Abstract
Rising prevalence of hypertension is pushing food industry towards the development of innovative food products with antihypertensive effects. The aim was to study the effect of reduced sodium content and 21 % addition of wholemeal wheat sourdough (produced by Lactobacillus brevis CECT 8183 and protease) on proximate composition, γ-aminobutyric acid (GABA) and peptide content of wheat bread. Angiotensin converting enzyme I (ACE) inhibitory and antioxidant activities were also evaluated. Sodium replacement by potassium salt did not affect chemical composition and biological activities of bread. In contrast, GABA and peptides <3 kDa contents in sourdough bread (SDB) were 7 and 3 times higher, respectively, than the observed in control. ACE inhibitory and antioxidant activities of the peptide fraction < 3 kDa from SDB was 1.7 and 2.6-3.0 times higher than control. Therefore, the combination of reduced sodium content with enriched concentrations of bioactive compounds in bread making may provide interesting perspectives for development of innovative breads towards blood pressure reduction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertension is a public health problem associated with cardiovascular complications affecting 25 % of adult population worldwide [1]. Reduction of sodium intake can shift the population distribution of hypertension [2]. Similarly, the consumption of healthy foods that contain bioactive compounds with antihypertensive activity may result effective in improving health outcomes of people with raised blood pressure (BP). Moreover, an increased intake of foods rich in antioxidants may ameliorate oxidative stress, and therefore, prevent from the development of hypertension and other cardiovascular complications [3]. Food industry must have an active role to solve this societal challenge through innovation focused on the development of foods with reduced sodium content and with antihypertensive and antioxidant properties. This approach will provide consumers with healthy foods that help in the prevention of hypertension.
Bread is considered to be the foodstuff that provides the most dietary salt (NaCl), therefore it is one of the key targets in any salt reduction strategy. A decrease in the salt content of bread is possible by replacing partially the salt content by other salts, mainly of potassium [4] without detrimental effects in terms of both technology and sensory acceptance [5]. Healthy and organoleptic features of bread can be additionally improved through sourdough fermentation by lactic acid bacteria (LAB) [6, 7]. Recent studies have shown that LAB metabolism may produce new nutritionally bioactive compounds with antihypertensive activity such as γ-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the mammalian nervous system, that is well known for lowering BP and its diuretic and tranquilizer effects [8, 9]. Angiotensin I converting enzyme (ACE)-inhibitory peptides and antioxidant peptides may be also synthesized by LAB during sourdough fermentation [10–12]. Inhibition of ACE hinders the formation of angiotensin II, a potent vasoconstrictor that results in BP decrease [13]. Antioxidant peptides may delay oxidative processes in vivo through radical scavenging, chelation of metal ion, inhibition of lipid peroxidation, etc. [12]. To the best of our knowledge no literature data deal with the development of a functional wheat bread from an innovative multi-strategic approach consisting of partial replacement of salt by potassium citrate, protease addition and sourdough fermentation by Lactobacillus brevis CECT 8183 strain (isolated from Spanish cheese) with proven high GABA-producing ability [14]. The effect of these approaches on nutritional composition, GABA and peptides production with ACE-inhibitory and antioxidant activities was evaluated.
Materials and Methods
Materials
Wholemeal wheat (14.9 % protein, 2.8 % fat, 67.6 % carbohydrates, 1.2 % ash, 13.5 % moisture) white wheat (15.3 % protein, 1.4 % fat, 69.9 % carbohydrates, 0.3 % ash, 13.1 % moisture) and roasted soybean flour (38.3 % protein, 21.6 % fat, 14.0 % carbohydrates, 3.0 % ash, 23.1 % moisture) were purchased from Harinas Polo (Zaragoza, Spain). Protease enzyme was from Puratos S.A. (Groot-Bijgaarden, Belgium) and potassium citrate was purchased from Quimidroga S. A. (Barcelona, Spain). Sourdough and the three types of bread were made at pilot scale. Each loaf of bread was grated on a laboratory knife mill (Grandomix GM 200) at 7,000 rpm for 10 s and stored at −20 °C.
Sourdough Fermentation and Bread Preparation
Lactobacillus brevis CECT 8183 isolated from an artisan Spanish cheese and with high proven capacity for GABA synthesis [14] was used as a starter for the sourdough fermentation. Four cryobeads of the microorganism were cultivated on Man, Rogosa and Sharpe (MRS) broth medium (AES Chemunex, Terrassa, Spain). After 48 h at 30 °C of incubation, the cells were recovered by centrifugation (10,000 × g for 15 min), washed twice in sterile water, and re-suspended in a 10 μM pyridoxal 5-phosphate solution at the minimum cell density of log 8 CFU/ml. Whole wheat flour (16.5 %), soya flour (2 %), protease (1.5 %) and distilled water (80 %) were used to prepare 5 L of sourdough (the dough yield was 500) with a continuous speed mixer (100 rpm) in a bioreactor (Biostat A plus, Sartorius, Germany) previously sterilized at 121 °C for 20 min. Sourdough fermentation was carried out at 30 °C for 48 h. In order to enhance GABA production, pH was maintained at 4.5 by the continuous addition of 2.5 M KHCO3. At the end of fermentation, protease was inactivated by increasing the temperature to 70 °C for 2 min. Sourdough fermentations were carried out ten times and analyzed in duplicate to check the GABA content.
Three types of bread (conventional wheat bread, CB; low-sodium wheat bread, LSB; low-sodium wheat sourdough bread, SDB) were manufactured according to the formulation shown in Table 1. After kneading and make-up, the bread dough was fermented at 27 °C for 90 min with a humidity level of 76 %. Baking was carried out in a rotary air oven Gashor (Zizurkil, Gipuzkoa, Spain) at 205 °C for 25 min.
Sodium Docecyl Sulphate Polyacrilamide Gel Electrophoresis (SDS-PAGE)
Proteins in breads and sourdough were extracted following the procedure of Dupont et al. [15] and separated under non-reducing conditions by SDS-PAGE on NuPAGE Novex 4−12 % Bis-Tris gels in the XCell-sure lock minicell system (Invitrogen, Madrid, Spain) at 200 V for 35 min. Proteins were stained with SimplyBlue SafeStain (Invitrogen), followed by destaining in deionized water. The molecular weights of poly- and oligopeptides were determined by comparison with the molecular weight marker® solution Sharp Novex unstained standard (Invitrogen).
Chemical Analysis
Proximate composition of breads and sourdough was determined following AOAC [16] methods and they include: moisture (method 925.10), protein (method 920.87), fat (method 922.06), ash (method 923.03) and total dietary fiber (method 991.43). Carbohydrates were calculated by difference: 100 – (% water –% protein – % total fat – % total ash – % total dietary fibre) [17]. Na and K were analysed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) using a Perkin-Elmer Optima 7300-DV spectrophotometer (Waltham MA, USA), following microwave digestion of the samples with nitric acid and hydrogen peroxide. The extract obtained was then diluted and absorbance was read at a wavelength of 589.59 nm for Na and 766.49 nm for K. Quantification of both elements was performed by external calibration. GABA was determined by reverse-phase high performance liquid chromatography, followed by UV detection after pre-column derivatization by 6-aminoquinolyl-N-hydroxy succinyl carbamate (AccQTag) according to Cohen and Michaud [18]. For peptides determination, breads and sourdough were ultrafiltrated through membranes of 3 kDa pore size (Millipore Corporation, Billerica, MA, USA). Permeates were analyzed by the DC protein assay (Biorad) using bovine serum albumin (BSA) as standard.
Biological Activity Testing
Antioxidant activity was analyzed by the oxygen radical absorbance capacity (ORAC-FL) method [19] in methanolic extracts and the water soluble 3 kDa peptide fraction of breads prepared according to Caceres et al. [20] and Garcia-Mora et al. [19], respectively. ACE-inhibitory activity of the 3 kDa peptide fraction of water-soluble extracts of samples was measured following the fluorescence-based protocol of Sentandreu and Toldrá [21]. IC50 was determined by dose–response curves using the non-linear regression sigmoidal curve fit function in GraphPad Prism 4.00 (Graphpad Software Inc., San Diego, CA, USA).
MALDI-TOF Analyses
Matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) analysis was performed in a Voyager-DE PRO mass spectrometer (Applied Biosystems, Foster City, CA) equipped with a pulsed 337 nm nitrogen laser (1 ns pulse width and 3 Hz frequency) which was operated in the reflectror mode for positive ions. The 3 kDa peptide fraction of breads was mixed with the matrix at a ratio of 1:5 (v/v), and 1 μL of this solution was spotted onto a flat stainless-steel sample plate and dried in air. Mass spectra were obtained at m/z range 500–4000. External mass calibration was applied using the monoisotopic [M + H] + values of des-Arg1 Bradykinin, Angiotensin I, Insuline, Glu1-Fibrino-peptide B, adrenocorticotropic hormone fragments 1–17, 18–39, and 7–38. Parameters of the instrument were as follows: time of flight analyser (1.3 m flight path) with an acceleration voltage of 20 kV, 76 % grid voltage, 0.001 % ion guide wire voltage, and a delayed extraction time of 400 ns.
Statistical Analysis
Data were subjected to one-way analysis of variance (ANOVA) by Statgraphics Centurion XVI software, version 16.1.17 (Statistical Graphics Corporation, Rockville, Md). Differences between samples were compared by using a Duncan’s multiple-range test at P ≤ 0.05 probability levels.
Results and Discussion
Proximate Composition, Protein and Peptide Profiles
The proximate composition (moisture, protein, fat, ash and carbohydrates) did not significantly differ (P > 0.05) between breads (Table 2), with the exception of dietary fiber that was significantly lower in CB. Potassium content was 2-fold higher while sodium content was 1.5-fold lower in low sodium breads (LSB and SDB) compared to control (P ≤ 0.05). These results are consistent with the replacement of sodium chloride by potassium citrate in the formulation of these breads.
GABA concentration in low-sodium wheat bread was similar (P > 0.05) to control (Table 2), therefore, replacement of sodium by potassium salt did not influence GABA content in experimental breads. GABA is produced during dough mixing and yeast’s fermentation by conversion of glutamic acid by glutamate decarboxylase of wheat and yeast [22]. In our study, GABA concentration in SDB increased 7 times by addition of sourdough in bread formulation (P ≤ 0.05). GABA content in SD reached a concentration 15-fold higher than those previously reported for sourdoughs of wheat, pseudocereals and legume flours started with a pool of selected LAB [23, 10]. Protease addition during sourdough fermentation increases the concentration of free amino acids [24] such as glutamine and glutamate improving the production yield of GABA during sourdough fermentation [25]. Additional protein degradation and glutamine and glutamate metabolism by lactobacilli glutaminases and glutamate decarboxilase give rise to GABA accumulation in sourdough [25]. GABA concentration in low-sodium SDB was much lower than the theoretical concentration expected from the addition of SD at a level of 21 % (1230.8 mg/100 g d.m.). GABA losses have been reported during bread making due to its consumption by yeast during proofing and thermal degradation by Maillard browning reactions that take place during baking [22]. An additional degradation of GABA might have occurred during thermal inactivation of protease (70 °C for 2 min) after sourdough fermentation. Human intervention studies have shown that a daily intake of 10–20 mg of GABA is able to prevent pre-hypertension [9, 26]. The results of the present study suggest that a daily consumption of 100 g of bread (fresh weight) would provide enough GABA (22.4 mg) to display the health benefits observed in the previous studies.
Bioactive peptides with ACE inhibitory and antioxidant activity have a molecular weight below 3 kDa, therefore, the quantification of peptides < 3 kDa was carried out in this study. Results in Table 2 show that LSB and CB had a similar concentration of peptides < 3 kDa (P > 0.05), unlike SDB with a concentration 3 times higher than those found in LSB and CB (P < 0.05). Sourdough fermentation is known to cause an increase in the concentration of peptides compared with bread dough [27] due to proteolysis by LAB proteases and peptidases. The use of commercial proteases during wheat flour sourdough fermentation may result in a more extensive proteolysis and, subsequently, in an increase of peptides yield. SDS-PAGE shows that protease addition and SD fermentation by L. brevis CECT 8183 gave rise to intense protein bands with a range of molecular masses between 30–40 and 58–70 kDa and small protein fragments at molecular masses below 20 kDa (Fig. 1; lane 4). In spite of no differences were observed by SDS-PAGE in the protein profile of SDB compared to LSB and CB (Fig. 1, lanes 1–3), a different peptide profile was found by MALDI-TOF between 3 kDa permeates from SDB and its respective control (LSB) (Table 3). Bolded peptide masses at m/z 519, 523, 537, 544, 549, 565, 609, 610, 613, 713, and 723 were only found in SDB, therefore, they could be released during sourdough fermentation. Comparing our results with reported SDS-PAGE profiles [28] and peptide content [10, 28] of wholemeal wheat sourdoughs fermented by different LAB strains (13.0–22.5 mg/g of sourdough d.m., Table 2) we found that addition of commercial protease during sourdough fermentation resulted in a more extensive proteolysis and a markedly enrichment of peptides < 3 kDa (57.7 mg/g of sourdough d.m.). Peptide concentrations in SDB (11.3 mg/g d.m., Table 2) were slightly lower than the theoretical concentration expected from the addition of SD at a level of 21 % (12.7 mg/g d.m.). This lower peptide concentration could be due to their degradation as consequence of Maillard reactions that take place during baking [29] and at the end of sourdough fermentation. Zhao et al. [29] reported a decrease in peptide content due to LAB enzymatic activity at the proofing and kneading stages. In our study this effect was unlikely to occur since a thermal treatment at 70 °C for 2 min was applied for enzymatic inactivation.
ACE Inhibitory and Antioxidant Activities
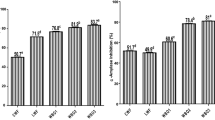
Table 4 shows ACE inhibitory and antioxidant activities of 3 kDa permeates of water-soluble extracts obtained from sourdough and experimental breads. ACE inhibitory and antioxidant activities of the peptide fraction < 3 kDa from SDB was 2 times and up to 3 times higher, respectively than those of LSB and CB (P ≤ 0.05). These results are due to bread supplementation with SD in which the release of amino acid sequences with antioxidant and ACE inhibitory activities from grain storage proteins may occur by protease and peptidase activities [27, 11, 12]. Positive correlation between peptide content and ACE inhibitory or antioxidant activities of breads supports this consideration (Table 4).
ACE inhibitory activity of the peptide fraction < 3 kDa from SD (0.09 mg peptide/mL) was between 2.1 and 26 times higher than those reported previously (0.19–0.54 mg/mL) for peptide fractions from wheat sourdoughs started by a pool of selected LAB [10]. Type of wheat grain variety, composition of starter culture, addition of protease, dough yield and processing conditions (fermentation time and temperature) could explain these differences. The comparison of IC50 values of 3 kDa permeates from SD (94.8 μg/mL) and SDB (127.2 μg/mL) indicated that ACE inhibitory activity remained stable after kneading, proofing and baking stages. Therefore, it can be assumed that ACE inhibitory peptides released during sourdough fermentation were not further degraded as consequence of yeast metabolism and thermal treatment in agreement with previous findings [29]. No previous studies have addressed the impact of bread making on the antioxidant activity of peptides.
Clinical trials with hypertensive humans indicate that consumption of 3–6 mg of peptides derived from milk proteins with ACE-inhibitory activity per day reduces systolic BP [13]. Although SDB was not evaluated in vivo, an initial assessment of their bioactivity can be made based on the comparison of IC50 (127.23 μg/mL) with the theoretical concentration of peptides reached in blood after consumption of one serving of fresh SDB. One serving of SDB (100 g fresh weight) contains 723 mg of peptides <3 kDa, therefore, it can be assumed that this amount may reach the absorption site in the intestine and further the blood stream at a concentration 2 times higher than the IC50 value of 3 kDa peptide fraction (241 μg/mL) in a reference person containing approximately 3 L of plasma. Although gastrointestinal digestion, absorption, transport and metabolism are likely to impact ACE inhibitory activity of bioactive peptides, results from the present study are promising and deserve further research to evaluate the antihypertensive effect of SDB in vivo.
Antioxidant activity was also analyzed in acidified methanolic extracts from sourdough and breads (Table 4). It is worth noting that ORAC values of methanolic extracts were higher than those found for the ≤3 kDa peptide fraction. Higher antioxidant capacity of methanolic extracts could be attributed to a better solubility and extraction of compounds with antioxidant activity such as phenolics [30]. Similarly to results found in the peptide fraction < 3 kDa, bread supplemented with 21 % wheat sourdough gave rise to ORAC values 1.5 and 1.9-fold higher than the observed in LSB and CB, respectively (Table 4). Cereal fermentation by LAB result in an increase and bioconversion of free phenolic compounds [31], effect that could be related with the increased ORAC values observed in SDB. Cereal and LAB enzymes are key contributors on the conversion of phenolic acids during fermentation [32] which have been found directly related to an increased antioxidant activity in fermented products [33]. Comparing SD to SDB, it is worth noting that the bread making process seems to have no impact on the antioxidant activity. However, the synthesis of substances with antioxidant properties, including certain Maillard reaction products that are accumulated in the bread crust, may mask the real decrease of antioxidants in bread as well as any loss in total antioxidant activity [34].
Conclusions
This study shows that bread supplemented with wheat sourdough produced by L. brevis CECT 8183 and a commercial protease markedly improved the total antioxidant activity of bread and its content in GABA and small peptides (<3 kDa) with ACE-inhibitory and antioxidant activity. Therefore, the combination of reduced sodium content with enriched concentrations of bioactive compounds such as GABA, ACE inhibitory and antioxidant peptides may provide interesting perspectives for development of innovative breads aimed at reducing blood pressure.
Abbreviations
- ACE:
-
Angiotensin I converting enzyme
- BP:
-
Blood pressure
- CB:
-
Conventional wheat bread
- GABA:
-
γ-aminobutyric acid
- LAB:
-
Lactic acid bacteria
- LSB:
-
Low-sodium wheat sourdough bread
- MALDI-TOF:
-
Matrix assisted laser desorption ionization time of flight
- ORAC:
-
Oxygen radical absorbance capacity
- SDB:
-
Low sodium wheat sourdough bread
- SD:
-
Sourdough
- SDS-PAGE:
-
Sodium dodecyl polyacrylamide gel electrophoresis
References
World Health Organization (2009) Causes of death; http://www.who.int/healthinfo/global_burden_disease/cod_2008_sources_methods. 2008. Accessed 12 April 2012
Webster JL, Dunford EK, Hawkes C, Neal BC (2011) Salt reduction initiatives around the world. J Hypertens 29(6):1043–1050
Adebiyi AP, Adebiyi AO, Yamashita J, Ogawa T, Muramoto K (2009) Purification and characterization of antioxidative peptides derived from rice bran protein hydrolysates. Eur Food Res Technol 228(4):553–563
Braschi A, Gill L, Naismith DJ (2009) Partial substitution of sodium with potassium in white bread: feasibility and bioavailability. Int J Food Sci Nutr 60(6):507–521
Quilez J, Salas-Salvado J (2012) Salt in bread in Europe: potential benefits of reduction. Nutr Rev 70(11):666–678
Arendt EK, Ryan LAM, Dal Bello F (2007) Impact of sourdough on the texture of bread. Food Microbiol 24(2):165–174
Bryszewska MA, Ambroziak W, Langford NJ, Baxter MJ, Colyer A, Lewis DJ (2007) The effect of consumption of selenium enriched rye/wheat sourdough bread on the body's selenium status. Plant Foods Hum Nutr 62(3):121–126
Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, Yamori Y (2004) Effect of a γ-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Nutr 92(3):411–417
Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H (2003) Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr 57(3):490–495
Rizzello CG, Cassone A, Di Cagno R, Gobbetti M (2008) Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and γ-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J Agric Food Chem 56(16):6936–6943
Hu Y, Stromeck A, Loponen J, Lopes-Lutz D, Schieber A, Gänzle MG (2011) LC-MS/MS quantification of bioactive angiotensin I-converting enzyme inhibitory peptides in rye malt sourdoughs. J Agric Food Chem 59(22):11983–11989
Coda R, Rizzello CG, Pinto D, Gobbetti M (2012) Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl Environ Microbiol 78(4):1087–1096
Ricci I, Artacho R, Olalla M (2010) Milk protein peptides with angiotensin I-Converting enzyme inhibitory (ACEI) activity. Cr Rev Food Sci Nutr 50(5):390–402
Diana M, Tres A, Quílez J, Llombart M, Rafecas M (2014) Spanish cheese screening and selection of lactic acid bacteria withhigh gamma-aminobutyric acid production. LWT - Food Sci Technol 56(2):351–355. doi:10.1016/j.lwt.2013.11.027
Dupont FM, Vensel WH, Tanaka CK, Hurkman WJ, Altenbach SB (2011) Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Sci 9:10. doi:10.1186/1477-5956-9-10
AOAC International (2005) Official Methods of Analysis of AOAC International, 18th edn. Gaithersburg, MD, USA
Food and Agriculture Organization of The United Nations (2003) Calculation of the Energy Content of Foods – Energy Conversion Factors. In: FAO (ed) Food energy – methods of analysis and conversion factors. Food and Nutrition Paper. 77. pp 18–37
Cohen SA, Michaud DP (1993) Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N- hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem 211(2):279–287
Garcia-Mora P, Peñas E, Frias J, Martínez-Villaluenga C (2014) Savinase, the most suitable enzyme for releasing peptides from lentil (Lens culinaris var. Castellana) protein concentrates with multifunctional properties. J Agric Food Chem 62(18):4166–4174
Cáceres PJ, Martínez-Villaluenga C, Amigo L, Frias J (2014) Maximising the phytochemical content and antioxidant activity of Ecuadorian brown rice sprouts through optimal germination conditions. Food Chem 152:407–414
Sentandreu MÁ, Toldrá F (2006) A fluorescence-based protocol for quantifying angiotensin-converting enzyme activity. Nat Protoc 1(5):2423–2427
Lamberts L, Joye IJ, Beliën T, Delcour JA (2012) Dynamics of γ-aminobutyric acid in wheat flour bread making. Food Chem 130(4):896–901
Coda R, Rizzello CG, Gobbetti M (2010) Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of γ-aminobutyric acid (GABA). Int J Food Microbiol 137(2–3):236–245
Diana M, Rafecas M, Quilez J (2014) Free amino acids, acrylamide and biogenic amines in gamma-aminobutyric acid enriched sourdough and commercial breads. J Cereal Sci 60(3):639–644
Stromeck A, Hu Y, Chen L, Gäzle MG (2011) Proteolysis and bioconversion of cereal proteins to glutamate and γ-aminobutyrate (GABA) in rye malt sourdoughs. J Agric Food Chem 59(4):1392–1399
Pouliot-Mathieu K, Gardner-Fortier C, Lemieux S, St-Gelais D, Champagne CP, Vuillemard JC (2013) Effect of cheese containing gamma-aminobutyric acid-producing lactic acid bacteria on blood pressure in men. Pharma Nutr 1(4):141–148
Nakamura T, Yoshida A, Komatsuzaki N, Kawasumi T, Shima J (2007) Isolation and characterization of a low molecular weight peptide contained in sourdough. J Agric Food Chem 55(12):4871–4876
Rizzello CG, Nionelli L, Coda R, Gobbetti M (2012) Synthesis of the cancer preventive peptide lunasin by lactic acid bacteria during sourdough fermentation. Nutr Cancer 64(1):111–120
Zhao CJ, Hu Y, Schieber A, Gänzle M (2013) Fate of ACE-inhibitory peptides during the bread-making process: quantification of peptides in sourdough, bread crumb, steamed bread and soda crackers. J Cereal Sci 57(3):514–519. doi:10.1016/j.jcs.2013.02.009
Bae H, Jayaprakasha GK, Crosby K, Jifon J, Patil B (2012) Influence of extraction solvents on antioxidant activity and the content of bioactive compounds in non-pungent peppers. Plant Foods Hum Nutr 67(2):120–128. doi:10.1007/s11130-012-0290-4
Kim D, Han GD (2011) Ameliorating effects of fermented rice bran extract on oxidative stress induced by high glucose and hydrogen peroxide in 3 T3-L1 adipocytes. Plant Foods Hum Nutr 66(3):285–290
Gänzle MG (2014) Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol 37:2–10
Rodríguez H, Curiel JA, Landete JM, De las Rivas B, de Felipe FL, Gómez-Cordovés C, Mancheño JM, Muñoz R (2009) Food phenolics and lactic acid bacteria. Int J Food Microbiol 132(2–3):79–90. doi:10.1016/j.ijfoodmicro.2009.03.025
Zhang M, Chen H, Li J, Pei Y, Liang Y (2010) Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT - Food Sci Technol 43(1):181–185. doi:10.1016/j.lwt.2009.06.020
Acknowledgments
Supported by Europastry S.A., St. Cugat del Valles, Barcelona, Spain and Ministry of Economy and Competitiveness (project number AGL2010-16310).
Conflict of interest
The authors claim no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peñas, E., Diana, M., Frias, J. et al. A Multistrategic Approach in the Development of Sourdough Bread Targeted Towards Blood Pressure Reduction. Plant Foods Hum Nutr 70, 97–103 (2015). https://doi.org/10.1007/s11130-015-0469-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-015-0469-6