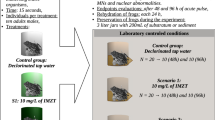
Abstract
Imazethapyr, a post-emergent herbicide used in worldwide soybean and corn crops, induces genetic and biochemical alterations in aquatic vertebrates. This study examined the relationship between biomarkers at different organization levels and imazethapyr real-life route exposure in Boana pulchella adults. Frogs were exposed to imazethapyr-based formulation Pivot® H (10.59%) at concentrations representing possible acute routes: field runoff (S1:10 mg.L−1), exposure after direct foliar application (S2:100 mg.L−1) and during direct foliar application (S3:1000 mg.L−1). Post-exposure, endpoints levels were evaluated: organism alterations, biochemical activities and cytogenetic assays. Forty-eight hours post-exposure, antioxidant enzymes decrease, micronuclei induction and DNA damage were observed in all scenarios, while cholinesterase activity increase and body condition reduction were observed in frog-exposed to S3. Ninety-six hours post-exposure, frogs showed glutathione-S-transferase inhibition in S1, micronuclei induction in S2 and S3, and DNA-damage increase in S3. Herbicides routes of exposures in real-life could indicate that authorized applications have a risk to amphibian populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are considered essential to maintain high agricultural output in modern agriculture based on genetically modified organisms. Nevertheless, pesticide products may cause a variety of adverse effects in non-target organisms through both direct and indirect actions (Howarth 2000; Köhler and Triebskorn 2013). The imidazolinones are a group of widely used broad-spectrum herbicides employed for selective pre- or post-emergence weed control in a variety of crops. These herbicides fall into the class of acetolactate synthase (ALS)-inhibitors, an enzyme involved in the synthesis of branched-chain amino acids (Tan et al. 2005; Kawai et al. 2007; Lin et al. 2007).
Imazethapyr (IMZT) [5-ethyl-2-(4-isopropyl-4-methyl-5-oxo-4,5-dihydroimidazol-1H-2-yl) nicotinicacid] is an imidazolinone herbicide widely used in soybeans and corn crops genetically modified to be resistant to these herbicides. The recommended application rate of IMZT ranges between 0.8 to 1 L/ha for both aerial and terrestrial foliar applications, respectively (CASAFE 2013). IMZT has been classified as a slightly toxic compound (Class III) by the USEPA (1989), and as a dangerous compound for the environment by the European Union (EU) (Kegley et al. 2020). Furthermore, they affirm that IMZT is an unsafe pesticide for the environment and is associated with adverse effects in humans such as eye irritation, skin lesions, and respiratory tract difficulties (Kegley et al. 2020).
At the same time, data from literature about IMZT-induced toxicity in non-target organisms is scarce. Briefly, reports showed that IMZT is nontoxic for fish (LC50 non-determinated) such as the channel catfish, the rainbow trout and the bluegill after acute exposures (Kegley et al. 2020). Furthermore, analyzing in aquatic vertebrates, Moraes et al. (2011) reported alterations on enzymes related to oxidative stress after 0.0148 mg/L exposure to both the active ingredient IMZT and to the IMZT-based commercial herbicide formulation Only® in hepatic tissues of the fish Cyprinus carpio. In addition, inhibition of acetylcholinesterase (AChE) was found after exposure to commercial formulations of IMZT in the Mozambique tilapia (Pasha and Singh 2005; Pasha 2013). Recently, in other aquatic vertebrates, our research group has demonstrated the toxicological effects at genetic, biochemical and individual levels in anurans such as Boana pulchella and Leptodactylus latinasus exposed to IMZT in their larval phase (Pérez-Iglesias et al. 2015; 2017; 2018; 2020). Particularly, we found alterations in several biomarkers such as primary DNA lesions and micronuclei frequency, glutathione-S-transferase alterations, morphological abnormalities and swimming performance. Thus, we were able to demonstrate that when the exposures of environmental stressors increase, IMZT-exposed frogs move from homeostasis toward a stress status (Pérez-Iglesias et al. 2015; 2017; 2018; 2020).
Due to their special characteristics such as their permeable skin, position in trophic webs, shell-less eggs, exposed embryogenesis, free-living aquatic larvae, dependence upon two environments throughout their life history, and a narrow home-range, amphibians have been widely used as bioindicators of environmental quality (Wake 1991; Blaustein et al. 2011). Furthermore, amphibians are declining and one of the proposed causes has been the increasing use of environmental contaminants (e.g., pesticides) (Wake 1991; Houlahan et al. 2000; Kiesecker et al. 2001; Beebee and Griffiths 2005; Mann et al. 2009). Several studies have demonstrated that amphibians are negatively affected at the physiological, histological, biochemical and cytogenetic levels by living in agroecosystems where pesticides are continuously introduced (Mann et al. 2009; Shutler and Marcogliese 2011; Hegde and Krishnamurthy 2014). Particularly in Argentina, several authors have reported evidences of alterations in anurans inhabiting the agricultural Pampa region where a variety of pesticides are extensively used (Cabagna et al. 2006; Attademo et al. 2007; 2011; 2014; Peltzer et al. 2008; Brodeur et al. 2011; 2012; Agostini et al. 2013; Sanchez et al. 2013; Guerra and Aráoz 2016). In this sense, amphibians are a particularly important and vulnerable group of non-target organisms in Pampasic agricultural landscapes, where they can be exposed directly or indirectly to a variety of pesticides (Mann et al. 2009; Brodeur et al. 2011; 2012; Suárez et al. 2016; Van Meter et al. 2014; 2019). Indeed, terrestrial exposure of amphibian to pesticides through dermal uptake may be a primary and a significant pathway (Van Meter et al. 2014; 2015; 2018; 2019). Adult amphibians are known to move across terrestrial landscapes in search of breeding ponds and/or overwintering habitats (Brühl et al. 2013; Van Meter et al. 2018; 2019). This situation, particularly in agriculturally intensive areas, leads to the terrestrial amphibians at risk of dermal exposure due to soils or vegetation contaminated with pesticides (Van Meter et al. 2018; 2019). The primary route of exposure to pollutants of amphibians in agricultural habitats is via skin contact with soil or water principally by direct application, and secondly by runoff or ingestion of insects previously exposed (Pérez-Iglesias et al. 2016). However, a limited number of studies employing a battery of bioassays evaluating the effects of pesticides after dermal exposure on adults’ anurans have been previously reported (Brühl et al. 2013; Van Meter et al. 2014; 2015; 2018; 2019). In Neotropical regions, some studies revealed that the anurans sampled in agricultural landscapes had lower body condition and enzymes alterations, which may have related to the presence of environmental stressors (Brodeur et al. 2011; 2012; 2021).
Environmental exposures to contaminants are quite complex and the implementation of a battery of biomarkers to monitor the status of amphibian populations is a valuable tool (Newman 2014). It is well documented that the use of biomarkers at different ecological levels allows researchers to associate environmental exposure with a wide range of deleterious biological responses of aquatic vertebrates (e.g. amphibians) and, the biomarkers are useful tools on which to base environmental decisions before irreversible damage occurs in the ecosystem (Van der Oost et al. 2003; Vasseur and Cossu-Leguille 2003; Sparling et al. 2010; Ossana et al. 2013).
In this context, the objective of the present study was to evaluate how IMZT affects the physiology and genetic material using several biomarkers in adults of the Neotropical anuran B. pulchella to simulations of potential real life terrestrial exposures under laboratory conditions to concentrations of the IMZT-based herbicide formulation Pivot® H simulating realistic scenarios.
Materials and methods
Chemical reagents and determinations
All chemicals, reactives and solvents of analytical grade were purchased from Sigma Chemical Co. (St. Louis, MO) while Pivot H® (10.59% w/v of IMZT, CAS 081335-77-5) was obtained from BASF Argentina S. A. Cyclophosphamide (CP, CAS 6055-19-2) was purchased from Sigma Chemical Co. (St. Louis, MO). IMZT concentrations present in bioassay solutions were analyzed by Waters Acquity Ultra Performance Liquid Chromatography (UPLC) system coupled to a Quattro Premier XE tandem quadrupole mass spectrometer (MS/MS), with an electrospray ionization (ESI) source equipment from the CIM Institute (National University of La Plata, La Plata, Argentina) according to the procedures described in Report 01-4134 of the U.S. Geological Survey (Furlong 2001). Samples from test nominal concentrations were taken and immediately measured after the solutions were prepared (0 h) and 24 h thereafter. Detection limit of IMZT was 0.5 μg/L.
Test organisms and specimen recollection
Boana pulchella (Duméril and Bibron, 1841), formerly named Hypsiboas pulchellus, is an arboreal anuran species from the Hylidae family with extensive distribution in neotropical South America, and which is very abundant in the Pampa region of Argentina (Cei 1980). Its natural habitats are dry low land grasslands, seasonally wet or flooded low land grasslands, intermittent freshwater lakes, intermittent fresh water marshes, and pasture lands (Kwet et al. 2004). This species lays its eggs in masses attached to the submerged stems of aquatic plants. This species is easy to acclimate to laboratory conditions, it has been previously employed as a bioindicator species (Lajmanovich et al. 2005; Agostini et al. 2009; Brodeur et al. 2011; 2012; Pérez-Iglesias et al. 2014; 2015; Brodeur and Vera Candioti 2017; Sansiñena et al. 2018) and its population status is considered as “least concern” (Vaira et al. 2012; IUCN 2020). Individuals of B. pulchella (n = 100) were obtained during night collections from a typical pampean unpolluted habitat, during breeding season (La Plata, Buenos Aires, Argentina - 35° 10′ S; 57° 51′ W). All frog collections were approved by the Buenos Aires Province government (permit number 22500-22339/13). All experimental procedures were performed according to “Reference Ethical Framework for Biomedical Research: Ethical Principles for Research with Laboratory, Farm, and Wild Animals” (CONICET 2005). Adult males of B. pulchella were transported to the laboratory to be weighted (average, 2.65 ± 0.52 g) and measured (snout-vent length average, 35.35 ± 2.58 mm). Afterwards, specimens were acclimated during 7 days in 2800 cm3 glass containers containing 200 cm3 of soil at the bottom. Photoperiod (16:8 h light/dark) and temperature (25.0 ± 1 °C) were maintained constant and animals were not fed during this period.
Experimental design and exposure protocol
Experiments were carried out reproducing plausible three exposure scenarios as recommended by Wang and Jia (2009) and Van Meter et al. (2015). The first scenario (S1) illustrated an exposure to field runoff water. The second scenario (S2) illustrated a situation where B. pulchella visited a leaf previously sprayed with a foliar application and in which one-tenth (1/10) of the applied concentration of IMZT would reach the frogs (100 mg IMZT/L) and finally, the third scenario was designed to illustrate a situation where frogs were directly sprayed with the recommended application rate (S3: 1000 mg IMZT/L). Briefly, before the start of the bioassay, frogs were placed in a clean 20 L glass aquarium for a 24 h period of dehydration. This dehydration period was intended to facilitate the movement of water and xenobiotics throughout the anuran dermis because the re-hydration would occur during the bioassay, according to Van Meter et al. (2018). For each scenario, adults’ frogs were exposed to an acute pulse of the IMZT-based herbicide formulation by immersing the entire specimens for 15 sec in each herbicide concentrations (10, 100 and 1000 mg IMZT/L). Test solutions were prepared according to procedures proposed by USEPA (1975). Also, negative control and positive groups were prepared consisting of 10 frogs immersed in dechlorinated tap water and cyclophosphamide, respectively; and run in parallel with herbicide-exposed specimens. Immediately after exposure, each frog was placed individually in 3 L glass flasks containing 200 mL of fertile soil at the bottom. Frogs were not fed throughout the experiment and were re-hydrated by spraying dechlorinated tap water every 24 h to avoid frogs’ death due to drying (Van Meter et al. 2018). Evaluation of the proposed endpoints was performed 48 and 96 h after the acute exposure pulse. At each sampling time, frogs were anesthetized, placed on ice and dissected according to directives and protocols detailed in the Guide for Care and Use of Laboratory Animals (Garber et al. 2011), and the ethical procedures of the Ethical Committee from the National University of La Plata (code11/N619), “Reference Ethical Framework for Biomedical Research” (CONICET 2005) and “Guide for Care and Use of Experimental Animals” (INTA 2008). Experiments were performed in triplicate and 10 individuals were employed each time.
Evaluations of endpoints
Individual endpoints
Frog body condition was assessed using a method described by Brodeur et al. (2011). This method consists of examining the residuals from a regression of body mass against snout-vent length where the regression line obtained establishes the average body weight for a given length. An individual with positive residuals is considered in a good condition whereas an individual with a negative residual is regarded as having low energy (Schulte-Hostedde et al. 2005; Brodeur et al. 2011). To evaluate behavioral sublethal endpoints, mobility and posture of the frogs were observed for 1 min in a polypropylene chamber (30 L). Frogs were then placed into a pool to examine swimming activity for another minute. Finally, the hepatosomatic index (HSI) was calculated as the ratio of liver weight with respect to total body weight. At the end of the experiment the liver was weighed to obtain the HSI index using a precision scale 0.001 g.
Biochemical endpoints
All procedures were performed as previously described by Brodeur et al. (2017, 2020). Briefly, livers were homogenized in ice-cold 50 mM tris buffer (1 mM EDTA acid, 0.25 M of sucrose, pH 7.4) with a Teflon-glass Potter-Elvehjem homogenizer. Then, the homogenates were centrifuged at 4 °C (10,000 × g, 10 min) to collect the supernatant while nuclei and cell debris were discarded. One portion of the supernatant was used for protein concentrations following the method of Lowry et al. (1951) using bovine serum albumin as a standard. All enzymatic reactions and protein calibration curves were performed on microplates, and posterior reads of the enzymatic activities as well as the protein concentration were carried out by using a microplate reader (SPECTROstar Nano, BMG Labtech, Ortenberg, Germany).
GST activity determination
The GST activity in the liver of B. pulchella was measured using 1-chloro-2, 4- dinitrobenzene (CDNB) as substrate. Determinations were performed in a reaction mixture containing 300 μL GST (30% m/v of GSH in PBS, pH 7), 10 µL CDNB (0.1 M) and 10 µL of sample (dilution, 1:25 of pure supernatant:PBS). The colorimetric reaction absorbance (340 nm) was recorded for 2 min (37 °C) and GST activity was calculated with molar extinction coefficient of 9.6 mM−1 cm−1.
AChE activity determination
Activity of hepatic acetylcholinesterase (AChE) was determined by the Ellman method (Ellman et al. 1961). The reaction mixture consisted of 200 µL of PBS (100 mM, pH 8), 10 μL of acetylcholine (1 mM), 10 μL of DTNB (0.5 mM), and 50 µl of sample (previously diluted 1/5; 200 μL homogenized sample in 800 μL of PBS). The kinetic absorbance (412 nm) was recorded for 3 min (37 °C) and AChE activity was calculated using a molar extinction coefficient of 14,150 M−1 cm−1.
CAT activity determination
The CAT activity in the liver of B. pulchella was determined by measuring the kinetic absorbance in microplates using a reaction mixture consisting of 300 µL of PBS (100 mM, pH 7), 10 µL of H2O2 (dilution 0,5% v/v, H2O2 99% in distilled water) and 10 µL of sample (dilution, 1:25 of pure supernatant:PBS). The change in the absorbance (240 nm) resulting from H2O2 consumption was recorded for 2 min (37 °C), using a molar extinction coefficient of 43.6 M−1 cm−1.
Cytogenetic endpoints
MNs induction and nuclear abnormalities
MN assay and blood analysis was conducted in accordance with the original protocol (Fenech 2007) with minor modifications for this species (Pérez-Iglesias et al. 2016). Slides of blood smears, by triplicate, were stained for 12 min with 5% of Giemsa solution for each treated group. MNs frequency was calculated in peripheral mature erythrocytes after acute pulse exposure in both scenarios. MNs were blind-scored from 1000 erythrocytes from each blood frog sample (×1000 magnification). While, the presence of other nuclear abnormalities in mature erythrocytes was evaluated for this species according to our previous procedures (Pérez-Iglesias et al. 2016; 2020). The following frequency of nuclear abnormalities were considered: notched nuclei (NNs), i.e., nuclei with vacuoles and appreciable depth into a nucleus without containing nuclear material; blebbed nuclei (BLs), i.e., cells with one nucleus presenting a relatively small evagination of the nuclear membrane which contains euchromatin; and erythroplastids (EPs), i.e., anucleated forms of circulating red blood cells. MNs and nuclear abnormalities frequencies are expressed as total number of alterations per 1000 cells and the examination criteria for MNs acceptance was determined following previously reports (Vera Candioti et al. 2010).
Comet assay
The same individuals employed for the MN assay (see Section 2.4.3.2) were also used for the comet assay. The comet assay was performed following the alkaline procedure described elsewhere for the species (Pérez-Iglesias et al. 2014; 2015; 2017; 2018). Briefly, blood samples were diluted in PBS, centrifuged (2000 rpm, 9 min), and resuspended in PBS (50 mL). An aliquot of diluted samples (30 mL) was mixed with low-melting-point agarose (70 mL, 0.5%) and was then layered on a slide precoated with normal-melting-point agarose (100 mL, 0.5%). The slide was placed at 4 °C. After solidification, the slide was covered with a third layer of low-melting-point agarose (50 mL, 0.5%). After that, the slides were immersed in ice-cold freshly prepared lysis solution and then lysed in darkness for a 1 h (4 °C). Then, slides were placed in an electrophoresis buffer (4 °C) to allow the cellular DNA to unwind, followed by electrophoresis in the same buffer (4 °C, 20 min, 25 V). Finally, the slides were neutralized with a solution comprising Tris–HCl (pH = 7.5) and stained with DAPI (4′,6-diamino-2-phenylindole, Vectashield Mounting Medium H1200; Vector Laboratories, Burlingame, CA, USA). Slides were examined under an OlympusBX50 fluorescence photomicroscope equipped with an appropriate filter combination. The extent of DNA damage was quantified by the length of DNA migration, which was visually determined in 100 randomly selected and non-overlapping cells. DNA damage was classified in four classes (0–I, undamaged; II, minimum damage; III, medium damage; IV, maximum damage), considering Cavaş and Könen (2007). Data are expressed as the mean number of damaged cells (sum of Classes II, III, and IV) and the mean comet score for each treatment group. The GDI was calculated for each test compound following Pitarque et al. (1999) using the formula GDI = [I(I) + 2(II) + 3(III) + 4(IV)/N(0–IV)], where 0–IV represents the nucleoid type, and N0–NIV represent the total number of nucleoids scored.
Statistical analysis
ANOVA one-way (analysis of variance) with post-hoc Dunnett test was performed to estimate the IMZT exposure- induced effects on body condition, organ index, liver enzymes activities (GST, CAT and AChE), frequencies of MN and others nuclear abnormalities, and GDI (response variables), at both exposure times evaluated (Zar 2010). ANOVA assumptions were corroborated with Barlett test for the homogeneity of variances and χ2 test for normality. Then, data were logarithmically transformed to meet assumptions, in those cases that did not meet the assumptions of normality, a Kruskal–Wallis test was performed (Zar 2010).
A principal component analysis (PCA) was performed considering each exposure scenarios as a grouping variable to improve interpretation of the results and obtain integral information of the biomarkers responses (or holistic vision). The integration of all biomarkers was made by using Components Principal Regression Analysis (Jackson 1993; Jolliffe and Cadima 2016). In addition, the relationship between biomarkers and IMZT was evaluated with a correlation matrix (Pearson product moment correlation coefficient) by using simple linear regression. Tests of significance of the regression and correlation coefficients were performed following Zar (2010). The level of significance chosen was α = 0.05 for all tests, unless indicated otherwise. Analyses were performed using the R software 6 v. 2.11.1 (R Core Team 2010).
Results
Chemical determinations
Chemical determinations demonstrated that concentrations of IMZT were stable over the 24 h period between daily renewals of the test solution (concentration range 98 ± 5% recovery). Specifically, HPLC analytic determinations of IMZT revealed a value equivalent to the real concentration of 10.86 IMZT mg/L ± 1.87 (SD), 98.62 IMZT mg/L ± 19.96 (SD) and 1043.0 IMZT mg/L ± 100.05 (SD), with respect to nominal concentration of 10, 100, and 1000 IMZT mg/L, respectively; without significant variations over the 24 h of analysis (p < 0.05).
Response of endpoints
The responses after 48 h showed a decrease in GST activity in all scenarios, an increase in body condition and AChE activity in animals exposed to S3, and an increase in CAT activity for S1 and S2 without increase in S3. Also, the cytogenetic biomarkers (MNs frequency and comet assay) increased in all concentrations 48 h after the exposure pulse. After 96 h, the only biochemical effects that remained was a decrease of GST activity in S1 while the cytogenetic alterations were observed in S2 and S3.
Individual endpoints
Evaluations of body condition in adults of B. pulchella after exposure to the herbicide IMZT revealed differences between frogs exposed in S3 respect to the control group after 48 h (p < 0.05) but not after 96 h (p > 0.05). However, no alterations in HSI were found in frogs exposed to IMZT scenarios after both exposure times evaluated (48 and 96 h).
Biochemical endpoints
Fig. 1 summarizes the results of the biochemical endpoints analyzed and the type of response observed with respect to the control group in each exposure scenario considered, and 48 and 96 h after the exposure. A decrease in GST activity was observed in frogs exposed to all scenarios (p < 0.05) at 48 h, but only for S1 and S2 (p < 0.05) at 96 h (Fig. 1a). Also, a concomitant decrease of CAT activity was observed in animals exposed to S2 and S3 scenarios (p < 0.05) (Fig. 1b), and a significant increase in the activity of AChE (p < 0.05) was seen in frogs of S1 and S3 after 48 h (Fig. 1c). However, the analysis did not reveal a significant difference in CAT or AChE activity when comparing all scenarios with respect to control group after 96 h (p > 0.05).
Biochemical biomarker response in B. pulchella adults exposed to IMZT in different scenarios. The graphs represent the enzymatic activities of GST (a), CAT (b) and AChE (c) evaluated at 48 h in CAT and AChE and in both times elapsed in GST, from the acute exposure pulse. Fig. a represents 48 h with dotted gray bars and 96 h with dotted black bars. CG (control group), S1 (scenario 1: 10 mg/L IMZT), S2 (scenario 2: 100 mg/L IMZT) and S3 (scenario 3: 1000 mg/L IMZT). *p < 0.05; significant differences with respect to the control values. The activity of each enzyme is expressed as mmol/min/mg protein in the case of CAT and in μmol/min/mg protein for GST and AChE. The lines with whiskers represent the ES
Cytogenetic endpoints
The results at the cellular level from the analysis of MNs and nuclear abnormalities revealed that IMZT is able to induce MNs at both times analyzed but not nuclear abnormalities in adults of B. pulchella. Particularly, a significant increase in the frequency of MNs was observed in S1 (p < 0.01), S2 (p < 0.05) and S3 (p < 0.01); and S2 (p < 0.05) and S3 (p < 0.05), when compared to the control group at 48 and 96 h, respectively. On the other hand, significant differences were not detected compared to the control group when frequency of nuclear abnormalities in erythrocytes were analyzed for the two scenarios and durations evaluated (p > 0.05). As expected, the positive control group (CP-exposed frogs) showed a significant increase in MNs frequencies with respect to control group (p < 0.05) after both 48 h and 96 h of treatment.
When DNA damage was assessed, we confirmed that IMZT is capable of inducing genetic lesions. Particularly, the results showed a significant increase in GDI in frogs exposed to S1 (p < 0.01), S2 (p < 0.01) and S3 (p < 0.05) after 48 h of the IMZT pulse, although GDI remained increased only in frogs exposed to S3 (p < 0.05) after 96 h (Fig. 2). The positive control group (CP treatment) showed a significant increase in MNs frequencies with respect to the control group (p < 0.05) for both times evaluated.
Integration of biomarkers by PCA analysis
The results obtained from the reduction of dimensionalities using the PCA revealed an evident separation between the groups treated with IMZT in different scenarios and the negative control, which is explained by the correlation and integral response between the biomarkers used 48 and 96 h after the IMZT pulse (Fig. 3). Specifically, when the response of the 6 biomarkers (body condition, CAT, GST, AChE, MNs and IDG) was correlated and analyzed at the different levels of organization (individual, biochemical and cytogenetic) after 48 h, the analysis showed that biomarkers are organized into 3 main components that explain 68.60% (PC1 = 27.90%, PC2 = 23.70% and PC3 = 17.00%) of the variability of the data. In addition to this, from the biomarker response observed, it was possible to determine two large subgroups characterized, one by the negative control and the other containing all the IMZT exposure scenarios (S1, S2 and S3) (Fig. 3). Specifically, the separation of the control group with respect to S1, S2 and S3 was due to a greater extent to the response of the biochemical and cytogenetic biomarkers that contribute to the variability of PC1 (Fig. 3). From these analyses, it was also possible to show significant positive correlations between GDI and body condition (r = 0.30; p < 0.05) and both biochemical biomarkers, CAT and GST (r = 0.32; p < 0.05).
Biplot representing the responses of each biomarker evaluated in Boana pulchella adults exposed to the simulating scenarios of exposure to the imazethapyr-based commercial herbicide Pivot® H after 48 h. The two gray white circles show the separation between control group from scenarios of IMZT exposure delimited by biomarkers response. Figure showed control group (stars), scenario or 10 mg IMZT/L (triangles), scenario 2 or 100 mg IMZT/L (squares) and direct spraying application or 1000 mg IMZT/L (circles) obtained by the correlation between the biomarkers. The length of the red arrow indicates the magnitude of the response of the different biomarkers. AChE acetylcholinesterase, CAT catalase, GST glutathione-S-transferase, GDI genetic damage index, MNs micronuclei
The analyses revealed that after 96 h the situation is similar to what occurs after 48 h, where there is a significant difference in the response of the biomarkers that explains PC1 and allow the negative control group to be separated from all exposure scenarios to IMZT such as S1, S2 and S3. The results obtained by the integration of biomarkers using the PCA show that the response to IMZT, after 96 h of the acute pulse, is due to 3 biomarkers (GST, MNs and IDG). This analysis shows that the response is organized into 2 main components that explain 80.40% (PC1 = 47.10% and PC2 = 33.30%) of the variability of the data. In the same way as what was seen at 48 h, it was possible to determine two large subgroups characterized by the negative control and the scenarios of greater exposure to IMZT (S2 and S3). Specifically, the separation of the control group from the exposure scenarios with high concentrations was largely due to the response of GDI (which separates the subgroups on the PC1 axis) and GST and MNs frequency (which separates the subgroups on the axis PC2). From these analyses, it was also possible to show significant positive correlations between biochemical and cytogenetic biomarkers such as MNs and GST (r = 0.48; p < 0.05).
Discussion
Real-life exposure scenarios in Boana pulchella adults demonstrate the potential adverse effect of IMZT on the health and fitness of anurans. Few laboratory studies have evaluated adverse biological effects under realistic exposure scenarios in post-metamorphic anurans (Leiva-Presa and Munro Jenssen 2006; Quaranta et al. 2009; Ezemonye and Tongo 2010; Edge et al. 2011; Brühl et al. 2013; Selcer and Verbanic 2014; Van Meter et al. 2014; 2015; 2018; Glinski et al. 2018; Abercrombie et al. 2020). However, studies in Neotropical anurans integrating several biomarkers are scarce and limited to field studies (Castillo et al. 1991; 2005; Brodeur et al. 2011; 2012; Lajmanovich et al. 2018). In this context, this study is the first to evaluate in a laboratory context a real scenario of exposure integrating the effects of pesticides at multiple biological scales using a species that inhabits agricultural environments.
Biochemical analysis showed that IMZT induces CAT, GST and AChE alterations in B. pulchella adults. Specifically, we do confirm that the antioxidant system is altered due to the decrease in CAT and GST activity. These results are in full agreement with Pasha and Singh (2005), Moraes et al. (2011) and Pasha (2013) who report that IMZT, by causing failures in the normal functioning of these enzymes, may cause an increase in ROS (reactive oxygen species). In this context, the imbalance due to excess ROS is known as oxidative stress. In short, regulating the balance between ROS production and the activation of antioxidant cell defenses (e.g., CAT and GST) is essential to maintain cell viability (Ferrari et al. 2008). Thus, the observed decrease in CAT and GST activity in B. pulchella adults could be considered a detrimental response to a stress situation by an increase in ROS. Specifically, GST activity is involved in phase II biotransformation of xenobiotics with the CYP-450-dependent monooxygenase system while CAT is involved in hydrogen peroxide uptake. In this way, they prevent oxidative damage after acute exposure in anurans (Attademo et al. 2007; Ferrari et al. 2008; 2011). In particular, the GST response to IMZT in these frogs agrees with that reported by Moraes et al. (2011) who found a decrease in the GST activity for Cyprinus carpio indicating that the action of the herbicide is not specific for a particular target enzyme. However, in these cases where inhibition of the enzymes involved with antioxidant systems occurs, the authors highlight it may be due to a physiological malfunction product of some type of dehydration associated with anoxia in frogs exposed and will end up compromising oxygenation in tissues and organs (Ferrari et al. 2011; Brodeur et al. 2012).
On the other hand, our studies revealed that adults’ frogs exposed to IMZT in a real situation causes AChE alterations, a response similar to that reported in C. carpio after being exposed to IMZT (Moraes et al. 2011). AChE plays an important role in the cholinergic system, causing the hydrolysis of the neurotransmitter acetylcholine at cholinergic synapses and neuromuscular junctions. In this sense, AChE has been used as a biomarker in many studies with anurans because it can be altered by the action of many pesticides (Attademo et al. 2011; Brodeur et al. 2012). In this context, in this study significant AChE increases were observed indicating that IMZT is affecting the cholinergic system and promoting undesirable adverse effects that may in the long-term lead to behavioral effects (Moraes et al. 2011; Pérez-Iglesias et al. 2015; 2020). Finally, our studies reveal that the AChE-enzyme evaluated here is an excellent biochemical biomarker of response to pesticides exposure not only in larvae of anurans but also in adults of B. pulchella, under experimental conditions.
Results also showed that IMZT-exposure at different scenarios produce cytogenetic damage. Specifically, MNs increased and DNA damage was observed for all possible exposure scenarios. It is important to highlight that genomic instability plays a fundamental role in the fitness decrease in the populations of aquatic vertebrates (Barni et al. 2007; Jha 2008). When DNA damage occurs, either due to an increase in MNs, nuclear alterations or damage directly to the DNA, this situation can end with cell death and lead to severe patho-physiological situations that increase physiological stress (Barni et al. 2007; Çavaş and Könen 2007; Jha 2008). Also, another scenario can occur if the damaged cells survive. In this case, damaged cells not repaired will also have immediate adverse effects on the health of these organisms, which may ultimately result in decreased survival and long-term population effects (Barni et al. 2007; Jha 2008). Finally, it is important to consider that both cytogenetic biomarkers are useful tools to be used not only for biomonitoring but also in these considered exposure modes more realistic to pesticides assessments. However, this work reports the first evaluation using the comet assay in adult Neotropical frogs experimentally exposed to pesticides.
In recent years, Newman (2014) highlighted the importance of evaluating the correlation of biomarkers as a whole and not treated separately. This information helps to understand not only the susceptibility of organisms to environmental stressors but also their mode of action and toxicity, which can later be used as early warning signals in environments that are disturbed or contaminated by the presence of environmental stressors. In particular, ecotoxicological evaluations focusing on biomarkers correlations at different scales are scarce. In this work, the multivariate analysis using a battery of biomarkers related at different levels of biological organization generates novel information not provided by the individual analysis of each biomarker. Briefly, we clearly observe the separation between those frogs exposed to IMZT from the control group, and also detect those biomarkers acting in each scenario. This situation corroborates that IMZT induces adverse effects and physiological alterations. Also, we observe a progression of effects at the levels of organization, from the cytogenetic and biochemical in low exposure scenarios (biomarkers) to irreversible effects at the individual level in conditions in high exposure scenarios, representing the concept of biomarkers proposed by Walker (2009). In this sense, a holistic approach of multivariate analysis allows the evaluation of multiple endpoints simultaneously and facilitates the general and particular understanding of the various effects of experimental exposure to an environmental stressor. This is an advantage for this type of analysis in contrast to the separate information provided by conventional analyses (Walker 2009; Newman 2014). In conclusion, we recommend an integrative biomarkers approach for ecotoxicological studies since it allows us to discern the groups of anurans that were exposed to environmental stressors from those that were not exposed and detect useful biomarkers for each exposure situation. It should be noted that this comprehensive and holistic analysis by IMZT-exposure had been reported in our previous work with this species in tadpoles (Pérez-Iglesias et al. 2020) and L. latinasus adults (Pérez-Iglesias et al. 2021), which shows that this analysis is useful when you want to evaluate integrative biomarker responses.
Considering exposure routes in this work is useful for making more realistic assessments of the ways pesticides enter and act on amphibians. Focusing on the ecological importance, we highlight that IMZT is one of the most present herbicides in rivers where Boana pulchella inhabits (Kuhn et al. 2021) which can also bioaccumulate in Neotropical frogs in high concentrations (Brodeur et al. 2021). In this study, the first scenario simulates surface runoff of pesticide that flows to the breeding sites (permanent and temporary lagoons) of the frogs. In this context, the highest concentrations of IMZT in runoff water occurs near the site of herbicide application and does not exceed 3% of what was initially applied (Dias Martini et al. 2013). In a second scenario, where the herbicide is applied directly on the leaves (where frogs rest and thermoregulate following its application), an estimated one-tenth of the applied concentration of IMZT reaches the exposed frog. A third scenario, considered the worst-case scenario exposure where a direct application to the frogs occurs with herbicide concentration equal to the recommendations of the manufacturer (e.g., frogs sprayed when actively foraging) (Van Meter et al. 2014). In these contexts, it is observed that after a realistic acute exposure of Boana pulchella, there was a significant increase in biochemical and cytogenetic damage in the proposed situation of surface runoff or non-direct application (S1 and S2) but the species presented more severe effects (cytogenetic damage, biochemical failures and individual alterations) in the worst exposure situation considered, direct exposure (S3). We understand that the cytogenetic biomarkers such as enzymes from antioxidant systems evaluated in this occasion are early signals of effect and exposure, responding to situations of low herbicide concentrations. In addition, we can understand that the combination of biochemical and cytogenetic biomarkers could be a valid tool to use in biomonitoring and laboratory studies with Boana pulchella. At this point, and as we pointed out previously (Pérez-Iglesias et al. 2015; 2017; 2018), IMZT could be a risk factor for Boana pulchella populations. Finally, further research evaluating integration of biomarkers should be developed to understand the impact of environmental stressors on anurans.
Abbreviations
- AChE:
-
acetylcholinesterase
- BL:
-
blebbed nucleus
- BN:
-
binucleated cell
- CAT:
-
catalase
- GST:
-
glutathione-S-transferase
- IMZT:
-
imazethapyr
- LB:
-
lobed nucleus
- MNs:
-
micronuclei
- NT:
-
notched nucleus
- PCA:
-
principal component analysis.
References
Abercrombie SA, de Perre C, Iacchetta M, Flynn RW, Sepúlveda MS, Lee LS, Hoverman JT (2020) Sublethal effects of dermal exposure to poly-and perfluoroalkyl substances on postmetamorphic amphibians. Environ Toxicol Chem 40:717–726. https://doi.org/10.1002/etc.4711
Agostini MG, Natale GS, Ronco AE (2009) Impact of endosulphan and cypermethrin mixture on amphibians under field use for biotech soya bean production. Int J Environ Health 3:379–389
Agostini MG, Kacoliris F, Demetrio P, Natale GS, Bonetto C, Ronco AE (2013) Abnormalities in amphibian populations inhabiting agroecosystems in northeastern Buenos Aires Province, Argentina. Dis Aquat Org 104:163–171. https://doi.org/10.3354/dao02592
Attademo AM, Peltzer PM, Lajmanovich RC, Cabagna M, Fiorenza G (2007) Plasma B-esterase and glutathione S-transferase activity in the toad Chaunus schneideri (Amphibia, Anura) inhabiting rice agroecosystems of Argentina. Ecotoxicology 16:533–539. https://doi.org/10.1007/s10646-007-0154-0
Attademo AM, Cabagna-Zenklusen MC, Lajmanovich RC, Peltzer PM, Junges CM, Bassó A (2011) B-esterase activities and blood cell morphology in the frog Leptodactylus chaquensis (Amphibia: Leptodactylidae) on rice agroecosystems from Santa Fe Province (Argentina). Ecotoxicology 20:274–282. https://doi.org/10.1007/s10646-010-0579-8
Attademo AM, Bionda CL, Peltzer PM, Lajmanovich RC, Seib SN, Basso A, Junges CM (2014) Edad, tamaño corporal en la madurez sexual, longevidad y potencial reproductivo de Leptodactylus latinasus y Leptodactylus mystacinus en un cultivo de soja y un bosque nativo del centro este de Argentina. Revista Mexicana de Biodiversidad 85:315–317. https://doi.org/10.7550/rmb.38361
Barni S, Boncompagni E, Grosso Bertone V, Freitas I, Fasola M, Fenoglio C (2007) Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquat Toxicol 81:45–54. https://doi.org/10.1016/j.aquatox.2006.10.012
Beebee TJ, Griffiths RA (2005) The amphibian decline crisis: a watershed for conservation biology. Biol Conserv 125:271–285. https://doi.org/10.1016/j.biocon.2005.04.009
Blaustein AR, Han BA, Relyea RA, Johnson PTJ, Buck JC, Gervasi SS, Kats LB (2011) The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Ann N Y Acad Sci 1223:108–119. https://doi.org/10.1111/j.1749-6632.2010.05909.x
Brodeur JC, Suarez RP, Natale GS, Ronco AE, Zaccagnini ME (2011) Reduced body condition and enzymatic alterations in frogs inhabiting intensive crop production areas. Ecotoxicol Environ Saf 74:1370–80. https://doi.org/10.1016/j.ecoenv.2011.04.024
Brodeur JC, Candioti JV, Soloneski S, Larramendy ML, Ronco AE (2012) Evidence of reduced feeding and oxidative stress in common tree frogs (Hypsiboas pulchellus) from an agroecosystem experiencing severe drought. J Herpetol 46:72–78. https://doi.org/10.1670/10-200
Brodeur JC, Damonte MJ, Vera Candioti J, Poliserpi MB, D’Andrea MF, Bahl MF (2020) Frog body condition: Basic assumptions, comparison of methods and characterization of natural variability with field data from Leptodactylus latrans. Ecol Indic 112:106098. https://doi.org/10.1016/j.ecolind.2020.106098
Brodeur JC, Damonte MJ, Rojas DE, Cristos D, Vargas C, Poliserpi MB, Andriulo AE (2021) Concentration of current-use pesticides in frogs from the Pampa region and correlation of a mixture toxicity index with biological effects. Environ Res 204:112354. https://doi.org/10.1016/j.envres.2021.112354
Brodeur JC, Vera Candioti J (2017) Impacts of agriculture and pesticides on amphibian terrestrial life stages: Potential Biomonitor/ Bioindicator Species for the Pampa Region of Argentina. In: Larramendy ML (ed) Ecotoxicology and genotoxicology-non-traditional terrestrial models. Royal society of chemistry, London, UK
Brühl CA, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure of amphibians: an underestimated cause of global decline? Sci Rep 3:1135. https://doi.org/10.1038/srep01135
Cabagna MC, Lajmanovich RC, Peltzer PM, Attademo AM, Ale E (2006) Induction of micronuclei in tadpoles of Odontophrynus americanus (Amphibia: Leptodactylidae) by the pyrethroid insecticide cypermethrin. Toxicol Environ Chem 88:729–737. https://doi.org/10.1080/02772240600903805
CASAFE (2013) Guía de Productos Fitosanitarios para la República Argentina. Cámara de Sanidad Agropecuaria y Fertilizantes, Buenos Aires
Castillo GA, Coviello A, Orce GG (1991) Effect of theophylline on the electrolyte permeability of the isolated skin of the toad Bufo arenarum. Arch Int Physiol Biochim Biophys 99:257–264. https://doi.org/10.3109/13813459109146932
Castillo GA, Chanampa Y, Orce GG (2005) Effect of mercuric chloride on electrical parameters and anion fluxes in the toad skin. Comp Biochem Physiol C Toxicol Pharmacol 140:21–27. https://doi.org/10.1016/j.cca.2004.12.003
Çavaş T, Könen S (2007) Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 22:263–268. https://doi.org/10.1093/mutage/gem012
Cei JM (1980) Amphibians of Argentina. Università degli Studi di Firenze, New Series Monografa
CONICET (2005) Reference Ethical Framework for Biomedical Research: Ethical Principles for Research with Laboratory, Farm, and Wild Animals. Consejo Nacional de Investigaciones Científicas y Técnicas. Exp. 344/99. Res. D. N° 1047. Secretaria de Ciencia, Tecnología e Innovación Productiva, Ministerio de Eduación, Ciencia y Tecnología, República Argentina. https://www.conicet.gov.ar/wp-content/uploads/OCR-RD-20050701-1047.pdf
Dias Martini LF, Mezzomo RF, de Avila LA, Massey JH, Marchesan E, Zanella R, Peixoto SC, Refatti JP, Cassol GV, Marques M (2013) Imazethapyr and imazapic runoff under continuous and intermittent irrigation of paddy rice. Agric Water Manag 125:26–34. https://doi.org/10.1016/j.agwat.2013.04.005
Edge CB, Gahl MK, Pauli BD, Thompson DG, Houlahan JE (2011) Exposure of juvenile green frogs (Lithobates clamitans) in littoral enclosures to a glyphosate-based herbicide. Ecotoxicol Environ Saf 74:1363–1369. https://doi.org/10.1016/j.ecoenv.2011.04.020
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Ezemonye L, Tongo I (2010) Sublethal effects of endosulfan and diazinon pesticides on glutathione-S-transferase (GST) in various tissues of adult amphibians (Bufo regularis). Chemosphere 81:214–217. https://doi.org/10.1016/j.chemosphere.2010.06.039
Fenech M (2007) Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084–1104. https://doi.org/10.1038/nprot.2007.77
Ferrari A, Lascano C, Pechen de D’Angelo AM, Venturino A (2011) Effects of azinphos methyl and carbaryl on Rhinella arenarum larvae esterases and antioxidant enzymes. Comp Biochem Physiol C Toxicol Pharmacol 153:34–39. https://doi.org/10.1016/j.cbpc.2010.08.003
Ferrari A, Anguiano L, Lascano C, Sotomayor V, Rosenbaum E, Venturino A (2008) Changes in the antioxidant metabolism in the embryonic development of the common South American toad Bufo arenarum: Differential responses to pesticide in early embryos and autonomous‐feeding larvae. J Biochem Mol Toxicol 22:259–267. https://doi.org/10.1002/jbt.20236
Furlong ET (2001) Methods of Analysis by the US Geological Survey National Water Quality Laboratory: Determination of Pesticides in Water by Graphitized Carbon-based Solid-phase Extraction and High-performance Liquid Chromatography/mass Spectrometry (No. 1). US Department of the Interior, US Geological Survey, Water-Resource Investigations Report 1-4134. https://nwql.usgs.gov/Public/pubs/WRIR01-4134.pdf
Garber JC, Barbee RW, Bielitzki JT (2011) Guide for the care and use of laboratory animals. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. National Academies Press, Washington, DC
Glinski DA, Henderson WM, Van Meter RJ, Purucker ST (2018) Effect of hydration status on pesticide uptake in anurans following exposure to contaminated soils. Environ Sci Pollut Res Int 25:16192–16201. https://doi.org/10.1007/s11356-018-1830-8
Guerra C, Aráoz E (2016) Amphibian malformations and body condition across an agricultural landscape of northwest Argentina. Dis Aquat Organ 121(2):105–116. https://doi.org/10.3354/dao03048
Hegde G, Krishnamurthy SV (2014) Analysis of health status of the frog Fejervarya limnocharis (Anura: Ranidae) living in rice paddy fields of Western Ghats, using body condition factor and AChE content. Ecotoxicol Environ Contam 9:69–76. https://doi.org/10.5132/eec.2014.01.009
Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL (2000) Quantitative evidence for global amphibian population declines. Nature 404:752–755. https://doi.org/10.1038/35008052
Howarth FG (2000) Non-target effects of biological control agents. In: Biological control: measures of success. Springer, pp 369–403
INTA (2008) Guía para cuidado y uso de animales para experimentación. Centro de Investigación en Ciencias Veterinarias, Instituto Nacional de Tecnología Agropecuaria (INTA), Secretaría de Agricultura, Ganadería, Pesca y Alimentación, República Argentina (Eds.)
IUCN (2020) The IUCN Red List of Threatened Species. Version 2020-3. Retrieved December 8, 2020, from https://www.iucnredlist.org
Jackson DA (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214. https://doi.org/10.2307/1939574
Jha AN (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23(3):207–221. https://doi.org/10.1093/mutage/gen014
Jolliffe IT, Cadima J (2016) Principal Component Analysis: A review and recent developments. Philosophical Transactions of the Royal Society, 374. https://doi.org/10.1098/rsta.2015.0202
Kawai K, Kaku K, Izawa N, Shimizu T, Fukuda A, Tanaka Y (2007) A novel mutant acetolactate synthase gene from rice cells, which confers resistance to ALS-inhibiting herbicides. Journal of Pesticide Science 32(2):89–98. https://doi.org/10.1584/jpestics.G06-40
Kegley SE, Hill BR, Orme S, Choi AH (2020) PAN Pesticide database. Pesticide action network. PAN557Pesticide database website, Oakland, CA. Retrieved March 19, 2020, from https://www.pesticideinfo.org
Kiesecker JM, Blaustein AR, Belden LK (2001) Complex causes of amphibian population declines. Nature 410:681–684. https://doi.org/10.1038/35070552
Köhler HR, Triebskorn R (2013) Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond. Science 341:759–765. https://doi.org/10.1126/science.1237591
Kuhn EC, Tavares Jacques M, Teixeira D, Meyer S, Gralha T, Roehrs R, Camargo S, Schwerdtle T, Bornhorst J, Silva Ávila D (2021) Ecotoxicological assessment of Uruguay River and affluents pre- and post-pesticides’ application using Caenorhabditis elegans for biomonitoring. Environ Sci Pollut Res 28:21730–21741
Kwet A, Aquino L, Lavilla E, di Tada I (2004) Hypsiboas pulchellus, Red List of Threatened Species. International Union for Conservation of Nature. Version 2012.2.
Lajmanovich RC, Cabagna M, Peltzer PM, Stringhini GA, Attademo AM (2005) Micronucleus induction in erythrocytes of the Hyla pulchella tadpoles (Amphibia: Hylidae) exposed to insecticide endosulfan. Mutat Res 587:67–72
Lajmanovich RC, Peltzer PM, Attademo AM, Colussi CL, Martinuzzi CS (2018) Blood biomarkers of common toad Rhinella arenarum following chlorpyrifos dermal exposure. Interdiscip Toxicol 11:148–154. https://doi.org/10.2478/intox-2018-0011
Leiva-Presa À, Munro Jenssen B (2006) Effects of p, p′-DDE on retinoid homeostasis and sex hormones of adult male European common frogs (Rana temporaria). J Toxicol Environ Health A 69:2051–2062. https://doi.org/10.1080/15287390600747676
Lin K, Xu C, Zhou S, Liu W, Gan J (2007) Enantiomeric separation of imidazolinone herbicides using chiral high‐performance liquid chromatography. Chirality 19:171–178. https://doi.org/10.1002/chir.20359
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: Review of the risks in a complex environment. Env Pol 157:2903–2927. https://doi.org/10.1016/j.envpol.2009.05.015
Van Meter RJ, Glinski DA, Purucker ST, Henderson WM (2018) Influence of exposure to pesticide mixtures on the metabolomic profile in post-metamorphic green frogs (Lithobates clamitans). Sci Total Environ 624:1348–1359. https://doi.org/10.1016/j.scitotenv.2017.12.175
Van Meter RJ, Adelizzi R, Glinski DA, Henderson WM (2019) Agrochemical mixtures and amphibians: the combined effects of pesticides and fertilizer on stress, acetylcholinesterase activity, and bioaccumulation in a terrestrial environment. Environ Toxicol Chem 38:1052–1061. https://doi.org/10.1002/etc.4375
Van Meter RJ, Glinski DA, Hong T, Cyterski M, Henderson WM, Purucker ST (2014) Estimating terrestrial amphibian pesticide body burden through dermal exposure. Environ Pollut 193:262–268. https://doi.org/10.1016/j.envpol.2014.07.003
Van Meter RJ, Glinski DA, Henderson WM, Garrison AW, Cyterski M, Purucker ST (2015) Pesticide uptake across the amphibian dermis through soil and overspray exposures. Arch Environ Contam Toxicol 69:545–556. https://doi.org/10.1007/s00244-015-0183-2
Moraes BS, Clasen B, Loro VL, Pretto A, Toni C, de Avila LA, Marchesan E, de Oliveira Machado SL, Zanella R, Reimche GB (2011) Toxicological responses of Cyprinus carpio after exposure to a commercial herbicide containing imazethapyr and imazapic. Ecotoxicol Environ Saf 74:328–335. https://doi.org/10.1016/j.ecoenv.2009.05.013
Newman MC (2014) Fundam Ecotoxicol Sci Pollut. CRC press, Boca Ratón, Florida
Van der Oost R, Beyer J, Vermeulen N (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/s1382-6689(02)00126-6
Ossana NA, Castañé PM, Salibián A (2013) Use of Lithobates catesbeianus tadpoles in a multiple biomarker approach for the assessment of water quality of the Reconquista River (Argentina). Arch Environ Contam Toxicol 65:486–497. https://doi.org/10.1007/s00244-013-9920-6
Pasha F (2013) Enzyme inhibition (AChE) in brain of Oreochromis mossambicus due to pesticidal pollution of herbicide Pursuit. J Biol Sci 1:91–100. https://doi.org/10.21608/EAJBSC.2013.16114
Pasha F, Singh R (2005) Enzyme inhibition (AChE) in muscles and skin of Oreochromis mossambicus due to pesticidal pollution of herbicide Pursuit. Asian. J Exp Sci 19:119–126
Peltzer PM, Lajmanovich RC, Sánchez-Hernandez JC, Cabagna MC, Attademo AM, Bassó A (2008) Effects of agricultural pond eutrophication on survival and health status of Scinax nasicus tadpoles. Ecotoxicol Environ Saf 70(1):185–197. https://doi.org/10.1016/j.ecoenv.2007.06.005
Pérez-Iglesias JM, Brodeur JC, Larramendy ML (2020) An imazethapyr-based herbicide formulation induces genotoxic, biochemical, and individual organizational effects in Leptodactylus latinasus tadpoles (Anura: Leptodactylidae). Environ Sci Pollut Res Int 27:2131–2143. https://doi.org/10.1007/s11356-019-06880-7
Pérez-Iglesias JM, Natale GS, Soloneski S, Larramendy ML (2018) Are the damaging effects induced by the imazethapyr formulation Pivot® H in Boana pulchella (Anura) reversible upon ceasing exposure? Ecotoxicol Environ Saf 148:1–10. https://doi.org/10.1016/j.ecoenv.2017.10.009
Pérez-Iglesias JM, Soloneski S, Nikoloff N, Natale GS, Larramendy ML (2015) Toxic and genotoxic effects of the imazethapyr-based herbicide formulation Pivot H® on montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol Environ Saf 119:15–24. https://doi.org/10.1016/j.ecoenv.2015.04.045
Pérez-Iglesias JM, Ruiz de Arcaute C, Natale GS, Soloneski S, Larramendy ML (2017) Evaluation of imazethapyr-induced DNA oxidative damage by alkaline Endo III-and Fpg-modified single-cell gel electrophoresis assay in Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol Environ Saf 142:503–508. https://doi.org/10.1016/j.ecoenv.2017.04.054
Pérez-Iglesias JM, Franco-Belussi L, Moreno L, Tripole S, de Oliveira C, Natale GS (2016) Effects of glyphosate on hepatic tissue evaluating melanomacrophages and erythrocytes responses in neotropical anuran Leptodactylus latinasus. Environ Sci Pollut Res Int 23:9852–9861. https://doi.org/10.1007/s11356-016-6153-z
Pérez-Iglesias JM, Ruiz de Arcaute CR, Nikoloff N, Dury L, Soloneski S, Natale GS, Larramendy ML (2014) The genotoxic effects of the imidacloprid-based insecticide formulation Glacoxan Imida on Montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol Environ Saf 104:120–126
Pérez-Iglesias JM, Fanali LZ, Franco-Belussi L, Natale GS, De Oliveira C, Brodeur JC, Larramendy ML (2021) Multiple Level Effects of Imazethapyr on Leptodactylus latinasus (Anura) Adult Frogs. Arch Environ Contam Toxicol 81:492–506
Pitarque M, Creus A, Marcos R, Hughes JA, Anderson D (1999) Examination of various biomarkers measuring genotoxic endpoints from Barcelona airport personnel. Mutat Res 440:195–204
Quaranta A, Bellantuono V, Cassano G, Lippe C (2009) Why amphibians are more sensitive than mammals to xenobiotics. PLoS ONE 4(11):e7699. https://doi.org/10.1371/journal.pone.0007699
R Core Team (2010) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Sanchez LC, Peltzer PM, Lajmanovich RC, Manzano AS, Junges CM, Attademo AM (2013) Reproductive activity of anurans in a dominant agricultural landscape from central-eastern Argentina. Revista Mexicana de Biodiversidad 84:912–926. https://doi.org/10.7550/rmb.32842
Sansiñena JA, Peluso L, Costa CS, Demetrio PM, Mac Loughlin TM, Marino DJ, Alcalde L, Natale GS (2018) Evaluation of the toxicity of the sediments from an agroecosystem to two native species, Hyalella curvispina (CRUSTACEA: AMPHIPODA) and Boana pulchella (AMPHIBIA: ANURA), as potential environmental indicators. Ecological indicators 93:100–110
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass–size residuals: validating body condition indices. Ecology 86:155–163. https://doi.org/10.1890/04-0232
Selcer KW, Verbanic JD (2014) Vitellogenin of the northern leopard frog (Rana pipiens): development of an ELISA assay and evaluation of induction after immersion in xenobiotic estrogens. Chemosphere 112:348–354
Shutler D, Marcogliese DJ (2011) Leukocyte profiles of northern leopard frogs, Lithobates pipiens, exposed to pesticides and hematozoa in agricultural wetlands. Copeia 301–307. https://doi.org/10.1643/CP-10-065
Sparling DW, Linder G, Bishop CA, Krest SK (2010) Ecotoxicology of amphibians and reptiles. SETAC Books, Boca Raton, FL
Suárez RP, Zaccagnini ME, Babbitt KJ, Calamari NC, Natale GS, Cerezo A, Codugnello N, Boca T, Damonte MJ, Vera-Candioti J (2016) Anuran responses to spatial patterns of agricultural landscapes in Argentina. Landsc Ecol 31:2485–2505. https://doi.org/10.1007/s10980-016-0426-2
Tan S, Evans RR, Dahmer ML, Singh BK, Shaner DL (2005) Imidazolinone‐tolerant crops: history, current status and future. Pest Manag Sci 61:246–257. https://doi.org/10.1002/ps.993
USEPA (1975) Methods for acute toxicity tests with fish, macroinvertebrates, andamphibians. In: Agency, U.E.P. (Ed.). USEPA. 660/3-75-009,62
USEPA (1989) Imazethapyr Herbicide Profle 3/89. Chemical Fact Sheet for Imazethapyr. United States Environmental Protection Agency, Ofce of Pesticide Programs, Washington DC. 196 pp
Vaira M, Akmentins M, Attademo M, Baldo D, Barrasso D, Barrionuevo S, Basso N, Blotto B, Cairo S, Cajade R, Céspedez J, Corbalán V, Chilote P, Duré M, Falcione C, Ferraro D, Gutierrez FR, Ingaramo MR, Junges C, Lajmanovich R, Lescano JN, Marangoni F, Martinazzo L, Marti L, Moreno L, Natale G, Pérez Iglesias JM, Peltzer P, Quiroga L, Rosset S, Sanabria E, Sanchez L, Schaefer E, Úbeda C, Zaracho V (2012) Categorización del estado de conservación de los anfibios de la República Argentina. Cuadernos de Herpetología 26:131–159
Vasseur P, Cossu-Leguille C (2003) Biomarkers and community indices as complementary tools for environmental safety. Environ Int 28:711–717. https://doi.org/10.1016/S0160-4120(02)00116-2
Vera Candioti J, Natale GS, Soloneski S, Ronco AE, Larramendy ML (2010) Sublethal and lethal effects on Rhinella arenarum (Anura, Bufonidae) tadpoles exerted by the pirimicarb-containing technical formulation insecticide Aficida®. Chemosphere 78:249–255. https://doi.org/10.1016/j.chemosphere.2009.10.064
Wake DB (1991) Declining amphibian populations. Science 253:860. https://doi.org/10.1126/science.253.5022.860
Walker CH (2009) Organic pollutants: an ecotoxicological perspective. CRC Press, Boca Raton, FL
Wang MZ, Jia XY (2009) Low levels of lead exposure induce oxidative damage and DNA damage in the testes of the frog Rana nigromaculata. Ecotoxicology 18:94–99. https://doi.org/10.1007/s10646-008-0262-5
Zar JH (2010) Biostatistical Analysis, 5 ed. Prentice Hall, New Jersey
Funding
This study was funded by Doctoral Internal Scholarships Granting Institution: National Commission for Scientific and Technical Research (CONICET). Grants File No. 11/N817 and 11/N847, Research Project at the National University of La Plata: “Empleo de herramientas bioanalíticas de respuesta temprana a la exposición de contaminantes emergentes en sistemas in vivo e in vitro. Facultad de Ciencias Naturales y Museo. Project I + D: 11/N746”, the project from Agencia Nacional de Promoción Científica y Tecnológica (FONCYT): PICT 2015-3137 “Desarrollo y aplicación de herramientas de diagnóstico de contaminación ambiental utilizando anuros autóctonos como indicadores”, and to the Research Project 2019-PE-E2-I054 Funded by INTA.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Pérez-Iglesias, J.M. (JMPI), Brodeur, J.C. (JCB), Natale, G.S. (GSN) and Larramendy, M.L (MLL). The first draft of the manuscript was written by JMPI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: JMPI, JCB, GSN and MLL; Methodology: JMPI, JCB, GSN and MLL; Formal analysis and investigation: JMPI, JCB, and MLL; Writing – original draft preparation: JMPI; Writing – review and editing: JCB and MLL; Funding acquisition and Resources: JCB, GSN and MLL; Supervision: JCB and MLL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
The authors have consented to the submission.
Consent to publish
The authors give their consent for the publication of identifiable details, which can include photograph(s) and/or videos and/or case history and/or details within the text to be published in the above Journal and Article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pérez-Iglesias, J.M., Natale, G.S., Brodeur, J.C. et al. Realistic scenarios of pesticide exposure alters multiple biomarkers in BOANA PULCHELLA (ANURA) Adult Frogs. Ecotoxicology 32, 309–320 (2023). https://doi.org/10.1007/s10646-023-02639-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02639-6