Iglesias et al. 2016; Lajmanovich et al. 2018). On each sampling time, frogs were anesthetized by immersion in benzocaine (2%), placed on ice and dissected according to guidelines detailed in Garber et al. (2011), CONICET (2005) and INTA (2008). The liver was weighed using a scale with a precision of 0.001 g. All procedures involving animals were of the institutional ethical committee from the National University of La Plata (permit #11/N619).
Evaluations of Endpoints
To assess the effects induced by IMZT under the two exposure scenarios, several endpoints were considered at different ecotoxicological levels, i.e., individual (e.g., body condition; hepatosomatic index, HSI and behavior alterations), histological (e.g., melanin; hemosiderin; hyper vascularization or congestion, HV; enlargement of sinusoids, ES; mononuclear cell infiltration, MI; vacuolization, V; and necrosis of hepatocytes), biochemical (e.g., acetyl cholinesterase, AChE; catalase, CAT; glutathione reduced, GSH; glutathione-S-transferase, GST) and cytogenetic (e.g., micronuclei, MNs; notched nuclei, NNs; blebbed nuclei, BLs; erythroplastids, EPs).
Individual Endpoints
Body condition was assessed using a method described by Brodeur et al. (2011, 2020). This method consists in examining the residuals from a regression of body mass against snout-vent length where the regression line obtained establishes the average body weight for a given length. Then, individuals with positive residuals are considered to be in a good condition, whereas individuals with negative residuals are regarded as having low energy reserves (Schulte-Hostedde et al. 2005; Brodeur et al. 2011, 2020). The hepatosomatic index (HSI) was calculated as the ratio of liver weight on total body weight. Finally, to evaluate behavior alterations, such as normal mobility, jump response to a stimulus and orientation position of the frogs, from each treatment were registered during 1 min in a polypropylene chamber (30 L) and, posteriorly, frogs were individually placed into a pool to examine swimming activity for another minute. These endpoints were recorded after 48 and 96 h of the acute pulse.
Histological Endpoints
Histological techniques were conducted as previously described by Pérez-Iglesias et al. (2016). Briefly, a half of the liver was extracted and used for biochemical procedures (“Liver tissue alterations” section), while the other portion was placed in Karnovsky fixative solution (0.1 M phosphate buffer, 5% paraformaldehyde and 2.5% glutaraldehyde) at pH 7.2 for 24 h (4 °C) for histological procedures. Then, each liver sample was rinsed with distilled water, placed in an alcohol series to dehydrate and embedded in historesin. Liver preparations were obtained by cutting randomly selected 2-μm-thick sections (~ 60 sections of each liver) using a Leica RM 2265 microtome. When mounting the slides, approximately 20 liver sections were selected, for a total of two slides per frog. One slide was stained with hematoxylin–eosin to evaluate liver alterations and melanin quantification, while the second slide was incubated in acidic ferrocyanide for 15 min and then stained with neutral red and eosin solutions for hemosiderin detection. Histological sections were observed under a Leica DM4000 B microscope equipped with an image capture system (Leica DFC 280).
Liver Tissue Alterations
Ten histological sections per sample were examined for liver tissue alterations according to Çakici (2015). The following elements were evaluated: hyper vascularization or congestion (HV), enlargement of sinusoids (ES), mononuclear cell infiltration (MI), vacuolization (V) and necrosis of hepatocytes.
Pigmentation of Liver Melanomacrophages (MMC)
For pigmentation evaluation, each liver sample (n = 25 micrographs) was analyzed using Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD). Based on the different staining intensity, the area occupied by melanin and hemosiderin pigment was quantified in the MMC (Pérez-Iglesias et al. 2016).
Biochemical Endpoints
All procedures were performed according to Brodeur et al. (2017). Livers were homogenized in ice-cold 50 mM tris buffer (1 mM EDTA acid, 0.25 M of sucrose, pH 7.4) with a Teflon-glass Potter–Elvehjem homogenizer. Then, the homogenates were centrifuged at 4 °C (10,000 × g, 10 min) to collect the supernatant, while nuclei and cell debris was discarded. One portion of the supernatant was used for direct determination of hepatic contents of GSH and the rest portion of supernatant was stored at freezer (220 °C) until enzyme activities determination. Protein concentrations were determined by the method of Lowry (1951) using bovine serum albumin as a standard. All enzymatic reactions and protein calibration curve were performed on microplates. Absorbance was read using a microplate reader (SPECTROstar Nano, BMG Labtech, Ortenberg, Germany).
CAT Activity Determination
The CAT activity in the liver of L. latinasus was determined by measuring the kinetic absorbance in microplates making reaction mixture of 300 µL of PBS (100 mM, pH 7), 10 µL of H2O2 (dilution 0,5% v/v, peroxide hydrogen 99% in distilled water) and 10 µL of sample (dilution, 1:25 of pure supernatant:PBS). The change in the absorbance (240 nm) resulting from H2O2 consumption was recorded during 2 min (37 °C), using a molar extinction coefficient of 43.6 M−1 cm−1 (Brodeur et al. 2011, 2017).
GSH Determination
Hepatic GSH contents were determined colorimetrically by quantifying the amount of 2-nitro-5-thiobenzoic acid resulting from the interaction of GSH with dithiobis-2-nitrobenzoic acid (DTNB). Three 60 µL aliquots of supernatant per sample were deproteinized separately by mixing with 120 µL of acetonitrile. First of the three deproteinized aliquots served as a blank and mixed with 10 µL of N-ethylmaleimide, 110 µL of acetonitrile and 60 µl of liver sample. Microtubes with solutions were centrifuged at 4 °C (10,000 × g, 10 min), and the supernatant was used for GSH determination. The reaction for GSH determination consisted of 200 µL of saline buffer or PBS (50 mM, pH 7.5), 10 µL of DTNB (0.5 mM) and 160 µL of supernatant with sample. Determination of GSH was made by using the average absorbance (412 nm) of replicates, after subtracting the absorbance of the blank and estimating the standard curve (Brodeur et al. 2011).
GST Activity Determination
The GST activity in the liver of L. latinasus was measured using 1-chloro-2, 4- dinitrobenzene (CDNB) as substrate. Determinations were performed in a reaction mixture containing 300 μL GST (30% m/v of GSH in PBS, pH 7), 10 µL CDNB (0.1 M) and 10 µL of sample (dilution, 1:25 of pure supernatant:PBS). The colorimetric reaction absorbance (340 nm) was recorded during 2 min (37 °C) and GST activity was calculated with molar extinction coefficient of 9.6 mM−1 cm−1 (Brodeur et al. 2011, 2017).
AChE Activity Determination
Activity of hepatic acetyl cholinesterase (AChE) was determined by the Ellman method (1961). The reaction mixture consisted of 200 µL of PBS (100 mM, pH 8), 10 μL of acetylcholine (1 mM), 10 μL of DTNB (0.5 mM) and 50 µL of sample (previously diluted 1/5; 200 μL homogenized sample in 800 μL of PBS). The kinetic absorbance (412 nm) was recorded during 3 min (37 °C) and AChE activity was calculated using a molar extinction coefficient of 14,150 M−1 cm1.
Cytogenetic Level. MNs and Nuclear Abnormalities on Erythrocytes
MN assay and blood analysis was conducted in accordance with original protocol (Fenech 2007) with minor modifications for this species (Pérez-Iglesias et al. 2016). Blood smears on microscope slides were confectioned by triplicate from each herbicide-exposed and non-exposed frogs stained during 12 min with 5% of Giemsa solution. MNs frequency was calculated in peripheral mature erythrocytes after acute pulse exposure in both scenarios. MNs were blind-scored from 1000 erythrocytes from each blood frog sample (× 1000 magnification). Also, the presence of other nuclear abnormalities in mature erythrocytes was evaluated for this species according our previous procedures (Pérez-Iglesias et al. 2016, 2020). The following frequency of nuclear abnormalities was considered: notched nuclei (NNs), i.e., nuclei with vacuoles and appreciable depth into a nucleus without containing nuclear material; blebbed nuclei (BLs), i.e., cells with one nucleus presenting a relatively small evagination of the nuclear membrane which contains euchromatin; and erythroplastids (EPs), i.e., anucleated forms of circulating red blood cells. MNs and nuclear abnormalities frequencies are expressed as total number of alterations per 1000 cells and the examination criteria for MNs acceptance were determined following previously reports (Vera-Candioti et al. 2010).
Statistical Analysis
ANOVA one-way (analysis of variance) with post hoc Dunnett test was performed to estimate the IMZT formulation-induced effects on body condition, HSI, liver tissue lesions, area occupied by melanin and hemosiderin pigment, liver enzymes activities (GST, CAT and AChE), hepatic GSH contents and frequencies of MNs and others nuclear abnormalities (response variables), at both times evaluated after the exposure (Zar 2010). ANOVA assumptions were corroborated with Bartlett test for the homogeneity of variances and χ2 test for normality. Then, data were logarithmic-transformed to meet assumptions, in those cases that did not meet the assumptions of normality, a Kruskal–Wallis test was performed (Zar 2010).
A principal component analysis (PCA) was performed considering each exposure scenario as a grouping variable to improve interpretation of the results and allows to obtain integral information of the biomarkers responses (or holistic vision). The integration of all biomarkers was made by using components principal regression analysis (Jackson 1993; Jolliffe and Cadima 2016). In addition, the relationship biomarkers and IMZT were evaluated with a correlation matrix (Pearson product moment correlation coefficient) by using simple linear regression. Tests of significance of the regression and correlation coefficients were performed following Zar (2010). The level of significance chosen was α = 0.05 for all tests, unless indicated otherwise. Analyses were performed using the R software 6 v. 2.11.1 (R Core Team 2010).
Results
Chemical Determinations
Analytic determinations of IMZT are presented in Table 1. Particularly, the values corresponding to the real concentrations were 10.86 IMZT mg/L ± 1.87 (SD) for scenario 1 (nominal 10 mg/L), and 1043.0 IMZT mg/L ± 100.05 (SD) for scenario 2 (nominal 1000 mg/L).
Response of Endpoints
Individual Endpoints
Evaluation of body condition and behavior alterations after exposure to the herbicide IMZT formulation did not reveal any differences between frogs exposed to S1 and S2 with respect to the control group at both times evaluated (p > 0.05). Nevertheless, it was observed that frogs exposed to IMZT under S1 and S2 scenarios exhibited a decrease in HSI 96 h after the exposure (p < 0.05), but not at 48 h (p > 0.05) (Table 2).
Histological Endpoints
Table 2 summarizes the results of all histological endpoints examined and the type of response observed for each of the two exposure scenarios. Liver tissue alteration was evident only HV and ES at both 48 and 96 h (Fig. 2). Statistical analysis performed revealed that these alterations occurred both at 48 and 96 h, but only in S2 (p < 0.05). Regarding the analysis of pigments on liver MMC, a decrease in melanin and hemosiderin was observed at 96 h (Table 2). Specifically, the area of melanin and hemosiderin in liver MMC did not show any significant differences when comparing frogs treated with IMZT formulation respect to control group (p > 0.05) after 48 h. However, a significant decrease in the melanin area was detected in both scenarios after 96 h (p < 0.05). On the other hand, a significant decrease in the pigmented area of hemosiderin in liver MMC was observed only in frogs submitted S1 (p < 0.05) but not in frogs of the S2 (p > 0.05) after 96 h.
Melanomacrophages (MMC) and liver sections in adults of Leptodactylus latinasus from treated groups at 48 (a) and 96 h (c), and non-exposed frogs to imazethapyr formulation at 48 (b) and 96 h (d). Arrows show some MMC present in the liver; double arrows show sections with enlargement of sinusoids (ES); asterisks show hyper vascularization (HV)
Biochemical Endpoints
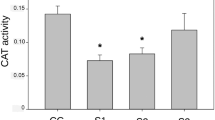
Table 2 and Fig. 3 summarize the results of biochemical endpoints analyzed and the type of response observed for each of the two exposure scenarios 48 and 96 after the exposure. IMZT formulation exposure did not have any effect on GSH activity (p > 0.05) 48 h after the exposure. However, at this time point, a significant decrease in the activity of GST was observed in frogs submitted to S1 (p < 0.05), whereas a significant increase in the activity of AChE (p < 0.05) was observed in frogs exposed to S2. No significant difference was observed in CAT activity when comparing both scenarios with respect to the control group (p > 0.05) 48 h after the exposure. Finally, 96 h after the exposure to IMZT formulation, the activity of GSH, GST and AChE was back to control levels in frogs exposed to both scenarios. Only the activity of CAT was modified at this time point, a significant increase in hepatic CAT activity levels being observed in frogs treated with S1 (p < 0.01).
Cytogenetic Level Endpoints
At the cellular level, a significant increase in the frequency of MNs was observed in the group treated with S1 (p < 0.01) and S2 (p < 0.05) when compared to the control group, but only at 96 h (Table 3). On the other hand, significant differences by IMZT-treatment were not detected with respect to the control group when the frequency of nuclear abnormalities in erythrocytes was compared to both exposure scenarios and both times points evaluated (p > 0.05) (Table 2).
Integration of Biomarkers by PCA Analysis
First, an exploratory PCA was carried out with all the evaluated endpoints that were standardized prior to the analysis. In a second stage, only those variables that showed significant differences (p < 0.05) with respect to the control groups after the ANOVA and Dunnett test and were selected and considered biomarkers.
The reduction of dimensionalities by means of the PCA revealed an evident differentiation between the IMZT-treated groups with respect to the control, which is explained by the correlation and integral response between the biomarkers at 48 and 96 h. In general, when analyzing the response of the 4 biomarkers (HV, ES, GST and AChE) at different levels of organization (histological and biochemical) and their correlation by the PCA at 48 h, the analysis showed that the biomarkers are organized into 3 main components that explain 86.71% (PC1 = 47.58% and PC2 = 39.13%) of the variability of the data. In addition to this, according to the response expressed by the biomarkers here evaluated, it was possible to determine an increasing gradient of concentrations along the acute pulse scenarios (from S1 to S2) with respect to the negative control (Fig. 4a). Specifically, the separation of the control group from S1 and S2 was due to a greater extent by the response of the biochemical biomarkers that contribute to the variability of PC1 and the histological biomarkers that contributed variability to the axis of PC2 (Fig. 4a). From these analyses, it was also possible to show significant positive correlations between histological biomarkers with each other (r = 0.52; p < 0.05) and between both biochemical biomarkers, AChE and GST (r = 0.88; p < 0.05). On the other hand, the analyses revealed that, at 96 h, there is a significant differentiation in the response of the biomarkers that explain the PC1 since they allow the negative control group to be separated from the S2 (p < 0.05), as well as the biomarkers that contribute to the PC2 axis that allow the control group to be separated from S1 (p < 0.05) and S2 (p < 0.001). Also, the correlation obtained by the PCA of the 7 biomarkers (HSI, ES, HV, melanin, hemosiderin, CAT and MNs) 96 h after the acute pulse of IMZT formulation at the different levels of organization (individual, histological, biochemical and cytogenetic), showed that these are organized into 3 main components that explain 63.77% (PC1 = 29.55%, PC2 = 20.29% and PC3 = 13.93%) of the variability of data. From the response biomarkers shown, it was possible to identify two large subgroups characterized by the negative control and the worst scenario (S2) (Fig. 4b). Here, the separation of the control group from S2 was largely due to the response of the MNs, melanin of the MMC, ES and HV (which separate the subgroups on the axis of PC1) and from HSI, CAT and hemosiderin in the MMC (which separate the subgroups on the axis of PC2) (Fig. 4b). From these analyses, it was also possible to show significant positive correlations between histological biomarkers such as ES and HV (r = 0.39; p < 0.05) as well as significant negative correlations between MNs and ES (r = -0.40; p < 0.05).
Biplot representing the responses of each biomarker evaluated in Leptodactylus latinasus adults exposed to the simulating scenarios of exposure to the IMZT-based commercial herbicide Pivot® H after 48 (a) and 96 h (b). The wide gray white arrow shows the increasing concentration gradient for control group and each scenario of IMZT exposure such as control group (squares), runoff simulation or 10 mg IMZT/L (triangles), and direct spraying application or 1000 mg/L (circles) obtained by the correlation between the biomarkers. Circles showed groupings of each treatment on an IMZT gradient. The length of the red arrow indicates the magnitude of the response of the different biomarkers. AChE, acetylcholinesterase; CAT, catalase; ES, enlargement of sinusoids; GST, glutathione-S-transferase; HSI, hepatosomatic index; HV, hyper vascularization; MNs, micronucleus
Discussion
Experiments simulating real-life exposure scenarios realized with adults of the frog L. latinasus demonstrated the potential adverse effect of IMZT formulation on the physiology of a Neotropical anuran. Particularly, considering scenarios of real field exposures, a few studies have been conducted worldwide to evaluate the adverse effects separately of environmental contaminants in juvenile and adult anurans from regions such as Africa (Ezemonye and Tongo 2010), Europe and Asia (Leiva-Presa and Munro Jenssen 2006; Quaranta et al. 2009; Brühl et al. 2013), and North America (Edge et al. 2011; Selcer and Verbanic 2014; Van Meter et al. 2014, 2015, 2018; Glinski et al. 2018; Abercrombie et al. 2020). The same situation was observed in studies carried out on Neotropical anurans (Castillo et al. 1991, 2005; Lajmanovich et al. 2018). In this sense, the current study is the first to evaluate and integrate the effects of a formulation of a herbicide (IMZT) at multiple biological levels in an adult Neotropical frog simulating real scenarios of exposure.
Overall, few impacts were observed at the individual level, no effect was observed on body condition and a decrease in HSI was observed only following exposure to S2 and only after 96 h. Overall, the lack of effects observed at the organismal level may be due to the short duration of the exposure. Indeed, it is probably necessary for the exposure to be longer-lasting and/or recurring for clear effects to be seen on body condition and HSI, as sometimes reported in field studies (Brodeur et al. 2011).
The histological effects of this study demonstrate for the first time that IMZT formulation produces liver lesions such as HV and AS and affects hepatic MMC in adult frogs. Specifically, IMZT in his commercial formulation induced alterations in the area occupied by the pigments melanin and hemosiderin in the liver of L. latinasus, corroborating the utility of using these pigments in MMC as histological biomarkers (Pérez-Iglesias et al. 2016). It is important to mention that other authors have highlighted the importance of determining the effects of environmental stressors in detoxification organs due to the maintenance of the homeostasis in aquatic vertebrates (McDiarmid and Altig 1999; Păunescu et al. 2010; Çakici 2015). In this study, we observed an increase in the frequency of liver alterations (HV and ES) in both scenarios. According to Gürkan and Hayretdağ (2012), this situation may be an indication of irreversible damage that consequently ends in hepatocellular degeneration. Several studies carried out in anurans support this idea since they have shown that high concentrations of pesticides cause damage to liver structures that lead to an increase in the rate of hepatic cell apoptosis and it has even been pointed out that principles of carcinogenesis may be associated with processes such as hypervascularization (Păunescu et al. 2010; Zaya et al. 2011; Çakici 2015; Pérez-Iglesias et al. 2016).
In addition, the analysis of the histological biomarkers in adults showed alterations in the pigments of the hepatic MMC by exposure to Pivot® H in S1 and S2 after 96 h exposure. These results are consistent with previous studies carried out in anurans that demonstrate the alteration produced in the hepatic pigmentary system (MMC) by environmental stressors (Păunescu et al. 2010; Zaya et al. 2011; Gürkan and Hayretdağ 2012; Franco-Belussi et al. 2013; Çakici 2015; Pérez-Iglesias et al. 2016; De Oliveira et al. 2017; Fanali et al. 2018). In general, MMC and its associated pigments such as melanin and hemosiderin are important in catabolic processes as an immune response, as detoxifiers and as protectors against ROS (Fenoglio et al. 2005; Franco-Belussi et al. 2013). However, an increase in MMC may be due to increased oxidative damage that occurs in cell membranes, increased cell death processes by the accumulation of lipofuscin within MMC (Couillard and Hodson 1996; Fenoglio et al. 2005; Franco-Belussi et al. 2013) or due to the ability of this cellular defense system to eliminate waste due to an increase in phagocytic activity (Agius and Roberts 2003; Franco-Belussi et al. 2013). Moreover, the results obtained in this work show that at 96 h there is a significant decrease in the area of the pigments melanin and hemosiderin in the hepatic MMC. According to previous studies, MMC pigments are physiological and cytologic biomarkers of the health status of amphibians and their decrease is related with a reduction in phagocytic and protective activity against oxidizing agents of MMC in the liver (Fenoglio et al. 2005; Pérez-Iglesias et al. 2016; Fanali et al. 2018; Franco-Belussi et al. 2020). Focusing on the response of the catabolic pigment hemosiderin in S1, this work again reports similar results to those previously described in this species after herbicide exposure (Pérez-Iglesias et al. 2016). The decrease in hemosiderin pigment, due to the herbicide exposure, may be related to more severe damages that involve alterations in the hepatic catabolic processes inhibiting the recycling of ferric compounds related to the erythrocatetic function (Agius and Roberts 2003). This statement can be corroborated by the significant increase in the frequency of MNs in erythrocytes of circulating blood cells, which would indicate alterations in erythrocateresis making cell renewal impossible, a function that depends on hepatic MMC in anurans (Agius and Roberts 2003; Fenoglio et al. 2005). The physiology and well-being or fitness of anurans exposed to pesticides would be negatively affected by the inability of the liver to eliminate or detoxify xenobiotics and damaged cellular components of the body (Pérez-Iglesias et al. 2016).
With regard to the biochemical responses observed, IMZT formulation was found to induce alterations in AChE activity in L. latinasus adults. In this sense, our results corroborate the same effects reported in fish by Pasha and Singh (2005), Moraes et al. (2011) and Pasha (2013), where IMZT commercial formulations alter the cholinergic system of local aquatic vertebrates. Oxidative stress refers to the imbalance due to excess ROS or oxidants over the cell’s ability to carry out an effective antioxidant response. ROS are highly reactive species that can affect membrane lipids and nucleic acids, so regulating the delicate balance between ROS production and the activation of antioxidant cell defenses, such as CAT and GST enzymes, is essential to maintain cell viability (because they participate in the elimination of ROS) (Ferrari et al. 2008). In particular, the significant increase in CAT in adults of L. latinasus suggests that this enzyme acts as one of the main antioxidant defenses for these organisms against the action of an herbicide (Ferrari et al. 2011) with pro-oxidant characteristics such as Pivot® H (Moraes et al. 2011). Added to this, the increase in CAT is considered a beneficial response in a stress situation since it prevents oxidation in cells (Ferrari et al. 2011). On the other hand, the decrease in GST activity demonstrates an enzymatic inhibition of the dominant antioxidant defense system of these organisms (Ferrari et al. 2011). The decrease in GST would lead to the deactivation of detoxifying systems that counteract the damaging effects of ROS (Attademo et al. 2007; Ferrari et al. 2008, 2011). In this case, the role of GST was inhibited at 48 h, which could lead to an increase in oxidative stress by herbicide exposure.
Finally, if we consider the response of histological biomarkers, we could think that since antioxidant enzyme systems do not respond (the case of CAT) or are inhibited (the case of GST) for that concentration and at that time, the antioxidant response would be in charge of MMC and its pigments, at least after 48 h of exposure. On the other hand, after 96 h, in S1 there is an increase in CAT that would indicate that this is the antioxidant system that prevails over the function of MMC since the concentration of the stressor is lower. However, the situation is different in S2 (higher concentration) since the antioxidant enzymes evaluated do not respond to the action of the herbicide, there is an inhibition in the cellular protection of liver MMCs evidenced by decreased melanin, and liver damages such as HV and AS were observed. In summary, the present study demonstrated that exposures of an anuran species to high concentrations of a formulation of IMZT resulted in liver damage. In a study of the effects of atrazine, Brodkin et al. (2007) interpreted similar responses as evidence that the anuran test species had lost their physiological response capacities to maintain cell renewal processes and antioxidant and detoxifying functions.
It should also be noted that exposure to IMZT formulation produces cytogenetic damage in L. latinasus adults, which is consistent with previous studies by our group (Pérez-Iglesias et al. 2015, 2017, 2018, 2020). In this context, genomic instability may be critical for affecting population health, causing one or both of two possible outcomes: 1) an increase in the physiological stress (Barni et al. 2007; Jha 2008); or 2) decreased organism survival and long-term population effects as a result of inability to repair the DNA damage (Barni et al. 2007; Çavaş and Könen 2007; Jha 2008).
In recent years, Newman (2014) highlighted the importance of correlating biomarker responses instead of evaluating them separately. This information helps to understand not only the susceptibility of organisms to environmental stressors but also their mode of action and toxicity, which can later be used as early warning signals in environments that are disturbed or contaminated by the presence of environmental stressors (Newman 2014; Pérez-Iglesias et al. 2020). In this sense, previous information evaluating correlations between adverse effects at different levels of biological organization is scarce. In this work, the multivariate analysis using a battery of biomarkers related to the responses at different levels of biological organization allows to generate novel information not provided by the individual and separate analysis of each biomarker. Furthermore, these results show that the endpoints evaluated respond to the concept of biomarkers proposed by Walker (2009) who affirms that the analyzed endpoints are useful biomarkers to use.
Added to this, the PCA analysis from this study clearly allowed us to observe the separation between those frogs exposed to IMZT formulation and the control group. This situation shows that the adverse effects of Pivot® H induce alterations in the physiological responses evidenced when evaluating biomarkers at different levels of biological organization. The holistic approach of multivariate analysis allows the evaluation of multiple endpoints simultaneously and facilitates the general and particular understanding of the various effects of experimental exposure to an environmental stressor. This is an advantage for this type of analysis in contrast to the separate information provided by conventional analyses. In conclusion, and in agreement with other authors (Van der Oost et al. 2003; Newman 2014), we recommend the use of this type of approach for ecotoxicological studies since it allows us to discern the groups of anurans that were exposed to environmental stressors from those that were not exposed.
Focusing on the ecological importance of experimental design, a first acute scenario (S1) simulate surface runoff of pesticide occurs and contaminated water income to breeding sites (caves in the ground) of the frogs. It is important to note that the highest concentrations of IMZT in runoff water occurred near the site of herbicide application, when the amount of IMZT transported by runoff did not exceed 3% and storage of rainwater inside the soil (Dias Martini et al. 2013). A second scenario (S2) considered the worst-case scenario exposure where a direct application to the frog occurs at a concentration of the herbicide approved for use on crops (e.g., the frog emerging from the cave and sprayed with the herbicide) (Van Meter et al. 2014). Our results prove that these realistic exposure scenarios can directly affect adults of L. latinasus. Indeed, S1 caused significant cytogenetic damage while more severe effects (biochemical damage, histological and individual) were observed in S2, the worst exposure situation considered. We understand that the cytogenetic biomarker evaluated on this occasion (MNs) is presented as an early signal, responding in situations of low herbicide concentrations, before irreversible damage occurs at higher levels of organization. In addition to this, if the particular life habits of this species are considered, we can understand that MNs in this case would be a valid tool to use in monitoring with L. latinasus given that a surface runoff event is the most likely exposure situation after runoff events due to heavy rains. Base on the information gather, IMZT exposure appears to be a risk factor for L. latinasus populations. However, we highlight what was previously mentioned (Pérez-Iglesias et al. 2015), it is important to know the identities of the additive compounds present in the commercial formulations because the acute toxicity of a technical active ingredient can differ significantly from that of the end-use formulation containing that active ingredient. Also, several investigations have demonstrated that the additive compounds present in pesticide commercial formulations have the ability to induce toxicity by themselves, separate from the active ingredient (Pérez-Iglesias et al. 2015). It should be mentioned that according to our Argentinean administration, the additive compounds (also called “moisturizers and inert ingredients”) present in any agrochemical are not required to be listed on the agrochemical data sheet and can be kept as a “trade secret,” as evidenced in the Pivot® H datasheet or letterhead. Further studies are required to reveal whether the adverse effects exerted by Pivot® H on the L. latinasus adults we observed are attributable to IMZT or result from the presence of xenobiotic (s) within the formulated technical formulation assayed in our study and to understand the impact of pesticides on local populations of anurans.
Abbreviations
- AChE:
-
Acetylcholinesterase
- BL:
-
Blebbed nucleus
- BN:
-
Binucleated cell
- CAT:
-
Catalase
- ES:
-
Enlargement of sinusoids
- GST:
-
Glutathione-S-transferase
- GSH:
-
Reduced glutathione
- HSI:
-
Hepatosomatic index
- HV:
-
Vascularization or congestion
- IMZT:
-
Imazethapyr
- LB:
-
Lobed nucleus
- MMC:
-
Melanomacrophages
- MNs:
-
Micronuclei
- NT:
-
Notched nucleus
- PCA:
-
Principal component analysis
References
Abercrombie SA, de Perre C, Iacchetta M, Flynn RW, Sepúlveda MS, Lee LS, Hoverman JT (2020) Sublethal effects of dermal exposure to poly- and perfluoroalkyl substances on postmetamorphic amphibians. Environ Toxicol Chem 40(3):717–726
Agius C, Roberts RJ (2003) Melano-macrophage centres and their role in fish pathology. J Fish Dis 26:499–509
Agostini MG, Kacoliris F, Demetrio P, Natale GS, Bonetto C, Ronco AE (2013) Abnormalities in amphibian populations inhabiting agroecosystems in northeastern Buenos Aires Province, Argentina. Dis Aquat Org 104:163–171
Agostini MG, Saibene PE, Roesler I, Bilenca D (2016) Amphibians of northwestern Buenos Aires province, Argentina: checklist, range extensions and comments on conservation. Check List 12:1998
Attademo AM, Peltzer PM, Lajmanovich RC, Cabagna M, Fiorenza G (2007) Plasma B-esterase and glutathione S-transferase activity in the toad Chaunus schneideri (Amphibia, Anura) inhabiting rice agroecosystems of Argentina. Ecotoxicology 16:533–539
Attademo AM, Cabagna-Zenklusen MC, Lajmanovich RC, Peltzer PM, Junges CM, Bassó A (2011) B-esterase activities and blood cell morphology in the frog Leptodactylus chaquensis (Amphibia: Leptodactylidae) on rice agroecosystems from Santa Fe Province (Argentina). Ecotoxicology 20:274–282
Attademo AM, Bionda CL, Peltzer PM, Lajmanovich RC, Seib SN, Basso A, Junges CM (2014) Edad, tamaño corporal en la madurez sexual, longevidad y potencial reproductivo de Leptodactylus latinasus y Leptodactylus mystacinus en un cultivo de soja y un bosque nativo del centro este de Argentina. Revista Mexicana De Biodiversidad 85:315–317
Babini MS, Bionda CL, Salas NE, Martino AL (2015) Health status of tadpoles and metamorphs of Rhinella arenarum (Anura, Bufonidae) that inhabit agroecosystems and its implications for land use. Ecotoxicol Environ Saf 118:118–125
Barni S, Boncompagni E, Grosso Bertone V, Freitas I, Fasola M, Fenoglio C (2007) Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquat Toxicol 81:45–54
Beebee TJC, Griffiths RA (2005) The amphibian decline crisis: a watershed for conservation biology? Biol Cons 125:271–285
Blaustein AR, Wake DB (1990) Declining amphibian populations: a global phenomenon? Trends Ecol Evol 5:203–204
Blaustein AR, Han BA, Relyea RA, Johnson PTJ, Buck JC, Gervasi SS, Kats LB (2011) The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Ann N Y Acad Sci 1223:108–119
Brodeur JC, Vera Candioti J (2017) Impacts of agriculture and pesticides on amphibian terrestrial life stages: Potential Biomonitor/Bioindicator Species for the Pampa Region of Argentina. In: Larramendy ML (ed) Ecotoxicology and genotoxicology-non-traditional terrestrial models. Royal society of chemistry, London, UK.
Brodeur JC, Suarez RP, Natale GS, Ronco AE, Zaccagnini ME (2011) Reduced body condition and enzymatic alterations in frogs inhabiting intensive crop production areas. Ecotoxicol Environ Saf 74:1370–1380
Brodeur JC, Vera-Candioti JV, Soloneski S, Larramendy ML, Ronco AE (2012) Evidence of reduced feeding and oxidative stress in common tree frogs (Hypsiboas pulchellus) from an agroecosystem experiencing severe drought. J Herpetol 46:72–78
Brodeur JC, Sanchez M, Castro L, Rojas DE, Cristos D, Damonte MJ, Poliserpi MB, D’Andrea MF, Andriulo AE (2017) Accumulation of current-use pesticides, cholinesterase inhibition and reduced body condition in juvenile one-sided livebearer fish (Jenynsia multidentata) from the agricultural Pampa region of Argentina. Chemosphere 185:36–46. https://doi.org/10.1016/j.chemosphere.2017.06.129
Brodeur JC, Damonte MJ, Vera-Candioti J, Poliserpi MB, D’Andrea MF, Bahl MF (2020) Frog body condition: basic assumptions, comparison of methods and characterization of natural variability with field data from Leptodactylus latrans. Ecol Indic 112:106098
Brodkin MA, Madhoun H, Rameswaran M, Vatnick I (2007) Atrazine is an immune disruptor in adult northern leopard frogs (Rana pipiens). Environ Toxicol Chem Int J 26:80–84
Brühl CA, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure of amphibians: an underestimated cause of global decline? Sci Rep 3:1135
Cabagna MC, Lajmanovich RC, Peltzer PM, Attademo AM, Ale E (2006) Induction of micronuclei in tadpoles of Odontophrynus americanus (Amphibia: Leptodactylidae) by the pyrethroid insecticide cypermethrin. Toxicol Environ Chem 88:729–737
Çakici Ö (2015) Histopathologic changes in liver and kidney tissues induced by carbaryl in Bufotes variabilis (Anura: Bufonidae). Exp Toxicol Pathol 67:237–243
CASAFE (2013) Guía de Productos Fitosanitarios para la República Argentina. In: Fertilizantes (ed.). CASAFE, Buenos Aires.
Castillo GA, Coviello A, Orce GG (1991) Effect of theophylline on the electrolyte permeability of the isolated skin of the toad Bufo arenarum. Arch Int Physiol Biochim Biophys 99:257–264
Castillo GA, Chanampa Y, Orce G (2005) Effect of mercuric chloride on electrical parameters and anion fluxes in the toad skin. Comp Biochem Physiol c Toxicol Pharmacol 140:21–27
Çavaş T, Könen S (2007) Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 22:263–268
Cei JM (1980) Amphibians of Argentina. Università degli Studi di Firenze, New Series Monografia
CONICET (2005) Reference Ethical Framework for Biomedical Research: Ethical Principles for Research with Laboratory, Farm, and Wild Animals. in: Council, N.S.a.T.R. (Ed.).
Couillard CM, Hodson PV (1996) Pigmented macrophage aggregates: a toxic response in fish exposed to bleached-kraft mill effluent? Environ Toxicol Chem Int J 15:1844–1854
De Oliveira C, Franco-Belussi L, Fanali LZ, Santos LR (2017) Use of melanin-pigmented cells as a new tool to evaluate effects of agrochemicals and other emerging contaminants in Brazilian anurans. Ecotoxicol Genotoxicol Non-Traditional Terr Models 32:125
Dias Martini LF, Mezzomo RF, de Avila LA, Massey JH, Marchesan E, Zanella R, Peixoto SC, Refatti JP, Cassol GV, Marques M (2013) Imazethapyr and imazapic runoff under continuous and intermittent irrigation of paddy rice. Agric Water Manag 125:26–34
Edge CB, Gahl MK, Pauli BD, Thompson DG, Houlahan JE (2011) Exposure of juvenile green frogs (Lithobates clamitans) in littoral enclosures to a glyphosate-based herbicide. Ecotoxicol Environ Saf 74:1363–1369
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ezemonye L, Tongo I (2010) Sublethal effects of endosulfan and diazinon pesticides on glutathione-S-transferase (GST) in various tissues of adult amphibians (Bufo regularis). Chemosphere 81:214–217
Fanali LZ, Franco-Belussi L, Bonini-Domingos CR, de Oliveira C (2018) Effects of benzo [a] pyrene on the blood and liver of Physalaemus cuvieri and Leptodactylus fuscus (Anura: Leptodactylidae). Environ Pollut 237:93–102
Fenech M (2007) Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084–1104
Fenoglio C, Boncompagni E, Fasola M, Gandini C, Comizzoli S, Milanesi G, Barni S (2005) Effects of environmental pollution on the liver parenchymal cells and Kupffer-melanomacrophagic cells of the frog Rana esculenta. Ecotoxicol Environ Saf 60:259–268
Ferrari A, Anguiano L, Lascano C, Sotomayor V, Rosenbaum E, Venturino A (2008) Changes in the antioxidant metabolism in the embryonic development of the common South American toad Bufo arenarum: Differential responses to pesticide in early embryos and autonomous-feeding larvae. J Biochem Mol Toxicol 22:259–267
Ferrari A, Lascano C, Pechen de D’Angelo AM, Venturino A (2011) Effects of azinphos methyl and carbaryl on Rhinella arenarum larvae esterases and antioxidant enzymes. Comp Biochem Physiol 153:34–39
Franco-Belussi L, de Lauro Castrucci AM, de Oliveira C (2013) Responses of melanocytes and melanomacrophages of Eupemphix nattereri (Anura: Leiuperidae) to Nle 4, D-Phe 7-α-melanocyte stimulating hormone and lipopolysaccharides. Zoology 116:316–324
Franco-Belussi L, Provete DB, Borges RE, De Oliveira C, Santos LR (2020) Idiosyncratic liver pigment alterations of five frog species in response to contrasting land use patterns in the Brazilian Cerrado. PeerJ 8:e9751
Furlong ET (2001) Methods of Analysis by the US Geological Survey National Water Quality Laboratory: Determination of Pesticides in Water by Graphitized Carbon-based Solid-phase Extraction and High-performance Liquid Chromatography/mass Spectrometry (No. 1). US Department of the Interior, US Geological Survey.
Garber JC, Barbee RW, Bielitzki JT (2011) Guide for the care and use of laboratory animals. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. National Academies Press, Washington, DC
Glinski DA, Henderson WM, Van Meter RJ, Purucker ST (2018) Effect of hydration status on pesticide uptake in anurans following exposure to contaminated soils. Environ Sci Pollut Res 25:16192–16201
Guerra C, Aráoz E (2016) Amphibian malformations and body condition across an agricultural landscape of northwest Argentina. Dis Aquat Org 121:105–116
Gürkan M, Hayretdağ S (2012) Morphological and histological effects of copper sulfate on the larval development of green toad, Bufo viridis. Turkish J Zool 36:231–240
Hegde G, Krishnamurthy SV (2014) Analysis of health status of the frog Fejervarya limnocharis (Anura: Ranidae) living in rice paddy fields of Western Ghats, using body condition factor and AChE content. Ecotoxicol Environ Contam 9:69–76
Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL (2000) Quantitative evidence for global amphibian population declines. Nature 404:752–755
Howarth FG (2000) Non-target effects of biological control agents. In: Biological control: measures of success. Springer, pp 369–403.
INTA (2008) Guia para cuidado y uso de animales para experimentación. Centro de Investigación en Ciencias Veterinarias, Instituto Nacional de Tecnología Agropecuaria (INTA), Secretaría de Agricultura, Ganadería, Pesca y Alimentación, República Argentina.
IUCN (2020) The IUCN Red List of Threatened Species. Version 2020–3. https://www.iucnredlist.org. Acceded on 30th december 2020.
Jackson DA (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214
Jha AN (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23:207–221
Jolliffe IT, Cadima J (2016) Principal component analysis: a review and recent developments. Philos Trans Royal Soc A Math Phys Eng Sci 374:20150202
Josende ME, Tozetti AM, Alalan MT, Mathies Filho V, da Silva XS, da Silva Júnior FMR, Martins SE (2015) Genotoxic evaluation in two amphibian species from Brazilian subtropical wetlands. Ecol Ind 49:83–87
Kawai K, Kaku K, Izawa N, Shimizu T, Fukuda A, Tanaka Y (2007) A novel mutant acetolactate synthase gene from rice cells, which confers resistance to ALS-inhibiting herbicides. J Pestic Sci 32:89–98
Kegley SE, Hill BR, Orme S, Choi AH (2020) PAN Pesticide Database. Pesticide Action Network. PAN Pesticide Database website, Oakland, CA. www.pesticideinfo.org. Acceded on 30th december 2020.
Kiesecker JM, Blaustein AR, Belden LK (2001) Complex causes of amphibian population declines. Nature 410:681–684
Köhler HR, Triebskorn R (2013) Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science 341:759–765
Lajmanovich RC, Peltzer PM, Attademo AM, Colussi CL, Martinuzzi CS (2018) Blood biomarkers of common toad Rhinella arenarum following chlorpyrifos dermal exposure. Interdiscip Toxicol 11:148–154
Leiva-Presa À, Munro Jenssen B (2006) Effects of p, p′-DDE on retinoid homeostasis and sex hormones of adult male European common frogs (Rana temporaria). J Toxicol Environ Health 69:2051–2062
Lin K, Xu C, Zhou S, Liu W, Gan J (2007) Enantiomeric separation of imidazolinone herbicides using chiral high-performance liquid chromatography. Chirality 19:171–178
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Magdaleno A, Gavensky MP, Fassiano AV, Ríos de Molina MC, Santos M, March H, Moretton J, Juárez ÁB (2015) Phytotoxicity and genotoxicity assessment of imazethapyr herbicide using a battery of bioassays. Environ Sci Pollut Res 22:19194–19202
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157:2903–2927
McDiarmid RW, Altig R (1999) Tadpoles: the biology of anuran larvae. University of Chicago Press
Medina RG, Ponssa ML, Aráoz E (2016) Environmental, land cover and land use constraints on the distributional patterns of anurans: Leptodacylus species (Anura, Leptodactylidae) from Dry Chaco. PeerJ 4:e2605
Moraes BS, Clasen B, Loro VL, Pretto A, Toni C, de Avila LA, Marchesan E, de Oliveira Machado SL, Zanella R, Reimche GB (2011) Toxicological responses of Cyprinus carpio after exposure to a commercial herbicide containing imazethapyr and imazapic. Ecotoxicol Environ Saf 74:328–335
Newman MC (2014) Fundamentals of ecotoxicology: the science of pollution. CRC Press, Boca Ratón, Florida
Pasha F (2013) Enzyme Inhibition (AChE) in Brain of Oreochromis mossambicus due to Pesticidal Pollution of Herbicide Pursuit. J Biol Sci 1:91–100
Pasha F, Singh R (2005) Enzyme inhibition (AChE) in muscles and skin of Oreochromis mossambicus due to pesticidal pollution of herbicide Pursuit. Asian J Exp Sci 19:119–126
Păunescu A, Ponepal CM, Drăghici O, Marinescu AG (2010) Histopathological responses of the liver tissues of Rana ridibunda to the champions 50 wp fungicide. Ann Food Sci Technol 11:60–64
Peltzer PM, Lajmanovich RC, Sánchez-Hernandez JC, Cabagna MC, Attademo AM, Bassó A (2008) Effects of agricultural pond eutrophication on survival and health status of Scinax nasicus tadpoles. Ecotoxicol Environ Saf 70:185–197
Pérez-Iglesias JM, Soloneski S, Nikoloff N, Natale GS, Larramendy ML (2015) Toxic and genotoxic effects of the imazethapyr-based herbicide formulation Pivot H® on montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol Environ Saf 119:15–24
Pérez-Iglesias JM, Franco-Belussi L, Moreno L, Tripole S, de Oliveira C, Natale GS (2016) Effects of glyphosate on hepatic tissue evaluating melanomacrophages and erythrocytes responses in neotropical anuran Leptodactylus latinasus. Environ Sci Pollut Res 23:9852–9861
Pérez-Iglesias JM, Ruiz de Arcaute C, Natale GS, Soloneski S, Larramendy ML (2017) Evaluation of imazethapyr-induced DNA oxidative damage by alkaline Endo III-and Fpg-modified single-cell gel electrophoresis assay in Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol Environ Saf 142:503–508
Pérez-Iglesias JM, Natale GS, Soloneski S, Larramendy ML (2018) Are the damaging effects induced by the imazethapyr formulation Pivot® H in Boana pulchella (Anura) reversible upon ceasing exposure? Ecotoxicol Environ Saf 148:1–10
Pérez-Iglesias JM, Brodeur JC, Larramendy ML (2020) An imazethapyr-based herbicide formulation induces genotoxic, biochemical, and individual organizational effects in Leptodactylus latinasus tadpoles (Anura: Leptodactylidae). Environ Sci Pollut Res 27:2131–2143
Quaranta A, Bellantuono V, Cassano G, Lippe C (2009) Why amphibians are more sensitive than mammals to xenobiotics. PLoS ONE 4:e7699
Reimche GB, Machado SLO, Oliveira MA, Zanella R, Dressler VL, Flores EMM, Gonçalves FF, Donato FF, Nunes MAG (2015) Imazethapyr and imazapic, bispyribac-sodium and penoxsulam: Zooplankton and dissipation in subtropical rice paddy water. Sci Total Environ 514:68–76
Sanchez LC, Peltzer PM, Lajmanovich RC, Manzano AS, Junges CM, Attademo AM (2013) Reproductive activity of anurans in a dominant agricultural landscape from central-eastern Argentina. Revista Mexicana De Biodiversidad 84:912–926
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass–size residuals: validating body condition indices. Ecology 86:155–163
Selcer KW, Verbanic JD (2014) Vitellogenin of the northern leopard frog (Rana pipiens): development of an ELISA assay and evaluation of induction after immersion in xenobiotic estrogens. Chemosphere 112:348–354
Shutler D, Marcogliese DJ (2011) Leukocyte profiles of northern leopard frogs, Lithobates pipiens, exposed to pesticides and hematozoa in agricultural wetlands. Copeia 2011:301–307
Stebbins RC, Cohen NW (1995) A natural history of amphibians. Princeton University Press, Princeton, New Jersey
Suárez RP, Zaccagnini ME, Babbitt KJ, Calamari NC, Natale GS, Cerezo A, Codugnello N, Boca T, Damonte MJ, Vera-Candioti J (2016) Anuran responses to spatial patterns of agricultural landscapes in Argentina. Landscape Ecol 31:2485–2505
Tan S, Evans RR, Dahmer ML, Singh BK, Shaner DL (2005) Imidazolinone-tolerant crops: history, current status and future. Pest Manag Sci 61:246–257
USEPA (1975). Methods for acute toxicity tests with fish, macroinvertebrates, and amphibians. in: Agency, U.E.P. (Ed.). USEPA 660/3-75-009,62.
USEPA (1989) Imazethapyr Herbicide Profile 3/89. Chemical Fact Sheet for Imazethapyr. United States Environmental Protection Agency, Office of Pesticide Programs, Washington DC. 196 pp.
Vaira M, Akmentins M, Attademo M, Baldo D, Barrasso D, Barrionuevo S, Basso N, Blotto B, Cairo S, Cajade R, Céspedez J, Corbalán V, Chilote P, Duré M, Falcione C, Ferraro D, Gutierrez FR, Ingaramo MR, Junges C, Lajmanovich R, Lescano JN, Marangoni F, Martinazzo L, Marti R, Moreno L, Natale GS, Pérez-Iglesias JM, Peltzer P, Quiroga L, Rosset S, Sanabria E, Sanchez L, Schaefer E, Úbeda C, Zaracho V (2012) Categorización del estado de conservación de los anfibios de la República Argentina. Cuadernos De Herpetología 26:131–159
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Van Meter RJ, Glinski DA, Hong T, Cyterski M, Henderson WM, Purucker ST (2014) Estimating terrestrial amphibian pesticide body burden through dermal exposure. Environ Pollut 193:262–268
Van Meter RJ, Glinski DA, Henderson WM, Garrison AW, Cyterski M, Purucker ST (2015) Pesticide uptake across the amphibian dermis through soil and overspray exposures. Arch Environ Contam Toxicol 69:545–556
Van Meter RJ, Glinski DA, Purucker ST, Henderson WM (2018) Influence of exposure to pesticide mixtures on the metabolomic profile in post-metamorphic green frogs (Lithobates clamitans). Sci Total Environ 624:1348–1359
Van Meter RJ, Adelizzi R, Glinski DA, Henderson WM (2019) Agrochemical mixtures and amphibians: the combined effects of pesticides and fertilizer on stress, acetylcholinesterase activity, and bioaccumulation in a terrestrial environment. Environ Toxicol Chem 38:1052–1061
Vera-Candioti J, Natale GS, Soloneski S, Ronco AE, Larramendy ML (2010) Sublethal and lethal effects on Rhinella arenarum (Anura, Bufonidae) tadpoles exerted by the pirimicarb-containing technical formulation insecticide Aficida®. Chemosphere 78:249–255
Walker CH (2009) Organic pollutants: an ecotoxicological perspective. CRC Press, Boca Raton, FL
Wang MZ, Jia XY (2009) Low levels of lead exposure induce oxidative damage and DNA damage in the testes of the frog Rana nigromaculata. Ecotoxicology 18:94–99
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice Hall, New Jersey
Zaya RM, Amini Z, Whitaker AS, Kohler SL, Ide CF (2011) Atrazine exposure affects growth, body condition and liver health in Xenopus laevis tadpoles. Aquat Toxicol 104:243–253
Acknowledgements
This study was supported by grants from the National University of La Plata (Grants 11/N817 and 11/N847) of Argentina. The National Council for Scientific and Technological Research (CONICET) from Argentina and Graduate Program of UNLP to provided funding for JMPI to carry out the investigations and doctoral studies. CO received a fellowship (304552/2019-4) from CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Iglesias, J.M., Fanali, L.Z., Franco-Belussi, L. et al. Multiple Level Effects of Imazethapyr on Leptodactylus latinasus (Anura) Adult Frogs. Arch Environ Contam Toxicol 81, 492–506 (2021). https://doi.org/10.1007/s00244-021-00880-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-021-00880-w