Abstract
In this study, the impact of hydration status on dermal uptake of pesticides in two species of amphibians is examined. Absorption of pesticides in anurans occurs primarily through a highly vascularized dermal seat patch; however, pesticides can also enter through the superficial dermis following exposure. Despite the growing body of literature on dermal exposure in amphibians, little is known on how hydration status influences uptake. Thus, the objective of this study was to investigate the influence of hydration status on absorption of pesticides (atrazine, triadimefon, metolachlor, chlorothalonil, and imidacloprid) in southern leopard frogs (Lithobates sphenocephala) and Fowler’s toads (Anaxyrus fowleri). Amphibian treatments included dehydration periods of 0, 2, 4, 6, 8, or 10 h prior to exposure to pesticide-contaminated soils for 8 h. Following exposure, soil and whole-body homogenates were extracted and analyzed by LC-MS/MS. Dehydration time was then regressed against post-exposure concentrations to infer the impact of dehydration on dermal pesticide uptake. Increased dehydration time resulted in significantly lowered pesticide concentrations in both species (F6, 293 = 67.66, p = 0.007) for the five pesticides studied. This phenomenon could be due to an energy and/or dilution effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental factors, including temporal weather patterns, are known to influence the absorption and evaporation of water in amphibians (Shoemaker et al. 1992; Shoemaker and Nagy 1977). Amphibians lack a hydrophobic barrier, allowing their skin to be extremely permeable to the surrounding environment, facilitating gas and water exchange (Hillman et al. 2009; Quaranta et al. 2009; Smith et al. 2007). Once an amphibian has morphed into its terrestrial form, however, it must remain hydrated to avoid desiccation (Smith et al. 2007). Thus, numerous adaptations for regulating water content and varying fluid osmolality in the amphibian body have been described (Barbeau and Lillywhite 2005; Churchill and Storey 1993; Heatwole et al. 1969; Lillywhite 2006; McClanahan 1967; Toledo and Jared 1993). One main route for terrestrial amphibians to uptake water and rehydrate themselves is to place the ventral pelvic region (seat patch) in contact with moist substrate (Taylor et al. 1999). In addition to water uptake, amphibians have been known to absorb metals and pesticides through their skin (James et al. 2004; Van Meter et al. 2014, 2015, 2016; Willens et al. 2006). Such passive absorption leads to transport across the skin as the most significant route for exposure to contaminants, compared to dietary and pulmonary routes (Smith et al. 2007).
Amphibians are extremely sensitive to environmental conditions that result in water loss, such as extreme arid conditions and diurnal and seasonal variations in climate. Amphibian bodies consist of 70–80% water by weight, and their daily water loss can range from 10 to 22% of total mass (Dole 1967; Hermes-Lima and Storey 1998; Thorson 1964). Due to this inherent susceptibility to dehydration, amphibians have developed both physiological and behavioral mechanisms to combat water loss. One physiological characteristic is the ability to reabsorb water from dilute urine in times of need, along with having a high storage capacity for urine (Ogushi et al. 2010a, b; Suzuki et al. 2007). Another mechanism is the use of a posteroventral seat patch that is highly vascularized and extremely permeable and responsive to water balance hormones that result in rapid rehydration of water (Bentley and Main 1972; Hillman et al. 2009; Ogushi et al. 2010b); this leads to the seat patch becoming the primary means for water absorption across the skin (Hillman et al. 2009).
Variation in skin permeability is a critical adaptation for amphibians. Bentley and Main (1972) observed that species from different habitats have distinct differences in seat patch permeability, with terrestrial species having more permeable skin than aquatic species. Aquatic amphibians have smooth ventral and dorsal skin, which is not very permeable, compared to the more granular skin of terrestrial amphibians (Hillman et al. 2009; Toledo and Jared 1993). The granulation or “epidermal sculpturing” of the ventral skin found in terrestrial species such as toads facilitates water absorption due to increased surface area, where this sculpturing can come in contact with a moist substrate that allows the skin to reabsorb water (Hillman et al. 2009; Lillywhite and Licht 1974).
Overall, terrestrial species have highly vascularized seat patches compared to aquatic and semi-terrestrial species (Hillman et al. 2009). The responsive hormones such as arginine vasotocin (AVT) and hydrins can also facilitate the permeability of the seat patch within amphibians (Bentley and Main 1972; Hillman et al. 2009). Arginine vasotocin is known to increase amphibian skin water permeability as well as slowing or halting the formation of urine, thereby conserving water (Hillman et al. 2009; Shoemaker and Nagy 1977). Furthermore, AVT and hydrin hormones enable absorption of water into the amphibian’s body by utilizing aquaporins (Hillman et al. 2009; Tracy and Rubink 1978). Aquaporins are water-conducting channels classified as integral membrane proteins (Ogushi et al. 2010a; Preston and Agre 1991). Arginine vasotocin induces the insertion of aquaporin containing vesicles into the apical membrane, allowing water to be more rapidly absorbed (Hillman et al. 2009; Suzuki et al. 2007). When water is absorbed, it moves into cutaneous capillaries via basolateral membranes, utilizing more aquaporins which can increase rehydration rates (Hillman et al. 2009, Suzuki et al. 2007).
Behavioral characteristics have also been observed that allow amphibians to prevent water loss such as higher activity levels at night than during the day: residing near or in aquatic habitats, burrowing, changing activity level or posture, aggregating together, or smearing lipids over their bodies (Barbeau and Lillywhite 2005; Heatwole et al. 1969; Lillywhite 2006; McClanahan 1967; Toledo and Jared 1993). Many amphibians will stop moving to conserve water, but some species locomote again when dehydration becomes too severe, which can lead to an increase in the amount of water lost (Heatwole et al. 1969; Pough et al. 1983; Putnam and Hillman 1977). Amphibians also prevent dehydration from air exposure with a water-conserving posture that places the ventral surfaces and head against a substrate while their legs are folded so the feet are under the body (Heatwole et al. 1969; Pough et al. 1983). Amphibians can also aggregate so that very little surface area is exposed to the environment as a means to conserve water. Last, to aid in slowing water loss, several species display a body-wiping behavior that puts an extra-epidermal water barrier comprised of lipids between the dermis and the environment to reduce evaporative water loss (Barbeau and Lillywhite 2005).
Many amphibian breeding grounds can be found within agricultural landscapes, leading to significant exposure potential to applied chemicals (Lenhardt et al. 2015). Terrestrial species are often mobile and must respond to changing water and habitat availability. Such changes can result in migration to other habitats, and irrigated agricultural fields may be preferable in water-scarce areas and seasons. Many species of amphibians have a terrestrial life stage which allows them to disperse far from their breeding grounds. These migration periods can lead to dehydration events and associated increases in conductivity that allows rapid rehydration when the amphibian does come in contact with water (Shoemaker and Nagy 1977). Rapid rehydration events may also allow exposure and absorption of fertilizers and pesticides when they occur on agricultural landscapes (Berger et al. 2012; Fryday and Thompson 2012). However, anuran breeding season frequently coincides with the planting of crops and application of pesticides to these agricultural areas (Berger et al. 2011, review in Fryday and Thompson 2012). This timing results in herbicides, fungicides, and insecticides being applied over fields, so that amphibians, along with other non-target species, can be exposed to these compounds. In Berger et al. (2011), during pesticide application, it was estimated that upwards of 85% of the population of spadefoot toads were active during this time, while during winter application of insecticides, only 20 to 40% of the spadefoot toad population was active. Additionally, modifications of climate patterns can also trigger habitat changes that may make agricultural landscapes a relatively desirable habitat for amphibians (U.S. EPA 2014). Many field surveys have observed a correlation between distance to agricultural fields and amphibian population decline (Bishop et al. 1999; Davidson 2004; Davidson and Knapp 2007; Davidson et al. 2002; Mann et al. 2009).
This study investigated how hydration status can affect whole-body concentrations of pesticides in terrestrial-phase amphibians. We investigated the influence of hydration status on dermal absorption of pesticides (atrazine, triadimefon, metolachlor, chlorothalonil, and imidacloprid) in two frog species, southern leopard frogs (Lithobates sphenocephala) and Fowler’s toads (Anaxyrus fowleri), by measuring uptake from predetermined pesticide exposures after variable periods of dehydration. Using these data, we infer whether duration of the dehydration period has a significant effect on pesticide uptake across five pesticides studied. Our null hypothesis was that the dehydration period would have no effect on pesticide uptake and our expectation was that longer dehydration periods could increase the uptake of pesticides during the rehydration period.
Materials and methods
All solvents used for pesticide extraction and analysis were of HPLC-grade purity and purchased from Fisher Scientific (Pittsburgh, PA, USA). Pesticide active ingredients and their metabolites were obtained from the U.S. Environmental Protection Agency’s National Pesticides Standard Repository (Fort Meade, MD, USA). Active ingredients (AI) and metabolites analyzed in the study were ≥ 98% purity for atrazine, chlorothalonil, imidacloprid, metolachlor, triadimefon, and their corresponding metabolites deisopropyl atrazine (DIA), desethyl atrazine (DEA), chlorothalonil metabolite, metolachlor ethane sulfonic acid (MESA), metolachlor oxanilic acid (MOXA), and triadimenol.
Dehydration experiments
Animal collection and rearing were described in detail in Van Meter et al. (2014). Egg masses or embryos collected from the University of Georgia’s Whitehall Forest in March 2014 were reared in 375 L wading pools at the U.S. EPA in Athens, GA. Amphibians were fed fish food ad libitum until metamorphosis, where they were moved to 600-L polyethylene tanks that contained sphagnum moss and leaf litter. All amphibians were fed cultured fruit flies and purchased crickets until 60–90 days post-metamorphosis. While soil collection and pesticide application to soils contained in Pyrex® bowls were detailed in Van Meter et al. (2016). Briefly, soil was collected from a control study site in Newton, GA, during July 2014 and sifted through a 2-mm sieve before being stored at 4 °C. A thin layer of Orangeburg loamy-sand (OLS) soil (~ 150 g) from Van Meter et al. (2016) was added to the bottom of each Pyrex® bowl, and individual pesticides were applied to the soil in 100% methanol (MeOH) using a compressed air propellant Spray Gun® canister attached to a graduated glass jar. To minimize spraying time and have as little error as possible between replicates, maximum application rates were scaled down to the area of six bowls at 1350 cm2 (each bowl 15 cm × 15 cm): atrazine 23.95 μg/cm2, chlorothalonil 44.30 μg/cm2, imidacloprid 5.39 μg/cm2, metolachlor 31.01 μg/cm2, and triadimefon 2.91 μg/cm2. After application, bowls were placed under a fume hood overnight to evaporate all MeOH, and the following morning, 50 mL of water was used to rehydrate the soil of each individual bowl using a standard spray bottle. Control bowls were sprayed with 100% MeOH and followed the same procedure as pesticide-applied bowls. Leopard frogs and Fowler’s toads used in the study were at least 60 days post-metamorphosis and had an average weight of ~ 1 g. Both amphibian species were last fed 48 h prior to exposure. Amphibians were dehydrated for 0, 2, 4, 6, 8, and 10 h before an exposure commenced with laboratory conditions: temperature ranged between 20 and 22 °C with 12 h light/12 h dark cycle. Dehydration data entailed taking an initial composite weight before frogs were moved to an empty glass aquarium with a screen lid to allow adequate air flow for dehydration; groups were n = 8 for leopard frogs and n = 6 for Fowler’s toads for each of the five dehydration time periods and pesticides tested. After the allotted dehydration time, composite amphibians from the same dehydration time point were reweighed to obtain an approximate percent of body weight lost during the dehydration period. Due to the lack of experimental resources, we were unable to obtain individual weights both pre- and post-dehydration. Subsequently, each amphibian was placed into a Pyrex® bowl and covered with a mesh screen that was secured with a rubber band for an 8 h exposure (n = 6 for leopard frogs and n = 5 for Fowler’s toads) and one control for each time point per pesticide exposure (total n = 7 for leopard frogs and n = 6 for Fowler’s toads). Exposures commenced at 08:00 and took place under laboratory conditions. At the end of the experiment, individual amphibians were placed in pre-labeled 50-mL centrifuge tubes and euthanized in an − 80 °C freezer in accordance with the approved animal use and care protocols. Soil samples were collected by randomly taking three small scoops of the top layer of soil and were placed into a pre-labeled 15-mL centrifuge tube. Final amphibian weights were taken after amphibians were thawed and before pesticide extraction. Rehydrated weights (difference in amphibian body weight between final weight and post-dehydration) during exposure studies were used to determine analytical body concentrations and burdens.
Amphibian and soil extraction
All pesticide extraction procedures have been previously described in Van Meter et al. (2014). Briefly, whole-body homogenates were spiked with 10 μL of 1000 μg/g internal standard (tetraconazole) and placed on a freeze-dryer overnight. Both amphibian and soil samples were extracted twice with MeOH, using both sonication and centrifugation. Methanol was then combined and evaporated down to ~ 1 mL using a gentle stream of nitrogen; next, a liquid-liquid extraction was done using methyl tert-butyl ether (MTBE). For chlorothalonil, 1 mL aliquot of the MTBE sample was taken and placed into a GC vial and analyzed on a GC/MS; for all other analytes, the 1-mL aliquot was blown dry under nitrogen, reconstituted with 30% MeOH (v/v), and analyzed on a LC-MS/MS. To obtain the whole-body concentration for each amphibian, the parent active ingredient was summed with its corresponding metabolite(s) and divided by the amphibian rehydrated weight. Rehydration rates were calculated by adding up the post-exposure weights of the individual amphibians; this resulted in the final post-experiment weight. Next, the difference between the post-dehydration weight and the final post-experiment weight was obtained. The difference between these two values was divided by the 8 h of exposure resulting in a rehydration rate per hour (g/h).
LC-MS/MS instrumentation
Active ingredients and metabolites were quantified on a Varian Prostar HPLC linked to a Varian 1200 L triple quadrupole mass spectrometer with an Eclipse XDB-C18 column (3.5 μm particle size, 3.0 × 150 mm; Agilent Technologies, CA, USA). Initial conditions were held for 2 min at 70% water with 0.1% formic acid (A) and 30% acetonitrile with 0.1% formic acid (B) then ramped to 90% B over 16 min and held there for 4 min before returning to starting conditions. The drying gas was set at 225 °C, and the capillary voltage was at 50 V for all compounds analyzed. MOXA, MESA, and chlorothalonil metabolite were the only analytes analyzed in negative mode; all other pesticides were analyzed in positive mode (Table 1).
Data analysis
The effects of dehydration time on the slope of post-exposure pesticide concentrations were tested with an ANCOVA. The analysis treated pesticide concentration as a dependent variable and tested for main effects with categorical variables that represented species and chemicals in addition to the ordinal dehydration time variable. Therefore, the ANCOVA decomposes the variance in the measured pesticide concentrations into the variance explained by the dehydration time treatment and the categorical chemical and species treatments. Continuous body weight was also tested as an independent variable but was discarded from the final model as uninformative. The sample design was complete for both species (n = 7 for leopard frogs; n = 6 for Fowler’s toads); treatments were conducted for each combination of chemical (atrazine, chlorothalonil metabolite, imidacloprid, metolachlor, and triadimefon) and dehydration time (0, 2, 4, 6, 8, and 10 h). ANCOVA test results are presented for the experiment as a whole for inference across the entire experiment by examining the common (pooled) fitted slope of pesticide concentration versus dehydration times across all the treatments. Chemical-conditional slopes are also examined for discussion purposes. All analyses were performed in R version 3.3 (R Core Team 2017).
Results
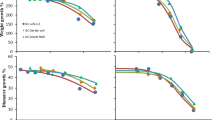
To determine the influence of hydration status on exposure to pesticides in amphibians, anurans were dehydrated for 0–10 h and exposed to a pesticide-contaminated soil for 8 h to observe differences in whole-body concentrations (Table 2). On average, dehydrated leopard frogs lost 12.5, 18.9, 19.8, 21, and 24.5% body weight when dehydrated for 2, 4, 6, 8, and 10 h, respectively. Fowler’s toads had slightly higher body loss with 11.4, 19.5, 25.7, 34.6, and 34.7% body weight for 2, 4, 6, 8, and 10 h of dehydration, respectively. There was no significant relationship between rehydration rate and dehydration time. However, hydration status significantly affected the absorption of pesticides in both leopard frogs and Fowler’s toads. The two amphibian species were considered to be statistically different from one another with Fowler’s toads having higher concentrations (p < 0.001; Table 2). Furthermore, when taking into account the five different pesticides and two amphibians species for each dehydration time point, there was a statistically significant decline in concentration with increase in dehydration time (F6, 293 = 67.66, p = 0.007). Although this relationship between concentration and dehydration time was significant across the entire experiment, all pesticides did not demonstrate monotonic declines in whole-body amphibian concentrations; therefore, each chemical is addressed individually below. However, all five chemicals tested individually showed statistical evidence for a negative relationship between dehydration time and amphibian pesticide concentration, with p values ranging from 0.01 to 0.17. The slopes of the five chemicals, which can be interpreted as the change in final pesticide concentration per dehydration hour, were for atrazine (− 0.7 μg/g/h; p = 0.01), chlorothalonil metabolite (− 0.002 μg/g/h; p = 0.04), imidacloprid (− 0.02 μg/g/h; p = 0.17), metolachlor (− 0.14 μg/g/h; p = 0.13), and triadimefon (− 0.02 μg/g/h; p = 0.14).
Leopard frogs exposed to atrazine-contaminated soils showed a decrease in pesticide concentrations as dehydration status increased (p < 0.01); the total body burden decreased from 8.84 ± 2.34 μg/g (S.E.) (0 h) to 7.65 ± 2.12 μg/g (10 h). The Fowler’s toads exposed to atrazine exhibited an initial increase in absorption from 0 h (20.59 ± 3.96 μg/g) to 2 h (27.44 ± 2.62 μg/g), followed by a steady decline in concentration as dehydration period increased (10 h, 9.62 ± 3.53 μg/g). Overall, Fowler’s toads demonstrated that dehydration periods had an effect on pesticide uptake (p = 0.01). Atrazine and its metabolites were found at higher concentrations in Fowler’s toads than in leopard frogs at each dehydration time point (p < 0.001; Fig. 1).
Tissue concentrations (μg/g) of exposed amphibians. Atrazine, metolachlor, and triadimefon are the summation of the parent and its corresponding metabolites. The two species are shown next to each other with body burdens presented as boxplot summaries for each of the dehydration time points within the pesticide treatment. Fowler’s toads (Anaxyrus fowleri) (blue) and leopard frogs (Lithobates sphenocephala) (red)
Exposure to chlorothalonil only resulted in the observation of its metabolite. For the chlorothalonil metabolite in leopard frogs, increase in dehydration time exhibited a decline in pesticide concentration from 0.074 ± 0.010 (S.E.) at 0 h to 0.038 ± 0.007 μg/g at 10 h (Table 2 and Fig. 1). In Fowler’s toads, there was also an inverse relationship between whole-body concentrations and dehydration time. Dehydration time was significant (p = 0.04), while species was close but does not meet the significant threshold (p = 0.1).
For the insecticide imidacloprid, the parent pesticide was the only compound analyzed; additionally, imidacloprid was only exposed to leopard frogs due to the limited number of Fowler’s toads. For leopard frogs, imidacloprid had a negative slope with dehydration time (p = 0.17; Fig. 1).
Both leopard frogs and Fowler’s toads exhibited decreases in metolachlor pesticide concentrations as a function of dehydration time with a p = 0.125 (Fig. 1). Overall, leopard frogs decreased from 4.48 ± 1.43 (S.E.) at 0 h to 1.75 ± 0.24 μg/g at 10 h (Table 2). For Fowler’s toads, metolachlor had an initial increase from 0 h (1.27 ± 0.45 μg/g) to 2 h (5.05 ± 2.82 μg/g), followed by a decrease in absorption as dehydration time increased to 10 h (2.16 ± 0.70 μg/g). There was no evidence for a difference in uptake between the species (p = 0.98).
For leopard frogs exposed to triadimefon, there was a negative slope indicating that increased dehydration periods resulted in lower pesticide body concentrations with a p = 0.14. At 0 h, the average was 0.98 ± 0.12 μg/g (S.E.) and at 10 h, the average was 0.59 ± 0.04 μg/g (Table 2). An upward, increasing trend was observed for the first few time points (≤ 4 h) in Fowler’s toads exposed to triadimefon; afterward, a decline was exhibited (4 h was 1.2 ± 0.50 μg/g and 10 h was 0.81 ± 0.13 μg/g). There was some evidence for a difference in uptake between the species (p = 0.2).
Rehydration rates (g/h) were determined for each pesticide and species combination at every time point (Fig. 2) to ensure that any time trends in pesticide concentrations were not confounded with changes in rehydration rates over the time of the experiment. For both leopard frogs and Fowler’s toads, there are no consistent trends for the dehydration time periods of 2–10 h (dropping the control of 0 h). Rehydration rates ranged from 0.12 to 0.79 g/h with a mean of 0.38 g/h. A linear regression of the rehydration rate as a function of dehydration time showed a slope that corresponded with a statistically insignificant increase in the change in the rehydration rate over the course of the experiment (mean effect of 0.01 g/h/h).
Discussion
Amphibian species have declined in the last several decades, and pesticides are one potential causative factor. A recent U.S. survey conducted by Gilliom et al. (2006) analyzed pesticides in water samples from agricultural, urban, and mixed land use areas, observing at least one pesticide 90% of the time. Similarly, Smalling et al. (2012) analyzed water and bed sediments for pesticides in 11 areas inhabited by amphibians throughout the USA, where 24 pesticides (in water) and 22 pesticides (in bed sediments) were identified and quantified. Moreover, several studies have found quantifiable amounts of current-use pesticides in frog tissues from various sites throughout the USA, presumably due to agricultural application (Battaglin et al. 2016; Smalling et al. 2013, 2015). Bradford et al. (2010) noted that pesticide body burden in amphibians from the Sierra Nevada mountains was linked to atmospheric transport of pesticides from the agricultural San Joaquin Valley. Pesticides therefore are present in most areas where amphibians live and breed, so coming into contact with pesticides can be a daily occurrence.
Dehydration tolerance has been well documented within amphibians and the more terrestrial species can withstand a higher loss in body water (Hillman 1982). This increased tolerance is due to overall skin thickness which correlates to what habitats they occupy (Toledo and Jared 1993). Aquatic frogs usually have smooth dorsal and ventral skin, making water less permeable; terrestrial species have granulations on their ventral skin for a greater surface area enabling them to uptake water quickly (Hillman et al. 2009; Lillywhite and Licht 1975; Toledo and Jared 1993). Overall, amphibian skin has a low resistance to water; however, studies indicate that higher skin resistance to evaporative water loss is more indicative of arboreal species than non-arboreal species, which is due to exposure to higher temperatures and radiation for the arboreal species (Lillywhite 2006) This skin permeability feature places anurans in one of four habitat groups: aquatic, semi-aquatic, terrestrial, or arboreal (Hillman et al. 2009).
Amphibians can tolerate up to 50–60% loss of their total body water due to dehydration (Hermes-Lima and Storey 1998). Since Fowler’s toads were expected to reabsorb water more quickly than leopard frogs, the hypothesis for this study was that concentrations and body burdens would be higher in Fowler’s toads. Moreover, we thought these concentrations would be higher at longer dehydration time points. Within the present study, Fowler’s toads had statistically higher concentrations at each dehydration time compared to leopard frogs. Increased hydration correlated with the overall uptake of pesticides in dehydrated frogs. While in Cusaac et al. (2017), the authors observed that dehydration did not influence mortality when amphibians were exposed to Headline AMP. In the current study, whole-body concentrations for each pesticide were shown to decrease with increasing dehydration time (Fig. 1); this was likely due to the decrease of aerobic metabolism when up to 30% of initial body weight was lost. Hillman (1978a) illustrated that anurans lost the ability to conduct aerobic metabolism when they were dehydrated, which resulted in increased glycolysis. Gatten Jr (1987) also observed that a major effect of dehydration was a decrease in ATP being synthesized via aerobic means. In amphibian livers, ATP was observed to drop by 44% in anurans dehydrated to 50% total body water (Churchill and Storey 1995). It is therefore possible that anurans in this study did not have enough energy to facilitate uptake of pesticides from the soil, or energy was utilized for more vital processes.
Dehydration in Xenopus laevis resulted in a decline in circulatory oxygen and cardiovascular capabilities that led to anoxia within the tissues (Hillman 1978a, 1980). This decrease in oxygen, measured as maximal oxygen consumption rates from dehydration, was due to osmotic effects in the tissues (Hillman 1978a). Loss of body water resulted in increased blood viscosity and hematocrit (cellular fraction of the blood); thus, it became more difficult to deliver oxygen which resulted in anoxic tissues (Hermes-Lima and Storey 1998; Hillman 1978a, b; Hillman et al. 2009; Shoemaker et al. 1992). The decline in oxygen pressure at rest due to dehydration decreases cardiac output which can lead to cells experiencing hypoxic conditions and ultimately death (Hillman 1978a; Hillman et al. 2009). It is stressful for the amphibian to rehydrate following dehydration, however, due to wide ranges in oxygen forming free radicals which can cause oxidative stress and compromise ATP production (Hermes-Lima and Storey 1998; Hillman et al. 2009). This stress occurs because dehydration lowers systemic O2 transport; once the amphibian rehydrates, reoxygenation of the tissues allows reactive oxygen species to form (Hermes-Lima and Storey 1998, Hillman et al. 2009).
Due to dehydration, tissues become hypoxic or anoxic from impairment of blood circulation throughout the body. It is therefore possible that pesticides were not accumulated in dehydrated amphibians at initiation of the experiment. Once the amphibian is rehydrated to a threshold value or targeted percent hydration, its aerobic metabolism and metabolic demand can facilitate uptake of pesticides into the body. This will result in lower concentrations of pesticides being observed in more dehydrated amphibians. Since hydrated anurans did not need to overcome a lack of metabolic demand on physiological processes, they continued to uptake pesticides at a regular rate.
Another mechanism that could have occurred in amphibians, in addition to the loss of energy, is a dilution scenario. Viborg and Rosenkilde (2004) have demonstrated that severely dehydrated amphibians have higher uptake rates of water than controls for at least a 2-h period, which would result in a higher amphibian mass leading to a lower pesticide tissue concentration for amphibians in our experiment with longer dehydration times. This has implications for pesticide uptake, since aquaporins are preferentially permeable to water and these channels would therefore essentially be too small to facilitate pesticide uptake through the amphibian dermis (Campbell and Reece 2005). Therefore, it is possible that the dilution scenario occurred for the shorter dehydration time periods (e.g., at 0, 2, and 4 h) based on Fig. 2. In the current study, during the 6–10 h dehydration time periods, a range of 13–35% body mass loss was observed for the amphibians prior to exposure. This loss of body mass could result in a decline in liver function and available ATP (Churchill and Storey 1995) which could therefore reduce available energy reserves at the onset of the experiment. Both of these mechanisms are possible and could operate simultaneously. In the current study, there was no indication of significant changes in rehydration rates as a function of the dehydration time, so there is no evidence for a dilution scenario effect. However, the highest observed percent body mass losses were at the threshold of reduced available ATP reserves observed in Churchill and Storey (1995); therefore, we cannot discount this possibility.
Several articles recently examined effects of pesticides on amphibian uptake. Van Meter et al. (2015) noted that dermal contact from pesticides to amphibians was the main route for exposure in anurans and simulated overspray scenarios resulted in higher concentrations compared to indirect contaminated soil. Van Meter et al. (2014) observed that water solubility and soil partition coefficients were the best indicators for uptake of pesticides in amphibians. Furthermore, Van Meter et al. (2016) showed that organic matter content of agricultural soil can significantly affect pesticide body burdens. This study built on past experiments by testing hydration status on terrestrial-phase amphibians and utilized a dehydration time of 10 h as a worst-case scenario. The current study demonstrates that more dehydrated amphibians have lower pesticide body burdens resulting after 8 h of exposure. Future experiments do not need to dehydrate amphibians for an extended period of time if the goal is to evaluate maximum uptake rates. These tissue concentrations calculated post-dehydration can also be incorporated into ecological risk assessments.
Climate change can result in weather patterns that make irrigated agricultural landscapes relatively desirable amphibian habitat in otherwise xeric landscapes or time periods (U.S. EPA 2014). In these situations, anurans may dehydrate faster, requiring them to search for water sources in order to remain hydrated, leading to a higher likelihood of being exposed to pesticides in agricultural areas. These interactions can add to the list of potentially interacting stressors that amphibian populations are confronting (Davidson 2004; Davidson et al. 2002). Results of this study help to address the interaction of hydration status and pesticide uptake in two different species of terrestrial-phase amphibians. In conclusion, our study demonstrates that hydration status plays a surprisingly important role in pesticide uptake within anurans: more severely dehydrated amphibians exhibited lower concentrations of pesticides, contrary to our initial expectation. Understanding the mechanisms behind this process and how it governs exposure of pesticides in amphibians is critical in estimating how hydration status interacts with other natural and anthropogenic stressors in specific landscapes (Purucker et al. 2007). Interactions of hydration status and pesticide exposure in amphibians also needs to be studied at levels beyond individual body burdens (biomarkers to population) for a broader understanding of the stressors being experienced by amphibians (Grant et al. 2016; Snyder et al. 2017).
References
Barbeau TR, Lillywhite HB (2005) Body wiping behaviors associated with cutaneous lipids in hylid tree frogs of Florida. J Exp Biol 208:2147–2156
Battaglin W, Smalling K, Anderson C, Calhoun D, Chestnut T, Muths E (2016) Potential interactions among disease, pesticides, water quality and adjacent land cover in amphibian habitats in the United States. Sci Total Environ 566:320–332
Bentley P, Main A (1972) Zonal differences in permeability of the skin of some anuran Amphibia. Am J Phys 223:361–363
Berger G, Pfeffer H, Kalettka T (2011) Amphibienschutz in kleingewässerreichen Ackerbaugebieten (Conservation of amphibians in agricultural landscapes rich in small water bodies), 384. Natur and Text, Rangsdorf
Berger G, Graef F, Pfeffer H (2012) Temporal coincidence of migrating amphibians with mineral fertiliser applications on arable fields. Agric Ecosyst Environ 155:62–69
Bishop C, Mahony N, Struger J, Ng P, Pettit K (1999) Anuran development, density and diversity in relation to agricultural activity in the Holland River watershed, Ontario, Canada (1990–1992). Environ Monit Assess 57:21–43
Bradford DF, Stanley K, McConnell LL, Tallent-Halsell NG, Nash MS, Simonich SM (2010) Spatial patterns of atmospherically deposited organic contaminants at high elevation in the southern Sierra Nevada mountains, California, USA. Environ Toxicol Chem 29:1056–1066
Campbell N, Reece J (2005) Biology 7th edition, AP. Pearson, Education Inc, San Francisco
Churchill TA, Storey KB (1993) Dehydration tolerance in wood frogs: a new perspective on development of amphibian freeze tolerance. Am J Physiol Regul Integr Comp Physiol 265:R1324–R1332
Churchill TA, Storey KB (1995) Metabolic effects of dehydration on an aquatic frog, Rana pipiens. J Exp Biol 198:147–154
Cusaac JPW, Mimbs WH, Belden JB, Smith LM, McMurry ST (2017) Factors influencing the toxicity of Headline® fungicides to terrestrial stage toads. Environ Toxicol Chem 36:2679–2688
Davidson C, Shaffer HB, Jennings MR (2002) Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conserv Biol 16:1588–1601
Davidson C (2004) Declining downwind: amphibian population declines in California and historical pesticide use. Ecol Appl 14:1892–1902
Davidson C, Knapp RA (2007) Multiple stressors and amphibian declines: dual impacts of pesticides and fish on yellow-legged frogs. Ecol Appl 17:587–597
Dole JW (1967) The role of substrate moisture and dew in the water economy of leopard frogs, Rana pipiens. Copeia 1967:141–149
Fryday S, Thompson H (2012) Toxicity of pesticides to aquatic and terrestrial life stages of amphibians and occurrence, habitat use and exposure of amphibian species in agricultural environments. Food and Environment Research Agency Supporting Publications EN-343
Gatten Jr RE (1987) Activity metabolism of anuran amphibians: tolerance to dehydration. Physiol Zool 60:576–585
Gilliom RJ, Barbash JE, Crawford CG, Hamilton PA, Martin JD, Nakagaki N, Nowell LH, Scott JC, Stackelberg PE, Thelin GP (2006) Pesticides in the nation’s streams and ground water, 1992-2001. Geological Survey (US)
Grant EHC, Miller DA, Schmidt BR, Adams MJ, Amburgey SM, Chambert T, Cruickshank SS, Fisher RN, Green DM, Hossack BR (2016) Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci Rep 6:25625
Heatwole H, Torres F, De Austin SB, Heatwole A (1969) Studies on anuran water balance—I. Dynamics of evaporative water loss by the coqui, Eleutherodactylus portoricensis. Comp Biochem Physiol 28:245–269
Hermes-Lima M, Storey KB (1998) Role of antioxidant defenses in the tolerance of severe dehydration by anurans. The case of the leopard frog Rana pipiens. Mol Cell Biochem 189:79–89
Hillman SS (1978a) The roles of oxygen delivery and electrolyte levels in dehydration death of Xenopus laevis. J Comp Physiol B 128:169–175
Hillman SS (1978b) Some effects of dehydration on internal distributions of water and solutes in Xenopus laevis. Comp Biochem Physiol A Physiol 61:303–307
Hillman SS (1980) Physiological correlates of differential dehydration tolerance in anuran amphibians. Copeia 1980:125–129
Hillman SS (1982) The effects of in vivo and in vitro hyperosmolality of skeletal muscle performance in the amphibians Rana pipiens and Scaphiopus couchii. Comp Biochem Physiol A Physiol 73:709–712
Hillman SS, Withers PC, Drewes RC, Hillyard SD (2009) Ecological and environmental physiology of amphibians. Oxford University Press, New York
James S, Little E, Semlitsch R (2004) Effects of multiple routes of cadmium exposure on the hibernation success of the American toad (Bufo americanus). Arch Environ Contam Toxicol 46:518–527
Lenhardt PP, Brühl CA, Berger G (2015) Temporal coincidence of amphibian migration and pesticide applications on arable fields in spring. Basic Appl Ecol 16:54–63
Lillywhite HB, Licht P (1974) Movement of water over toad skin: functional role of epidermal sculpturing. Copeia 1974:165–171
Lillywhite HB, Licht P (1975) A comparative study of integumentary mucous secretions in amphibians. Comp Biochem Physiol A Physiol 51A:937–941
Lillywhite HB (2006) Water relations of tetrapod integument. J Exp Biol 209:202–226
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157:2903–2927
McClanahan L (1967) Adaptations of the spadefoot toad, Scaphiopus couchi, to desert environments. Comp Biochem Physiol 20:73–99
Ogushi Y, Akabane G, Hasegawa T, Mochida H, Matsuda M, Suzuki M, Tanaka S (2010a) Water adaptation strategy in anuran amphibians: molecular diversity of aquaporin. Endocrinology 151:165–173
Ogushi Y, Kitagawa D, Hasegawa T, Suzuki M, Tanaka S (2010b) Correlation between aquaporin and water permeability in response to vasotocin, hydrin and beta-adrenergic effectors in the ventral pelvic skin of the tree frog Hyla japonica. J Exp Biol 213:288–294
Pough FH, Taigen TL, Stewart MM, Brussard PF (1983) Behavioral modification of evaporative water loss by a Puerto Rican frog. Ecology 64:244–252
Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A 88:11110–11114
Purucker S, Welsh C, Stewart R, Starzec P (2007) Use of habitat-contamination spatial correlation to determine when to perform a spatially explicit ecological risk assessment. Ecol Model 204:180–192
Putnam RW, Hillman SS (1977) Activity responses of anurans to dehydration. Copeia 1977:746–749
Quaranta A, Bellantuono V, Cassano G, Lippe C (2009) Why amphibians are more sensitive than mammals to xenobiotics. PLoS One 4:e7699
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Shoemaker V, Hillman S, Hillyard S, Jackson D, McClanahan L, Withers P, Wygoda M (1992) Exchange of water, ions, and respiratory gases in terrestrial amphibians. In: Feder ME, Burggren WW (eds) Environmental physiology of the amphibians. The University of Chicago Press, Chicago, pp 125–150
Shoemaker VH, Nagy KA (1977) Osmoregulation in amphibians and reptiles. Annu Rev Physiol 39:449–471
Smalling KL, Orlando JL, Calhoun D, Battaglin WA, Kuivila KM (2012) Occurrence of pesticides in water and sediment collected from amphibian habitats located throughout the United States, 2009–2010. US Geological Survey Data Series 707: 36
Smalling KL, Kuivila KM, Orlando JL, Phillips BM, Anderson BS, Siegler K, Hunt JW, Hamilton M (2013) Environmental fate of fungicides and other current-use pesticides in a Central California estuary. Mar Pollut Bull 73:144–153
Smalling KL, Reeves R, Muths E, Vandever M, Battaglin WA, Hladik ML, Pierce CL (2015) Pesticide concentrations in frog tissue and wetland habitats in a landscape dominated by agriculture. Sci Total Environ 502:80–90
Smith PN, Cobb GP, Godard-Codding C, Hoff D, McMurry ST, Rainwater TR, Reynolds KD (2007) Contaminant exposure in terrestrial vertebrates. Environ Pollut 150:41–64
Snyder MN, Henderson WM, Glinski DA, Purucker ST (2017) Biomarker analysis of American toad (Anaxyrus americanus) and grey tree frog (Hyla versicolor) tadpoles following exposure to atrazine. Aquat Toxicol 182:184–193
Suzuki M, Hasegawa T, Ogushi Y, Tanaka S (2007) Amphibian aquaporins and adaptation to terrestrial environments: a review. Comp Biochem Physiol A Mol Integr Physiol 148:72–81
Taylor K, Mayer LP, Propper CR (1999) Intra- and extracellular dehydration-induced thirst-related behavior in an amphibian. Physiol Behav 65:717–721
Thorson TB (1964) The partitioning of body water in amphibia. Physiol Zool 37:395–399
Toledo R, Jared C (1993) Cutaneous adaptations to water balance in amphibians. Comp Biochem Physiol A Physiol 105:593–608
Tracy CR, Rubink WL (1978) The role of dehydration and antidiuretic hormone on water exchange in Rana pipiens. Comp Biochem Physiol A Physiol 61:559–562
U.S. EPA (2014) Climate change indicators in the United States, 2014. EPA 430-R-14-004. www.epa.gov/climatechange/indicators
Van Meter RJ, Glinski DA, Hong T, Cyterski M, Henderson WM, Purucker ST (2014) Estimating terrestrial amphibian pesticide body burden through dermal exposure. Environ Pollut 193:262–268
Van Meter RJ, Glinski DA, Henderson WM, Garrison AW, Cyterski M, Purucker ST (2015) Pesticide uptake across the amphibian dermis through soil and overspray exposures. Arch Environ Contam Toxicol 69:545–556
Van Meter RJ, Glinski DA, Henderson WM, Purucker ST (2016) Soil organic matter content effects on dermal pesticide bioconcentration in American toads (Bufo americanus). Environ Toxicol Chem 35:2734–2741
Viborg A, Rosenkilde P (2004) Water potential receptors in the skin regulate blood perfusion in the ventral pelvic patch of toads. Physiol Biochem Zool 77:39–49
Willens S, Stoskopf MK, Baynes RE, Lewbart GA, Taylor SK, Kennedy-Stoskopf S (2006) Percutaneous malathion absorption by anuran skin in flow-through diffusion cells. Environ Toxicol Pharmacol 22:255–262
Acknowledgments
Thanks to Fran Rauschenberg, Mike Cyterski, and Ed Odenkirchen for manuscript review and edits. This research was supported in part by an appointment to the Research Participation Program for the U.S. Environmental Protection Agency, Office of Research and Development, administered by the Oak Ridge Institute for Science and Education through interagency agreement between the U.S. Department of Energy and EPA. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. EPA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This IACUC protocol (A2012 05–018-Y1-A0) received approval from the University of Georgia Institutional Animal Care and Use Committee
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Glinski, D.A., Henderson, W.M., Van Meter, R.J. et al. Effect of hydration status on pesticide uptake in anurans following exposure to contaminated soils. Environ Sci Pollut Res 25, 16192–16201 (2018). https://doi.org/10.1007/s11356-018-1830-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1830-8