Abstract
Wholegrain foods are becoming increasingly popular as a high fiber dietary supplement recommended for people with diabetes. In Indonesia, the incidence of diabetes mellitus has almost doubled recently and poses a significant health risk with the high prevalence of obesity and cardiovascular diseases. The present research aimed to develop cookie bars from foxtail millet, arrowroot flour, and kidney beans. The physical, chemical, and sensory properties were evaluated by selecting the best formula to test the glycemic index. Three formulae of cookie bars, which had different percentages of foxtail millet, kidney beans, and arrowroot flour were evaluated. The results showed that the three formulae (F1, F2, F3) had °Hue values of 53.77, 58.46, and 58.31, and breaking force of 8.37, 10.12, and 5.87 N, respectively. While all other nutritional content were significantly different between formulae, the total crude fat was not. The F2 cookie bar was selected and evaluated for the glycemic index because it has the best sensory properties, lowest total sugar and available carbohydrate content. F2 cookie bars that contain 15% foxtail millet, 15% arrowroot flour, and 30% of kidney beans have a glycemic index of 37.6 hence it could be classified as a low glycemic index cookie bar. In conclusion, our findings indicated that F2 cookie bars can be further developed as a suitable diabetic food since it has the best physico-chemical properties, sensory properties, and low glycemic index.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an increasing interest in functional food development from local materials. Interest in the effect of specific foods on blood glucose levels has also increased in line with the increasing of diabetes mellitus prevalences. The prevalences of diabetes mellitus (DM) per 1000 population in Indonesia is increasing from 1.1 in 2007 to 2.1 in 2013 (Basic Health Research Survey 2013). People with diabetes need to consume certain foods that contain low sugar and are in high dietary fiber. On the other hand, there are many foods that have low glycemic index (GI) such as boiled kidney beans and boiled arrowroot that have GI as low as 29 and 14 respectively (Robbins 2015; Marsono 2001), whereas biscuits from foxtail millet have a GI as low as 50.8 (Anju and Sarita 2010). Based on this property, the development of low GI cookie bars from this raw material is promising.
The glycemic index (GI) is a method of ranking carbohydrates in foods on the basis of the blood glucose response they produce for a given amount of carbohydrate. The GI ranks foods based on their postprandial blood glucose response with respect to an equivalent carbohydrate portion of references such as glucose or bread. Another definition of GI was released by the Food and Agriculture Organization (FAO) and World Health Organization (WHO) as “the incremental area under the blood glucose response curve for a 50-g carbohydrate portion of a test food expressed as a percent of the response to the same amount of carbohydrate from a standard food taken by the same subject” (FAO/WHO 1998).
Foxtail millet (Setaria italica) is one of the small millets grown in semi arid regions; it requires warm weather and matures quickly in summer. In most areas in Indonesia, it is considered as a crop of poor people. However in Maluku island, it became popular with all the people. It has good nutritional value and is comparable to staple cereals such as rice and wheat in terms of protein, fiber, minerals, and vitamin content. The potency of foxtail millet for the low GI food has been unexploited. Considered as a high non starch polysaccharide resource, it can be developed for a functional food especially for its low GI.
Arrowroot (Maranta arundinacea) is a locally grown tuber crop in Indonesia. Arrowroot starch is commonly used as a thickener in many foods such as puddings and sauces, cookies, and other baked goods. Arrowroot flour is mainly used in cookies and baked goods. The arrowroot tubers contain plenty of total dietary fiber as much as 9.79–13.70% dry basis (Kumalasari et al. 2012). According to Marsono (2001), boiled arrowroot has a low GI as little as 14. One recent study suggested that the arrowroot flour is a potential source of prebiotics and has an immunomodulatory effect (Kumalasari et al. 2012).
Kidney beans are a very good source of dietary fibers as much as 24.9% (USDA National Nutrient Database for Standard Reference 2015) so that it could prevent blood sugar levels from rising too rapidly after a meal, making these beans an especially good choice for individuals with diabetes, insulin resistance or hypoglycemia. Hence, according to Robbins (2015), boiled kidney beans have a low GI as low as 29. Kidney beans are also an excellent source of trace minerals. Kidney beans were introduced in Asia by Spanish and Portuguese traders. Today, the largest commercial producers of dried kidney beans are India, China, Indonesia, Brazil and the United States.
Today, there are various food bars on the market; some that are specifically formulated to prevent hypoglycemia and others that are designed to lessen hyperglycemia. This food usually is referred to as diabetic food bars. This food bar contains certain nutrients and physiologically active components that play a role in improving glycemic control and reducing risk factors for development of diabetes complications. Functional characteristics of this product include resistant starch and dietary fiber. High fiber diets have been shown to lower the post prandial blood glucose rise, improve insulin sensitivity, and reduce hyperinsulinemia (Marlett and Slavin 1997; Krauss et al. 2000; Chandalia et al. 2000). The objectives of this study were to develop cookie bars from foxtail millet, arrowroot flour, and kidney beans. In the process, we evaluated the physical, chemical, and sensory properties; and then selecting the best formula to test the glycemic index.
Materials and methods
Raw material and cookies bar formulation
Foxtail millet, arrowroot flour, and kidney beans were purchased from the local market. The foxtail millet were dehusked and pulverised, then sieved with an 80-mesh sieve. The arrowroots were first washed, cleaned of the paper-like scale, pulverised, dried, ground, and then sieved with an 80-mesh sieve. The kidney beans were pulverised along with seed coat, then sieved with an 80-mesh sieve. The recipe of the cookie bars consisted of 100 g of mixed main ingredients (foxtail millet, kidney beans, and arrowroot flour), margarine, maltitol, egg yolks, and salt. The formula of the cookie bars can be seen in Table 1. The steps for processing the cookie bars were as follows: foxtail millet flour; arrowroot flour; and kidney beans flours were mixed thoroughly, then other ingredients (margarine, egg yolks, maltitol, and salt) were added and mixed. Next the dough was put into a baking pan and then baked at 140 °C for 30 min. Finally the baked cookie bars were cut into rectangular sized shapes measuring 2 cm × 5 cm.
Physical analysis (colour and texture measurement)
The surface colour of the cookie bars was evaluated using the Hunter method (HunterLab 1996; Hutchings 1999). Total colour difference (ΔE) is a parameter that quantifies the overall colour difference of a given sample compared to a reference sample. Mean L, a, and b values were used to determine the ΔE between the formulae of the cookie bars (Hutchings 1999). The smaller the value of ΔE, the closer the samples are in colour. Values of ΔE between 0 and 0.2 indicate an imperceptible colour difference, 0.2–0.5 for a very small difference, 0.5–1.5 for a small difference, 1.5–3.0 for distinct, 3.0–6.0 for very distinct, and 6.0–12.0 for great and values >12 for a very great difference (Drlange 1994; Young and Whittle 1985; Verma et al. 2015).
The hardness of the cookie bars was measured with texture analyzer XT2i. Hardness was determined from the maximal force (Fmax) recorded until the sample breakage in Newton (N) unit.
Chemical analysis
Proximate analysis (moisture, protein, fat, ash) was conducted according to the methods of AOAC (2005), whereas carbohydrate was determined by difference. The energy of cookie bar samples was calculated by multiplying the percentage of crude protein and carbohydrate with 4 and crude fat with 9. The values were then converted to calories per 100 g of the sample. Total sugars and reducing sugars were determined using the Nelson–Somogyi methods (AOAC 2005). Total dietary fibers were determined using the enzymatic–gravimetric methods according to Asp et al. (1983), whereas resistant starches were determined using the methods described by Goni et al. (1996).
Sensory analysis using preference test
Evaluation of sensory characteristics of the cookie bars was performed by 26 semi trained panelists who were undergraduate students of the Nutrition and Health Department, Faculty of Medicine, Universitas Gadjah Mada, Indonesia. Three formulae of cookie bars were randomly assigned to each panelist. In a preference test, the panelists were asked to tell which of three samples that they preferred. Ranking test was one of the commonly used preference tests. The panelist were asked to rank 3 samples in ascending order according to preference or liking (Lawless and Heymann 2010). A 3 point scale was used where 1 was dislike, 2 was like slightly, and 3 was like.
GI determination
Twelve healthy volunteers between 21 and 22 years of age with a body mass index (BMI) between 18.5 and 25 kg/m2 participated in the study. The exclusion criteria were fasting capillary blood glucose concentrations of ≥110 mg/dL as well as DM, pregnancy, or kidney problems.
The subjects arrived at the Nutrition Laboratory in the morning. They were assigned for fasting 8–10 h prior to the GI test. They had already filled out the informed consent form before the GI test. All rules and procedures regarding the test were explained to them. Firstly, their fasting capillary blood glucose concentrations were determined. The subjects then consumed the test meal (glucose or cookie bars) within 10 min. Pure glucose was used as food reference, and given by dissolving 25 g in 150 ml of water and consumed in <10 min. The test food was tested in the next week. The subjects had to consume 85 g of cookie bars within 10 min. Both food reference (glucose) and test food had 25 g of available carbohydrate.
Exactly 30 min from when they started to eat, the capillary blood glucose concentration was measured, and then it was measured again at 60, 90, and 120 min. Blood glucose responses after consumption of glucose or cookie bars were then calculated for the GI. Firstly, the area under the blood responses curve was calculated with the following formula (Wolever and Jenkins 1986):
where, L, the area under the curve; t, time interval blood sampling; Δ30, the difference in blood glucose level 30 min after the load with fasting; Δ60, the difference in blood glucose level 60 min after the load with fasting; Δ90, the difference in blood glucose level 90 min after the load with fasting; Δ120, the difference in blood glucose levels 120 min after the load with fasting.
Based on the area under the curve, the glycemic index can be calculated using the following formula (Brouns et al. 2005):
The Medical and Health Research Ethics Committee (MHREC), Faculty of Medicine, Universitas Gadjah Mada has approved the nutritional studies on subjects (KE/FK/1105/EC) dated October 7, 2014. All subjects gave signed informed consent to participate in the study.
Statistical analysis
Each result is expressed as mean ± standard deviation (SD). One-way ANOVA or Krusskal Wallis test was used to assess the statistical significance of differences between formulae of cookie bars. Each value of p < 0.05 is considered to be statistically significant.
Results and discussion
Physical properties
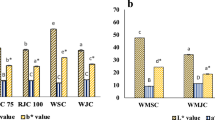
The physical properties of the cookie bars are shown in Table 2 with the L value representing the lightness on a scale of 0 (dark) to 100 (white). L values of the cookie bars were significantly different between formulas. The higher the addition of arrowroot flour, the lighter it tended to be. The a value represented +a for redness and −a for greenness. The a values of the cookie bars were not different between formulas. The b value represented +b for yellowness and −b for blueness. The b values of the cookie bars were not different between formulas. The °Hue values between formulas also were not different. However the colour interpretations were different. F1 colour could be interpreted as red, whereas F2 and F3 were yellow red. Total colour difference (∆E) between F1 and F2 was 4.33, whereas ∆E between F1 and F3 was 5.95. This result indicated that F1 was very distinct compared to F2 and F3. However ∆E between F2 and F3 was 1.79 so that F2 was distinct compared to F3. F3 had the lighter color since it contain more arrowroot flour compare to other formulae. The protein content of F3 was lower than other formulae hence the Maillard reaction between amino acid and reducing sugar in F3 was lesser and the colour appearance was less browning. The darker colour of F1 and F2 was due to the red pigmented ingredient i.e. foxtail millet and red kidney bean.
The texture of the cookie bars is shown as Fmax, where Fmax is the maximal force recorded until the sample breakage. It was shown that the higher addition of kidney beans resulted in the harder cookie bars. Similar with this finding, Lee and Beuchat (1991) reported that more strength was needed to break cookies incorporated with legumes flour. On the contrary, the higher addition of arrowroot flour resulted in the more easily crumbled texture since this product contains the highest dietary fiber and resistant starch. According to Sajilata et al. (2006), resistant starch improves product characteristics including crispness, expansion, mouthfeel, color, and flavor. Sharma et al. (2016) also found that substitution in cookies with gelatinized-retrograded starch and extruded starch have lower rupture force compared to non-substituted cookies. This finding could be attributed to the higher resistant starch content in substituted cookies. Hoojjat and Zabik (1984) found that wheat flour cookies that contain higher protein content tend to have harder texture. This might be due to the incorporation of protein rich flour which need more water to obtain good cookie dough, and the cookies prepared from high-absorption dough tend to be extremely hard.
Chemical properties
Table 3 shows that the total fat of the cookie bars was not significantly different. On the contrary, the moisture content, ash, crude protein, crude fibers, and the total carbohydrate were significantly different. Addition of different flours in the cookie bars affected the chemical properties. The reducing sugar and total sugar were significantly different between formulae (Table 3). Formula F1 has the lowest reducing sugar, whereas F2 has the lowest total sugar. The dietary fiber of F3 formula was the highest as well as the resistant starch content. The best product that is suitable for a diabetic person contains less sugar and more complex carbohydrate including dietary fiber and resistant starch.
The moisture content of the cookie bars play an important role in determining the shelf life of the product. Additionally, it can affect the texture, appearance, and taste of the food product. The carbohydrate content of a food product will affect its physical characteristics including color, taste, and texture so that it will further affect the acceptability, palatability, and the shelf life.
High dietary fiber meals are needed for the diet of a person who is diabetic. Dietary fiber can modulate glucose blood levels and increase insulin’s sensitivity (DeVries 2012). According to the Indonesian Consensus of Management and Prevention for Non-Insulin Dependent Diabetes Mellitus (NIDDM) (Perkumpulan Endokrinologi Indonesia (PEI) 2011), people with NIDDM are strongly recommended to consume ±25 g of dietary fiber every day. The F2 cookie bars contain 14.48% dietary fiber and 9.67% resistant starch hence consumption of 100 g or approximately 3 pieces of cookie bars will fulfil the daily need of dietary fiber. According to the American Association of Cereal Chemists (AACC Report 2001), resistant starch is included as dietary fiber. Dietary fiber and resistant starch are complex carbohydrates recommended for preventing NIDDM. According to Indonesian Endocrinology Association in Consensus of Management and Prevention of NIDDM (Perkumpulan Endokrinologi Indonesia (PEI) 2011), carbohydrate must be consumed for only 45–65% of daily energy intake. Snack food usually supplies 15% of daily energy needs, which is approximately 300 kcal. It was assumed that consumption of 3 pieces of cookie bars will fulfil the need of energy, protein, fat, carbohydrate, and dietary fiber as much as 18.29, 15.54, 35.75, 12.58, and 8.79% respectively. Thus, the fat content of this cookies bar is slightly higher than the daily recommendation which is only 30% of total energy consumption.
Sensory properties
The total sensory scores of the cookie bars was presented in Fig. 1. Cookies prepared from 15% foxtail millet, 15% arrowroot flour, and 30% of kidney beans were rated high in colour, flavor, taste, and texture hence it was more acceptable than other formulae. Sensory attributes could affect the palatability and quality of the food product.
Glycemic index of selected food bar
The F2 formula was selected for further GI determination. The F2 formula has the best characteristics according to the following criteria: (1) the physical characteristics, i.e. colour lightness and texture, (2) the chemical characteristics, i.e. lowest total sugar and available carbohydrate content, and (3) the sensory characteristics. The 3 formulae of cookie bars were ranked according to 7 parameters, i.e. L value, texture, total sugar, dietary fiber, resistant starch, available carbohydrate, and sensory attributes. Each parameter has a value of 1, 2, or 3 where 1 is fair, 2 is good, and 3 is excellent. The F1, F2, and F3 cookie bars have total score of 12, 16, and 13 respectively hence F2 was selected for the final GI determination.
Twelve healthy volunteers between 21 and 22 years of age with average body mass index (BMI) of 20.92 participated in the study. Figure 2 showed that blood glucose responses after ingestion of pure glucose were higher than the cookie bars. The area of the glucose curve is 3612.5, whereas the area of cookie bars curve is 1357.5. The GI can be calculated based on this data. The GI of the cookies bar is 37.6. This GI value is considered as a low GI value since the value is below 55.
Glycemic index of certain food depends on its digestibility and glucose absorption. Low GI foods are digested slowly, thus glucose absorption in the small intestine is slow. As a result, the fluctuation of blood sugar levels will be relatively lower. On the other hand, foods with a high GI are digested rapidly so that the glucose absorption is fast. The value of the glycemic index of food is influenced by several factors i.e. nature of the monosaccharide components (glucose, fructose, galactose), nature of the starch (amylose, amylopectin, starch–nutrient interaction, resistant starch), cooking or food processing (degree of starch gelatinization, particle size, food form, cellular structure), and other food components (fat and protein, dietary fiber, antinutrients, organic acids) (FAO/WHO 1998).
The dietary fiber content of foods will affect blood sugar levels, thus the GI value is low. This result is due to the characteristics of the dietary fiber which inhibit the digestion rate of food and the activity of the enzymes. Low GI foods reduce both postprandial blood glucose and insulin responses. The digestibility of the carbohydrate in low GI foods is generally less than that of high GI foods. Thus, low GI foods increase the amount of carbohydrate entering the colon and increase colonic fermentation and short chain fatty acid production (FAO/WHO 1998). In addition to dietary fiber, resistant starch in the diet tend to retard starch degradation and delay gastric emptying rate in the small intestine which leads to lower glycemic response (Odenigbo et al. 2012).
Amylose and amylopectin content varied in starch depending upon source. High amylose content in food will slow the rate of food digestion, especially the digestion of starch. This result is due to the amylose’s structure and the hydrogen bond of amylose so that amylose is more difficult to hydrolyze by digestive enzymes (Behall and Hallfrisch 2002). In addition, the larger size of amylopectin provides more open and wide surface for enzymatic attack as compared to smaller amylose (Bennion and Scheule 2000; McWilliams 2001). The presence of amylose–lipid content also reduces the amylose digestion rate (Hu et al. 2004).
Digestibility of starch is the easiness of certain types of starch to be hydrolyzed by the digestive enzymes into simple sugars. Starch digestion is influenced by intrinsic and extrinsic factors. Intrinsic factors are related to the characteristics of starch, such as granule size, and presence of food matrices, as well as the number and size of pores on the surface of the starch. Extrinsic factors are related to the duration of digestion in the stomach, the activity of amylase in the intestine, and the amount of starch, as well as the presence of other food components. The higher the digestibility of the starch, the more easily it will be converted into glucose thus raising the blood sugar level rapidly.
The existence of fat and protein in the diet also affects the GI value of a food. Foods with high fat and protein content will slow the rate of gastric emptying (Moghaddam et al. 2006; Odenigbo et al. 2012), so that digestion of food in the small intestine will proceed slowly. Food with high protein content is expected to stimulate the secretion of insulin which controls the blood glucose levels in the body. Foods that are high in protein and fat will tend to have a lower GI (Pi-Sunyer 2002).
According Englyst et al. (1992), the GI value of a food can be influenced by the way of the food processing such as heating (steaming, boiling, frying) and milling. The heating process will affect the gelatinization of starch and the starch structure, resulting in the formation of retrograded starch that are digested more slowly. Boiling is believed to induce gelatinization, thereby permanently disrupting the amylose–amylopectin structure of the starch complex, thus making it more readily accessible by digestive enzymes. At the same time retrograded amylose is indigestible due to the presence of stronger hydrogen bonding in comparison with retrograded amylopectin. The smaller the particle size of the food, especially the size of the starch, will increase the surface area and thus contact with the digestive enzymes will be optimal. Furthermore the food digestibility will increase and the absorption of glucose is faster so that the GI value will be higher.
The F2 cookie bars contain a low amount of total sugar whereas the total protein, fat, and dietary fiber were high hence the glycemic index of the cookie bars with these characteristics tends to be low. Protein, fat, and dietary fiber will inhibit the sugar absorption. The number of available carbohydrates in F2 cookies was approximately 19.76%, however the type of available carbohydrate (amylose and amylopectin) was not determined.
Conclusion
Substitution with 30% foxtail millet in the ingredients of the cookie bars resulted in the darker color and slightly harder texture and showed higher protein content. Substitution with 30% kidney beans in the cookie bars resulted in the harder texture and showed higher ash content and lower total sugar content. Substitution with 30% arrowroot flour in the cookie bars resulted in the lighter color and the more easily crumbled texture and showed higher total dietary fibers and resistant starch content. F2 cookie bars which contain 30% of kidney beans have a glycemic index of 37.6 hence it could be classified as low GI cookie bars.
References
AACC Report (2001) The definition of dietary fiber. Report of the Dietary Fiber Definition Committee to the Board of Directors of the American Association of Cereal Chemists. Cereal Foods World 46(3):112–126
Anju T, Sarita S (2010) Suitability of foxtail millet (Setaria italica) and barnyard millet (Echinochloa frumentacea) for development of low glycemic index biscuits. Malays J Nutr 16(3):361–368
AOAC (2005) Official methods of analysis, 18th edn. Association of Official Analytical Chemists, Washington
Asp NG, Johansson CG, Halmer H, Siljestrom M (1983) Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem 31:476–482
Basic Health Research Survey (2013) Riset Kesehatan Dasar (Riskesdas). Kementerian Kesehatan Republik Indonesia, Jakarta
Behall KM, Hallfrisch J (2002) Plasma glucose and insulin reduction after consumption of breads varying in amylose content. Eur J Clin Nutr 56:913–920
Bennion M, Scheule B (2000) Introductory foods, 11th edn. Prentice-Hall, New York
Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TMS (2005) Glycemic index methodology. Nutr Res Rev 18:145–171
Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ (2000) Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 342:1392–1398
DeVries JW (2012) Total dietary fiber. Medallion Laboratories, Minneapolis
Drlange (1994) Color review. Drlange application report, vol 8.0e. Drlange
Englyst HN, Kingman SM, Cummings JH (1992) The glycemic index of potatoes: the effect of variety, cooking method and maturity. Eur J Clin Nutr 53:249–254
FAO/WHO (1998) Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation (FAO Food and Nutrition Paper 66) Food and Agriculture Organization: April 14–18, 1997. Rome
Goni I, Garcia-Diz L, Manas E, Saura-Calixto F (1996) Analysis of resistant starch: a methods for foods and food products. Food Chem 56(4):445–449
Hoojjat P, Zabik ME (1984) Sugar-snap cookies prepared with wheat-navy bean-sesame seed flour blends. Cereal Chem 61:41–44
Hu P, Zhao H, Duan Z, Linlin Z, Wu D (2004) Starch digestibility and the estimated glycemic score of different types of rice differing in amylose contents. J Cereal Sci 40:231–237
HunterLab (1996) Insight on color. Application note, vol 8, no 7. HunterLab
Hutchings JB (1999) Food color and appearance, 2nd edn. Aspen Publishers Inc.
Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckel-baum RJ, Erdman JW Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL (2000) AHA Dietary Guidelines: revision 2000: a statement for health-care professionals from the Nutrition Committee of the American Heart Association. Circulation 102:2284–2299
Kumalasari ID, Harmayani E, Lestari LA, Raharjo S, Asmara W, Nishi K, Sugahara T (2012) Evaluation of immunostimulatory effect of the arrowroot (Maranta arundinacea. L) in vitro and in vivo. Cytotechnology 64(2):131–137
Lawless HT, Heymann H (2010) Sensory evaluation of food—principles and practices, 2nd edn. Springer, New York
Lee C, Beuchat LR (1991) Functional and sensory properties of muffins and cookies containing dried fermented peanut milk. Lebensm-Wiss Technol 24:528–534
Marlett JA, Slavin JL (1997) Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc 97(10):1157–1159. doi:10.1016/S0002-8223(97)00279-4
Marsono Y (2001) Glycemic index of selected Indonesian starchy foods. Indones Food Nutr Progr 8:15–20
McWilliams M (2001) Foods: experimental perspectives, 4th edn. Prentice-Hall, Englewood Cliffs
Moghaddam E, Vogt JA, Wolever TMS (2006) The effects of fat and protein on glycemic responses in nondiabetic humans vary with waist circumference, fasting plasma insulin, and dietary fiber intake. J Nutr 136:2506–2511
Odenigbo A, Rahimi J, Ngadi M, Amer S, Mustafa A (2012) Starch digestibility and predicted glycemic index of fried sweet potato cultivars. Funct Foods Health Dis 2(7):280–289
Perkumpulan Endokrinologi Indonesia (PEI) (2011) Konsensus Pengelolaan dan Pencegahan Diabetes Mellitus Tipe 2 di Indonesia. Jakarta, pp. 11–64
Pi-Sunyer FX (2002) Glycemic index and disease. Am J Clin Nutr 76(Suppl):S290S–S298S
Robbins S (2015) Glycemic Index (GI) food chart. http://www.hflsolutions.com/healthtips/weightloss/GI_foodchart.pdf
Sajilata MG, Singhal RS, Kulkarni PR (2006) Resistant starch—a review. Compr Rev Food Sci Food Saf 5:1–17
Sharma S, Singh N, Katyal M (2016) Effect of gelatinized-retrograded and extruded starches on characteristics of cookies, muffins, and noodles. J Food Sci Technol 53(5):2482–2491
USDA National Nutrient Database for Standard Reference (2015) Full Report (All Nutrients) 19015, Snacks, granola bars, hard, plain. http://ndb.nal.usda.gov
Verma M, Singh J, Kaur D, Mishra V, Rai GK (2015) Effect of various dehydration methods and storage on physicochemical properties of guava powder. J Food Sci Technol 52(1):528–534
Wolever TMS, Jenkins DJA (1986) The use of glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 43:167–172
Young KW, Whittle K (1985) Colour measurement of fish minces using Hunter L, a, and b values. J Sci Food Agric 36:383–392
Acknowledgements
I would like to thank the Faculty of Medicine, Universitas Gadjah Mada (UGM) for the funds given through the Research Grant (Hibah Dana Masyarakat Dosen - Mahasiswa) in 2014. Thanks to the native speaker and professional editor in Klinik Bahasa (Language Clinic) Consultation, Office of Research & Publication, Faculty of Medicine, UGM for reviewing this article. I would like to express my gratitude to my students (Jap Kristianto Ade Cahyono, Aldino Dwi Caesar, and Titus Rian Pradita) who support this research as research assistants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lestari, L.A., Huriyati, E. & Marsono, Y. The development of low glycemic index cookie bars from foxtail millet (Setaria italica), arrowroot (Maranta arundinacea) flour, and kidney beans (Phaseolus vulgaris). J Food Sci Technol 54, 1406–1413 (2017). https://doi.org/10.1007/s13197-017-2552-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2552-5