Abstract
Response surface methodology was used to predict optimum conditions for hot air roasting of barley grains (temperature, time, and amount). Antioxidant capacity in the grains was highest under optimum conditions of 250 °C, 63.5 min and 42 g (one and a half layers). A correlation of R 2 = 0.74 (p < 0.05) was found between 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and total phenolic contents. Ethanol and aqueous extracts were prepared from grains roasted under optimum conditions and assessed for antioxidant capacity. Antioxidative compounds in the extracts were then identified using GC–MS. The IC50 value of ethanol extract was significantly lower (11.45 μg mL–1) than that of aqueous extract (33.54 μg mL–1) and α-tocopherol (12.6 μg mL–1) but higher than BHT (9.59 μg mL–1). The same trend was observed in linoleic acid assay. In reducing power, the ethanol extract and α-tocopherol were not significantly different. Phenolic acids p-hydroxybenzaldehyde, vallinic and gallic acids were identified as the major compounds in the extracts. The results obtained from this study show that it is possible to optimize antioxidant capacity in barley grains during roasting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exogenous chemical and endogenous metabolic processes in the human body or in the food system might produce highly reactive free radicals. Oxidation damage caused by these free radicals can theoretically contribute to chronic diseases, such as cancer, cardiovascular disease, age-related macular degeneration, and aging [1].
Barley (Hordeum vulgare L.) is an ancient cereal grain, which upon domestication has evolved from largely a food grain to a feed and malting grain. Barley is gaining renewed interest as an ingredient for production of functional foods due to its concentration of bioactive compounds. Modest quantities of non-alcoholic drinks based on barley and malt are consumed in various parts. Both barley and malt are roasted and hot water infusion of the whole or ground products are consumed. Examples of such beverages are ‘malt coffee’ `barley tea’ or ‘barley coffee’. The main antioxidative components in grain are classified as phenolic compounds [2]. In barley most of the free phenolics are flavanols and tocopherols, whereas the bound phenolics are mainly phenolic acids, ferulic acid, and p-coumaric acid [3]. There have been studies on the antioxidant capacity and phenolic content of barley [4–6]. The abundant contents of phenolic compounds in barley reveal that barley may serve as an excellent dietary source of natural antioxidants for disease prevention and health promotion.
Radical scavenging properties of some roasted nuts and grains have been studied, and it was demonstrated that roasted wheat germ, coffee, hazelnut, sweet almond [7], peanut [8], coffee brews [9–11], and barley [12, 13] have antiradical properties at certain levels. Roasted barley grains are valued due to the presence of antioxidant compounds [14]. Barley tea has been reported to provide a protective effect against gastric stress ulcers and helps reduce blood pressure which may reduce the risk of circulatory diseases related to lifestyle [15]. More and more consumers are recognizing beverage as a source of health-promoting nutrients which presents an opportunity for the introduction of novel products, such as healthy grain-based beverages.
Although barley grain has been proven to exhibit antioxidant capacities to some degree of roasting, no attempts have been undertaken to optimize hot air roasting and antioxidant capacity in the roasting process. The objective of this study was therefore to use a hot air oven to optimize antioxidant capacity in barley grains. The other objective was to prepare roasted barley grains under optimum conditions and examine the antioxidant capacity of their extracts in different in vitro model systems. The antioxidative compounds in the extracts were also identified using GC–MS analysis.
Materials and methods
Materials
Whole barley grains were purchased from a local market in Nanjing, China. Linoleic acid (99%), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azobis(2-amidinopropane)hydrochloride (AAPH) were obtained from Wako Pure Chemical Industries Ltd. (Osaka, Japan); α-tocopherol was purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Other reagents were of analytical grade and were purchased from Nanjing Chemical Industry (Nanjing, China).
Roasting of barley grains
Barley grains containing 6.5% moisture were weighed (30, 45 or 60 g) and placed evenly in a pyrex petri dish (diameter 12.5 cm). The petri dish was placed in the middle of a pre-heated hot air oven measuring 31 × 27 × 31 cm internal dimensions. The grains were roasted at different treatment combinations according to the experimental design obtained using the software Design Expert (Version 6.0.5, Stat-Ease Inc., Minneapolis, MN, USA). The grains were then allowed to cool to room temperature before grinding to pass through a sieve of size 0.9 mm.
Preparation of crude barley extracts
Barley flour was extracted in 80% ethanol or distilled water in a 1:10 w/v ratio at 45 °C in a shaking water bath for 2 h under dark conditions. The supernatant and the residue were separated by vacuum filtration. The residue was then re-extracted under the same condition and the extraction solutions were combined. The obtained extraction solutions were stored in the dark at −20 °C and analyzed within 24 h or concentrated in a rotary evaporator under vacuum at 45 °C, lyophilized and stored at −20 °C.
Determination of total phenolic content (TPC)
Total phenolic content of the extracts was determined according to the Folin–Ciocalteu spectrophotometric method [16] with slight modifications. Briefly, 0.5 mL diluted barley extract solution was mixed with 2.5 mL of tenfold diluted Folin–Ciocalteu’s phenol reagent and allowed to react for 5 min. Then 2 mL of 7.5% sodium carbonate (Na2CO3) solution was added, and the final volume made up to 10 mL with deionized water. After reaction for 1 h at room temperature, the absorbance at 760 nm was determined. The calibration curve was performed with gallic acid, and the results were expressed as mg of gallic acid equivalents per gram of dry weight (mg GAE/g dw).
Antioxidant capacity
DPPH radical scavenging activity
The radical scavenging activity of the barley extracts was determined according to the method described by Gaulejac et al. [17] with slight modifications. Every barley extract (0.1 mL) was added to 2.9 mL of 6 × 10−5 M ethanolic solution of DPPH and mixed thoroughly. The absorbance at 517 nm was measured after reaction in the dark at 30 °C for 30 min. Lower absorbance of the reaction mixture indicates high free radical scavenging activity. All tests were performed in triplicate, and the percentage inhibition of the radicals due to the antioxidant properties of the extracts was calculated as shown below. The IC50 value, the concentration of antioxidant needed to decrease (by 50%) the initial substrate concentration, was then determined.
where A Sample and A Control are reactions of DPPH with and without sample after 30 min, respectively.
Reducing power
The reducing power of roasted barley grains was measured by direct electron donation in the reduction of [Fe(CN)6]3− to [Fe(CN)6]4− according to the method of Oktay et al. [18]. Briefly, 1 mL of different concentrations of the extracts (50–200 μg mL–1) were mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of K3Fe(CN)6 (1%, w/v). The mixture was incubated at 50 °C for 20 min (during which ferricyanide was reduced to ferrocyanide). Then 2.5 mL of 10% (w/v) trichloroacetic acid (TCA) was added to terminate the reaction. After centrifugation for 10 min at 3,000g, 2.5 mL of the supernatant was added to the tubes containing 2.5 mL distilled water and 0.5 mL FeCl3 (0.1%, w/v). The absorbance of the resulting solution was measured at 700 nm against a blank. BHT and α-tocopherol served as standards. Increased absorbance of the reaction mixture indicated increased reducing power of the sample.
Linoleic acid system
The antioxidant capacity of ethanolic and aqueous extracts of roasted barley grains was assayed using a linoleic acid system. One milliliter of 50 μg mL–1 sample, 2 mL of 2.51% (w/v) linoleic acid in ethanol, 4 mL of 0.05 M phosphate buffer (pH 7.0), and 2 mL of distilled water were mixed in a tube with a screw cap. The oxidation was initiated by the addition of 0.417 mL of 0.1 M 2,2′-azobis(2-amidinopropane)dihydrochloride (AAPH) and kept in a 37 °C water bath in the dark. The above mixture (0.1 mL) was added to 9.7 mL of 75% (v/v) ethanol and 0.1 mL of 30% (w/v) ammonium thiocyanate. After 5 min, 0.1 mL of 0.02 M ferrous chloride in 3.5% (v/v) hydrochloric acid was added to the above mixture and then mixed. The absorbance of the mixture was recorded at 500 nm after 50, 100, and 200 min. The degree of oxidation was measured according to the ferric thiocyanate (FTC) method, described in detail by Kikuzaki and Nakatani [19]. Linoleic acid mixture without the addition of sample was used as the control. BHT and α-tocopherol at the same concentration served as the reference antioxidants.
Experimental design
The roasting conditions were optimized with Box–Behnken design for a higher yield of antioxidant capacity from barley grains. Response surface methodology (RSM) was used to investigate the effect of three independent variables temperature, time, and amount on the responses of total phenolic content and antioxidant capacity. The independent variables and their levels were selected based on preliminary experiments carried out in our laboratory. The factors and levels investigated in the study are shown in Table 1. Experimental design, data analysis, and quadratic model building were conducted using the software Design Expert (Version 6.0.5, Stat-Ease Inc., Minneapolis, MN, USA). A Box–Behnken design with three variables was used to determine the response pattern and then establish a model [20, 21]. The Box–Behnken matrix of the factors is given in Table 2 along with the experimental values. Five replicates at the center of the design were used to allow for estimation of a pure error sum of squares. Experiments were randomized to maximize the effects of unexplained variability in the observed responses due to extraneous factors. The response function (Y) was partitioned into linear, quadratic, and interactive components.
where β 0 is defined as the constant, B i the linear coefficient, B ii the quadratic coefficient and B ij the cross-product coefficient. X i and X j are levels of the independent variables while k equals to the number of the tested factors (k = 3).
GC–MS analysis of antioxidative compounds
GC–MS analysis was carried out on an Agilent 6890 gas chromatography with a mass selective detector MSD 5973 (both from Agilent, Palo Alto, CA, USA) using helium at 1.0 mL min–1 as the carrier gas. A 30 m × 0.25 mm i.d. × 0.25 μm film thickness fused silica column coated with SPB-1 was used. The mass spectrometer was operated in the electron impact (EI) mode at 70 eV scanning the 20–550 m/z range. Acquisition was done using HP ChemStation software (Hewlett Packard Palo Alto, CA, USA). In order to obtain the phenolic compounds, the extracts were concentrated to smaller volumes and then partitioned using ethyl acetate. The ethyl acetate was evaporated from the sample by a rotary vacuum evaporator until dryness. GC–MS analysis was done by injecting 1 μL of the sample which was dissolved in methanol at the concentration of 1 mg mL–1. Injection was carried out at 250 °C. The oven temperature was programed at 100 °C for 5 min and then raised to 280 °C at 4 °C min–1. The final temperature was held for 5 min. The compounds were identified from spectral comparison with Wiley registry 7th edition with the US National Institute of Standards and Technology (NIST) 2005 mass spectra library.
Statistical analysis
For statistical analysis all computations were performed by SAS (version 8.0). The data are presented as means of three determinations. Analysis of variance followed by Student’s t test was used to determine the differences among various groups for each concentration. Multiple comparisons of means were done by LSD (least significant difference) test. A probability value of p < 0.05 was considered significant.
Results and discussion
Effects of roasting conditions on antioxidant capacity
The antioxidant capacity was evaluated using DPPH, a stable free radical that shows maximum absorbance at 517 nm in ethanol. DPPH has been widely accepted as a tool for estimating free radical scavenging activities of antioxidants [22]. When DPPH encounters a proton-donating substance such as an antioxidant, the radical is scavenged and the absorbance reduced. Thus, the antioxidant capacity of the roasted barley grain can be expressed as its ability in scavenging the DPPH free radical.
A correlation of R 2 = 0.74 (p < 0.05) was found between DPPH radical scavenging activity and total phenolic content of roasted barley grain. This result suggests that the phenolic compounds in roasted barley grains could be the major contributors of DPPH radical scavenging activity. A study on malting barley varieties by Zhao et al. [6] reported that the total phenolic content of barley was significantly correlated with the antioxidant capacity, as measured by the DPPH and ABTS assays.
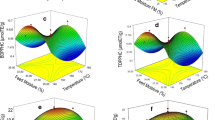
The effect of roasting temperature, time, and amount of grain on the RSA of extracts from roasted barley grain and their interactions are shown in Fig. 1. The effect of interaction between time and temperature and that of time and amount were more significant than the interaction between temperature and amount at p < 0.05. An increase in temperature resulted in a higher DPPH radical scavenging activity. The activity reached the maximum when the temperature was at a certain level with no significant further improvement thereafter (Fig. 1a, b). Time had a similar effect on radical scavenging activity as temperature (Fig. 1a, c). This is attributed to the liberation of insoluble bound phenolic compounds in barley grain during roasting which leads to an increase in the amount of active compounds in the extract. Phenolic compounds are found in both free and bound forms in cereals. The majority are in the insoluble bound form which is bound to cell wall material, such as ferulic acid and its derivatives. Studies by Jeong et al. [23, 24] showed that simple heat treatment converts insoluble phenolic compounds to soluble phenolics. They reported that roasting sesame seeds at 200 °C for 60 min significantly increased the total phenolic content, radical scavenging activity, reducing power, and antioxidant activity of sesame meal extract. Increase in antioxidant capacity during roasting of barley grain could also have been due to formation of novel compounds having antioxidant capacity from the Maillard reaction. Maillard reaction products (MRPs) have been reported to have antioxidant capacity [13].
Response surface plots showing effects of three independent variables: temperature, time, and amount of barley grains on the radical scavenging activity (RSA) of roasted barley grains using DPPH discoloration assay. a Amount was held constant at 45 g, b time was held constant at 60 min, and c temperature was held constant at 225 °C
The decline in antioxidant capacity observed with increase in roasting temperature beyond a certain point (Fig. 1a, b) was due to degradation of polyphenols because of the high temperature. Phenolic compounds, both natural and newly formed, can be degraded due to high temperatures, and total phenolic content may decrease. Duh et al. [12] found that the antioxidant efficiency of water extracts of roasted barley samples was weaker than that of unroasted samples because of the reduction of antioxidant components with increasing roasting temperature. This can be attributed to the high roasting temperatures that were used in this study. The MRPs which appear in excessive roasting conditions have also been reported to have less oxygen scavenging capacity or pro-oxidant effects [25].
Different effect on RSA was shown for the amount of grain used (Fig. 1b, c). There was an optimum value for amount to obtain the highest DPPH radical scavenging activity. Higher or lower values led to a decrease in the scavenging activity.
Model fitting
The Box–Behnken design matrix of the factors is given in Table 2 along with the experimental values of antioxidant capacity and total phenolic content of roasted barley grains. The mathematical model representing the RSA of barley grain as a function of the independent variables within the region under investigation was expressed by the following equation:
where Y is the RSA of extracts from roasted barley grains and X 1, X 2, and X 3 are the coded variables for temperature, time, and amount, respectively.
In general, exploration and optimization of a fitted response surface may produce poor or misleading results, unless the model exhibits a good fit, which makes checking the model adequacy essential. By use of analysis of variance (ANOVA), the p value of the model was <0.0001 (Table 3), which indicated that the model fitness was significant. The lack of fit value of the model was 0.7819, which was not significant, indicating that the model equation was adequate for predicting the yield under any combination of values of the variables.
Coefficient (R 2) of determination is defined as the ratio of the explained variation to the total variation, and is a measurement of the degree of fitness. A small value of R 2 indicates a poor relevance of the dependent variables in the model. The model can fit well with the actual data when R 2 approaches unity. The high coefficient of determination value (R 2 = 0.9989) obtained indicated that the model as fitted can explain 99.8% of the RSA variability.
By computation, the optimal conditions to obtain the highest RSA of roasted barley grains were determined as follows: temperature of 250 °C, time of 63.5 min and 42 g of grains. In terms of layers this amount of grain formed one and a half layers in the petri dish (one layer was formed by 30 g).
Antioxidant capacity
Antioxidant compounds present in barley extracts are complex, and their activities and mechanisms would largely depend on the composition and conditions of the test system. Many authors have stressed the need to perform more than one type of antioxidant capacity measurement to evaluate the antioxidant capacity of plants [26, 27]. Hence, the following measurements were adopted in order to assess the antioxidative potential of extracts from barley grain roasted under optimum conditions.
Measurement of reducing power
It has been reported that reducing power is associated with antioxidant capacity, and may serve as a significant reflection of the antioxidant capacity [18]. The reducing power of ethanolic and aqueous extracts of roasted barley grains was concentration dependent (Fig. 2a). As the concentration increased from 50 to 200 μg mL–1, there was an increase in absorbance with all the extracts. For all the tested concentrations, the ethanol extract had a reducing power significantly higher than the aqueous extract but significantly lower than BHT (p > 0.05). There was no significant difference between ethanol extract and α-tocopherol in all the concentrations tested. The power of certain antioxidants is associated with their reducing power [28], which is associated with the presence of reductones. The reducing power of extracts from roasted barley grain was from their antioxidant capacity. The phenolic compounds in the extracts acted in a similar way as the reductones.
DPPH radical scavenging activity
Relatively stable organic radical DPPH has been used widely for the determination of antioxidant capacity of pure antioxidant compounds as well as different cereal extracts [29, 30]. The IC50, the concentration of antioxidant needed to decrease (by 50%) the initial substrate concentration, is a parameter widely used to measure antiradical efficiency. The lower the IC50, the higher is the antioxidant power. The IC50 values of barley extracts, BHT, and α-tocopherol were compared (Table 4). The ethanol extract was more efficient by the lower IC50 value of 11.45 ± 0.43 μg mL–1 compared to the aqueous extract (33.54 ± 0.39 μg mL–1) and α-tocopherol (12.6 ± 0.45 μg mL–1). The IC50 values of BHT were significantly lower (9.59 ± 0.12 μg mL–1) than both the roasted barley grain extracts and the reference antioxidant α-tocopherol at p < 0.05.
Linoleic acid system
Figure 2b displays the inhibitive activity of extracts from roasted barley grains and reference antioxidants against linoleic acid peroxidation. A low absorbance is an indication of a low concentration of the formed peroxides and a high level of antioxidant capacity. Both the ethanol and aqueous extracts delayed the oxidation of linoleic acid, on the basis of low absorbance values, and exhibited higher antioxidant capacity than control. In particular, the ethanolic extracts showed significantly stronger antioxidant capacity than the aqueous extracts and the positive control of α-tocopherol. The antioxidant capacity followed the order: control < aqueous extracts < tocopherol < ethanol extracts < BHT. The same pattern of activity was revealed in the DPPH radical scavenging activity.
Antioxidative compounds in roasted barley grain extracts
Antioxidative compounds were analyzed by GC–MS, and several phenolic compounds attributed to the antioxidant capacity of roasted barley grain were identified (Table 5). The major phenolic compounds in the ethanol extract, which had a higher antioxidant capacity compared to the aqueous extract, were p-hydroxybenzaldehyde (34.27%), vanillic acid (15.98%), and gallic acid (15.4%). The aqueous extract was high in vanillic acid (29.8%) and gallic acid (16.75%). The difference in antioxidant capacity can be attributed to the polarity of the two solvents used with some phenolic compounds being extracted in the ethanol and not in the aqueous extract. This included compounds, such as p-hydroxybenzaldehyde, p-hydroxyacetophenone, 3,4-dihydroxybenzaldehyde, and p-hydroxybenzyl alcohol, which have been reported as phenolic compounds present in barley grain [14]. Gallic acid, vanillic acid, catechin, and syringic acid together with other phenolic compounds have been identified in barley and malt extracted with hot water at 45 °C [31]. All identified compounds had similitude index of more than 800.
Conclusion
From response surface plots, three factors (temperature, time, and amount of grain) significantly influenced the radical scavenging activity of barley grain, independently and interactively. Roasting increased both antioxidant capacity and polyphenolic content in barley to an extent thereafter having a decrease. These results show that it is possible to optimize antioxidant capacity during roasting using response surface methodology. Under optimum conditions the results indicated that the ethanol extract from roasted barley grain possesses significantly higher capacity than the aqueous extract and α-tocopherol in all the tested assays except reducing power. GC–MS analysis revealed more compounds in the ethanol than in the aqueous extract which is the reason for the high antioxidant capacity of the former. Phenolic compounds p-hydroxybenzaldehyde, gallic acid, and vanillic acid were found to be the major compounds in the roasted grains responsible for antioxidant capacity. The differences in the ethanol and the aqueous extracts are due to differences in the polarity of the extraction solvents used. This influenced the type of compounds that were extracted and thus the antioxidant capacity. However, more research is required to find out the best extraction for aqueous extract in order to increase the phenolic content and antioxidant capacity of the extract for health purposes. This can be based on factors such as the amount of water to be used, its temperature, and the amount of grain to be extracted.
References
Seitz HK, Stickel F (2006) Biol Chem 387:349–360
Martínez-Tomé M, Murcia MA, Frega N, Ruggieri S, Jiménez AM, Roses F, Parras P (2004) J Agric Food Chem 52:4690–4699
Holtekjøen AK, Kinitz C, Knutsen SH (2006) J Agric Food Chem 54:2253–2260
Bonoli M, Marconi E, Caboni MF (2004) J Chromatogr 1057:1–12
Liu Q, Yao HY (2007) Food Chem 102:732–737
Zhao H, Fan W, Dong J, Lu J, Chen J, Shan L, Lin Y, Kong W (2008) Food Chem 107:296–304
Krings U, Berger RG (2001) Food Chem 72:223–229
Hwang JY, Shue YS, Chang HM (2001) Food Res Int 34:639–647
Nicoli MC, Anese M, Manzocco L, Lerici CR (2997) Lebenson Wiss Technol 30:292–297
Borrelli RC, Visconti A, Mennella C, Anese M, Fogliano V (2002) J Agric Food Chem 50:6527–6533
Charurin P, Ames JM, Castiello MDD (2002) J Agric Food Chem 50:3751–3756
Duh P, Yen G, Yen W, Chang L (2001) J Agric Food Chem 49:1455–1462
Adele P, Maria D, Camilla A, Milena Q, Cesarina G, Gabriella G (2006) J Agric Food Chem 54:1209–1216
Etoh H, Murakami K, Yogoh T, Ishikawa H, Fukuyama Y, Tanaka H (2004) Biosci Biotech Biochem 68:2616–2618
Suganuma H, Inakuma T, Kikuchi Y (2002) J Nutr Sci Vitaminol 48:165–168
Singleton VL, Rossi JA (1965) Am J Enol Vitic 16:144–158
Gaulejac NS-C, Provost C, Vivas N (1998) J Agric Food Chem 47:425–431
Oktay M, Gülçin I, Küfrevioğlu Őİ (2003) Lebenson Wiss Technol 36:263–271
Kikuzaki H, Nakatani N (1993) J Food Sci 58:1407–1410
Box GEP, Behnken DW (1960) Technometrics 2:455–475
Wang L, Pan B, Sheng J, Xu J, Hu Q (2007) Food Chem 105:36–41
Sānchez-Moreno C (2002) Food Sci Tech Int 8:121–137
Jeong SM, Kim SY, Kim DR, Nam KC, Ahn DU, Lee SC (2004) J Food Sci 69:377–381
Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU, Lee SC (2004) J Agric Food Chem 52:3389–3393
Nicoli MC, Anese M, Parpinel M (1999) Trends Food Sci Technol 10:94–100
Frankel EN, Meyer AS (2000) J Sci Food Agric 80:1925–1941
Wong SP, Leong LP, Koh JHW (2006) Food Chem 99:775–783
Jayaprakasha GK, Singh RP, Sakariah KK (2001) Food Chem 73:285–290
Goupy P, Hugues M, Boivin P, Amiot J (1999) J Sci Food Agric 79:1625–1634
Yu L, Zhou K (2004) Food Chem 90:311–316
Dvořáková M, Douanier M, Jurková M, Kellner V, Dostálek P (2008) J Inst Brew 114:150–159
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Omwamba, M., Hu, Q. Antioxidant capacity and antioxidative compounds in barley (Hordeum vulgare L.) grain optimized using response surface methodology in hot air roasting. Eur Food Res Technol 229, 907–914 (2009). https://doi.org/10.1007/s00217-009-1128-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-009-1128-7