Abstract
Wheat, barley or wheat + barley and herbs (Terminalia chebula, Terminalia bellerica and Emblica officinalis) based low-glycemic-index (low-GI) foods were developed and studied α-amylase, α-glucosidase and DPP-IV inhibition property in vitro and in the streptozotocin-induced diabetic rats. The GI of products ranged from 47 to 53 than control white bread (GI = 95). Total phenolic (20.1 ± 1 mg gallic acid/g dry wt.) and flavonoids (15.2 ± 1 mg quercetin/g dry wt.) were higher in wheat + barley than barley (17.2 ± 1; 13.6 ± 2) and wheat (16.9 ± 1; 14.9 ± 2) products. The in vitro α-amylase (4–10%), α-glucosidase (5–17%) and DPP-IV (3–26%) inhibition (IC50) of methanol extracts were higher than the aqueous extracts. The fasting blood glucose (50.85, 33.22 and 24.52%) and oral glucose tolerance (AUC = 32.1, 36.04, and 27.73%) was lower in barley, wheat, and wheat + barley fed diabetic groups than diabetic control group (1571.5 ± 13.5 mg/dL/120 min). Feeding wheat, barley, and W + B foods for 60 days inhibited the intestinal α-amylase (1.2, 1.1 and 1.5-folds), α-glucosidase (1.3, 1.2 and 1.7-folds) and DPP-IV (1.6, 1.5 and 2.1-folds) activity compared to diabetic control. Low-GI foods lower the systemic glucose level, inhibit the glycolytic enzymes and DPP-IV activity and hence desirable for diabetes management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is associated with hyperglycemia due to defective insulin action/secretion and one of the cause factors for macro-vascular (cardiovascular disease) and microvascular (nephropathy, retinopathy and neuropathy) complications (Naveen and Baskaran 2018). Food plays a vital role in DM management, including insulin resistance (Forouhi et al. 2018). Hence, currently functional food therapy is receiving attention among diabetic subjects. In this regard, traditional food formulations are well recommended to mitigate DM than synthetic drugs owing to no side effects. Cereals, wheat (Triticum dicoccum) and barley (Hordeum vulgare) and blend of dried fruits of herbs (Terminalia chebula, Terminalia bellerica and Emblica officinalis) used in the present study (described in traditional Indian food/medicine), are practiced globally testify their hypoglycemic nature (Bano et al. 2015). Phytochemicals found in the cereals and herbs used in this study also reported displaying antioxidant, antibacterial, antidiabetic, anti-inflammatory, immunomodulatory, anti-mutagenic, antineoplastic and chemo-protective effects (Naveen and Baskaran 2018; Salunke et al. 2019).
Terminallia chebula, used from ancient time, considered as an antidiabetic fruit as showed reduction of blood glucose in diabetic rats by reducing the oxidative stress and has inhibitory activity to glycolytic enzymes and reduce cardiovascular risk in type 2 diabetic patients (Shasidharan et al. 2012; Pingali et al. 2020). Terminalia bellecira, a bioactive rich fruit exhibited α-amylase and α-glucosidase inhibitory potency and reduce oxidative stress in alloxan induced diabetic rats (Gupta et al. 2020). Emblica officinalis, known as Indian goosbarry used in herbal medicine from ancient time. Supplementation of fruit powder lowers the glucose and lipid profile in diabetic volunteer (Akhtar et al. 2011). However, the mixture of these herbal fruits found to be potentially effective for several clinical uses as antioxidant, anti-inflammatory, antibacterial, hypoglycemic, chemoprotective and other metabolic disorders (Peterson et al. 2017).
Currently, therefore, importance has been given towards economically viable processing of cereal and herbs based antidiabetic foods. In addition, low carbohydrate (low-glycemic index) foods for glycemic control have gained acceptance among clinicians, dietitians and the public (Tay et al. 2015). Although there are cereal and herbal blends in practice since ancient times, scientific evidence on efficiency, nutraceutical profile and therapeutic value are not available completely (WHO 2019). Hence, examining the functionality of such preparations can ensure their effective use against diabetes. Therefore, in the present study, wheat, barley, wheat + barley with herbal based low-GI foods were prepared. The products were validated for their antidiabetic effect in vitro; streptozotocin induced diabetic rat model and nutrient, functional and sensory properties. Products were examined for α-amylase, α-glucosidase and DPP-IV inhibition property. The outcome of the study is expected to provide new insight into the effective application of wheat, barley and herbal-based low GI products to manage diabetes.
Materials and methods
Materials
Herbs—Harad (Terminalia chebula), Bahira (Terminalia bellerica) and Indian gooseberry (Emblica officinalis) and cereals—wheat (Triticum dicoccum) and barley (Hordeum vulgare); turmeric (Curcuma longa), ghee, and sesame oil were purchased from a local supermarket for natural produce (Mysore, Karnataka, India). HPLC, MS-grade solvents and Folin-Ciocalteu-reagent were purchased from Sisco Research Laboratories (Mumbai, India). Gallic acid, quercetin, α-amylase (porcine pancreas type IV-B), α-glucosidase (intestinal acetone powder from rats), and DPP-IV/CD26 (human recombinant, untagged), streptozotocin, p-nitrophenol, maltose and p-nitroaniline were obtained from Sigma-Aldrich (St. Louis, USA). Analytical grade chemicals mentioned elsewhere in the text were purchased from Hi-Media Laboratories (Mumbai, India). Casein, vitamin and mineral mix and corn starch were purchased from MP Biomedical India Pvt. Ltd., (Mumbai, India). Food grade lard was procured from Coorg Meat House (Mysore, India).
Cereal and herb-based food preparation
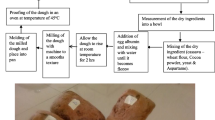
Dried fruit of T. chebula, T. bellerica and E. officinalis were deseeded, ground into a fine powder and mixed in the ratio of 1:2:4 (herb mixture). Wheat and barley were washed, dried and roasted at 100 °C in a roaster (Shri Venkateshwara Industries, Mysore, India) for 20 min and pulverized into fine flour using a hammer mill (Pilot Smith India Pvt. Ltd., India). Products were prepared with wheat flour (60%) or barley flour (60%) or wheat flour + barley flour (30 + 30%, W + B product) and herb mixture (20%), ghee (10%), sesame oil (10%), turmeric powder (0.2%), black salt (for taste), made a slurry with distilled water, homogenized (Fryma mill, Germany), and drum dried (M/s Escher-Wyss, Germany) using steam (Supplementary Fig. 1). Products were stored in airtight container at 4 ºC for further analysis.
Proximate composition analysis
Products (n = 3/product) were analyzed for moisture, protein, fat and ash content as per AOAC methods (AOAC 2005). Protein was estimated by the nitrogen content (6.25 factor was used to calculate protein). Soluble, insoluble and total dietary fiber was estimated as per Englyst et al. (1996). Carbohydrate content (%) was calculated as: 100−(protein + fat + ash + dietary fiber).
Total phenolic, flavonoid and tannins extraction and quantification
Total polyphenols were extracted (n = 3/product) from products (5 g) with 1% acidic methanol at 80 °C for 2 h repeatedly, filtered, pooled, concentrated by rotary evaporator (Hahn Shin Scientific Co, South Korea) and used for total phenols, flavonoid and tannin estimation (Hithamani and Srinivasan 2014). To an aliquot (50 μL) of methanolic extract of each sample and standard gallic acid (100 μg/mL), added distilled water, 1 N FC reagent and 20% sodium carbonate followed by vortexed and read at 765 nm (Spectrophotometer; UV-1800, Shimadzu, Japan) for total phenols (expressed as milligram gallic acid equivalent/g dry product). For total flavonoids, methanolic extracts (50 μL) of samples were made up to 5 mL with distilled water followed by added 3% NaNO2, 10% of AlCl3 and of 1 M NaOH and made up to 10 mL with distilled water, vortexed and read at 510 nm (expressed as milligram catechin equivalent/g dry product). For total tannins, vanillin-HCl reagent (5 mL) was added to acidified methanolic extracts (0.1 mL), absorbance was read at 500 nm, (expressed as milligram of catechin equivalent/g dry product).
Characterization of phenolics by UHPLC-HRMS
Phenolics were characterized using C18 column (30 × 2.1 mm 1.7 μm, Phenomenex) connected to Ekspert Ultra LC 100-XL system (Eksigent Technologies, CA, USA) at 35 °C. The mobile phase include two solvents: A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile and methanol, 8:2, v/v) with the following gradient program: 0 min: A-95%; 3 min: A-95%; 15 min: 5%; 18 min: A-5%; 18.10 min: A-95%; 25 min: 95% maintained at a flow rate of 0.2 mL/min. MS analysis was performed using Triple Tof 5600 + MS (Sciex, MA, Canada) in negative mode calibrated with APCI(−ve) calibration solution (AB Sciex, MA, Canada) with the conditions: nitrogen as gas source, temperature set at 400 °C, Ion Spray Voltage Floating (ISVF) set to 4500 and decluttering potential 100. The mass range was set from 100 to 2000 m/z for TOF MS. MS/MS was performed using Information Dependent Acquisition (IDA) experiment with selection of three most intense ions with normalization collision energy at 45 and CES at 20. MS data was evaluated using Peak View TM 2.1 (Sciex, MA, Canada) and phenolics were identified tentatively based on their respective calculated formula using the Master view, ChemSpider and MS fragmentation patterns.
Starch digestibility
Starch digestibility in vitro of products were estimated as per Englyst et al. (1996). Briefly, defatted products (100 mg each, n = 3) were mixed separately with distilled water (15 mL) containing α-amylase (100 µL) and incubated in a water bath (100 °C) for 30 min, followed by added glycine–HCl buffer containing porcine stomach pepsin (10 mg) and continued the incubation for 2 h at 37 °C, pH was adjusted to 4.8, added acetate buffer (15 mL) containing amyloglucosidase (20 mg) and incubated at 55 °C for 2 h. The solution was made up to 100 mL with distilled water and measured glucose (0.1 mL sample) using glucose oxidase–peroxidase kit (Auto span, Span Diagnostic Limited, India), at 510 nm by UV-spectrophotometer. The conversion of glucose from starch was calculated using the 0.9 factor.
Estimation of glycemic index
The glycemic index (GI) of products (n = 3) was estimated based on hydrolysis index (HI) and starch hydrolysis curve (0–120 min) as the percentage of glucose released over 2 h digestion compared to that glucose released from control (white bread). The estimated glycemic index (EGI) was calculated (Goni et al. 1997), by using the formula: EGI = 39.71 + (0.549 × HI). Where, HI = Hydrolysis Index.
Sensory analysis of products
Sensory and acceptability of products were evaluated with the trained panel. Products (6% each) were dispersed separately in lukewarm water and served to ten sensory expert panelists for qualitative and quantitative evaluation. The evaluation was performed in sensory booths designed, to reduce the panelist biases (Stone and Sidel 1998). Booths are air-conditioned (20 ± 2 °C) and maintained under white fluorescent light with relative humidity at 50 ± 5%. Scorecard was prepared using “free-choice profiling” method by selecting suitable terminologies associated with the product. A standard sensory method, "Quantitative Descriptive Analysis" was designed and used to assess the products' quality characteristics. Each dispersion (25 mL) was served to panelist for tasting and instructed to cleanse their mouth with water thoroughly between each test.
Antioxidant properties
Total antioxidant activity of products was determined by phosphomolybdenum method (Rajesh et al. 2013). In brief, methanolic extract of products (20, 30, 40, 50, 60 µL) was mixed with 3 mL of reagent mixture (0.6 M sulfuric acid, 4 mM ammonium molybdate and 28 mM of sodium phosphate), incubated for 90 min at 95 °C, cooled, read at 695 nm against the blank and antioxidant capacity was expressed as vitamin C equivalents.
The total reductive capacity of methanol extracts of each product was analyzed (Rajesh et al. 2013) at 10, 20, 30, 40 µL concentrations mixing separately with 2.5 mL phosphate buffer (0.2 M, pH 6.5) and 2.5 mL potassium ferricyanide (1%) and incubated for 20 min at 50 °C, followed by added 2.5 mL trichloroacetic acid (10%), centrifuged for 10 min at 3000 rpm and to the supernatant (2.5 mL), 0.5 mL of ferric chloride (0.1%) and 2.5 mL of millipore water was added and read at 700 nm against blank. Reducing capacity is expressed as quercetin (μg) equivalents.
Radical scavenging property of products was assessed based on scavenging ability of stable DPPH radical (Rajesh et al. 2013). In brief, methanolic extracts (2, 4, 6, 8 µL) were added to 3 mL of DPPH (0.004% DPPH in 95% ethanol), incubated for 30 min in dark at 27 °C and measured the absorbance at 517 nm against ascorbic acid standard. The IC50 value was calculated using the formula: IC50 = [(ΣC/ΣI) × 50], where, ΣC = sum of the concentration of extracts; ΣI = sum of the percentage of inhibition of extract at different concentrations.
Hypoglycemic property of water and methanolic extracts in vitro
Products (5 g each) dissolved separately in distilled water (50 mL) were agitated in a magnetic stirrer overnight, centrifuged (15 min) at 5000 rpm and the supernatant (water extract) was used for α-amylase, α-glucosidase and DPP-IV assay in vitro. For methanol extract, products (5 g each) dissolved separately in 15 mL methanol, incubated (3 h) in a shaking water bath (50 °C), centrifuged (15 min) at 5000 pm and the pooled supernatant (3-time extracted) was used for α-amylase, α-glucosidase and DPP-IV assays in vitro.
Effect of aqueous and methanolic extract of products (n = 3) on α-amylase inhibition in vitro was measured (Gopal et al. 2017). In brief, extracts (100, 200, 300, 400 µL) with 3.3 units of α-amylase was incubated with pre-gelatinized starch solution (1%) and CaCl2 (0.01 M) dissolved in sodium citrate buffer (0.1 M, pH 6.9) for 30 min at 37 °C. The reaction was stopped by adding dinitrosalicylic acid (1.0 mL) and subsequent boiling for 5 min. The quantity of reduced sugars released was measured spectrophotometrically (UV-1800, Shimadzu, Shimadzu UK limited) at 540 nm. Acarbose was used as positive control.
The α-glucosidase inhibition of aqueous and methanolic extract of products (n = 3) was determined using 4-nitrophenyl α-D-glucopyranoside as substrate (Gopal et al. 2017). The reaction mixture contained 120 µL of 0.05 M phosphate buffer (pH 6.8), 3 mM PNPG (50 µL), aqueous extract (20, 30, 40, 50 µL) and methanol extract (20, 30, 40, 50 µL) and 50 µL of α-glucosidase solution (25 mg/mL) in a 96-well plate, incubated for 45 min at 37 °C, and the reaction was stopped by the 0.67 M Na2CO3 (50 µL). Enzyme activity was determined against the absorbance of p-nitrophenol at 405 nm released from the 4-nitrophenyl α-D-glucopyranoside calculated percent inhibition relative to control with no added extract.
The DPP-IV enzyme inhibition capacity of products was examined by the releases of yellow-colored p-nitroaniline (Al-masri et al. 2009). Aqueous (10, 20, 30 µL) and methanol (10, 15, 20, 25 µL) extracts (n = 3) in 35 μL of 50 mM Tris–HCl buffer (pH 7.5) was pre-incubated with 15 μL of DPP-IV (0.05 U/mL) for 10 min at 37 °C, followed by, added 50 μL of the substrate (0.2 mM Gly-Pro-p-nitroanilide), incubated at 37 °C for 30 min, the reaction was stopped by adding 25% glacial acetic acid (25 μL), and the release of p-nitroaniline was calculated from the absorbance at 405 nm using a microtiter plate reader (Varioskan Flash Multimode Reader, Thermo Scientific, USA.
Animal experiment
Animal experiment was carried out with male Wistar rats (52 ± 5 g) after the approval of the Committee for Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC No.122/2018) of CSIR-CFTRI. Rats were divided randomly into five groups (n = 12/group) and housed individually in polypropylene cages at 28 ± 2 °C with 12 h light: dark cycle. Group 1 (normal control) fed diet with 7% fat (7 g lard/100 g diet). Group 2 received a high fat diet (35 g lard /100 g diet) with no products and considered as diabetic group. While group 3, 4 and 5 received high fat diet (35 g lard/100 g diet) along with barley, wheat and W + B products (20 g/100 g diet). The energy value of the diet fed to all the groups were isocaloric (Reeves 1997). After 30 days of feeding high fat diet, group 2, 3, 4 and 5 were induced diabetes by intraperitoneal injection of streptozotocin (30 mg/kg body weight) (Reed et al. 2000). The experiment was continued further for 60 days. Upon termination of experiments, rats were fasted overnight and sacrificed under carbon dioxide (5%) anesthesia. Blood was collected by cardiac puncture, intestine was collected and stored in − 80 °C until further analyses.
Oral glucose tolerance test
The glucose tolerance of rats was examined by the oral glucose tolerance test (OGTT). The OGTT was performed with four animals from each group. Animals were fasted for 12 h and blood was drawn from the caudal vein and measured fasting blood glucose level. Subsequently, glucose solution (2 g/kg body weight) was intubated, blood was collected from tail vein at 30, 60 and 120 min interval and glucose level was measure-using glucometer (Arkray Glucocard mini 01 Mini-auto coding glucometer kit). Blood glucose level (mg/dL) versus time (min) intervals were constructed and area under curve (AUC) was calculated by using GraphPad Prism 5.00 software.
α-Amylase, α-glucosidase and DPP-IV assay and serum glucose level
At the termination of the experiments, the intestine was collected from each group (n = 8/group), washed with ice-cold phosphate buffer saline (pH = 7.4), cut opened, mucosa was scrapped and homogenized with 10% in 0.9% saline (enzyme source). α-Amylase, α-glucosidase and DPP-IV activity were measured as described earlier under in vitro analysis section. Tissue protein level was measured as per Lowry et al. (1951). Also, estimated the serum glucose level by using the commercial kit (Agappe Diagnostic, Kerala, India).
Results
Nutritional composition of low-GI foods
Table 1 demonstrates the nutritional compositions of low-GI foods prepared. Moisture content is slightly lower in barley product compared to wheat and W + B products. The protein content is higher (13.1 ± 0.5%) in the wheat product compare to barley (7.5 ± 0.3%) and W + B (10.4 ± 0.51%) products. The products’ total dietary fiber content ranged from 15.4–18.3%, with significantly higher in barley product (18.3%). Besides, the insoluble and soluble fiber content of products are 11.4 and 4% (barley product), 12.4 and 5.9% (wheat product) and 13.6 and 3.7% (W + B product), respectively. The total lipid content ranged as 15–18%. No significant difference in the total ash content of products. The total carbohydrate content is slightly higher in the barley (53.11 ± 0.2%) compared to wheat (48.5 ± 0.1%) and W + B (48.7 ± 0.1%) products. The energy (kcal/100 g) value is 400.5 (wheat product), 381.9 (barley product) and 395.7 (W + B product), respectively. Results indicate that products are nutritionally superior.
Total phenolic, flavonoid and tannin
Table 1 show slightly higher (3%) phenolic and (2%) flavonoid content in W + B product than the wheat and barley products (17.5 ± 1.1; 15.2 ± 1.8 mg/g). However, tannin level is higher in barley product (0.2 ± 0.01 mg/g) compared to wheat (0.08 ± 0.01 mg/g) and W + B (0.08 ± 0.01 mg/g) products (Table 1). Further, the extract was characterized by UPLC-HRMS and MS/MS) and identified phenolics (peak number 1 to 19 in Fig. 1) with their molecular mass, formula and fragmentation pattern are listed in Supplementary Table 1.
Starch digestibility
The in vitro starch digestibility of foods ranged between 61 and 65% compared to white bread (control, 94.65%), signifying lower starch digestibility. The slight difference in the starch digestibility of products may be due to variation in particle size of starch granules and gelatinization during drying processing.
Estimated glycemic index (GI) of products
Glycemic index is the plasma glucose and insulin responses after ingestion of carbohydrate-containing food. EGI of the barley product is the least (47.3) than the wheat (49.2) and W + B (53.8) products compared to control (100). The hydrolysis index of barley product is least than the other two products (Table 1).
Sensory analysis
Sensory attributes like appearance, dispensability, saltiness, bitter taste, astringency, mouth-feel, aroma, texture, overall acceptability and overall quality of the products were analyzed. All the products exhibited almost similar textural and sensory properties with no significant difference. However, W + B product found to score higher overall acceptability (9.6) followed by wheat (8.9) and barley (9.0) products on a 15 cm Quantitative Descriptive Analysis scale (Fig. 2).
Antioxidant property of products
Wheat product exhibited higher total antioxidant capacity at 46.1 mg (60 µL methanolic extract), which is equivalent to 59.3 ± 0.7 µg of ascorbic acid. In case of barley and W + B products, the total antioxidant activity was higher at 39.9 mg and at 33.3 mg, which are equivalent to 56.9 ± 0.7 µg and 70.2 ± 1.1 µg of ascorbic acid. The products' total reductive capability against the quercetin revealed that wheat product (40 µL of extract) exhibited the highest reductive capacity at 30.7 mg (equivalent to 52.9 ± 1.0 µg of quercetin). Barley and W + B products exhibited the maximum activity at 26.64 mg and 22.2 mg which is equivalent to 50.85 ± 0.5 µg and 93.7 ± 0.9 µg quercetin activity, demonstrating superior antioxidant activity of product’s extract compared to ascorbic acid and quercetin. The hydrogen donating ability of the products was studied by DPPH assay and detected at 5.33, 6.15 and 4.44 mg of wheat, barley and W + B products respectively. The percent radical scavenging activity of products (5 mg) was 67.8%, 82.7% and 81.6%, respectively. The IC50 value of products (5 mg) was ranged between 4.3 and 3.6 ± 0.8 µg of ascorbic acid equivalent.
α-Amylase, α-glucosidase and DPP-IV inhibition in vitro
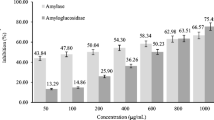
Table 2 shows that the α-amylase inhibition is concentration-dependent on water and methanol extracts of products. The IC50 values for the methanol extracts of wheat, barley and W + B products were 8.1, 5.1 and 6.2 mg/mL, respectively, whereas IC50 values for water extracts were 12.2, 16.7 and 10.6 mg/mL, respectively. However, IC50 values for the products are lower than acarbose (IC50 8.1 ± 0.14 µg/mL). The inhibitory effect of methanol and water extract of products against α-glucosidase was a dose-dependent. The IC50 inhibitory concentration of extracts was ranged as 2–12 mg/mL. However, IC50 of acarbose is 4.1 ± 0.48 µg/mL. The DPP-IV inhibitory effect of methanol and water extracts of products was dose-dependent (Table 2). Although no difference in the inhibitory effect (IC50 4.3–4.6 mg/mL) among methanol extract of products, water extract of the mixed product showed higher inhibitory activity (IC50 5.6 mg/mL) than barley and wheat products (IC50 7.2 and 30.5 mg/mL); however, IC50 value of drug sitagliptin is significantly higher (0.145 ± 2.3 µg/mL).
Effect of products on oral glucose tolerance
The blood glucose level showed significant change after oral loading (2 g/kg body weight) of glucose (Fig. 3). The level of blood glucose in diabetic group was significantly higher throughout the measurement period (120 min) compared to the normal control group. However, diabetic groups those fed on wheat, barley and W + B showed significantly lower level compared to diabetic group. Similarly, the AUC values (mg/dL/120 min) of groups received wheat (1067 ± 5.7), barley (1005 ± 9.9) and wheat + barley (1135 ± 7.1) products showed lower value by 47.7, 36 and 27.7% respectively than the diabetic control group (1571.5 ± 13.5 mg/dL/120 min).
Effect of wheat (W), barley (B) and wheat+barley (W+B) products on oral glucose tolerance in diabetic rats (a). The area under curve of the blood glucose values (b). Values are mean ± SD (n = 4/group). *P < 0.05 vs diabetic, ** P < 0.05 vs experimental groups. Note: C: control, D: diabetic, B: barley product, W: wheat product, W+B: wheat+barley product
Serum glucose level, α-amylase, α-glucosidase and DPP-IV inhibition in vivo
Streptozotocin induced diabetic group 2 found to have a blood glucose level higher than 200 mg/dL within 7 days of induction and it was 271.15 ± 5.34 mg/dL at the end of the experimental run compared to normal control group 1 (99.25 ± 8.25 mg/dL). Whereas, the blood glucose level (mg/dL) was significantly lowered which received wheat (181.08 ± 4.63), barley (133.27 ± 8.88) and W + B (204.67 ± 4.64) compared to group 2. Intestinal α-amylase and α-glucosidase activity were elevated significantly in diabetic group 2 by 2.23 folds and 1.79 folds when compared to normal group (404.84 ± 4.8nmole maltose/min/mg protein and 57.92 ± 1.64 nmole PNP/min/mg protein). However, feeding diet with wheat, barley and W + B products resulted in inhibition of α-amylase activity (476.54 ± 4.2, 434.48 ± 2.3 and 595.81 ± 4.3) and α-glucosidase activity (73.79 ± 1.3, 67.15 ± 3.1 and 95.89 ± 2.6) compared to diabetic control group 2. Similarly, DPP-IV enzyme activity also increased by 3.29 folds (1.4 ± 0.2 p moles PNP/ min/ mg protein) in diabetic group 2 compare to normal control group (0.43 ± 0.01). However, its activity (p moles PNP/min/mg protein) decreased upon feeding diet with wheat (0.71 ± 0.01), barley (0.64 ± 0.02) and W + B (0.91 ± 0.08) products compared to diabetic group 2 (Fig. 4).
α-Amylase (a), α-glucosidase (b) and DPP4 (c) enzyme activity in rat intestine and serum glucose level (d). α-Amylase activity was determined by measuring the maltose released per minute. α-glucosidase activity measured by estimating p-nitrophenol release per minutes and DPP4 enzyme activity expressed by p-nitoaniline equivalent released per min. Values are Mean ± SD (n = 4/group). Note: C: control, D: diabetic, B: barley product, W: wheat product, W + B: wheat + barley product
Discussion
In recent years, cereal and poly-herbal preparations are highly endorsed to control diabetics. However, not enough scientific validations are available on their action mechanism with diabetic targets. Hence, in the present study, cereal and herbal-based low-GI antidiabetic products were prepared adopting drum drying technique. We have chosen the herbs (Terminalia chebula, Terminalia bellerica and Emblica officinalis) used in this study based on the available evidence in the classical medicine on their use for treating diabetic in the form of triphala (Gurjar et al. 2012; Peterson et al. 2017). The dried herbs were ground well into a fine powder and was blended homogeneously with finely grounded wheat and barley as per the ratio standardized. Wheat and barley contribute protein, minerals, dietary fiber and phytochemicals in addition to low GI. Herbal fruits and ghee used in this study were reported to lower blood glucose in a diabetic model (Chouhan et al. 2013) and help stimulating biliary secretion and emulsification process. Sesame oil is rich in antioxidants sesamin, sesamolin and sesaminol and they scavenge radicals in hyperglycaemia (Sankar et al. 2011). Owing to pharmacological properties of wheat, barley, herbal mix, ghee, sesame oil and turmeric, low-GI foods were prepared to manage diabetes.
Products found to contribute phenolic, lipids, dietary fiber, and carbohydrate (Table 1). Drum drying process adopted did not affect the nutrient profile of products; however, improved acceptability. The moisture content of the products indicating longer storage stability to reduce microbial load. Incorporation of herbal mix with cereals reduced starch digestibility of the products than the white bread. Total fat content of the products (15–18%) is mainly from sesame oil (10%) and ghee (10%). Foods prepared in this study contribute 8 to 13% of protein which is more than one third of Recommended Dietary Allowance (RDA).
Polyphenols exhibits antidiabetic property. The herbal mixture (20%) used in this study contained 38% of total polyphenol in which gallic acid, ellagic acid and gallic acid derivatives are the major phenolics. Comparatively, W + B product contain higher phenolic than the wheat and barley-based product. Products were also rich in flavonoid and tannin content, indicating intense antioxidants that suppress the circulating systematic glucose in diabetes (Pfundstein et al. 2010). In addition, heat processing of products affects physicochemical property of starch by gelatinization that lowers the GI of the product and loss of organized structure, granule swelling, loss of birefringence, and crystallinity (Cooke and Gidley 1992).
Glycemic index infers the rise of blood glucose after ingestion of carbohydrate-rich foods. Hence, low-GI foods are prerequisite to manage diabetes (Lopes et al. 2019). In this study, products prepared to have low EGI (i.e., < 55) compared to white bread. Barley product exhibited the least GI (EGI = 47.2) than wheat and W + B products. It is reported that millet based food products reduces the starch hydrolysis and glycemic load effectively (Lestari et al. 2017). Hence, these products can be appropriate to manage diabetes (AD 2020). From ancient time, preparations with combination of two cereals considered as nutritionally superior and hence given importance in the classical medicine like Ayurveda. Barley with β-glucan and wheat (Emmer type) with higher protein content along with difference in dietary fiber found to contribute low-glycemic index value (Nilsson et al. 2008) apart from rich in phenolic compounds like caffeic acid and velonic acid. However, in the present study, wheat + barely product displayed higher GI than individual cereals. The sensory quality of the product showed overall acceptability and better palatability, may be due to the mixture of cereals and herbs, and gelatinization. All three products are stable and can be stored for 6 months at room temperature. No changes were observed in terms of sensory and nutritional characteristics during storage (data not shown). Although there are many low glycemic diabetic food formulations available in the market, there is no cereal and herbal foods based on traditional knowledge and their usage for lowering blood sugar and no added additives and preservatives available commercially. So, the present study provides scientific evidences with respect to inhibition of glycolytic enzymes and DPP-IV and hence will have application.
In addition to low GI, antioxidant dense foods recommended to manage oxidative stress in diabetes. The present result shows that products exhibited higher antioxidant and reductive capability because of polyphenols, flavonoids and tannins. Pfundstein et al. (2010) have reported that phenolic of Terminalia fruits rendered higher antioxidant activity. Omwamba and Hu (2009) suggested that roasting of grains increases antioxidant capacity, as observed in the present study.
α-Amylase and α-glucosidase are the most targeted markers of clinical research in the diabetes management. Herbal extracts inhibited α-amylase and α-glucosidase activity (Li et al. 2009). The present in vitro study (Table 2) show that the methanol extract of products significantly inhibited the α-amylase and α-glucosidase activity compared to aqueous extracts. However, the values are comparable with methanolic extracts (IC50 values of 0.966 and 2.431 mg/mL) of herb Potentilla discolor Bge (Cheng et al. 2020). The IC50 values of products on α-glucosidase inhibition are higher than the extract of Emblica Officinalis (IC50 106.7 µg/mL) (Majeed et al. 2020) and lower than the barley extract (128.82 mg/mL) (Ha et al. 2016). Gallic acid and ellagic acid reported to inhibit the α-amylase and α- glucosidase activity (Kam et al. 2013; Oboh et al. 2016). Penta-O-gallyol β-D glucose from T. chebula inhibited α-glucosidase activity. Polyphenolic compounds found in the products could be the reason for the inhibition of glycolytic enzymes and DPP-IV. As observed in the in vitro study (Table 2), wheat, barley and W + B products found to inhibit intestinal α-amylase, α-glucosidase and DPP-IV in the diabetic rats, which may be due to low GI, blood glucose-lowering property and higher soluble fiber content of products. The lower AUC values for blood glucose tolerance in diabetic rats fed on low GI foods further supports the antidiabetic property. The level of blood glucose in diabetic group was significantly higher throughout the measurement period (120 min) compared to the normal control group. However, diabetic groups those fed on wheat, barley and W + B showed significantly lower level compared to diabetic group. Similarly, the AUC values (mg/dL/120 min) of groups received wheat (1067 ± 5.7), barley (1005 ± 9.9) and wheat + barley (1135 ± 7.1) products showed lower value by 47.7, 36 and 27.7% respectively than the diabetic control group (1571.5 ± 13.5 mg/dL/120 min). Results clearly indicate the significant difference with respect to OGT in wheat and barley based products fed groups compared to wheat + barley fed group. Further, the DPP-IV inhibitory effect of methanol extract of barley product (IC50 4.3 mg/mL) was higher, compared to W + B and wheat products, which may be due to higher phenolic and flavonoids. Further, polyphenols, flavonoids and tannins present in the cereals and herbs used in this study could be another reason for blood glucose-lowering effect, inhibition of glycolytic and DPP-IV enzymes. Hydroalcoholic extract of barley seeds has a blood glucose-lowering effect in streptozotocin induced diabetic rats. Therefore, ingestion of high dietary fiber and antioxidants flavonoids rich foods decreases blood glucose by increased insulin secretion in diabetic rats and patients with type 2 diabetes (Chandalia et al. 2000; Minaiyan et al. 2014). Between the cereals in combination with herbs, barley product was found to inhibit more significantly the glycolytic and DPP-IV enzymes that may be due to higher β-glucan and the soluble fiber content of the product (Minaiyan et al. 2014). Although, all the three products found to inhibit glycolytic enzymes and DPP-IV activity. The efficiency of wheat + barley product on DPP-IV found to be higher compared to α-amylase and α-glucosidase which may be due to higher levels of active component (phenolic). Hence, it can be considered that wheat + barley product is effective in controlling blood glucose. Further, result of the study demonstrates that cereal + herb mixture inhibits strongly all the enzymes studied compared to with no added herb. The lower IC50 values of product wheat + barley further support and justifies the findings. Additional, lower starch digestibility of foods prepared in this study (61–65%) compared to white bread (control, 94.65%), signify one of the reasons for higher inhibition of enzymes studied and blood glucose lowering potential of products.
Conclusion
Nutritional therapy accepts food(s) to reverse diabetes. Hence, it is vital to deliver proper nutrient and antioxidant-dense foods to diabetic subjects to manage diabetes. Wheat and barley products were found to inhibit α-amylase, α-glucosidase and DPP-IV activity and serve as a functional food for regulating blood glucose. Hence, foods developed in the present study serve as a health food to manage diabetes. However, studies on the mechanism of action in human subjects are required to support the results.
Abbreviations
- DM:
-
Diabetes mellitus
- GI:
-
Glycemic index
- DPP-IV:
-
Dipeptidyl peptidase-4
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl; di(phenyl)-(2,4,6-trinitrophenyl) iminoazanium)
- OGTT:
-
Oral glucose tolerance test
- EGI:
-
Estimated glycemic Index
- W + B:
-
Wheat + barley product
- HI:
-
Hydrolysis index
- UV:
-
Ultra violate
- MS:
-
Mass spectroscopy
- UHPLC:
-
Ultra high performance liquid chromatography
- HRMS:
-
High resolution mass spectroscopy
- APCI:
-
Atmospheric pressure chemical ionization
- AUC:
-
Area under curve
References
Akhtar MS, Ayesha R, Amanat A, Maqsood A (2011) Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int J Food Sci Nutr 62(6):609–616
Al-masri IM, Mohammad MK, Tahaa MO (2009) Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J Enzyme Inhib Med Chem 24:1061–1066
AOAC (2005) Official methods of analysis 18th Edition. Pub AOAC International Maryland
Association AD (2020) 6. Glycemic targets: standards of medical care in diabetes- 2020. Diabetes Care 43:S66–S76
Bano T, Goyal N, Tayal PK (2015) Innovative solar dryers for fruits, vegetables, herbs and ayurvedic medicines drying. Int J Eng Res General Sci 3:883–888
Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ (2000) Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 342(19):1392–1398. https://doi.org/10.1056/NEJM200005113421903
Cheng D, Wang P, Huang J et al (2020) Antioxidant, antidiabetic and identification of phenolic constituents from potential discolor Bge. Eur Food Res Technol 246:2007–2016
Chouhan B, Kumawat RC, Kotecha M et al (2013) Triphala: A comprehensive ayurvedic review. Int J Res Ayurveda Pharm 4:612–617
Cooke D, Gidley MJ (1992) Loss of crystalline and molecular order during starch gelatinisation: origin of the enthalpic transition. Carbohyd Res 227:103–112
Englyst HN, Veenstra J, Hudson GJ (1996) Measurement of rapidly available glucose (RAG) in plant foods: a potential in vitro predictor of the glycemic response. Br J Nutr 75:327–337
Forouhi NG, Misra A, Mohan V et al (2018) Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 361:k2234
Goñi I, Garcia-Alonso A, Saura-Calixto F (1997) A starch hydrolysis procedure to estimate glycemic index. Nutr Res 17:427–437
Gopal SS, Lakshmi MJ, Sharavana G et al (2017) Lactucaxanthin–a potential anti-diabetic carotenoid from lettuce (Lactuca sativa) inhibits α-amylase and α-glucosidase activity in vitro and in diabetic rats. Food Funct 8:1124–1131
Gupta A, Kumar R, Pandey AK (2020) Antioxidant and antidiabetic activities of Terminalia bellirica fruit in alloxan induced diabetic rats. S Afr J Bot 130:308–315
Gurjar S, Pal A, Kapur S (2012) Triphala and its constituents ameliorate visceral adiposity from a high-fat diet in mice with diet-induced obesity. Altern Ther Health Med 18:38–45
Ha KS, Jo SH, Mannam V, Kwon YI, Apostolidis E (2016) Stimulation of phenolics, antioxidant and α-glucosidase inhibitory activities during Barley (Hordeum vulgare L.) seed germination. Plant Foods Hum Nutr 71(2):211–217
Hithamani G, Srinivasan K (2014) Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Chem 164:55–62
Kam A, Li KM, Valentina RN, Srinivas N, Jeffrey S, Kelvin C, Li G (2013) A comparative study on the inhibitory effects of different parts and chemical constituents of pomegranate on a-amylase and a-glucosidase. Phytother Res 27:1614–1620
Lestari LA, Huriyati E, Marsono Y (2017) The development of low glycemic index cookie bars from foxtail millet (Setaria italica), arrowroot (Maranta arundinacea) flour, and kidney beans (Phaseolus vulgaris). J Food Sci Technol 54:1406–1413. https://doi.org/10.1007/s13197-017-2552-5
Li YQ, Zhou FC, Gao F et al (2009) Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J Agric Food Chem 57:11463–11468
Lopes CO, Barcelos MFP, Vieira CNG et al (2019) Effects of sprouted and fermented quinoa (Chenopodium quinoa) on glycemic index of diet and biochemical parameters of blood of Wistar rats fed high carbohydrate diet. J Food Sci Technol 56:40–48
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Majeed M, Majeed S, Mundkur L et al (2020) Standardized Emblica officinalis fruit extract inhibited the activities of α-amylase, α-glucosidase, and dipeptidyl peptidase-4 and displayed antioxidant potential. J Sci Food Agric 100:509–516
Minaiyan M, Ghannadi A, Movahedian A, Hakim-Elahi I (2014) Effect of Hordeum vulgare L. (Barley) on blood glucose levels of normal and STZ-induced diabetic rats. Res Pharm Sci 9:173
Naveen J, Baskaran V (2018) Antidiabetic plant-derived nutraceuticals: a critical review. Eur J Nutr 57:1275–1299
Nilsson A, Ostman E, Preston T, Björck I (2008) Effects of GI vs content of cereal fibre of the evening meal on glucose tolerance at a subsequent standardized breakfast. Eur J Clin Nutr 62:712–720
Oboh G, Opeyemi BO, Mariam DO, Stephen AA (2016) Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. J Food Drug Anal 24:627–634
Omwamba M, Hu Q (2009) Antioxidant capacity and antioxidative compounds in barley (Hordeum vulgare L.) grain optimized using response surface methodology in hot air roasting. Eur Food Res Technol 229:907–914
Peterson CT, Kate D, Deepak C (2017) Therapeutic uses of triphala in ayurvedic medicine. J Altern Complement Med 23:607–614
Pfundstein B, El Desouky SK, Hull WE et al (2010) Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry 71:1132–1148
Pingali U, Sukumaran D, Nutalapati C (2020) Effect of an aqueous extract of Terminalia chebula on endothelial dysfunction, systemic inflammation, and lipid profile in type 2 diabetes mellitus: a randomized double-blind, placebo-controlled clinical study. Phytother Res 34:3226–3235
Rajesh KP, Manjunatha H, Krishna V, Swamy BK (2013) Potential in vitro antioxidant and protective effects of Mesua ferrea Linn. bark extracts on induced oxidative damage. Ind Crops Prod 47:186–198
Reed MJ, Meszaros K, Entes LJ et al (2000) A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism-Clin Exp 49:1390–1394
Reeves PG (1997) Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 127:838S-841S
Salunke M, Banjare J, Bhalerao S (2019) Effect of selected herbal formulations on anthropometry and body composition in overweight and obese individuals: a randomized, double blind, placebo-controlled study. J Herb Med 17:100298
Sankar D, Ali A, Sambandam G, Rao R (2011) Sesame oil exhibits synergistic effect with anti-diabetic medication in patients with type 2 diabetes mellitus. Clin Nutr 30:351–358
Sasidharan IA, Sundaresan VM, Nisha MS, Raghu Kirishna KG, Jayamurthy P (2012) Inhibitory effect of Terminalia chebula Retz. fruit extracts on digestive enzyme related to diabetes and oxidative stress. J Enzyme Inhib Med Chem 27(4):578–586
Stone H, Sidel JL (1998) Quantitative descriptive analysis: developments, applications, and the future. Food Technol 52:48–52
Tay J, Luscombe-Marsh ND, Thompson CH et al (2015) Comparison of low-and high-carbohydrate diets for type 2 diabetes management: a randomized trial. Am J Clin Nutr 102:780–790
World Health Organization (2019) WHO global report on traditional and complementary medicine 2019. World Health Organization
Acknowledgements
The authors are grateful to Dr. N. S. Mahendrakar, Ex-chief editor, Journal of Food Science and Technology, for editing the manuscript for its English language.
Funding
Arpita Das acknowledges the University Grant Commission (UGC), Government of India, for financial assistance (Award No. 1108/(SC) (NET-JULY 2016).
Author information
Authors and Affiliations
Contributions
AD: Formal analysis, investigation, methodology, writing of original draft; NJ: Methodology, validation, visualization, review and editing. YNS: Validation, data curation, visualization; BSGK: Validation, data curation. VB: Project administration, supervision, resources, writing—review and editing, conceptualization, validation, visualization.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Ethical approval
The rats were all treated in strict accordance with Committee for Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC No. 122/2018) of CSIR-CFTRI guidelines for the care and use of laboratory animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Das, A., Naveen, J., Sreerama, Y.N. et al. Low-glycemic foods with wheat, barley and herbs (Terminalia chebula, Terminalia bellerica and Emblica officinalis) inhibit α-amylase, α-glucosidase and DPP-IV activity in high fat and low dose streptozotocin-induced diabetic rat. J Food Sci Technol 59, 2177–2188 (2022). https://doi.org/10.1007/s13197-021-05231-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05231-0