Abstract
The imazethapyr herbicide (formulation Verosil®) was evaluated for phytotoxicity and genotoxicity using a battery of bioassays: (1) the growth inhibition of the green alga Pseudokirchneriella subcapitata, (2) the root growth and germination of the higher plant Lactuca sativa, (3) the genetic damage using the Salmonella/microsome test, and (4) the aneugenic and clastogenic effects on Allium cepa. The Verosil® formulation was highly toxic to the non-target green alga (median effective concentration (EC50) = 1.05 ± 0.05 mg active ingredient (a.i.) L−1), and concentrations above 10 mg a.i. L−1 inhibited root elongation in lettuce: relative growth index (RGI) between 0.28 ± 0.01 and 0.66 ± 0.10. No genotoxic effect was observed in S almonella typhimurium at 100 mg a.i. L−1, either with or without the microsomal fraction. However, significant differences in the frequency of chromosomal aberrations in anaphases and telophases (bridges, chromosome fragments, and vagrants) were observed in A. cepa at concentrations between 0.01 and 1 mg a.i. L−1 with respect to the control. The frequencies of micronuclei showed significant differences with respect to the control at concentrations between 0.001 and 0.1 mg a.i. L−1. A very high mitotic index (MI = 93.8 ± 5.8) was observed associated with a high number of cells in the prophase stage at 100 mg a.i. L−1, indicating cytotoxicity. These results showed that imazethapyr is toxic to the non-target populations in both aquatic and terrestrial ecosystems. This herbicide might also exert clastogenic and aneugenic mitotic damage in higher plants. Therefore, the imazethapyr formulation may constitute an environmental risk to plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Imazethapyr is a selective systemic herbicide widely used throughout the world due to its high activity at low application rates and wide spectrum of weed control (Tan et al. 2005). It belongs to the imidazolinone herbicides and is commonly applied pre-planting and pre- or post-emergence on terrestrial crops. The imidazolinones are absorbed by both foliage and roots inhibiting the acetohydroxy acid synthase, an enzyme responsible for the biosynthesis of branched chain amino acids (isoleucine, leucine, and valine) in plants. This inhibition causes a disruption in protein synthesis which, in turn, could slow down the rate of cell division and eventually lead to the death of the cell (Shaner et al. 1984). As animals do not synthesize these amino acids via this pathway, the imidazolinone herbicides generally exhibit very low toxicity in these organisms (NYS DEC 2003; Fragiorge et al. 2008). However, imazethapyr can be used alone or in co-formulation with other components, such as surfactants, which could produce highly toxic effects. Grisolia et al. (2004) showed that the surfactant nonylphenol ethoxylate in formulation with imazethapyr is the most toxic compound to invertebrates.
In Argentina, there are extensive areas, particularly in the central Pampean Region, where different herbicide formulations are used alone or in combination with other formulations. The intensive use of herbicides in this country has caused environmental concern due to contamination of non-target sites, such as terrestrial and aquatic ecosystems near to agricultural areas. In general, when herbicides are applied to soils, they can enter water bodies through runoff or by drift during aerial application and once in the water may have harmful effects on non-target organisms. For example, recently published papers have shown the effects of glyphosate on the growth and metabolism of the native freshwater alga Chlorella kessleri (Romero et al. 2011) and the juvenile crayfish Cherax quadricarinatus, which is commonly cultured in Argentina (Avigliano et al. 2014). Imazethapyr is one of the herbicides used in recent years in many agricultural areas of this region, mainly due to glyphosate resistance developed by many weeds.
Due to the anionic structure and high solubility of imazethapyr at near-neutral pH conditions (140 mg/mL), this compound frequently shows high mobility in soils and water, allowing its presence in water bodies. So, this chemical has been detected in groundwater supplies and surface water courses at concentrations of 0.369 and 0.69 μg L−1, respectively (Battaglin et al. 2000; Espy et al. 2011). However, these concentrations in water supplies are lower than the lethal dose 50 (LD50) values obtained in rats by the oral route (LD50 > 5,000 mg/kg), indicating that imazethapyr is unlikely to present acute hazard in normal use (WHO 2005). On the other hand, a Canadian Report (PRVD-02 2010) indicates that imazethapyr does not pose a risk to terrestrial invertebrates, mammals, or birds. However, it is highly toxic to non-target terrestrial plants, such as Allium cepa, constituting a probable risk to other organisms located directly adjacent to the application field. It has also been reported that imazethapyr exerts toxicity in aquatic organisms, such as fish, freshwater algae, and aquatic vascular plants. The median effective concentration (EC50) for several representative aquatic species under laboratory conditions showed that 0.1 μg L−1 is the baseline concentration that exerts a toxic effect (Battaglin et al. 2000; Silveira Moraes et al. 2011).
Laboratory bioassays are useful tools for estimating the damage caused by toxic pollutants in both terrestrial and aquatic organisms. In particular, the phytotoxicity tests using plants present several advantages. These tests are simple, quick and reliable, inexpensive, and do not require major equipment. Many tests on plant species were shown to be more sensitive to environmental stress than tests on other organisms (Dutka 1989; Wang and Freemark 1995; Bowers et al. 1997; Charles et al. 2011; Abreu et al. 2014). Lactuca sativa is among the 10 plant species recommended by the US Environmental Protection Agency and the Organisation for Economic and Co-operation Development for the determination of ecological effects of toxic substances (US EPA 1996; OECD 2006). On the other hand, phytoplanktonic microalgae are also important models for studying the phytotoxicity of aquatic pollutants in vivo. Due to its importance as dominant primary producers in most aquatic ecosystems, the algae are used in test batteries for environmental hazard assessment (Magdaleno et al. 1997; Blaise et al. 1998; Franklin et al. 2002; Vendrell et al. 2009). In particular, the green microalga P. subcapitata is one of the most widely used species in toxicity tests due to its sensitivity to different pollutants, easy maintenance in laboratory cultures, and its relatively short life cycle (Lewis 1995; US EPA 2002; Magdaleno et al. 2015).
Many different herbicides have been tested for genotoxicity using different test systems: the induction of DNA damage in erythrocyte cells of fish exposed to glyphosate (Gholami-Seyedkolaei et al. 2013), the chromosomal aberrations (CA) in cells in vitro and micronuclei (MN) in mice in vivo exposed to imazaquin (Losi-Guembarovski et al. 2004; Grisolia et al. 2004), CA in A. cepa and Vicia fava exposed to atrazine (Srivastava and Mishra 2009), and dominant lethal mutations in the freshwater snail Biomphalaria glabrata exposed to 2,4-D (Estevam et al. 2006).
The Salmonella/microsome mutagenicity assay (Ames test) is a widely accepted short-term bacterial reverse mutation assay specifically designed to identify chemical substances that can produce genetic damage. The test employs several histidine-dependent Salmonella typhimurium strains each carrying different mutations in various genes in the histidine operon. New mutations at the site of these pre-existing mutations, or nearby in the genes, can restore the gene’s function and allow the cells to synthesize histidine. The incorporation of the microsome fraction (mammalian liver enzymes) is used to mimic mammalian metabolic conditions, so the mutagens could be mutagenic or carcinogenic by extension in laboratory animals, or represent a risk of cancer to humans (Mortelmans and Zeiger 2000). Aspects ranging from gene mutations to chromosome damage and aneuploidies can be identified by analysis of eukaryotes.
Higher plants present characteristics that make them suitable genetic models for assessing environmental pollution (Grant 1999). Various tests have been performed with a variety of plant species, e.g., Tradescantia pallida (Ma 1981), V icia faba (Kanaya et al. 1994), Zea mays (Grant and Owens 2006), and A. cepa (Grant 1982). The A. cepa test is known as an excellent genetic assay due to its high sensitivity, good correlation with other test systems, easy handling, and low cost. Additionally, this organism has the advantage of having a low number (2n = 16) of large chromosomes (Fiskesjǒ 1985; Rank and Nielsen 1997).
The aim of this work was to investigate the toxicity and genotoxicity of a commonly used imazethapyr formulation, Verosil®, using a battery of toxic and genotoxic bioassays: (1) the growth inhibition test with the green alga P. subcapitata, (2) the L. sativa seed germination and root elongation inhibition test, (3) the Salmonella/microsome mutagenicity test, and (4) the A. cepa assay.
Materials and methods
Chemicals
The herbicide used in this study was the commercially available formulation Verosil® Agrofina S.A. (Buenos Aires, Argentina), containing 10.59 % (p/v) imazethapyr (2-[4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1H-imidazol-2-yl]-5-ethyl-3-pyridinecarboxylic acid ammonium salt) (CAS No. 101917-66-2) and unknown coadjuvants. Bioassays were carried out using dilutions from a stock solution (5 g a.i. L−1). Methyl methane sulfonate (MMS, CAS No. 66-27-3), sodium azide (SAZ, CAS No. 26628-22-8), and 2-aminofluorene (2AF, CAS No. 153-78-6), used as positive controls in A. cepa and Salmonella tests, were obtained from Sigma-Aldrich.
The initial imazethapyr concentrations in solution were verified through high-performance liquid chromatography (HPLC)-tandem mass spectrometry (MS/MS). The analysis was performed on a Waters Quattro Premier XE spectrometer (Waters, Milford, MA, USA) equipped with a Waters 2695 binary pump plus an autosampler. The HPLC separation was carried out using a XTerra MS C18 column (Waters, Milford, MA, USA, 100 mm × 2.1 mm, 3.5 μm). The mobile phase consisted of (A) water/acetonitrile (90:10 with 0.1 % formic acid) and (B) acetonitrile/0.1 % formic acid. An increasing linear gradient (v/v) of solvent B was used [(t(min), %B): (0, 20), (4.5, 40)] with a flow rate of 0.3 mL/min and a column temperature of 40 °C. The injection volume was 10 μL. The mass instrument was operated in the electrospray positive ion mode, the source voltage was kept at 3 kV, the cone voltage at 30 V, and the source temperature was 150 °C. Nitrogen was used for desolvation as well as the cone gas. The detection and quantification of imazethapyr were performed using the multiple reaction monitoring (MRM) mode and ion precursor-product ion of 290–177 m/z. The collision gas was argon with a flow rate of 0.2 mL/min, and the collision energy was 25 eV. The actual values were 100 ± 8 % of their nominal values.
Algal growth inhibition test
The microalga P. subcapitata (Koršhikov) Hindak (previously named Selenastrum capricornutum Printz), obtained from the Culture Collection of Algae and Protozoa, UK (CCAP No. 278/4), is currently kept in the Culture Collection of the laboratory of Protists Biology, Departamento de Biodiversidad y Biología Experimental, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. The axenic stock alga was cultivated in 125-mL Erlenmeyer flasks, containing 50 mL sterilized Bold’s Basal Medium (BBM, Archibald and Bold 1970) and agitated on a shaker at 80 rpm, under continuous cool-white fluorescent light (80 μmol photons m−2 s−1). The flasks were maintained at 22 ± 2 °C for 7 days to obtain the inoculum in the exponential growth phase (approximately 2.5 × 106 cells mL−1).
The experimental treatments were prepared according to algal growth inhibition tests standards using sterile 96-well microplates (Environmental Canada 2007). The bioassays were conducted in four replicate wells containing 200 μL of BBM with different concentrations of herbicide and an initial cell density of 2.5 × 104 cells mL−1. Eight serial concentrations (between 1 and 10 mg a.i. L−1) of the herbicide formulation were prepared from a stock solution of 5 g a.i. L−1. Eight replicates of BBM culture medium without any herbicide were used as a control. The microplates were incubated under the same conditions as the inoculum cultures. Cell densities were estimated by absorbance at 620 nm after 96-h culture. The EC50 was obtained by plotting the percentages of algal growth inhibition with respect to the control at each concentration. Curve data were fitted to a classical sigmoidal equation, Y = A 2 + [(A 1 − A 2) / (1 + (x/x 0)p)], where p is the slope parameter, x 0 is the center point of the curve, and A 1 and A 2 are the upper and lower asymptotes, respectively. Graphics and equations were obtained using the OrigenPro 8 program (OrigenLab Corporation, Northampton, MA, USA). Three independent experiments were carried out. One-way analysis of variance (ANOVA) followed by a Dunnett’s post hoc test were performed to evaluate significant differences between each herbicide concentration and the control. A p value less than 0.05 was considered statistically significant.
L. sativa test
Organically grown seeds of L. sativa (variety Gallega) with 97 % germination, from INTA, La Consulta, Mendoza, Argentina, were used for testing. Six serial concentrations (between 10 and 250 mg a.i. L−1) of the herbicide formulation were prepared from a stock solution of 5 g a.i. L−1. The toxicity end points assessed were seed germination (EC50) and root elongation (relative growth index = RGI and germination index = GI). A seed was considered germinated when visible appearance of the radicle was detected. The assay was considered valid when the mean control survival was at least 90 % germinations and the coefficient of variation for root elongation below 30 % (Sobrero and Ronco 2004; OECD 2006). Tests were carried out in 90-mm-diameter Petri dishes lined with filter paper, with 20 seeds each, containing 4 mL of the herbicide dilution or distilled water as a control. The Petri dishes were kept in darkness in an incubator at 22 ± 2 °C for 120 h. Three replicates were performed for each concentration and the control. The number of germinated seeds was used to calculate the EC50 of the imazethapyr formulation using probit analysis (Finney 1971). The radicle lengths were measured by using a digital caliper, and elongation data was used to calculate the GI and the RGI, according to Young et al. (2012). The calculation of these phytotoxicity indexes is shown in the following equations:
where RLI is the radicle length of the imazethapyr-treated seeds, RLC is the radicle length of the control seeds, GSI is the number of germinated seeds in the imazethapyr concentration, and GSC is the number of germinated seeds in the control. The RGI values were differentiated into three categories according to the toxic effects observed:
-
Inhibition of root elongation (I): 0 < RGI < 0.8
-
No significant effects (NSE): 0.8 ≤ RGI ≤ 1.2
-
Stimulation of root elongation (S): RGI > 1.2
Three independent experiments were carried out. Statistical analysis of the data was performed using the analysis of variance (ANOVA). When the F values of the ANOVA were significant (p < 0.05), the means of the treatments were compared by Tukey’s test.
Salmonella mutagenicity test
The tester strains TA 98 and TA 100 were obtained from the University of California, Berkeley, USA. These two strains allow the detection of reading frame shift and base substitution mutations, respectively. The assay was conducted using the plate incorporation procedure described by Maron and Ames (1983). Herbicide concentrations ranging from 1 to 100 mg a.i. L−1 were assayed, with and without the hepatic S9 fraction. SAZ (5 μg plate 1) and 2AF (10 μg plate 1) were used as a positive control for TA 100 without the S9 fraction and TA 98 and TA 100 with the S9 fraction, respectively. All plates were run in triplicate. Sterile distilled water was used as a negative control. Plates were then inverted and placed in a dark incubator for 72 h at 37 °C. We considered positive results when the number of revertants obtained in the plates exposed to the herbicide were twofold or greater than the spontaneous reversion rates in the negative control, according to Mortelmans and Zeiger (2000).
A. cepa test
Organically grown seeds of A. cepa (2n = 16), variety Valcatorce, with more than 80 % germination, from INTA, La Consulta, Mendoza, Argentina, were used for testing. The seeds were genetically and physiologically homogenous. The assays were performed according to a modified version of Grant’s protocol (Matsumoto et al. 2006). One hundred onion seeds were germinated in 90-mm-diameter Petri dishes lined with filter paper containing 4 mL of the herbicide formulation dilution (concentrations between 0.0001 and 100 mg a.i. L−1). Distilled water and MMS (12 mg L−1) were used as negative and positive controls, respectively. The Petri dishes were kept in darkness in an incubator at 22 ± 2 °C for 96 h. After this period, the seeds were collected and the roots fixed in alcohol–acetic acid (3:1) for 24 h. The fixed roots were stored in 70 % ethyl alcohol until microscopic analysis. To prepare the slides, the meristematic regions were covered with coverslips and carefully squashed in a drop of 2 % acetic orcein solution. The mitotic index (MI) was calculated by counting all stages of mitotic cells with respect to the total number of cells. For the CA analyses, several aberrations such as fragments, vagrants, and bridges in the anaphase and telophase were analyzed. All these categories were placed into one category in order to evaluate the CA as a single endpoint, following the criteria used by Hoshina and Marin-Morales (2009). The MN induction was recorded by observing the interphase cells. The analyses were performed by scoring 5,000 cells per treatment, i.e., 1,000 cells per slide and a total of 5 slides. Toxicity was evaluated based on the seed germination index, which was calculated as the ratio of the number of germinated seeds to the total seeds allowed to germinate. Cytotoxicity was assessed based on MI values, and genotoxicity was evaluated based on the CA and MN frequencies, as frequency = (A / B) × 100, where A is equivalent to the total number of cells with a parameter to be analyzed (CA or MN), and B corresponds to the entire number of analyzed cells (200 telophases and anaphases and 1,000 interphases, respectively). Statistical analysis was performed using the Kruskal–Wallis test at a significance level of 0.05.
Results and discussion
The number of germinated seeds in L. sativa in all test concentrations (between 10 and 250 mg a.i. L−1) was similar to the control, showing no significant differences between the percentage germination and the control (Table 1). Therefore, the EC50 value could not be estimated. However, the phytotoxicity analysis using the RGI parameter indicated that the Verosil® formulation significantly inhibited root elongation at all concentrations tested. According to Young et al. (2012), values of RGI between 0 and 0.8 indicate an inhibition effect. Our results were between 0.28 ± 0.01 and 0.66 ± 0.10; therefore, all herbicide concentrations tested inhibited the root elongation in L. sativa (Table 1). These results were reinforced by the significant reduction of the GI% at all herbicide concentrations with respect to the control. Similarly, Radetski et al. (2000) showed that biomass (root elongation) is one of the most sensitive parameter for herbicide toxicity evaluation in L. sativa assay. Moreover, other studies carried out with aquatic plants, such as Lemna minor and Myriophyllum sibiricum, showed that root elongation is a suitable parameter for assessing the sensitivity to different herbicides including the imodazolinones (Peterson et al. 1994; Roshon et al. 1999).
Toxicity, cytotoxicity, and genotoxicity were evaluated in A. cepa, by quantifying seed germination, mitotic cells, and CA and MN frequencies, respectively. The percentage of seeds germinated in all test concentrations (between 10 and 100 mg a.i. L−1) and in the controls were between 82 and 91 %, showing no significant differences among them. Therefore, the EC50 could not be estimated. However, high MIs were observed at 100 mg a. i. L−1 (MI = 93.8 ± 5.8), being significantly higher than the negative control (Table 2). According to Leme and Marin-Morales (2009), elevated MIs are the result of an increase in cell division, which can be harmful to the cells, leading to a disordered cell proliferation and even to the formation of tumor tissues. In this particular case, the high MI was due to the high number of cells in the prophase stage that did not complete the mitotic cycle (no cells in metaphase, anaphase, or telophase stage were observed). So, the Verosil® formulation exerts cytotoxicity to A. cepa at 100 mg a. i. L−1. MI in the other concentrations tested (between 0.0001 and 10 mg a. i. L−1) showed no significant differences with respect to the control. Different results were obtained in a recent publication (Liman et al. 2015), showing that pure imazethapyr at 10, 20, and 40 ppm significantly reduced the MIs compared to negative control and MMS. These differences could be due to the presence of coadjuvants in the Verosil® formulation. It has been proposed that surfactants and coadjuvants in formulations could modify the herbicide toxicity (Lewis 1990; Grisolia et al. 2004). On the other hand, we observed a clear delay between prophase and metaphase stages at 100 mg a. i. L−1. One explanation could be that imazethapyr blocks the mitotic process at the end of prophase, producing the accumulation of dividing cells at this stage. Similar results were observed by Liman et al. (2015) at 10 ppm pure imazethapyr.
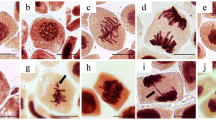
Significant differences with respect to the negative control were observed in the CA analysis at concentrations between 0.01 and 1 mg a. i. L−1 and in the MN analysis at concentrations between 0.001 and 0.1 mg a.i. L−1. However, no significant differences with respect to the control were observed at 10 mg L−1, so the formulation was not genotoxic at that concentration. Contrary to the results obtained in this work, Liman et al. (2015) observed an increase in the CA at 10 ppm pure imazethapyr. Again, these differences could be due to the presence of coadjuvants in the Verosil® formulation. High frequencies of CA and MN were observed at 0.1 mg a.i. L−1 (Table 2). When the meristematic cells are exposed to chemical agents, changes in either chromosomal structure or in the total number of chromosomes may occur. The CA may be induced by DNA breaks, inhibition of DNA synthesis and replication of altered DNA (chromosome bridges and breaks), or abnormal segregation of chromosomes (vagrants), being indicators of clastogenic and aneugenic actions, respectively (Leme and Marin-Morales 2009). Cells in anaphase with more than one bridge and fragment were observed mainly at 0.1 and 1 mg a. i. L−1 (Fig. 1). The presence of MN in F1 interphases is the result of damages derived from chromosome breaks and vagrants in the parental cells, which are observed in daughter cells. Therefore, the A. cepa test indicated that the Verosil® formulation exerts genotoxicity through both aneugenic and clastogenic action.
Chromosome aberration (CA) observed in A. cepa meristematic cells exposed to Verosil® formulation. a Normal anaphase; b 1 mg a.i. L−1, anaphase with various bridges (arrow) and two fragments (arrowhead); c 1 mg a. i. L−1, anaphase with bridge and ring (arrow); and d 0.1 mg a. i. L−1, anaphase with two bridges (arrow) and one fragment (arrowhead)
Mutagenic effects have also been observed in other plants, such as Z. mays exposed to imazethapyr (Grant and Owens 2006) and V. faba exposed to the Pursuit formulation (El-Nahas 2000). In the latter case, a variety of chromosomal abnormalities were observed in both the mitotic and meiotic divisions represented by stickiness, disturbance, laggards, bridges, multipolar, C-metaphase, breaks, and micro and multinuclei. This author also showed that the frequency of these types of abnormalities decreases when the formulation herbicide was combined with urea fertilizer. Another study using A. cepa showed differences between the active technical imazapyr ingredient and the surfactant nonylphenol ethoxylate with respect to toxicity and genotoxicity, indicating that surfactants and other so-called inert components generally increase the toxicity of the herbicide formulations (Grisolia et al. 2004).
It has been reported that imazethapyr shows high selectivity to annual and perennial grasses and broadleaved weeds, showing low toxicity to mammals (Tan et al. 2005). The imidazolinone herbicides were developed to control weeds by inhibiting the enzyme acetohydroxyacid, a critical enzyme for the biosynthesis of branched chain amino acids in plants which is not found in mammals. So, according to the mode of action, imazethapyr is not expected to pose an acute or chronic hazard to mammals (PRVD-02 2010). In this work, we performed the Ames test to evaluate a possible mutagenic effect of the Verosil® formulation (imazethapyr plus coadjuvants). Before performing the genotoxicity test, we analyzed the cytotoxicity of Verosil® at 100 mg a.i. L−1, showing that this formulation was not toxic to S. typhimurium. The genotoxicity assay showed no genotoxic effect in S. typhimurium (TA98 and TA100 strains) at 100 mg L−1, either with or without the microsomal fraction (Table 3). The incorporation of the microsomal S9 hepatic fraction in the Ames test enables the evaluation of the mutagenic potential of those chemicals that require metabolic activation to become mutagenic. Therefore, the S9 fraction was added to the Ames test in order to obtain mutagenic products of the herbicide metabolization, due to the action of the cytochrome P450 enzymes. Our results showed that Verosil® formulation exerts no mutagenic activity on the test with S9 incorporation, showing that this formulation could not be a promutagenic product.
Herbicides applied in the terrestrial environment can enter water bodies through runoff or by drift through the air, producing potential harmful effects on non-target aquatic organisms. To analyze the possible impact of imazethapyr on single-cellular algae, we selected the green alga P. subcapitata. We selected algae to evaluate ecotoxicity because these organisms represent the base of the trophic chain in aquatic ecosystems and changes in the algal population growth may influence the community structure and energy transference to the upper trophic levels. The percentage of algal growth inhibition at different concentrations of the herbicide was plotted in order to obtain the sigmoidal curve (y = 84.69 + (13.33–84.69) / (1 + (x / 1.31)3) (Fig. 2). According to this curve, the EC50 was 1.05 ± 0.05 mg a.i. L−1. The EU Directive 93/67/EEC (Commission of the European Communities 1996) classifies substances according to their EC50 for aquatic organisms as follows: very toxic (EC50, <1 mg L−1), toxic (EC50, 1–10 mg L−1), and harmful (EC50, 10–100 mg L−1); the Verosil® formulation was found to be toxic–very toxic to P. subcapitata.
The Verosil® formulation (10.59 % of the technical-active ingredient imazethapyr) showed higher toxicity to algae compared to the 2ASU formulation (22.2 % of the technical-active ingredient imazethapyr): EC50 22.4 mg L−1 for the green alga P. subcapitata, EC50 4.8 mg L−1 for the cyanobacteria Anabaena flos-aquae, and EC50 22.9 mg L−1 for the diatom Navicula pelliculosa) (PRVD-02 2010). These differences could be related to the quantity and quality of surfactants or coadjuvants contained in the herbicide formulations (Siemering et al. 2008). It has been shown that surfactants or coadjuvants are toxic per se and can increase herbicide toxicity (Lewis 1990; Tsui and Chu 2003; Grisolia et al. 2004).
In Argentina, there have been few studies of herbicide concentrations in waters, sediments, and soils. Peruzzo et al. (2008) reported levels of glyphosate in waters ranging from 0.10 to 0.70 mg L−1 and in sediments and soils between 0.5 and 5.0 mg kg−1. However, no data of imazethapyr concentrations have been reported in this country. According to Battaglin et al. (2000), the concentrations of imidazolinone herbicides measured in surface waters in the USA were lower than 1 μg L−1. These concentrations would not represent a risk for freshwater algae considering the EC50 for P. subcapitata obtained in our study, which was 1,000 times greater than that concentration. However, imazethapyr is degraded slowly in soils, having a half-life between 30 and 60 days under laboratory conditions and between 2.6 and 10.6 months under field conditions (Ramezani et al. 2010). While there is no information about its degradability in water, it would be relatively slow as in soil and thus may remain for some time in the aquatic ecosystems, increasing algae exposure. On the other hand, soil characteristics may also exacerbate this scenario. For example, large amounts of organic matter causing greater adsorption of imazethapyr by the soil, together with high rainfall, might allow a percentage of the herbicide to run off into surface waters (Espy et al. 2011). Besides, it was recently reported that imazethapyr reduces both the microbial biomass and microbial community structure in soils and shows inhibitory effects on many enzymes, such as dehydrogenase, phosphatase, protease, and catalase (Zhang et al. 2010). Therefore, the biodegradability in soil would also be reduced.
According to our results, the Verosil® formulation of imazethapyr did not show mutagenic effect. However, environmental impact is an issue to be considered, because this product is applied in soils and then it could enter surface water through runoff from soils. This formulation showed toxic and genotoxic effect on non-target terrestrial plants and toxic effect on freshwater algae. These effects could occur at higher concentrations than it could be found in terrestrial and aquatic environment. However, with the lack of knowledge about the half-life and persistence in aquatic ecosystems, potential risks cannot be excluded.
Conclusion
We studied the toxic and genotoxic effects of an imazethapyr formulation (Verosil®) frequently used in Argentina, using four tests: the growth inhibition of the green algae P. subcapitata, the root growth and germination of L. sativa, the Salmonella/microsome Ames test, and the A. cepa test. The combination of these four assays allowed us to determine that the Verosil® formulation is toxic–highly toxic to the non-target green alga, and it also exerts an inhibitory effect on plants at concentrations above 10 mg a.i. L−1. On the other hand, the formulation is not genotoxic to S. typhimurium (TA98 and TA100 strains) at 100 mg a. i. L−1, either with or without the microsomal fraction. However, the Verosil® formulation shows genotoxic activity in the non-target plant A. cepa producing high frequencies of CA and MN in the meristematic cells of roots at concentrations as low as 0.001 mg a. i. L−1. Therefore, the imazethapyr formulation could probably constitute an environmental risk in both the aquatic and terrestrial ecosystems.
References
Abreu MM, Lopes J, Santos ES, Magalhães MCF (2014) Ecotoxicity evaluation of an amended soil contaminated with uranium and radium using sensitive plants. J Geochem Explor 142:112–121
Archibald PA, Bold HC (1970) Phycological studies. XI. The genus Chlorococcum meneghini. Univ Texas, Austin, 86 p, Public N° 7015
Avigliano L, Fassiano AV, Medesani DA, Ríos de Molina MC, Rodríguez EM (2014) Effects of glyphosate on growth rate, metabolic rate and energy reserves of early juvenile crayfish, Cherax quadricarinatus M. Bull Environ Contam Toxicol 92(6):631–635
Battaglin WA, Furlong ET, Burkhardt MR, Peter CJ (2000) Occurrence of sulfonylurea, sulfonamide, imidazolinone, and other herbicides in Rivers, reservoirs and ground water in the Midwestern United States, 1998. Sci Total Environ 248:123–133
Blaise C, Férard JF, Vasseur P (1998) Microplate toxicity tests with microalgae: a review. In: Wells P, Lee K, Blaise C (eds) Microscale testing in aquatic toxicology. Advances, techniques, and practice. CRC, Boca Raton, pp 269–288
Bowers N, Pratt JR, Beeson D, Lewis M (1997) Comparative evaluation of soil toxicity using lettuce seeds and soil ciliates. Environ Toxicol Chem 16:207–213
Charles J, Sancey B, Morin-Crini N, Badot PM, Degiorgi F, Trunfio G, Crini G (2011) Evaluation of the phytotoxicity of polycontaminated industrial effluents using the lettuce plant (Lactuca sativa) as a bioindicator. Ecotoxicol Environ Saf 74:2057–2064
Commission of the European Communities (1996) Technical guidance document in support of Commission Directive 93/67/EEC on risk assessment for new notified substances and Commission Regulation (EC) No 1488/94 on risk assessment for existing substances. Part II. Environmental risk assessment. Office for Official Publications of the European Communities, Luxembourg
Dutka B (1989) Short-term root elongation toxicity bioassay. Methods for toxicological analysis of waters, wastewaters and sediments. National Water Research Institute (NWRI). Environment Canada, Canada
El-Nahas AI (2000) Mutagenic potential of imazethapyr herbicide (Persuit*) on Vicia faba in the presence of urea fertilizer. Pak J Biol Sci 3(5):600–905
Environmental Canada (2007) Biological test method: Growth inhibition test using a freshwater algae. EPS 1/RM/25, Environmental Science and Technology Centre, Science and Technology Branch, Environment Canada. Environmental Protection Series, Second Edition, p 53
Espy R, Pelton E, Opseth A, Kasprisin J, Nienow AM (2011) Photodegradation of the herbicide imazethapyr in aqueous solution: effects of wavelength, pH, and natural organic matter (NOM) and analysis of photoproducts. J Agric Food Chem 59:7277–7285
Estevam EC, Nakano E, Kawano T, Pereira CAB, Amancio FF, Melo AMMA (2006) Dominant lethal effects of 2,4-D in Biomphalaria glabrata. Mutat Res 611:83–88
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge, UK
Fiskesjǒ G (1985) The Allium test as a standard in environmental monitoring. Hereditas 102:99–112
Fragiorge EJ, Alves A, de Rezende A, Graf U, Spanó MA (2008) Comparative genotoxicity evaluation of imidazolinone herbicides in somatic cells of Drosophila melanogaster. Food Chem Toxicol 46:393–401
Franklin NM, Stauber JL, Lim RP, Petocz P (2002) Toxicity of metal mixtures to a tropical freshwater alga (Chlorella sp.): the effect of interactions between copper, cadmium, and zinc on metal cell binding and uptake. Environ Toxicol Chem 21(11):2412–2422
Gholami-Seyedkolaei SJ, Mirvaghefi A, Farahmand H, Kosari AA, Gholami-Seyedkolaei SJ, Gholami-Seyedkolaei SJ (2013) Optimization of recovery patterns in common carp exposed to roundup using response surface methodology: evaluation of neurotoxicity and genotoxicity effects and biochemical parameters. Ecotoxicol Environ Saf 98:152–161
Grant WF (1982) Chromosome aberration assays in Allium. A report of the US Environmental Protection Agency Gene-Tox Program. Mutat Res 99:273–291
Grant WF (1999) Higher plant assays for the detection of chromosomal aberrations and gene mutation—a brief historical background on their use for screening and monitoring environmental chemicals. Mutat Res 426:107–112
Grant WF, Owens ET (2006) Zea mays assays of chemical/radiation genotoxicity for the study of environmental mutagens. Mutat Res 613(1):17–64
Grisolia CK, Bilich MR, Menezes Formigli L (2004) A comparative toxicologic and genotoxic study of the herbicide arsenal, its active ingredient imazapyr, and the surfactant nonylphenol ethoxylate. Ecotoxicol Environ Saf 59:123–126
Hoshina MM, Marin-Morales MA (2009) Micronucleus and chromosome aberrations induced in onion (Allium cepa) by a petroleum refinery effluent and by river water that receives this effluent. Ecotoxicol Environ Saf 72:2090–2095
Kanaya N, Gill BS, Grover IS, Murin A, Osiecka R, Sandhu SS, Anderson HC (1994) Vicia faba chromosomal aberration assay. Mutat Res 310:231–247
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res 682:71–81
Lewis MA (1990) Chronic toxicity of surfactants and detergent builders to algae: a review and risk assessment. Ecotoxicol Environ Saf 20:123–140
Lewis MA (1995) Use of freshwater plants for phytotoxicity testing: a review. Environ Pollut 87:319–336
Liman R, Ciğerci IH, Öztürk NS (2015) Determination of genotoxic effects of Imazethapyr herbicide in Allium cepa root cells by mitotic activity, chromosome aberration, and comet assay. Pestic Biochem Physiol 118:38–42
Losi-Guembarovski R, Santos FV, Dias FL, Frederico RG, Cólus IMS (2004) Assessment of the ability of Imazaquin herbicide to induce chromosomal aberrations in vitro in cultured Chinese hamster ovary cells and micronuclei in vivo in mice. Food Chem Toxicol 42:1245–1249
Ma TH (1981) Tradescantia micronucleus bioassay and pollen tube chromatid aberrations test for in situ monitoring and mutagen screening. Environ Health Perspect 37:85–90
Magdaleno A, Gómez CE, Vélez CG, Accorinti J (1997) Preliminary toxicity tests using the green alga Ankistrodesmus falcatus. Environ Toxicol Water Qual 12(1):11–14
Magdaleno A, Saenz ME, Juárez AB, Moretton J (2015) Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata. Ecotoxicol Environ Saf 113:72–78
Maron D, Ames BN (1983) Revised methods for the Salmonella mutagenicity test. Mutat Res 113:173–215
Matsumoto ST, Mantovani MS, Malagutti MIA, Dias AL, Fonseca IC, Marin-Morales MA (2006) Genotoxicity and mutagenicity of water contaminated with tannery effluents, as evaluated by the micronucleus test and comet assay using the fish Oreochromis niloticus and chromosome aberrations in onion root-tips. Genet Mol 29:148–158
Mortelmans K, Zeiger E (2000) The Ames Salmonella/microsome mutagenicity assay. Mutat Res 455:29–60
New York State Department of Environmental Conservation (2003) Imazamox (Raptor) NYS DEC Letter—active ingredient registration 3/03. New York State Department of Environmental Conservation. March 13, 2003. New York. <http://pmep.cce.cornell.edu/profiles/herb-growthreg/fatty-alcohol-monuron/Imazamox/Imazamox_reg_303.html> (Accessed 12.04.2014)
OECD (2006) OECD guideline for the testing of chemicals. Proposal for updating guideline 208. Terrestrial plant test: 208: seedling emergence and seedling growth test. OECD Publications Service, Paris
Peruzzo PJ, Porta AA, Ronco AE (2008) Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environ Pollut 156:61–66
Peterson HG, Boutin C, Martin PA, Freemark KE, Ruecker NJ, Moody MJ (1994) Aquatic phyto-toxicity of 23 pesticides applied at expected environmental concentrations. Aquat Toxicol 28(3):275–292
PRVD-02 (2010) Imazethapyr. Pest Management Regulatory Agency Health Canada Editors, Otawa, 118 p
Radetski CM, Cotelle S, Férard JF (2000) Classical and biochemical endpoints in the evaluation of phytotoxic effects caused by the herbicide trichloroacetate. Environ Exp Bot 44(3):221–229
Ramezani MK, Oliver D, Kookana RS, Lao W, Gill G, Preston C (2010) Faster degradation of herbicidally-active enantiomer of imidazzolinones in soils. Chemosphere 79:1040–1045
Rank J, Nielsen MH (1997) Allium cepa anaphase-telophase root tip chromosome aberration assay on N-methyl-N-nitrosourea, maleic hydrazide, sodium azide, and ethyl methanesulfonate. Mutat Res 390:121–127
Romero DM, Ríos de Molina MC, Juárez AB (2011) Oxidative stress induced by a commercial glyphosate formulation in a tolerant strain of Chlorella kessleri. Ecotoxicol Environ Saf 74:741–747
Roshon RD, McCann JH, Thompson DG, Stephenson GR (1999) Effects of seven forestry management herbicides on Myriophyllum sibiricum, as compared with other nontarget aquatic organisms. Can J For Res 29(7):1158–1169
Shaner DL, Anderson PC, Stidham MA (1984) Imidazolinones. Potent inhibitors of acetohydoxyacid synthase. Plant Physiol 76:545–546
Siemering GS, Hayworth JD, Greenfield BK (2008) Assesment of potential aquatic herbicide impacts to California aquatic ecosystems. Arch Environ Contam Toxicol 55:415–431
Silveira Moraes B, Clasen B, Loro VL, Pretto A, Toni C, de Avila LA, Marchesan E, de Oliveira Machado SL, Zanella R, Boschmann Reimche G (2011) Toxicological responses of Cyprinus carpio after exposure to a commercial herbicide containing imazethapyr and imazapic. Ecotoxicol Environ Saf 74:328–335
Sobrero C, Ronco A (2004) Ensayo de toxicidad aguda con semillas de L. sativa. In: Castillo G (ed) Ensayos toxicológicos y métodos de evaluación de calidad de aguas: estandarización, intercalibración. Resultados y Aplicaciones, México, pp 71–79
Srivastava K, Mishra KK (2009) Cytogenetic effects of commercially formulated atrazine on the somatic cells of Allium cepa and Vicia faba. Pestic Biochem Physiol 93:8–12
Tan S, Evans RR, Dahmer ML, Singh BK, Shaner DL (2005) Imidazolinone-tolerant crops: history, current status and future. Pest Manag Sci 61:246–257. doi:10.1002/ps.993
Tsui MT, Chu LM (2003) Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 52:1189–1197
U.S. EPA (1996) Seed germination/root elongation toxicity test. In: Ecological effects test guidelines. Office of Prevention, Pesticides, and Toxic Substances, Series 850, Washington, D.C. http://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryID=47927
U.S. EPA (United States Environmental Protection Agency) (2002) Selenastrum capricornutum growth test. In: Short-term method for estimating the chronic toxicity of effluents and receiving water to freshwater organisms http://water.epa.gov/scitech/methods/cwa/wet/disk3_index.cfm
Vendrell E, de Barreda G, Ferraz D, Sabater C, Carrasco JM (2009) Effect of glyphosate on growth of four freshwater species of phytoplankton: a microplate bioassay. Bull Environ Contam Toxicol 82:538–542
Wang W, Freemark K (1995) The use of plants for environmental monitoring and assessment. Ecotoxicol Environ Saf 30:289–301
WHO (2005) The WHO recommended classification of pesticides by hazard and guideline to classification. Switzerland, Geneva
Young BJ, Riera NI, Beily ME, Bres PA, Crespo DC, Ronco AE (2012) Toxicity of the effluent from an anaerobic bioreactor treating cereal residues on Lactuca sativa. Ecotoxicol Environ Saf 76:182–186
Zhang C, Xu J, Liu X, Dong F, Kong Z, Sheng Y, Zheng Y (2010) Impact of imazethapyr on the microbial community structure in agricultural soils. Chemosphere 81:800–806
Acknowledgments
This study was financially supported by the Buenos Aires University, Argentina, under Projects UBACYT 01/W484 and 01/W9985. The authors are grateful to Mr. Ricardo J. Piccolo for kindly providing the L. sativa and A. cepa seeds to perform the toxicity and genotoxicity assays.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Rights and permissions
About this article
Cite this article
Magdaleno, A., Peralta Gavensky, M., Fassiano, A.V. et al. Phytotoxicity and genotoxicity assessment of imazethapyr herbicide using a battery of bioassays. Environ Sci Pollut Res 22, 19194–19202 (2015). https://doi.org/10.1007/s11356-015-5103-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5103-5