Abstract
Activity of B-esterases (BChE: butyrylcholinesterase and CbE: carboxylesterase using two model substrates: α-naphthyl acetate and 4-nitrophenyl valerate) in a native frog, Leptodactylus chaquensis from rice fields (RF1: methamidophos and RF2: cypermethrin and endosulfan sprayed by aircraft) and non-contaminated area (pristine forest) was measured. The ability of pyridine-2-aldoxime methochloride (2-PAM) to reactivate BChE levels was also explored. In addition, changes in blood cell morphology and parasite infection were determined. Mean values of plasma BChE activities were lower in samples from the two rice fields than in those from the reference site. CbE (4-nitrophenyl valerate) levels varied in the three sites studied, being highest in RF1. Frog plasma from RF1 showed positive reactivation of BChE activity after incubation with 2-PAM. Blood parameters of frogs from RF2 revealed morphological alterations (anisochromasia and immature erythrocytes frequency). Moreover, a major infection of protozoan Trypanosoma sp. in individuals from the two rice fields was detected. We suggest that integrated use of several biomarkers (BChE and CBEs, chemical reactivation of plasma with 2-PAM, and blood cell parameters) may be a promising procedure for use in biomonitoring programmes to diagnose pesticide exposure of wild populations of this frog and other native anuran species in Argentina.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus (OP) and carbamate (CB) insecticides affect invertebrates that damage crop plants by inhibition of cholinesterase enzyme (ChE), essential for normal functioning of the central and peripheral nervous system (Grue et al. 1997; Parsons et al. 2000). These anticholinesterase chemicals, despite of their low bioaccumulation in the environment, can also have similar detrimental effects on non-target wildlife species and their populations (Chambers and Levi 1992). Particularly, exposure to these pesticides can be deleterious to amphibian species and may become an important factor in population declines in agricultural landscapes (Sparling et al. 2001; Davidson et al. 2002; Mann et al. 2009).
In the last decade, rice crops have substantially increased in Argentina, particularly in Santa Fe, Entre Ríos, and Corrientes Provinces; where 90% of the total rice of the country is produced (Aranguren 1998; Begenesic 1998). Such intensive agriculture has led to an increase in the use of OP, CB, and pyrethroid (PYT) compounds (CASAFE 2005). Rice fields are supplemental habitats for reproduction of amphibians and provide water sources during their adult stage (Bambaradeniya et al. 2004; Duré et al. 2008), increasing their vulnerability to exposure to pesticides.
In this context, plasma B-esterase activity has been used in several studies to monitor vertebrate wildlife exposed to pesticides (McCarthy and Shugart 1990; Walker 1998; Chuiko et al. 2003; Sánchez-Hernández 2006; Wheelock et al. 2008). Particularly, butyrylcholinesterase (BChE) and carboxylesterase (CbE) were recommended as useful indicators of amphibian exposure to anti-ChE chemicals (Lajmanovich et al. 2004, 2008; Attademo et al. 2007). PYT, CB, and OP pesticides usually inhibit these enzymes (Dettbarn et al. 1999; Sogorb and Vilanova 2002; Wheelock et al. 2004, 2008; Sánchez-Hernández 2006). Moreover, chemical reactivation techniques based on oximes (e.g., pyridine-2-aldoxime methochloride [2-PAM]) can be used to evaluate exposure to OP pesticides and has been suggested as an indicator of exposure to anti-ChE pesticides (Soler-Rodríguez et al. 1998; Parsons et al. 2000; Maul and Farris 2005). Accordingly, reactivation of phosphorylated BChE activity using 2-PAM has been applied in laboratory and field studies using amphibians and reptiles (Sánchez-Hernández et al. 2004; Attademo et al. 2007; Lajmanovich et al. 2008).
In addition, haematological parameters such as erythrocyte and leukocyte morphologies, can also be used as biomarkers and have potential applications in terrestrial and aquatic ecotoxicological studies (Cabagna et al. 2005; Marques et al. 2009). The blood of amphibians is a very plastic tissue (Barni et al. 2007), and variations in the morphology of several blood cells in anurans have frequently been reported as a response to stress in agroecosystems (Stansley and Roscoe 1996; Cabagna et al. 2005; Barni et al. 2007). Similarly, studies by Johnson and Chase (2004) suggested that agricultural runoff might intensify parasite infection and the frequency of malformations in amphibians. Few field studies in Argentina have demonstrated significant relationships between enzyme activity and haematological parameters of amphibians, and application of pesticides (Lajmanovich et al. 2004; Attademo et al. 2007).
In the present study, we hypothesized that different levels of enzymatic activity and blood cell parameters occur in frogs Leptodactylus chaquensis inhabiting rice fields compared with those from a reference site (pristine forest) in the Mid-eastern Argentina. Measurements of BChE, CbE (using two model substrates; α-naphthyl acetate and 4-nitrophenyl valerate), and blood cell morphology have been selected as stress indicators in frog for this study. In addition, we investigated the potential recovery of BChE activity in the presence of 2-PAM. Parasite infections were also evaluated. This study forms part of a project to investigate adverse effects of exposure the pesticides on native populations of anurans in Argentine agroecosystems.
Materials and methods
Study area
The study area is situated in Santa Fe Province in Mid-eastern Argentina. The area belongs to the Espinal ecoregion (Burkart et al. 1999); average annual rainfall in the area is 800 mm, and mean annual temperature is 18°C. The area is dominated by wetlands, remnants of fluvial forests, and intensively managed agricultural lands (cultivated with soybean, maize, wheat, and rice) where the use of pesticides is constant throughout the year (Silva et al. 2005; Jergentz et al. 2005).
In Santa Fe Province, rice production is located mainly on the floodplains of the Paraná River (Alvisio 1998), occupying a north–south area of approximately 15–20 km wide and 100 km long (Ruiz 1998) (the rice cultivation area reached 17,624 ha in 2008). The expansion of the rice crop involves deforestation and destruction of the Espinal forests. Rice cultivation in Santa Fe alternates with extensive cattle grazing, with farms ranging between 150 and 500 ha (Begenesic 1998).
Fields survey
To compare the effects of chemical exposure we selected three areas in Santa Fe Province, Argentina (Fig. 1). The reference site (RS) was located within a pristine forest (700 ha) in Garay department (31° 18′ 52.35″ S; 60°16′ 37.01″ W). We considered it free of anticholinesterase chemicals because no agricultural activities or pesticide uses have been observed in this area. Agricultural sampling sites were two rice fields (RF1 = 120 ha and RF2 = 287 ha) in San Javier department (RF1: 30° 00′ 36.8′′ S–59° 50′ 27.5′′ W and RF2: 30° 00′ 31.8′′ S–59° 52′ 19.4′′ W), that differed in pesticide treatment and application. Methamidophos (1,000 cc/ha of Me) was sprayed by aircraft in RF1, while cypermethrin (200 cc/ha of Cy) and endosulfan (800 cc/ha of E) were sprayed by airplane in RF2. Pesticides were applied on 4 and 6 January 2008 in RF1 and RF2, respectively. These pesticide doses are the recommended concentrations for treatment of rice pests (e.g.: Hemiptera: Pentatomidae; Tibraca limbativentris and T. obscurata) (CASAFE 2005; Olmos 2006).
Animal sampling
Fifty-one adult male L. chaquensis were collected by hand from sampling areas (RS = 12, RF1 = 20, and RF2 = 19) in January 2008, 7 days after aerial spraying. This species of frog was selected because it is frequently found in rice fields (Duré et al. 2008) and individuals were commonly observed in unpaved roads across rice fields during the night (2200 and 2400 h). After capture, animals were rapidly transported to the laboratory in darkened buckets containing water to minimize stress. Blood samples (0.5 ml approximately) were collected by cardiac puncture using a heparinized syringe (Lajmanovich et al. 2004; Attademo et al. 2007). Anaesthesia was not used because it might have interfered with enzymatic activity (Vernadakis and Routledge 1973). Busk et al. (2000) also reported that anaesthesia might be more stressful to amphibians and reptiles than cardiac puncture. Likewise, Tyler (1999) suggested that cardiac puncture is a reliable method. Therefore, we considered the practical experience of our personnel with amphibians, the health of animals after blood sampling, and minimum blood volume required for experimental purposes as the criteria for selecting cardiac puncture as the most appropriate blood sampling technique in the frogs studied. Blood was centrifuged at 10,000 rpm for 15 min, and the plasma was separated and immediately frozen (−25°C). Snout-vent length and body mass were recorded, and a condition factor (CF) for each animal, expressed as 100 × [body weight (g)]/[length (cm)]3 was then calculated (Bagenal and Tesch 1978). Frogs were toe-clipped for later individual identification, and after determining their health status, they were released at the sites where they have been captured.
B-esterase assays
Plasma BChE activity was determined colorimetrically by the Ellman et al. (1961). The reaction medium included 1,870 μl 25 mM Tris–HCl, 1 mM CaCl2 (pH = 7.6), 100 μl 5,5′-dithiobis-2-nitrobenzoic acid (3 × 10−4 M, final concentration), 20 μl butyrylthiocholine iodide (2 × 10−3 M, final concentration) and 10 μl of plasma. The variation in optical density was recorded at 410 nm for 1 min at 25°C using a Jenway 6405 UV–VIS spectrophotometer. Kinetic was carried out in duplicate. Plasma BChE activity was expressed as μmol of substrate hydrolyzed min−1 ml−1 of plasma using a molar extinction coefficient of 13.6 × 103 M−1 cm−1. We did not determine the plasma AChE activity because BChE is the enzyme that primarily contributes to total plasma ChE activity in many vertebrate species (Thompson and Walker 1994; Sánchez-Hernández and Moreno-Sánchez 2002; Bain et al. 2004).
We used two different substrates for CbE analysis because this esterase activity comprises multiple isozymes. Carboxylesterase activity using the substrates α-naphthyl acetate (α-NA) and 4-nitrophenyl valerate (4-NPV), and enzyme activity was expressed as μmol min−1 ml−1 of plasma. Carboxylesterase activity using α-NA was measured by the Gomori method (1953) as adapted by Bunyan and Jennings (1968). The reaction solution (final volume; F.V. = 1,940 μl) contained 25 mM Tris–HCl, 1 mM CaCl2 (pH = 7.6) and the sample (10 μl of plasma; 1/5 dilution). The reaction was initiated with the addition of 50 μl α-naphthyl acetate (1.04 mg/ml in acetone) after a preincubation period of 5 min at 25°C. The formation of naphthol was stopped after 10 min by addition of 500 μl 2.5% sodium dodecyl sulphate and subsequently 500 μl 0.1% Fast Red ITR in 2.5% Triton X-100 in water (freshly prepared). The samples were left in darkness for 30 min to develop, and the absorbance of the complex was read at 530 nm (using a molar extinction coefficient of 33.225 × 103 M−1 cm−1). Hydrolysis of 4-NPV by CbE was determined as described by Carr and Chambers (1991). Samples (20 μl of plasma; 1/50 dilution) were preincubated in 50 mM Tris–HCl (pH = 7.5) for 5 min at 25°C (F.V. = 1,960 ml), and the reaction was initiated by the addition of 20 μl 4-NPV (5 × 10−4 M, final concentration). After 10 min, the reaction was stopped by the addition of a solution 0.5 ml 2% (w/v) SDS and 0.5 ml of 2% (w/v) Tris base. The 4-nitrophenol liberated was read at 405 nm and quantified by a calibration curve (5–100 mM).
Chemical reactivation of BChE
Previous experiments have indicated 2-PAM-induced reactivation of OP-inhibited BChE activity of three anuran species after 60-min incubation of the sample at 25°C in the presence of the oxime (Lajmanovich et al. 2008). In the current study, we used two aliquots of each plasma sample from the three sampling sites for testing reactivation of BChE activity in the presence of 2-PAM. An aliquot was incubated in the presence of 2-PAM (1/5, dilution factor) to yield a final concentration of 1.7 × 10−3 M for 60 min at 25°C. A second aliquot of plasma was diluted with dH2O (1/5 factor dilution) and assayed for BChE activity and used as a control. The BChE activity of the control was compared. We assumed that BChE activity was inhibited by pesticide compounds when an increase of esterase activity after 2-PAM incubation was greater than 5% of controls.
Blood cell morphology and parasite infection
Two blood smears for each frog were prepared on clean slides, fixed, and stained by the May–Grunwald–Giemsa method (Dacie and Lewis 1984). Red blood cells (RBC) were evaluated for evidence of anisocytosis (AS) and anisochromasia (AC) through the determination of macrocytes (MA), microcytes (MI), polychromatophils (PY), and hypochromic (HY) frequencies. Furthermore, immature erythrocyte (IE), which consists in erythroblast and PY frequency (Marques et al. 2009), was also determined. Those frequencies were expressed as percentage (%) of mature erythrocyte counted. Genotoxicity was tested using the presence of erythrocyte nuclear abnormalities (ENA), carried out in mature peripheral erythrocytes according to the procedures of Guilherme et al. (2008) by determination of the frequency of the following nuclear lesions: micronuclei (MN), lobed nuclei (L), binucleates or segmented nuclei (S), kidney shaped nuclei (K), and notched nuclei (N). The results were expressed as ENA frequency, the mean value (%) of the sum (MN + L + S + K + N) for all the lesions observed. The appearance of different leukocytes was assessed before performing a differential leukocyte count (heterophils, eosinophils, basophils, lymphocytes, and monocytes). Moreover, the heterophil/lymphocyte (H/L) ratio was determined as a response estimator of stress (Davis et al. 2008). Slides were also examined for the presence of blood parasites under ×40 and ×100 magnification. We defined parasite prevalence as the number of hosts infected by a particular hematozoan species taxon divided by the total number of hosts examined and it was expressed as percentages (DeJong and Muzzall 2000).
Statistical analysis
Data are presented as the mean ± SEM. Because we could not meet the assumption of normality (Kolmogorov–Smirnov and Levene test) to perform ANOVA, we ran non-parametric Kruskal–Wallis tests, followed by Dunn post-hoc test to determine differences between sampling sites for enzymatic activity (BChE and CbEs), body condition factor, and blood cell morphology. We evaluated prevalence of parasite infection between sites using χ2. A value of P < 0.05 was considered significant.
Results
B-esterase activity
The mean condition factor (CF) values for L. chaquensis collected from rice fields were 9.23 ± 2.32 (RF1) and 11.24 ± 2.50 (RF2), which were similar to those for frogs from the reference site (RS = 10.86 ± 1.67) (Kruskal–Wallis KS test H = 1.90; P = 0.18).
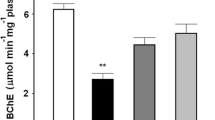
The BChE activity was statistically lower for frogs collected from the two rice fields (RF1 and RF2) respect to reference site (RS, H = 5.20; P = 0.04). The Dunn post-hoc multiple comparisons test detected significantly greater BChE activity in frogs captured at RS (7.06 ± 1.38 μmol min−1 ml−1 plasma) compared with those in the two rice fields (RF1 = 4.29 ± 0.75 and RF2 = 3.67 ± 0.56 μmol min−1 ml−1 plasma) (Fig. 2).
The CbE (4-PNV) activity was significantly different among frogs sampled from the study sites (H = 6.16; P = 0.03, Fig. 3). The Dunn test for post-hoc multiple comparisons detected significantly lower CbE (4-PNV) activity in frogs from RS (RS = 5.73 ± 0.85 μmol min−1 ml−1 plasma) than in those captured in RF1 (10.27 ± 1.13 μmol min−1 ml−1 plasma). However, CbE (4-PNV) values from RS and RF2 (7.09 ± 0.96 μmol min−1 ml−1 plasma) were similar. The CbE (α-NA) activity was similar among frogs sampled from rice fields (RF1 and RF2) and RS (H = 0.93; P = 0.64, Fig. 4).
Chemical reactivation of BChE
The reactivation of BChE activity using 2-PAM was not possible for all samples of agricultural sites (RF1 = 35%; RF2 = 50%) due to plasma volume limitations. Positive oxime reactivation was determined only in 30.75% of samples from RF1; no reactivation was detected in the samples from RF2. The percentage of plasma BChE activity increased from 6 to 44%, after 2-PAM incubation.
Blood cell morphology and parasite infection
The blood cell parameters of frogs from agricultural sites (RF1 and RF2) did not differ significantly from those of RS (P > 0.05, Table 1), with the exception of AC and IE from RF2 (Fig. 5). The prevalence of protozoan parasite infection (Trypanosoma sp., Fig. 6) was higher in frog blood samples from rice fields (RF1 = 60% and RF2 = 84.2%) than those from RS (25%) (χ2, P = 0.0001).
Discussion
Plasma BChE of L. chaquensis was significantly inhibited in the two agricultural sites (RF1 and RF2) compared with enzyme activity of individuals the reference site (RS). Mean BChE from RF1 and RF2 ranged between 4.29 ± 0.75 and 3.67 ± 0.56 μmol min−1 ml−1 plasma, respectively. These values are higher than BChE values reported for a sympatric species (Rhinella schneideri, 1.18 ± 0.26 and 1.02 ± 0.19 μmol min−1 ml−1 plasma) from rice agroecosystems (Attademo et al. 2007). However, a similar decreasing trend of BChE activity was observed in cropped sites with respect to RS. Notably, reduction in BChE activity in frogs from RF2, where no organophosphorus pesticide (OP) was sprayed, is attributed to the application of cypermethrin (Cy). These observation is in agreement with findings of Khan et al. (2003) and Khan (2005), who determined that pyrethroid pesticides inhibit BChE activity of anuran (Rana cyanophlystis) and reptile (Calotes versicolor) species. Moreover, another possible explanation for inhibition of BChE levels of frogs from this agricultural site is that endosulfan (E) and Cy can act in synergism to inhibit BChE activity. Recently, Mor and Ozmen (2010) demonstrated cholinesterase activity inhibition induced by E in rabbit. Moreover, plasma samples from frogs collected from RF1 treated with 2-PAM presented positive reactivation (between 6 and 44%), in contrast to samples collected from RF2, where no reactivation was observed. This finding allowed us to identify organophosphates (methamidophos) as the agrochemicals responsible for plasma BChE depression in the animals collected from RF1. Furthermore, the lowest recovery values are in agreement with Sánchez-Hernández et al. (2004) and Lajmanovich et al. (2008). These authors reported that serum BChE activity of herpetofauna previously inhibited by OP did not recover totally in the presence of 2-PAM. Likewise, Sánchez-Hernández (2003) and Sánchez-Hernández et al. (2004) observed similar increments of BChE activity reactivation after exposure to pesticides in Gallotia galloti (between 8 and 60%) and G. galloti palmae (between 5 and 53%) from agroecosystems. Accordingly, Parsons et al. (2000) and Iko et al. (2003) indicated that chemical reactivation of plasma phosphorylated ChE activity has been used for complementary diagnosis of OP intoxication.
On the other hand, Wheelock et al. (2008) recommended the use of a battery of substrates to establish CbE activity in field monitoring. In this sense, we used two different substrates (α-NA and 4-PNV) to evaluate CbE activity, considering that CbE reflects different sensitivity to OP and CB pesticides (Laguerre et al. 2009). We found a marked difference in CbE activity, depending on the substrate and pesticide treatment (RF1: Me and RF2: E and Cy). Plasma CbE (4-PNV) activity was higher in L. chaquensis collected from RF1 than in specimens from the reference sites and RF2. Sánchez-Hernández (2006) and Wheelock et al. (2008) suggested that higher levels and isozyme abundance of CbEs may contribute to pesticide tolerance and are able to bind to the OP, therefore decreasing the effective concentration of pesticide (Maxwell and Brecht 2001). Considering CbE (4-PNV) activities observed in the three study sites, it seems that values for CbE (4-PNV) activity in RF2 were not statistically different from RS, implying a biological response. However, this assumption needs to be tested. According to these results, it seems that inhibition of plasma CbE activity might depend on a number on factors related to the chemicals used, such as concentration, toxicity, field application rate, and type of pesticide. Likewise, CbE level might depend on intra-specific and inter-specific variation. Based on the known role of CbE in detoxification of pesticides, we propose that over-expression or abundance of this enzyme (CbE, substrate 4-PNV) may be involved in the mechanism of resistance to Me, but would exhibit low or no resistance to E and Cy insecticides in the different populations of L. chaquensis sampled 7 days after application. By contrast, Sánchez et al. (1997) reported that a maximum inhibition of CbE activity was recorded in the bird Serinus canaria 17 days after spraying. Conversely, field studies demonstrated significant reductions in BChE activity 24 h after pesticide exposure (pirimicarb or dimethoate); and no significant inhibition was observed for CbE activity (Cordi et al. 1997). In addition, CbE (α-NA) activity was similar in the three study sites, with values lower than those of CbE (4-PNV) activity. As mentioned above, these variations in CbEs levels dependent on substrates used may indicate that periodic exposure to adverse environmental factors (type of pesticide, timing and application form, doses) may evoke particular adaptive responses allowing the animal to maintain the optimal level of life activity (Kornienko et al. 1965).
In addition, we observed few immature erythrocytes (IE) and frequency of anisochromasia (AC) in frogs collected from RF2, fumigated with Cy and E. These results are in agreement with studies of Kaplan and Glaczenski (1965), Arshad et al. (2001), and Hii et al. (2007), who demonstrated that Cy, E, and OP could affect haematological parameters in different vertebrate species. Similarly, haematological alterations were reported in amphibians (Rhinella arenarun and Rana esculenta) commonly found in soybean, rice, and horticultural crops (Cabagna et al. 2005; Barni et al. 2007). Although the variations we found may be common in amphibians during summer (Varela and Sellarés 1938), the decrease in IE and AC parameters may be related to the development of non-regenerative anaemia (Cabagna et al. 2005; Allender and Fry 2008). Accordingly, the evaluation of the general morphology observed here does not contribute to the characterization of this type of anaemia, since no hypochromia or size changes were found in red blood cells of L. chaquensis. In addition, when blood volume is not adequate packed cell volume and haemoglobin concentration should be measured to determine this type of anaemia, Conversely, there were no differences in frequency of erythrocyte nuclear abnormalities (ENA), indicating no genotoxicity effects. The frequency of nuclear lesions may be altered by several factors, such as erythropoiesis (Udroiu 2006). Erythropoiesis decrease may be related to IE decline (Varela and Sellarés 1938; Marques et al. 2009), this situation being observed in RF2. In fact, ENA frequency might have been falsely reduced. Furthermore, differential leukocyte count and heterophile/lymphocyte (H/L) ratio were not different between the two agroecosystem fields, which could be explained by the fact that time lag required for the leukocyte stress response should be longer than that spanned between pesticide application and sample extraction (Pough 1980).
Furthermore, we observed a greater infection by the protozoan Trypanosoma sp. in L. chaquensis from the two rice fields. Although systemic parasite infestations rarely causes disease in wildlife amphibians, chemical stress in the form of high level of exposure to pesticides may have reduced the host frog ability to resist infection, resulting in higher parasite loads (Peltzer et al. 2008; Marcogliese et al. 2009). In addition, low exposure to E and Cy might produce deleterious effects on the immune system, even when there are few or no detectable effects (Pistl et al. 2001) and such health condition could increase individual vulnerability to parasite infection (Arshad et al. 2001). Nevertheless, little is known about the relationship between OP and parasites (Kaplan and Glaczenski 1965). In agreement with Marcogliese et al. (2009), relationships between a B-esterase enzymes, pesticides, and parasite infection are difficult to explain and require further studies.
Overall, our results demonstrate that: (1) BChE of L. chaquensis was inhibited in rice crops, (2) 2-PAM chemical procedure reactivated samples of individuals exposed to methamidophos, (3) CbE exhibited different responses depending on the substrate (α-NA or 4-PNV) used and pesticide exposition, (4) individuals occurring in the rice field sprayed with endosulfan and cypermethrin exhibited a tendency to increased immature erythrocytes and anisocromasia, (5) parasite infection was higher in individuals from the two rice fields. Although these results could be more valuable if pesticide residues in environmental matrices (soil, water) had been done, we suggest that integrated use of several biomarkers (BChE and CBEs, chemical reactivation of plasma with 2-PAM, and blood cell parameters) may be a promising procedure for use in biomonitoring programmes to diagnose pesticide exposure of wild populations of this frog and other native anuran species in Argentina.
References
Allender MC, Fry MM (2008) Amphibian hematology. Vet Clin N Am 11:463–480. doi:10.1016/j.cvex.2008.03.006
Alvisio A (1998) Arroz. Modelos zonales de producción en el movimiento CREA: Región Litoral Norte. Cuad Act Téc 61:141–145
Aranguren JD (1998) Evolución del cultivo del arroz en el movimiento CREA. Cuad Act Téc 61:6–8
Arshad N, Yunus S, Adan GE (2001) Endosulfan induced changes in hematological and some immunological parameters in mice. Punjab Univ J Zool 16:147–154
Attademo AM, Peltzer PM, Lajmanovich RC, Cabagna M, Fiorenza G (2007) Plasma B-esterases and glutathione S-transferase activities in the toad Chaunus schneideri (Amphibia, Anura) inhabiting rice agroecosystems of Argentina. Ecotoxicology 16:533–539. doi:10.1007/s10646-007-0154-0
Bagenal TB, Tesch FW (1978) Methods for assessment of a fish production in fresh 376 waters. In: Bagenal TB (ed) Age and growth, vol 377. Blackwell Scientific Publications, Oxford, pp 101–136
Bain D, Buttemer WA, Astheimer L, Fildes K, Hooper MJ (2004) Effects of sublethal fenitrothion ingestion on cholinesterase inhibition, standard metabolism, thermal preference, and prey capture ability in the Australian central bearded dragon (Pogona vitticeps: Agamidae). Environ Toxicol Chem 23:109–116. doi:10.1897/02-555
Bambaradeniya CNB, Edirisinghe JP, De Silva DN, Gunatilleke CVS, Ranawana KB, Wijekoon S (2004) Biodiversity associated with an irrigated rice agroecosystem in Sri Lanka. Biodivers Conserv 13:1715–1753. doi:0.1023/B:BIOC.0000029331.92656.de
Barni S, Boncompagni E, Grosso A, Bertone V, Freitas I, Fasola M, Fenoglio C (2007) Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquat Toxicol 81:45–54. doi:10.1016/j.aquatox.2006.10.012
Begenesic F (1998) Arróz. Panorama Agrícola. Informe de la Secretaría de Agricultura, Ganadería, Pesca y Alimentación 2:1–47
Bunyan PJ, Jennings DM (1968) Organophosphorus poisoning; some properties of avian esterase. J Agric Food Chem 16:326–331
Burkart R, Barbaro NO, Sánchez RO, Gómez DA (1999) Eco-regiones de la Argentina. PRODIA, Buenos Aires
Busk M, Jensen FB, Wang T (2000) Effects of feeding on metabolism, gas transport and acid base balance in the bullfrog Rana Catesbiana. Am J Physiol Regul Integr Comp Physiol 278:85–95
Cabagna M, Lajmanovich RC, Stringhini G, Peltzer PM (2005) Hematological studies in the common toad (Bufo arenarum) in agrosystems of Argentina. Appl Herpetol 2:373–380. doi:10.1163/157075405774483085
Carr RL, Chambers JE (1991) Acute effects of the organophosphate paraoxon on schedule-controlled behaviour and esterase activity in rats: dose–response relationships. Pharmacol Biochem Behav 40:929–936. doi:10.1016/0091-3057(91)90108-E
CASAFE (2005) Cámara de sanidad agropecuaria y fertilizantes de la República Argentina. Guía de Productos Fitosanitarios para la República Argentina, Buenos Aires
Chambers JE, Levi PE (1992) Organophosphates: chemistry, fate and effects. Academic Press, San Diego
Chuiko GM, Podgornaya VA, Zhelnin YY (2003) Acetylcholinesterase and butyrylcholinesterase activities in brain and plasma of freshwater teleosts: cross-species and cross-family. Comp Biochem Physiol 135:55–61. doi:10.1016/S1096-4959(03)00048-4
Cordi B, Fossi C, Depledge MH (1997) Temporal biomarker responses in wild passerine birds exposed to pesticide spray drift. Environ Toxicol Chem 16:2118–2124. doi:10.1897/1551-5028(1997)016<2118:TBRIWP>2.3.CO;2
Dacie JV, Lewis SM (1984) Practical hematology. Churchill Livingstone, New York
Davidson C, Shaffer HB, Jennings MR (2002) Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conserv Biol 16:1588–1601. doi:10.1046/j.1523-1739.2002.01030.x
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772. doi:10.1111/j.1365-2435.2008.01467.x
DeJong RJ, Muzzall PM (2000) Hematozoa of waterfowl from the Kellogg Biological Station area in southwestern Michigan. J Wildl Dis 36:767–773
Dettbarn WD, Yang ZP, Milatovic D (1999) Different role of carboxylesterases in toxicity and tolerance to paraoxon and DFP. Chem Biol Interact 120:445–454. doi:10.1016/S0009-2797(99)00057-5
Duré MI, Kehr AI, Schaefer EF, Marangoni F (2008) Diversity of amphibians in rice fields from north-eastern Argentina. Interciencia 33:523–527
Ellman GL, Courtney KD, Andreas V Jr, Featherstone RM (1961) A new and rapid calorimetric determination of cholinesterase activity. Biochem Pharmacol 7:88–95. doi:10.1016/0006-2952(61)90145-9
Gomori G (1953) Human esterases. J Lab Clin Med 142:445–453
Grue CE, Gilbert PL, Seeley ME (1997) Neurophysiological and behavioral changes in nontarget wildlife exposed to organophosphate and carbamate pesticides: thermoregulation, food consumption, and reproduction. Am Zool 37:369–388
Guilherme S, Válega M, Pereira ME, Santos MA, Pacheco M (2008) Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental mercury contamination gradient. Ecotoxicol Environ Saf 70:411–421. doi:10.1016/j.ecoenv.2007.08.016
Hii YS, Lee MY, Chuah TS (2007) Acute toxicity of organochlorine insecticide endosulfan and its effect on behaviour and some hematological parameters of Asian swamp eel (Monopterus albus, Zuiew). Pestic Biochem Physiol 89:46–53. doi:10.1016/j.pestbp.2007.02.009
Iko WM, Archuleta AS, Knopf F (2003) Plasma cholinesterase levels of mountain plovers (Charadrius montanus) wintering in central California, USA. Environ Toxicol Chem 22:119–125. doi:10.1897/1551-5028(2003)022<0119:PCLOMP>2.0.CO;2
Jergentz S, Mugni H, Bonetto C, Schulz YR (2005) Assessment of insecticide contamination in runoff and stream water of small agricultural streams in the main soybean area of Argentina. Chemosphere 61:817–826. doi:10.1016/j.chemosphere.2005.04.036
Johnson PT, Chase JM (2004) Parasites in the food web: linking amphibian malformations and aquatic eutrophication. Ecol Lett 7:521–526. doi:10.1111/j.1461-0248.2004.00610.x
Kaplan HM, Glaczenski SS (1965) Hematological effects of organophosphate insecticides in the frog (Rana pipiens). Life Sci 4:1213–1219. doi:10.1016/0024-3205(65)90335-8
Khan MZ (2005) Effects of agro pesticides cypermethrin and malathion on cholinesterase activity in liver and kidney of Calotes versicolor Daudin (Agamidae: Reptilia). Turk J Zool 29:77–81
Khan MZ, Maria Z, Fatima F (2003) Effect of Lambda cyhalothrin (pyrethroid) and Monocrotophos (organophosphate) on cholinesterase activity in liver, kidney and brain of Rana cyanophlystis. Korean J Biol Sci 7:165–168
Kornienko IA, Maslov SP, Shilov IA (1965) Some general principles of adaptation in biological systems. Zh Obshch Biol 1:121–126
Laguerre C, Sánchez-Hernández JC, Kohler HR, Triebskorn R, Capowiez Y, Rault M, Mazzia C (2009) B-type esterases in the snail Xeropicta derbentina: an enzymological analysis to evaluate their use as biomarkers of pesticide exposure. Environ Pollut 157:199–207. doi:10.1016/j.envpol.2008.07.003
Lajmanovich RC, Sánchez-Hernández JC, Stringhini G, Peltzer PM (2004) Levels of serum cholinesterase activity in the Rococo toad (Bufo paracnemis) in agrosystems of Argentina. Bull Environ Contam Toxicol 72:586–591. doi:10.1007/s00128-004-0284-5
Lajmanovich RC, Sánchez-Hernández JC, Peltzer PM, Attademo AM, Fiorenza GS, Cabagna MC, Bassó A (2008) Levels of plasma B-esterases and glutathione-S-transferase activities in three South American toad species. Toxicol Environ Chem 90:1145–1161. doi:10.1080/02772240801923107
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157:2903–2927. doi:10.1016/j.envpol.2009.05.015
Marcogliese DJ, King KC, Salo HM, Fournier M, Brousseau P, Spear P, Champoux L, McLaughlin JD, Boily M (2009) Combined effects of agricultural activity and parasites on biomarkers in the bullfrog, Rana catasbeiana. Aquat Toxicol 91:126–134. doi:10.1016/j.aquatox.2008.10.001
Marques SM, Antunes SC, Pissarra H, Pereira ML, Gonçalves F, Pereira R (2009) Histopathological changes and erythrocytic nuclear abnormalities in Iberian green frogs (Rana perezi, Seoane) from a uranium mine pond. Aquat Toxicol 91:187–195. doi:10.1016/j.aquatox.2008.04.010
Maul JD, Farris JL (2005) Monitoring exposure of northern cardinals, Cardinalis cardinalis, to cholinesterase-inhibiting pesticides: enzyme activity, reactivations, and indicators of environmental stress. Environ Toxicol Chem 24:1721–1730. doi:10.1007/s00128-004-0480-3
Maxwell DM, Brecht KM (2001) Carboxylesterase: specific and spontaneous reactivation of an endogenous scavenger for organophosphorus compounds. J Appl Toxicol 21:103–107. doi:10.1002/jat.833
McCarthy J, Shugart L (1990) Biological markers of environmental contamination. In: McCarthyand J, Shugart L (eds) Biomarkers of environmental contamination. Lewis Publishers, Boca Raton, pp 3–14
Mor F, Ozmen O (2010) Endosulfan-induced neurotoxicity and serum acetylcholinesterase inhibition in rabbits: the protective effect of Vit C. Pestic Biochem Physiol 96:108–112. doi:10.1016/j.pestbp.2009.10.004
Olmos S (2006) Prácticas para el manejo de arroz Cátedra de Cultivos II. Facultad de Ciencias Agrarias, UNNE, Corrientes, Argentina
Parsons KC, Matz AC, Hooper MJ, Pokras MA (2000) Monitoring wading bird exposure to agricultural chemicals using serum cholinesterase activity. Environ Toxicol Chem 19:1317–1323. doi:10.1897/1551-5028(2000)019<1317:MWBETA>2.3.CO;2
Peltzer PM, Lajmanovich RC, Sánchez-Hernández JC, Cabagna MC, Attademo AM, Bassó A (2008) Assessment of agricultural pond eutrophication on survival and health status of the Scinax nasicus tadpoles. Environ Ecotoxicol Saf 70:185–197. doi:10.1016/j.ecoenv.2007.06.005
Pistl J, Kovalkovicova N, Kacmar P, Kosava I, Mikula I, Sutiakova I (2001) Effect of endosulfan on peripheral sheep leukocytes in vitro. Vet Hum Toxicol 43:78–82
Pough F (1980) The advantages of ectothermy for tetrapods. Am Nat 115:92–112
Ruiz A (1998) Caracterización del área arrocera de la Región CREA Litoral Norte. CREA. Cuad Act Téc 61:10–13
Sánchez JC, Fossi MC, Focardi S (1997) Serum B esterases as an nondestructive biomarker for monitoring the exposure of reptiles to organophosphorus insecticides. Ecotoxicol Environ Saf 38:45–52. doi:10.1006/eesa.1997.1560
Sánchez-Hernández JC (2003) Evaluating reptile exposure to cholinesterase-inhibiting agrochemicals by serum butyrylcholinesterase activity. Environ Toxicol Chem 22:296–301
Sánchez-Hernández JC (2006) Ecotoxicological perspectives of B-esterases in the assessment of pesticide contamination. In: Plattenberg RH (ed) Environmental pollution: new research. Nova Science Publishers, New York
Sánchez-Hernández JC, Moreno-Sánchez B (2002) Lizard cholinesterases as biomarkers of pesticide exposure: enzymological characterization. Environ Toxicol Chem 21:2319–2325. doi:10.1897/1551-5028(2002)021<2319:LCABOP>2.0.CO;2
Sánchez-Hernández JC, Carbonell R, Henríquez Pérez A, Montealegre M, Gómez L (2004) Inhibition of plasma butyrylcholinesterase activity in the lizard Gallotia galloti palmae by pesticides: a field study. Environ Pollut 132:479–488. doi:10.1016/j.envpol.2004.05.008
Silva C, Boia C, Valente J, Borrego C (2005) Pesticides in Esteros del Iberá (AR): evaluation of impacts and proposal of guidelines for water quality protection. Ecol Model 186:85–97. doi:10.1016/j.ecolmodel.2005.01.018
Sogorb MA, Vilanova E (2002) Enzymes involved in the detoxification of organophosphorus, carbamatos and pyrethroid insecticides through hydrolysis. Toxicol Lett 128:215–228. doi:10.1016/S0378-4274(01)00543-4
Soler-Rodríguez F, Miguez-Santiyan MP, Reja-Sánchez A, Roncero-Cordero V, Garcia-Cambero JP (1998) Recovery of brain acetylcholinesterase and plasma cholinesterase activities in quail (Coturnix coturnix) after chlorpyriphos administration and effect of pralidoxime treatment. Environ Toxicol Chem 17:1835–1839. doi:10.1897/1551-5028(1998)017<1835:ROBAAP>2.3.CO;2
Sparling DW, Fellers GM, McConnell L (2001) Pesticides and amphibian population declines in California USA. Environ Toxicol Chem 20:1591–1595. doi:10.1897/1551-5028(2001)020<1591:PAAPDI>2.0.CO;2
Stansley W, Roscoe DE (1996) The uptake and effects of lead in small mammals and frogs at a trap and skeet range. Arch Environ Contam Toxicol 30:220–226. doi:10.1007/BF00215801
Thompson HM, Walker CH (1994) Blood esterases as indicators of exposure to organophosphorus and carbamate insecticides. In: Fossi MC, Leonzio C, Chelsea MI (eds) Non-destructive biomarkers in vertebrates. Lewis Publishers, Boca Raton, pp 37–62
Tyler MJ (1999) Frogs and toads as experimental animals. ANZCCAR News 12:1–4
Udroiu I (2006) The micronucleus test in piscine erythrocytes. Aquat Toxicol 79:201–204. doi:10.1016/j.aquatox.2006.06.013
Varela ME, Sellarés ME (1938) Variations annuelles du sang du crepaud Bufo arenarum Hensel. C R Soc Biol 129:1248–1249
Vernadakis A, Routledge CO (1973) Effects of ether and phenobarbital anaesthesia on the activities of brain acetylcholinesterase and butyrylcholinesterase in young adult rats. J Neurochem 20:1503–1504. doi:10.1111/j.1471-4159.1973.tb00267.x
Walker CH (1998) The use of biomarkers to measure the interactive effects of chemicals. Ecotoxicol Environ Saf 40:65–70. doi:10.1006/eesa.1998.1643
Wheelock CE, Miller JL, Miller MG, Shan G, Gee SJ, Hammock BD (2004) Development of toxicity identification evaluation (TIE) procedures for pyrethroid detection using esterase activity. Environ Toxicol Chem 11:2699–2708. doi:10.1897/03-544
Wheelock CE, Phillips BM, Anderson BS, Miller JL, Miller MJ, Hammock BD (2008) Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). Rev Environ Contam Toxicol 195:117–178. doi:10.1007/978-0-387-77030-7_5
Acknowledgments
We wish to thank Mateo R. and Chuiko G. for critical comments and valuable suggestions that improved the manuscript. We thank Elberg G. for help during sample collection and farmers for permission to work in their rice fields. This work was supported by PICT-SECYT No. 1148 (Director: Dr. Rafael C. Lajmanovich).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attademo, A.M., Cabagna-Zenklusen, M., Lajmanovich, R.C. et al. B-esterase activities and blood cell morphology in the frog Leptodactylus chaquensis (Amphibia: Leptodactylidae) on rice agroecosystems from Santa Fe Province (Argentina). Ecotoxicology 20, 274–282 (2011). https://doi.org/10.1007/s10646-010-0579-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0579-8