Abstract
B-esterase (BChE: butyrylcholinesterase and CbE: carboxylesterase) and glutathione S-transferase (GST) activity were measured in the plasma of Chaunus schneideri collected in rice fields and surrounding environments and in a reference pristine forest. The chemical criterion based on in-vitro reactivation of BChE activity using pyridine-2-aldoxime methochloride (2-PAM) was also determined. Mean values of plasma BchE, CbE, and GST activity for samples from agricultural areas were different from those for samples from pristine forest. Plasma samples from the two agricultural areas showed positive reactivation of BChE activity after incubation with 2-PAM. Based on our experimental evidence we suggest B-esterases and gluthatione S-transferases can be used in field monitoring as biomarkers of exposure of wildlife to pesticides, because the analysis in non-destructive and is sensitive to anti-ChE agrochemicals. Chemical reactivation of BChE is also a complementary method for assessing the effects of pesticides on toads inhabiting rice fields. Further studies are urgently needed to investigate adverse effects of massive exposure to pesticides experienced by native populations of anurans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticide application is regarded as an important factor in the decline in amphibian populations in agricultural landscapes (Kiesecker et al. 2001; Sparling et al. 2001; Davidson et al. 2002). The deleterious effects of pesticides on anural survival and health have been well documented (Bridges and Boone 2003; Relyea et al. 2005; Relyea 2006). In the last five years, rice crops have increased substantially in Argentina, particularly in Santa Fe and Entre Ríos Province, which account for approximately 90% of national production (Trimboli et al. 2003). Rice fields with their aquatic and dry-land habitats comprise a heterogeneous mosaic of rapidly changing ecotones that harbor a rich animal biodiversity characterized by rapid colonization, and by rapid reproduction and growth of some organisms (Elphik 2000). Rice fields are also potential supplemental habitats and amphibian breeding sites and water sources during their adult lives (Bambaradeniya et al. 2004). These highly human-impacted ecosystems are, however, affected by autogenic (nutrients) and allogenic (agrochemicals) inputs, resulting in temporal disturbances of the populations of this opportunistic biota. In this context, the allogenic inputs comprise a wide range of pesticides, especially organophosphates (OPs) and carbamates (CBs) (CASAFE 1999). These pesticides inhibit B-esterases, i.e. cholinesterases (ChE) and carboxylesterases (CbE). CbE activity can also hydrolyze CBs, pyrethroids, and some OPs pesticides (Gruber and Munn 1998; Dettbarn et al. 1999; Sogorb and Vilanova 2002; Wheelock et al. 2004).
Glutathione S-transferase (GSTs) is also a common biomarker implicated in the detoxification of pesticides and in the mechanism of resistance to pesticides by over-expression of this enzyme (Ladagic et al. 1994; Bucciarelli et al. 1999; Eaton and Bammler 1999; Greulich and Pflugmacher 2004).
Endangered species or those with a low population densities in the study area pose a serious problem in assessment of the effects of pesticides. Use of biochemical data from blood or other non-destructive materials has become a tool for analyzing the effects of pesticides on biota (McCarthy and Shugart 1990; Fossi and Leonzio 1994; Walker 1998). Venturino et al. (2003) recently suggested that the use of biomarkers could increase understanding of the adverse effects of environmental contaminants on amphibian species.
Plasma B-esterase (ChE and CbE) and GST activity are useful indicators of exposure to pesticides of toads in agroecosystems (Lajmanovich et al. 2004; Venturino et al. 2003; Cabagna et al. 2005). Sparling et al. (2001) reported that insecticides inhibited ChE activity in the larval frog and that this may have contributed to their decline. Only a few field studies in Argentina have demonstrated significant relationships between enzyme activity (e.g. butyrylcholinesterase, BChE) of amphibians and application of pesticides (Lajmanovich et al. 2004); even fewer have provided information about the role of GST and CbE activity in these vertebrates.
One objective of this study was to measure B-esterase and GST activity in the native toad, Chaunus schneideri, collected in a rice agroecosystem (rice-crop field and farm town) and in a pristine forest in the Litoral Fluvial areas of Argentina. We also investigated the ability of pyridine-2-aldoxime methochloride (2-PAM), a known potent reactivator of phosphorylated ChEs, to reverse OP-inhibited plasma BChE activity. This work forms part of a project to investigate adverse effects of massive exposure to pesticides on native populations of anurans in Argentina (Lajmanovich et al. 2004; 2005a b; Cabagna et al. 2005).
Materials and methods
Study area and animal sampling
The study area was situated in mid-eastern Argentina (Fig. 1), a region dominated by wetlands and fluvial forests (Bertonatti and Corcuera 2000). Because intensive agriculture was introduced to this area at the beginning of the 20th century, small plots of wetlands, fluvial forests, and semi-xerophitic vegetation are included in the agricultural matrix (e.g. rice, soybean, sorghum, and corn fields). Climatically, this region has an average annual rainfall of 800 mm and the mean annual temperature is 18°C.
A total of 24 male adults of C. schneideri were collected by hand from three sampling areas (n = 8 for each site) during January 2006. The reference site was located within a pristine forest (Parque General San Martin: PGSM, 31°44′36′′ S; 60°19′40′′ W). The two agricultural sampling sites were a rice field (RF: locality “Pájaro Blanco” 29°44′57″ S; 59°58′34′′ W) and a farm town (FT) located in the center area of rice fields (29°50′30′′ S, 59°48′28.8″ W). After capture, animals were quickly transported to the laboratory in darkened buckets, with water, to minimize stress. Blood samples were collected by cardiac puncture using a heparinized syringe (1 mL) (Lajmanovich et al. 2004). Anesthesia was not used because it could have interfered with enzymatic activity (Vernadakis and Routledge 1973). Blood was centrifuged at 3,500 rpm for 15 min and the plasma was separated and immediately frozen. Snout vent length and body mass were recorded, and a condition factor (CF) for each animal, expressed as 100 × [body weight (g)]/[length (cm3)] was calculated (Bagenal and Tesch 1978). After blood samples had been taken the toads were immediately released at the sites where they had been captured.
B-esterase assays
Plasma BChE activity was determined colorimetrically by the method of Ellman et al. (1961). The reaction medium included 1,870 μL 25 mmol L−1 Tris-HCl, 1 mmol L−1 CaCl2 (pH = 7.6), 100 μL 5,5′-dithiobis-2-nitrobenzoic acid (3 × 10−4 mol L−1, final concentration), 20 μL butyrylthiocholine iodide (2 × 10−3 mol L−1, final concentration), and 10 μL plasma. The variation in optical density was recorded at 405 nm for 1 min at 25°C. Kinetics was measured in triplicate. Plasma BChE activity was expressed as μmol substrate hydrolyzed 10 min−1 mL−1 plasma using a molar extinction coefficient of 13.6 × 103 mol−1 L cm−1.
Plasma CbE activity was measured by the Gomori method (1953) as adapted by Bunyan and Jennings (1968). The assay was carried out with 1,940 μL 25 mmol L−1 Tris-HCl, 1 mmol L−1 CaCl2 (pH = 7.6), and 10 μL plasma at 25°C. The reaction was initiated by addition of 50 μL α-naphthyl acetate (1.04 mg mL−1 in acetone) as substrate, and stopped after 10 min by addition of 500 μL 2.5% sodium dodecyl sulfate and subsequently 500 μL 0.1% Fast Red ITR in 2.5% Triton X-100 in water (freshly prepared). The samples were left in darkness for 30 min to develop, and the absorbance of the complex was read at 530 nm. Plasma CbE activity was expressed as μmol of substrate hydrolyzed 10 min−1 mL−1 plasma using a molar extinction coefficient of 33.225 × 103 mol−1 L cm−1.
Glutathione S-transferase activity
Plasma GST activity was determined spectrophotometrically by the method described by Habig et al. (1974). The reaction medium was 1,850 μL 100 mmol L−1 Na-phosphate buffer (pH = 6.5), 40 μL 2 mmol L−1 1-chloro-2,4-dinitrobenzene, 100 μL 5 mmol L−1 reduced glutathione, and 10 μL plasma. Kinetics were measured for 1 min at 340 nm in duplicate. Plasma GST activity was expressed as nmol min−1 mL−1 of plasma using a molar extinction coefficient of 9.6 × 103 mol−1 L cm−1.
Chemical reactivation of BChE
We used two aliquots of each plasma sample for assaying reactivation of BChE activity in the presence of 2-PAM. An aliquot was spiked with 1.7 × 10−3 mol L−1 2-PAM, and the second aliquot of plasma was diluted with an equal volume of distilled H2O (control). After incubation for 60 min at 25°C, BChE activity of both aliquots was measured. Inhibition of BChE activity by OPs was assumed when the increase of esterase activity was higher than 10% compared with the corresponding controls.
Statistical analysis
Data are expressed as mean ± S.E.M. Because we could not meet the assumption of normality to perform ANOVA, we ran non-parametric Kruskal–Wallis tests to determine whether means of enzyme activity (BChE, CbE, and GST) were statistically different. Pairwise comparisons between plasma samples from three sites were tested by use of the Dunn post-hoc multiple comparison test. The percentage increase of BChE activity (angularly transformed data) after 2-PAM treatments was also tested by Kruskal–Wallis analysis between three sampling sites. A value of P < 0.05 was regarded as statistically significant.
Results
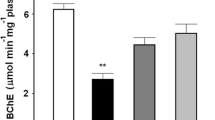
The mean (±S.E.M.) CF values for toads collected from agricultural sites were 14.06 ± 1.08 (RF: locality “Pájaro Blanco”) and 13.82 ± 1.17 (FT: farm town), which were similar to those for toads from the reference site (PGSM: Parque General San Martin, 14.72 ± 1.33) (Kruskal–Wallis KS test = 0.35; P > 0.05). Figure 2 shows the mean (±S.E.M.) BChE activity for the toads from the three sampling sites. The BChE activity was statistically lower for toads collected from the agricultural sites than for those from the reference site (Kruskal–Wallis KS test = 10.87, P < 0.05). The Dunn post-hoc multiple comparisons test detected significant differences among BChE activity found in animals captured in the PGSM (2.47 ± 0.49 μmol min−1 mL−1 plasma) and agricultural sites (FT = 1.18 ± 0.26; and RF = 1.02 ± 0.19 μmol min−1 mL−1 plasma).
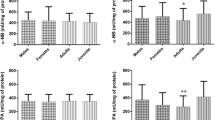
Mean CbE activity was significantly different among toads sampled from the three sites; that for RF was statistically different (Kruskal–Wallis KS test = 7.02; P < 0.05, Fig. 3). The Dunn test for post-hoc multiple comparisons detected significant differences between mean CbE activity found in the animals from PGSM (2.60 ± 0.31 μmol 10 min−1 mL−1 plasma) and those captured in RF (1.49 ± 0.17 μmol 10 min−1 mL−1 plasma). RF and FT (2.15 ± 0.24 μmol 10 min−1 mL−1 plasma) were also different.
GST activity was different among the toads sampled from the three sites (Kruskal–Wallis KS test = 15.16; P < 0.05; Fig. 4). Dunn’s test detected significant differences between GST activity in animals from the two agricultural areas (FT = 44.61 ± 0.57 nmol min−1 mL−1 plasma; and RF = 41.88 ± 0.93 nmol min−1 mL−1 plasma) and that in toads from the reference site (PGSM: 29.85 ± 1.05 nmol min−1 mL−1 plasma).
Oxime-induced reactivation of plasma BChE activity was observed in 81.2% of toads from the two agriculture sites (Table 1) and no reactivation were observed in samples from the reference site. The percentage increase of plasma BChE activity in samples from the RF site varied from 12 to 93%, whereas for all the samples from the FT agricultural site the increase was at least 51% after 2-PAM incubation. Although the percentages of BChE reactivation were higher in the samples from FT (77.00 ± 6.28) compared with those from the RF site (59.32 ± 12.45), the differences were not significant (KS = 2.04; P = 0.18).
Discussion
In our study, plasma B-esterase activity was significantly inhibited in toads collected from both agricultural sites (FT and RF) compared to those from the reference site (PGSM). This was corroborated by the 2-PAM reactivation assay, which enabled us to identify organophosphates (OPs) as the agrochemicals responsible for plasma BChE depression in the animals collected from the agricultural sites; this suggestion do not, however, rule out the possibility that carbamates (CBs) also were involved. Chemical reactivation of plasma phosphorylated ChE activity has been used for complementary diagnosis of OP intoxication in birds (Parson et al. 2000; Iko et al. 2003) and reptiles (Sánchez-Hernández, 2003; Sánchez-Hernández et al. 2004) (Table 1). Our data show that OP pesticides are an important class of pesticide to amphibians in rice fields. We further speculate that fenitrothion, clorpyrifos, and methamidofos might be the OPs responsible for plasma B-esterase inhibition because empty containers of these pesticides were observed adjacent to the agricultural sampling sites during the study. Moreover, interviews with farmers reinforced this premise. The rice fields in the Santa Fe Province harbor numerous noxious species of arthropod and pesticide applications occur frequently. In particular, these three pesticides were applied twice in January just before our field samplings (CASAFE 1999).
In the other hand, 81.2% of plasma samples of toads showed BChE reactivation after 2-PAM treatment. Increments of BChE activity after chemical reactivation suggest that pesticide applications in agricultural areas have affected toads, at least sublethally. Similarly, Sánchez-Hernández et al. (2004) found similar increments of BChE reactivation in an herpetofauna species (Gallotia galloti) in Canarian Island (Table 1).
Moreover, in our study, higher levels of GST activity were observed in toad plasma samples from agricultural areas (FT and FR) than in those from the reference site. It is important to note that the role of amphibian GST in an ecotoxicological context has received little attention (Greulich and Pflugmacher 2004). These authors suggested that this enzyme seems to be involved in the detoxication of cypermethrin in amphibian larvae. Although there are not enough reports regarding GST in amphibian’s blood, the values these authors found and presented here are in agreement with those reported for the blood of mammals (Ognjanović et al. 2003). Similar results were obtained by Kaaya et al. (1999) and Wilczek et al. (1997) in bivalves living in a contaminated environment with communal sewage and industrial waste, and in number of spider species collected along a gradient of pollution.
The reduction in B-esterase activity and induction of GST activity observed in toads from farm town (FT) may be attributed to application of OP pesticides around and within rice fields. Large amounts of pesticides are dispersed from agricultural target sites as a result of drift, runoff, volatilization, off-gassing, and other processes (Moses 1989; Plimmer 1991). House dust in agricultural areas can accumulate several parts per million of pesticides in an environment in which they are not readily degraded by sun, rain, or soil microbes (Simcox et al. 1995). In the same sense, a study conducted in agricultural areas of Entre Ríos and Santa Fe Province revealed residues of pesticides in amphibian tissues in non-agricultural areas (Lajmanovich et al. 2002; 2005b). Likewise other authors (Angermann et al. 2002, Fellers et al. 2004) found pesticide residues in north American frogs, and linked this finding with declines in amphibian populations (Sparling et al. 2001).
In conclusion, based on experimental evidence obtained here we can suggest that B-esterases and GST can be useful biomarkers in field monitoring of the effects of pesticide exposure on wildlife, because of their non-destructive nature and their sensitivity to anti-ChE agrochemicals. Indeed, we recommend that when samples are low (≥8 individuals) chemical reactivation of plasma with 2-PAM may be a valuable method for detecting effects of anti-cholinesterase compounds on wildlife. Further studies are necessary, however, to investigate the adverse effects of massive exposure to pesticides on this and other native populations of anurans.
References
Angermann JE, Fellers GM, Matsumura F (2002) Polychlorinated biphenyls and toxaphene in Pacific tree frog tadpoles (Hyla regilla) from the California Sierra Nevada, USA. Environ Toxicol Chem 21(10):2209–2215
Bagenal TB, Tesch FW (1978) Methods for assessment of a fish production in fresh 376 waters. In: Bagenal TB (ed) Age and growth, vol 377. Blackwell Scientific Publications, Oxford, UK, pp 101–136
Bambaradeniya CNB, Edirisinghe JP, De Silva DN, Gunatilleke CVS, Ranawana KB, Wijekoon S (2004) Biodiversity associated with an irrigated rice agroecosystem in Sri Lanka. Biodiv Conserv 13:1715–1753
Bertonatti C, Carguera J (2000) Situación ambiental Argentina 2000. Fundación vida silvestre Argentina, Buenos Aires
Bridges CM, Boone MD (2003) The interactive effects of UV-B and insecticide exposure on tadpole survival, growth and development. Biol Conserv 113:49–522
Bucciarelli T, Sacchetta P, Pennelli A, Cornelio L, Romagnoli R, Melino S (1999) Characterisation of toad glutathione transferase. Biochim Biophys Acta 1431:189–198
Bunyan PJ, Jennings DM (1968) Organophosphorus poisoning; some properties of avian esterase. J Agric Food Chem 16:326–331
Cabagna M, Lajmanovich RC, Stringhini G, Peltzer PM (2005) Hematological studies in the common toad (Bufo arenarum) in agrosystems of Argentina. Applied Herpetol 2:373–380
CASAFE (1999) Cámara de sanidad agropecuaria y fertilizantes de la República Argentina. Buenos Aires, Guía de Productos Fitosanitarios para la República Argentina, pp 635
Davidson C, Shaffer HB, Jennings MR (2002) Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conserv Biol 16:1588–1601
Dettbarn WD, Yang ZP, Milatovic D (1999) Different role of carboxylesterases in toxicity and tolerance to paraoxon and DFP. Chem Biol Interact 120:445–454
Eaton DL, Bammler TK (1999) Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci 49:156–164
Elphick CS (2000) Functional equivalency between Rice fields and seminatural wetland habitats. Conserv Biol 14:181–191
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fellers GM, McConnell LL, Pratt D, S Datta (2004) Pesticides in mountain yellow-legged frogs (Rana muscosa) from the Sierra Nevada Mountains of California, USA. Environ Toxicol Chem 23(9):2170–2177
Greulich K, Pflugmacher S (2004) Uptake and Effects on detoxication enzymes of Cypermethrin in embryos and tadpoles of amphibians. Arch Environ Contam Toxicol 47:489–495
Gruber SJ, Munn MD (1998) Organophosphate and carbamate insecticides in agricultural waters and cholinesterase (ChE) inhibition in common carp (Cyprinus carpio). Arch Environ Contam Toxicol 35:391–396
Gomori G (1953) Human esterases. J Lab Clin Med 42:445–453
Fildes K, Astheimer LB, Store P, Buttemer W, Hooper MJ (2006) Cholinesterase response in native birds exposed to fenitrothion during locust control operations in eastern Australia. Environ Toxicol Chem 25:2964–2970
Fossi MC, Leonzio C (1994) Nondestructive biomarkers in vertebrates. Lewis Publishers, CRC Press, USA, pp 345
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Iko WM, Archuleta AS, Knopf F (2003) Plasma cholinesterase levels of mountain plovers (Charadrius montanus) wintering in Central California, USA. Environ Toxicol Chem 22:119–125
Kaaya A, Najimi S, Ribera D, Narbonne JF, Moukrim A (1999) Characterisation of glutathione-S-transferases (GST) activities in Perna perna and Mytilus galloprovincialis used as biomarkers of pollution the Agadir Marine Bay (South of Morocco) Bull Environ Contam Toxicol 62:623–629
Kiesecker JM, Blaustein AR, Belden LK (2001) Complex causes of amphibian population declines. Nature 410:681–684
Lagadic L, Caquet T, Ramade F (1994) The role of biomarkers in environmental assessment Invertebrate populations and communities. Ecotoxicology 3:193–208
Lajmanovich RC, Lorenzatti E, De la Sierra P, Marino F, Peltzer PM (2002) First registrations of organochlorine pesticides residues in amphibians of the Mesopotamic region, Argentina. Froglog 54:4–5
Lajmanovich RC, Sánchez-Hernández JC, Stringhini G, Peltzer PM (2004) Levels of serum cholinesterase activity in the rococo toad (Bufo paracnemis) in agrosystems of Argentina. Bull Environ Contam Toxicol 72:548–591
Lajmanovich RC, Cabagna M, Peltzer PM, Stringhini GA, Attademo AM (2005a) Micronucleus induction in erythrocytes of the Hyla pulchella tadpoles (Amphibia: Hylidae) exposed to insecticide endosulfan. Mutat Res 587:67–72
Lajmanovich R, De La Sierra P, Marino F, Peltzer P, Lenardón A, Lorenzatti E (2005b) Determinación de residuos de organoclorados en vertebrados silvestres del litoral fluvial de Argentina. In: FC Aceñolaza (ed) Temas de la biodiversidad del litoral fluvial Argentino II, INSUGEO, Miscelánea, Tucuman, Argentina, pp 255–262
Maul JD, Farris JL (2004) Monitoring exposure of passerines to acephate, dicrotophos, and malathion using cholinesterase reactivation. Bull Environ Contam Toxicol 73:682–689
Maul JD, Farris JL (2005) Monitoring exposure of northern cardinals, Cardinalis cardinalis, to cholinesterase-inhibiting pesticides: enzyme activity, reactivations, and indicators of environmental stress. Environ Toxicol Chem 24:1721–1730
McCarthy J, Shugart L (1990) Biological markers of environmental contamination. In: McCarthyand J, Shugart L (eds) Biomarkers of environmental contamination. Lewis, Boca Raton, FL, pp 3–14
McInnes P, Andersen DE, Hoff DJ, Hooper MJ, Kinkel LL (1996) Monitoring exposure of nestling songbirds to agricultural application of an organophosphorus insecticide using cholinesterase activity. Environ Toxicol Chem 15:544–552
Moses M (1989) Pesticide-related health problems and farmworkers. AAOHN J 37:115–130
Ognjanović BI, Pavlović SZ, Maletić SD, Zikić RV, Stajn AS, Radojičić RM, Saičić ZS, Petrović VM (2003) Protective Influence of Vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol Res 52: 563–570
Parsons K, Matz AC, Hooper MJ, Pokras MA (2000) Monitoring wading bird exposure to agricultural chemicals using serum cholinesterase activity. Environ Toxicol Chem 19:1317–1323
Plimmer JR (1991) Dissipation of pesticides in the environment. In: JL Schnoor (ed) Fate of pesticides and chemicals in the environment. Wiley, New York, pp 79–91
Relyea RA (2006) The effects of pesticides, pH, and predatory stress on amphibians under mesocosm conditions. Ecotoxicology 15:503–511
Relyea RA, Schoeppner NM, Hoveman JT (2005) Pesticides and amphibians: the importance of community context. Ecol Applic 15:1125–1134
Sánchez-Hernández JC (2003) Evaluating reptile exposure to cholinesterase-inhibiting agrochemicals by serum butyrylcholinesterase activity. Environ Toxicol Chem 22:296–301
Sánchez-Hernández JC, Carbonell R, Henríquez-Pérez A, Montealegre M, Gómez L (2004) Inhibition of plasma butyrylcholinesterase activity in the lizard Gallotia galloti palmae by pesticides: a field study. Environ Pollut 132:479–488
Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA (1995) Pesticides in household dust and soil: Exposure pathways for children of agricultural families. Environ Health Perspect 103:1126–1134
Sparling DW, Fellers GM, McConnell L (2001) Pesticides and amphibian population declines in California USA. Environ Toxicol Chem 20:1591–1595
Sogorb MA, Vilanova E (2002) Enzymes involved in the detoxification of organophosphorus, carbamatos and pyrethroid insecticides through hydrolysis. Toxicol Lett 128:215–228
Trimboli G, Vicino R, Peart M (2003) Análisis de la producción de Arroz en la provincia Centro Regional Santa Fe. Estación Experimental Agropecuaria Reconquista (INTA). Agencia de Extensión Rural San Javier. Información para Extensión No 77, pp 11
Venturino A, Rosenbaum E, Caballero de Castro A, Anguiano OL, Gauna L, Fonovich de Schroeder T, Pechen de D’Angelo AM (2003) Biomarkers of effect in toads and frogs. Biomarkers 8:167–186
Vernadakis A, Routledge CO (1973) Effects of ether and Phenobarbital anaesthesia on the activities of brain acetylcholinesterase and butyrylcholinesterase in young adult rats. J Neurochem 20:1503–1504
Walker CH (1998) The use of biomarkers to measure the interactive effects of chemicals. Ecotoxicol Environ safety 40:65–70
Wheelock CE, Miller JL, Miller MG, Shan G, Gee SJ, Hammock BD (2004) Development of toxicity identification evaluation (TIE) procedures for pyrethroid detection using esterase activity. Environ Toxicol Chem 11:2699–2708
Wilczek G, Majkus Z, Migula P, Bednarska K, Swierczek E (1997) Heavy metals and detoxifying enzymes in spiders from coal and metallurgic dumps near Ostrava (Czech Republic). Proceedings of the 16th European colloidal arachnology siedlce, pp 317–328
Acknowledgements
We wish to thank Donald Sparling, S. Pflugmacher, and Juan C. Sánchez-Hernandez for helpful comments and for checking the language. We thank farmers for permission to work in their fields. This work was supported in part by grants SEED GRANT DAPFT-2005 (Director: Dra. Paola M. Peltzer) and PIP-CONICET no. 5618−2006 (Director: Dr Rafael C. Lajmanovich).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attademo, A.M., Peltzer, P.M., Lajmanovich, R.C. et al. Plasma B-esterase and glutathione S-transferase activity in the toad Chaunus schneideri (Amphibia, Anura) inhabiting rice agroecosystems of Argentina. Ecotoxicology 16, 533–539 (2007). https://doi.org/10.1007/s10646-007-0154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-007-0154-0