Abstract
Yellow -fleshed cassava flour (CF) and wheat flour (WF) composite bread was prepared in various proportions of 100:0 (100% CF), 50:50 (50% CF:50% WF), 20:80 (20% CF:80% WF), 10:90 (10% CF:90% WF), and 0:100 (100% WF) and flavored with 2 g of cocoa powder (FL). Forty male Wistar rats, weighing 170-200 g were divided into eight groups of five animals each. Rats in the control group were fed with the basal diet, and other rat groups were fed with high fat diet (30% fat) for two weeks prior to injection with 25 mg/kg body weight of streptozotocin (STZ) for the induction of type-2 diabetes. Thereafter, the diabetic rats were subsequently placed on different formulated breads for two weeks, while the positive control rats were placed on acarbose (25 mg/kg/body weight) in addition to the composite bread for the time the experiment lasted. The blood glucose of the rats was checked every four days, and the rats were sacrificed using cervical dislocation at the end of the experiment. The blood was rapidly collected through a heart puncture, and the plasma was rapidly separated while the liver was excised, rinsed, and subsequently homogenized prior to the assessment of the lipid profile, and the pancreas histopathology was carried out. The result revealed that 100% CF bread + FL (98.00 mg/dl) significantly (p < 0.05) lowered blood glucose levels in diabetic rat as compared to the acarbose (240.50 mg/dl) and the control rats. Furthermore, there were significant (p < 0.05) elevation in HDL-cholesterol and a concomitant decrease in LDL-cholesterol in the plasma of diabetic rats fed with 100% CF bread + FL as compared to the control. This study suggests that composite bread from vitamin-A enriched cassava and wheat flour may represent safe and effective functional foods with antihyperglycemic and hypolipidemic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent reports have confirmed the fast increase in the debilitating conditions of diabetes throughout the worlds especially in developing nations [1,2,3]. Diabetes mellitus is a chronic metabolic disease resulting from elevated blood glucose resulting from defects in insulin secretion, insulin action, or both [4,5,6]. Diabetic mellitus may increase the risk of both microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular (ischemic heart disease, stroke, and peripheral vascular disease) damage, with associated reduced life expectancy and diminished quality of life [7,8,9,10,11]. Type-2 diabetes Mellitus is a prevalent metabolic condition globally, primarily resulting from a combination of two primary factors: defective insulin secretion by pancreatic β-cells and the inability of insulin-sensitivity tissues to respond to insulin [12,13,14,15].
Elevated prostprandial hyperglycemia may result from the rapid degradation of these complex carbohydrates, and possible inhibition of the enzymes may represent a key target of minimizing these effects [16,17,18,19]. Acarbose and miglitol are synthetic oral antidiabetic agents that are currently in use for the management of type-2-diabetes and to elicit their antihyperglycemic effects and inability to reverse or alleviate diabetic complications [20], Decreasing hyperglycemia could be one of the therapeutic strategies towards the management of diabetes [19, 21, 22].
Precise matching of endogenous glucose synthesis or dietary glucose administration and utilization is necessary to maintain normal plasma glucose levels. Glucose is delivered from three sources: the intestinal absorption that follows the digestion of dietary carbohydrate, glycogenolysis, and gluconeogenesis. Glucose is transported into the cells through multiple metabolic pathways; it may be stored as glycogen; it may undergo glycolysis to pyruvate; and finally, it may be released into circulation by the liver and kidney, the sole organs continuing the glucose phosphate-6-phosphatase enzyme necessary for the release of glucose into the circulation [23, 24].
Insulin is the dominant glucoregulatory hormone. Higher amounts, mainly through inhibiting hepatic glucose synthesis, control the plasma glucose concentration during fasting, such as those found after meals, are required to stimulate glucose utilization [25, 26]. Glucagon is a potent hyperglycemic hormone that acts almost exclusively on the liver to increase hepatic glucose production within minutes. Ingestion of carbohydrates elicits a rise in insulin concentration and a decrease in glucagon concentration. The increase in insulin concentration, which occurs before the rise in arterial glucose concentrations, is thought to be mediated largely via hormone signals arising in the gastrointestinal tract [25, 26]. Early insulin released allows increased glucose disposal during absorption and prevents hyperglycemia [25].
Pancreatic β-cells selectively absorb streptozotocin (STZ), also chemically known as N-(methylnitrosocarbamoyl)-α-d-glucosamine, a naturally occurring substance generated by streptomycetes achromogenes having antibiotic qualities, causing its destruction [27, 28]. After its intraperitoneal or intravenous administration, STZ behaves as a glucose analogue and is transported mainly by the glucose transporter subtype-2 (GLU-2) [29] into the pancreatic β-cell, where it induces toxicity, mainly by producing DNA alkylation [27].
Natural substances, such as steroids, terpenoids, polyphenols, alkaloids, and tannins, have a range of pharmacological activities that target multiple metabolic pathways [30]. These spontaneously produced molecules' improved intercellular medication distribution is crucial for producing effective therapeutic effects [31, 32]. Plant-derived chemical agents, such as polyphenols, have the ability to enhance the intercellular drug transport mechanism, which is crucial for establishing favorable treatment outcomes for diabetes mellitus [33, 34].
Cassava is a major source of carbohydrate in local diets, and when biofortified with increased levels of carotenoids, it provides many other nutritional health benefits, such as improvements in visual ability and the immune system [34, 35]. The new variety of vitamin-A, or yellow-fleshed cassava, was introduced by the Federal Government of Nigeria under Harvest plus, which is 25% richer in β-carotene (a pro-vitamin A) than those earlier released in 2011 [35,36,37]. The characteristic yellow color associated with vitamin-A cassava variety is due to its higher vitamin A content [38]. Vitamin A deficiency is a major public health challenge in sub-Saharan countries [39].
Every population agrees that bread is a very practical food source that is essential. It is a good source of nutrients, such as macronutrients (carbohydrates, protein, and fat) and micronutrients (minerals and vitamins), that are essential for human health [40]. Efforts have been made to promote the use of flour from high protein seeds and locally grown crops to replace wheat flour used in baking bread, thereby decreasing the demand for imported wheat and leading to the production of protein-enriched bread [41]. A composite flour used to create bread has been made from the majority of tropical cereal grains and several tubers [42].
The nutritional importance arising from cassava/wheat flour bread’s blends will be harnessed to promote well-being and healthy living for the consumer with associated health challenges. According to the research, there may be new avenues for addressing the devastating effects of cardiovascular diseases, hyperlipidemia, and other ailments brought on by everyday dietary consumption that endanger human health and survival. Therefore, this research is designed to investigate the effect of the composite bread blend’s blend produced from yellow cassava flour (CF)/wheat flour (WF) flavored with cocoa powder on the blood glucose level), enzymes inked with Type-2 diabetes (α-amylase and α-glucosidase), Lipid profile (total cholesterol, HDL-cholesterol, LDL-cholesterol, triglyceride), and histopathology of the pancreas sections of HFD/STD induced Type-2 diabetic and normal rats.
Materials and methods
Materials
Yellow-fleshed cassava, also known as Vitamin-A enriched Cassava was obtained from the International Institute of Tropical Agriculture, IITA Ibadan, Oyo State, Nigeria. It was peeled, washed, cut into smaller pieces and oven dried in a cabinet dryer at 45 °C for 72 h before it was milled to flour while wheat flour (Golden penny), cocoa powder, yeast, and eggs were purchased from Waso market a major market in Ogbomoso city, in Oyo State. Aspartame was purchased from a chemical supplier in Ilorin, Kwara State. All the chemicals used were analytical grade while the water was glass- distilled.
Production of cocoa powdered, yellow-fleshed cassava/wheat composite flour bread
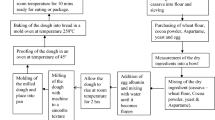
Yellow fleshed cassava was peeled, washed clean, cut into smaller pieces, and then dried in the cabinet dryer at 45 °C for 72 h. The dried cassava was milled into cassava flour (CF). The composite yellow fleshed cassava flour (CF) and wheat flour (WF) are in the ratios of 100:0 (100% CF and 0% WF), 50:50 (50% CF and 50% WF), 20:80 (20% CF and 80% WF), 10:90 (10% CF and 90% WF), and 0:100 (0% CF and 100% WF). In the process of making composite breads, aspartame was used as a sweetener, egg albumin served as an emulsifier, cocoa powder served as a flavoring agent (FL) and an extra source of phenol, and water was added to taste. It was baked for thirty minutes at 250 °C in a gas oven.
Diabetic experiment
Forty [40] Albino rats weighing 170–200 g were used in the experiment. The animals were acclimatized for two weeks and given commercial feed and water ad libitum. The animals were grouped into two groups after acclimatization. Group I (the control) consists of five animals, and Group II consists of other rats. Animals in group I (control) were fed a basal diet, and those in group II were fed a high fat diet (30% fat) for 2 weeks (basal diet compositions are; corn flour, skimmed milk, mineral and vitamin premixed, and vegetable oil, while high fat diet compositions are; Corn flour, skimmed milk, mineral and vitamin premixed, and lard). Then, at the expiration of the second week, the group II animals were induced with 25 mg/kg/body weight of streptozotocin (STZ) according to the method described by Adefegha, et al. [43]. After 72 h of induction with STZ, the blood glucose level of the animals was checked using the Fine Test Glucometer.
Animals with blood glucose (≥ 250 mg/dl) were regarded as diabetic. Then, the diabetic group was divided into seven groups, groups II–VIII of five rats each in a group. The rats were fed with the formulated composite breads for two weeks. Furthermore, group III was treated with Acarbose (25 mg/kg/body weight) in addition to the composite bread for the periods during which the experiment lasted, and the blood glucose of all the rats was examined at 4-days intervals using a Fine Test Glucometer by collecting the blood through the tails of the animals. The animals were subjected to a night fast a day prior to the sacrifice by removing composite bread and water. The animals were sacrificed using cervical dislocation, and blood was rapidly collected through a heart puncture. Plasma was rapidly separated from the blood, and the liver was also excised, rinsed in cold saline (0.9%), and homogenized in 0.1 M phosphate buffer (pH 7.4) for further biochemical analyses (Table 1).
Feed formulation and group treatments
-
Group 1: Normal rats fed a basal diet + 100% WF bread.
-
Group II: Diabetic rats fed a high- fat diet + 25 mg/kg/body weight STZ + 100% WF bread.
-
Group III: Diabetic rats fed a high- fat diet + 25 mg/kg/body weight STZ + 25 mg/kg/body weight Acarbose + 100% WF bread.
-
Group IV: Diabetic rats fed a high- fat diet + 25 mg/kg/body weight STZ + 100% CF bread + cocoa powder.
-
Group V: Diabetic rats fed a high- fat diet + 25 mg/kg/body weight STZ + 50% CF + 50% WF bread + cocoa powder.
-
Group VI: Diabetic rats fed a high- fat diet + 25 mg/kg/body weight STZ + 20% CF + 80% WF bread + cocoa powder.
-
Group VII: Diabetic rats fed a high- fat diet + 25 mg/kg/body weight STZ + 10% CF + 90% WF bread + cocoa powder.
-
Group VIII: Diabetic rats fed a high- fat diet + 25 mg/kg/body weight STZ + 100% WF bread + cocoa powder.
Preparation of plasma
At the conclusion of the feeding trial, the rats were sacrificed and the whole blood was collected into an EDTA container. To separate the plasma, the blood sample was centrifuged for 10 min at 3000 rpm. In preparation for additional analysis, the plasma was decanted into a plain sample vial and kept at 4 °C.
Preparation of pancreas and small intestine homogenate
The rats were sacrificed, and the pancreas and small intestine were rapidly isolated, placed on ice, and weighed. These tissues were subsequently rinsed in cold 0.9% normal saline and homogenized in 0.1 M phosphate buffer (pH 7.4). The homogenates were centrifuged at 3000 rpm for 10 min to yield pellet that was discarded, and low-speed supernatant (SI) was kept for the determination of α- amylase and α-glucosidase activities.
Determination of α-amylase activity
The activity of α- amylase was determined according to the method of Ali et al. [44] with slight modifications. Briefly, 250 µl of phosphate buffer was added to 50 µl of the tissue homogenate and incubated at 25 °C for 10 min. The reaction was started by the addition of 50 µl of potato starch (0.5%) dissolved in 20 mM phosphate buffer at pH 6.9. The reaction mixture was incubated for a further 10 min at 25 °C and the catalytic reaction was terminated by the addition of 200 µl of DNSA reagent (1% 3,5-dinitrosalicylic acid and 12% sodium potassium tartarate in 0.4 M NaOH). The reaction mixture was heated for 5 min at 100 °C. 2 ml of distilled water was added, and α-amylase activity was determined by measuring the absorbance at 540 nm.
Determination of α-glucosidase activity
This was determined according to the method described by Kim et al. [45] with a slight modification. About 15 µl of Glutathione was added to 15 µl of tissue, then, 445 µl of 20 mM phosphate buffer pH 6.9 was added and incubated for 10 min at 37 °C. Para-nitrophenyl-glucopyranoside (PNPG) (3 mM) already dissolves in 20 mM phosphate buffer, pH 6.9 was added to start the reaction. The reaction mixture was further incubated at 37 °C for 10 min and stopped by the addition of 2 ml of 0.1 M Na2CO3. The α-glucosidase activity was determined by measuring the absorbance of the yellow-colored P-Nitrophenol release from PNPG at 400 nm in the spectrophotometer.
Determination of plasma total cholesterol
About 1.0 ml of the reacting mixture containing 4-aminoantipyrine, phenol, peroxidase, cholesterol esterase, cholesterol oxidase, and 80 mM pipe buffer pH 6.8 was mixed with 10 µl of plasma and incubated for 5 min at 37 °C. The absorbance at 546 nm was then taken against the reagent blank within 60 min. The concentration of cholesterol in the sample was subsequently calculated against a standard.
Determination of plasma triacylglycerides.
About 10 µl of the sample was mixed with 1.0 ml of pipe reagent (40 mM phosphate buffer. 5.5 mM 4-chlorophenol, and 1.75 mM Mg2+) and enzyme reagent (phosphate oxidase and peroxidase). Thereafter, the mixture was incubated for 5 min at 37 °C and the absorbance at 546 nm was taken against the blank within 60 min. The triglyceride concentration was subsequently calculated.
Determination of plasma LDL-cholesterol
The LDL-cholesterol concentration of the plasma samples was determined according to the equation of Friedewald et al. [46], expressed:
where LDL = low-density lipoprotein, TC = total cholesterol, HDL = high density lipoprotein, and TG = triglycerides.
Determination of HDL-cholesterol
The precipitation was carried out according to the method of Lopes-Virella et al. [47] as described in the kit’s manufacturer’s (Randox Laboratories Ltd.) manual. Briefly, 200 µl of plasma was mixed with 500 µl of the precipitant (0.55 mM phosphotungstic chloride) and allowed to sit for 10 min at room temperature. Then, the mixture was centrifuged for 10 min at 800×g. Thereafter, the clear supernatant was separated off and subjected to the same procedure for the determination of cholesterol described above. The HDL-cholesterol was thus calculated using a standard.
Histopathological examination of the pancreas using heamatoxylin and eosin staining methods
Histopathological assay of pancreas was carried out according to the method of Bancroff and Gamble [48]. The procedures of heamatoxylin and eosin stains were carried out; the pancreas section of the normal and HFD/STZ induced Type-2 diabetic rats were de-waxed and dehydrated. Paraffin wax was also removed from the section by immersing the slides in xylene for 2–3 min. This process was intensified by first warming the sections over a 60 °C flame until the paraffin wax began to melt. Afterwards, the slides were rehydrated by immersing them in descending grades of alcohol (2 changes of absolute alcohol, 90%, and 70% alcohol) and finally in distilled water. The slide was further stained thoroughly in Harris hematoxylin for 10 min, which was later washed in running tap water for 5–10 min. Then the slides were stained with eosin for 1–2 min, which were later washed in running water until excess Eosin was removed. The slides were further dehydrated with ascending grades of alcohol. The absolute alcohol was finally removed, and the slides were cleared in xylene. The slides were then mounted and covered slip using Dibutylpthalate xylene (DPX)
Results and discussion
High fat diet was formulated (Table 1) and fed to rats prior to induction with low dose of streptozotocin according to the method described by Adefegha et al. [43]. The flow chart represents how the yellow fleshed cassava -wheat flour composite bread was produced (Fig. 1) and the bread was presented in Plate 1. Figure 2 revealed the effect of yellow fleshed cassava/wheat flour composite bread on the blood glucose levels of the normal rats and high- fat diet (HFD)/STZ induced Type-2-diabetes rats presented in mg/dl. From the result, it was observed that there was a significant (p < 0.05) increase in the blood glucose of Type-2 diabetic control rats (STZ + 100 WF bread) as compared to the normal control group (100% WF bread). However, treatment of the diabetic acarbose group with acarbose brought a significant (p < 0.05) reduction to the blood glucose level, but not as low as the group fed 100% CF bread + FL. The trend of blood glucose lowering ability of yellow fleshed composite bread in the blood of the normal and high-fat diet (HFD)/STZ-induced Type-2 diabetic groups is as follows: STZ + 100% CF Bread + FL (98.00a ± 0.67 mg/dl) < Basal diet + commercial bread (120.33b ± 1.46 mg/dl) < STZ + Acarbose + 100% WF bread (240.50c ± 0.83 mg/dl) < STZ + 50% CF + 50% WF brad + FL (266.50d ± 0.25 mg/dl) < STZ + 20% CF + 80% WF bread + FL (344.50e ± 0.33 mg/dl) < STZ + 10% CF + 90% WF bread + FL (362.50f ± 0.95 mg/dl) < STZ + 100% WF bread + FL (470.67 g ± 0.09 mg/dl) < STZ + 100% WF bread [diabetic control group (522.00 h ± 1.84 mg/dl)]. Diabetes mellitus is a serious, chronic, and complex illness characterized by hyperglycaemia that results from the pancreatic β-cells generating deficient insulin (a hormone that regulates blood glucose) when the body cannot efficiently use either insulin or both of them [49,50,51]. Inhibition of starch digestive enzymes or glucose transporters can reduce glucose release and absorption in the small intestine [14, 52, 53]. It was discovered that an increased substitution of yellow fleshed cassava in composite bread fed by normal and high- fat diet/STZ Type-2 diabetic rats contributed to the higher blood glucose lowering ability of the composite bread. This could be attributed to the regeneration of the damaged β-cell of the pancreas by yellow fleshed cassava /wheat flour composite bread, which contributed to more insulin secretion. Also, previous studies that reported that yellow fleshed cassava/wheat composite bread is higher in polyphenol and flavonoid contents [54, 55], high crude fibre, amylose/amylopectin ratio, potassium contents [54, 56, 57] and low glycaemic index [54, 58, 59] may have contributed to blood lowering ability. It is worth nothing that the blood glucose level in the high- fat diet/STZ induced Type-2 diabetic rats fed 100% CF bread + FL [STZ + 100% CF bread + FL (98.00a ± 0.67 mg/dl)] was much lower than the group treated with the Acarbose diabetic drug [STZ + Acarbose + 100% WF bread (240.50c ± 0.83 mg/dl) as well as the control group (120.33b ± 1.46 mg/dl)]. This result is in agreement with the works reported by Shewaita, et al., [60] on the role of Alhagi maurorum extract on the change in oxidative stress and antioxidant enzyme activities in streptozotocin-induced diabetes mellitus in rats and Emran et al. [61], on antidiabetic potential of leaf extract of Centella asiatica in Alloxan-induced diabetic rats.
Flow Chart representing the production of yellow fleshed cassava-wheat flour composite breads and Plate 1. Yellow fleshed Cassava-wheat composite bread. Key: A = 100% yellow-fleshed cassava bread + cocoa powder; B = 50% yellow-fleshed cassava + 50% wheat flour bread + cocoa powder; C = 20% yellow-fleshed cassava + 80% wheat flour bread + cocoa powder; D = 10% yellow-fleshed cassava + 90% wheat flour bread + cocoa powder; E = 100% wheat flour bread + cocoa powder; X = 100% yellow-fleshed cassava bread without cocoa powder; Y = 100% wheat flour bread without cocoa powder
Determination of Blood glucose level of Normal control and Type-2-diabetic rats fed with composite breads. Values represent mean ± standard deviation (n = 5). Key: Group I-Normal Rats fed with Basal diet + commercial bread; Group II- Diabetic Rats fed with High fat diet + 25 mg/kg/body weight STZ + 100% WF bread without cocoa powder; Group III-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 25 mg/kg/body weight Acarbose + 100% WF bread without cocoa powder; Group IV-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% CF bread + cocoa powder; Group V-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 50% CF + 50% WF bread + cocoa powder; Group VI-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 20% CF + 80% WF bread + cocoa powder; Group VII-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 10% CF + 90% WF bread + cocoa powder; Group VIII-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% WF bread + cocoa powder
Moreover, the result of the effect of yellow fleshed cassava/wheat flour composite bread on the α-amylase activity of the pancreas of the normal control and HFD/STZ induced Type-2 diabetic rats is presented in Fig. 3. The result showed that there was a significant (p < 0.05) increase in the activity of α-amylase in the pancreas of the diabetic group control rats (STZ + 100% WF bread) as compared to the control. However, treatment of the Type-2 diabetic Acarbose group with Acarbose brought a significant (p < 0.05) reduction in its activity but not as lower as the Type-2 diabetic rats fed with 100% CF bread + FL. This is the order of reduction in the activity of the α-amylase; STZ + 100% CF bread + FL < Basal diet + 100% WF bread < STZ + 50% CF + 50% WF bread + FL < STZ + 20% CF + 80% WF bread + FL < STZ + Acarbose + 100% WF bread < STZ + 10% CF + 90% WF bread + FL < STZ + 100% WF bread + FL. < STZ + 100% WF bread. Figure 4 shows the effect of yellow fleshed cassava/wheat flour composite bread on the activity of the α-glucosidase in the intestines of the normal control and HFD/STZ induced Type-2 diabetic rats. From the results, it was observed that the activity of α-glucosidase in the intestine of the diabetic group control rats (STZ + 100% WF bread) was significantly (p < 0.05) higher as compared to the normal control. However, treatment of the Type-2 diabetic Acarbose group with Acarbose brought a significant (p < 0.05) reduction in its activity but not as lower as the Type-2 diabetic rats fed with 100% CF bread + FL. This is the trend in the reduction of the activity of the α-glucosidase; STZ + 100% CF bread + FL < STZ + 50% CF + 50% WF bread + FL < STZ + 20% CF + 80% WF bread + FL < STZ + Acarbose + 100% WF bread < Basal diet + 100% WF bread < STZ + 10% CF + 90% WF bread + FL < STZ + 100% WF bread + FL < STZ + 100% WF bread. Inhibition of enzymes involved in carbohydrate hydrolysis such as α-amylase and α-glucosidase has attracted the interest of researchers for some time now as therapeutic approaches for lowering hyperglycemia [30]. It is worthy of noting that the group fed STZ + 100% CF bread + FL has the highest lowering effect of α-amylase and β-glucosidase activities. This report is in agreement with what was reported earlier in the previous in vitro study by Ajani et al. [42] and on the free polyphenol contents, antioxidant activity, and inhibition of enzymes linked to type-2 diabetes in cassava/wheat flour composite bread, Prasathkumar et al. [62], on anti-pathogenic, anti-diabetic, anti-inflammatory, antioxidant and wound healing efficacy of Datura metel L. leaves and Shahin et al. [63], on Comparative assessment of three medicinal plants against diabetes and oxidative stress.
The effect of vitamin-A enriched cassava / wheat flour composite bread on α-amylase activity of the pancreas of normal control and Type- 2-diabetic rats. Values represent mean ± standard deviation (n = 5). *Value are significantly (p < 0.05) different from normal and control group. Key: Group 1-Normal Rats fed with Basal diet + 100% WF bread without cocoa powder; Group II- Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% WF bread without cocoa powder; Group III-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 25 mg/kg/body weight Acarbose + 100% WF bread without cocoa powder; Group IV-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% CF bread + cocoa powder; Group V-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 50% CF + 50% WF bread + cocoa powder; Group VI-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 20% CF + 80% WF bread + cocoa powder; Group VII-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 10% CF + 90% WF bread + cocoa powder; Group VIII-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% WF bread + cocoa powder
The effect of vitamin-A enriched cassava / wheat flour composite bread on α-glucosidase activity of the intestine of normal control and Type-2-diabetic rats. Values represent mean ± standard deviation (n = 5). *Value are significantly (p < 0.05) different from normal and control group. KEY: Group 1-Normal Rats fed with Basal diet + 100% WF bread without cocoa powder; Group II- Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% WF bread without cocoa powder; Group III-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 25 mg/kg/body weight Acarbose + 100% WF bread without cocoa powder; Group IV-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% CF bread + cocoa powder; Group V-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 50% CF + 50% WF bread + cocoa powder; Group VI-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 20% CF + 80% WF bread + cocoa powder; Group VII-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 10% CF + 90% WF bread + cocoa powder; Group VIII-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% WF bread + cocoa powder
The results in Table 2 reveal the values of HDL, total cholesterol, triglyceride and HDL in the plasma of normal and HFD/STZ induced Type-2 diabetic rats fed with yellow fleshed cassava/wheat composite bread. The result showed that there was a significant (p < 0.05) increase in the value of HDL in the plasma of the diabetic rats (STZ + 100% CF bread + FL) when compared to its value in the diabetic control group and normal rats. The trend of the results is as follows; STZ + 100% CF bread + FL (264.43h ± 1.90 mg/dl) > STZ + 50% CF + 50% WF bread + FL (210.47g ± 2.55 mg/dl) > STZ + Acarbose + 100% WF bread (206.88f ± 2.77 mg/dl) > Basal diet + 100% WF bread (185.25e ± 2.45 mg/dl) > STZ + 20% CF + 80% WF bread + FL (170.60d ± 1.65 mg/dl) > STZ + 10% CF + 90% WF bread + FL (166.45c ± 1.82 mg/dl) > STZ + 100% WF bread (157.68b ± 2.50 mg/dl) > STZ + 100% WF bread + FL (145.67a ± 0.96 mg/dl). In addition, the results of total cholesterol in the plasma of normal and diabetic rats are presented in mg/dl in Table 2. The result revealed that there was a significant (P < 0.05) decrease in the value of total cholesterol in rats’ group STZ + 100% CF + FL when compared to the diabetic control and normal rats. The order of the value was sequential decrease in total cholesterol, following this order; STZ + 100% CF bread + FL < STZ + 50% CF + 50% WF bread + FL < Basal diet + 100% WF bread < STZ + Acarbose + 100% WF bread < STZ + 20% CF + 80% WF bread + FL < STZ + 10% CF + 90% WF bread + FL < STZ + 100% WF bread FL < STZ + 100% WF bread with these corresponding results: 41.35a ± 0.77 mg/dl, 48.58b ± 1.37 mg/dl, 51.99c ± 1.72 mg/dl, 54.02d ± 1.36 mg/dl, 54.13e ± 1.07 mg/dl, 61.36f ± 1.32 mg/dl, 103.55 g ± 1.31 mg/dl, 144.79 h ± 1.71 mg/dl. In addition, the results of triglyceride are also presented in Table 2. From the result, it was observed that the value of triglyceride was significantly (P < 0.05) lower in the rat’s group STZ + 100% CF bread + FL as compared to diabetic rats group treated with Acarbose (STZ + Acarbose + 100% WF bread) and normal control rats (Basal diet + 100% WF bread). The level of the decrease was in this order; STZ + 100% CF bread + FL (75.36a ± 1.21 mg/dl) < Basal diet + 100% WF bread (90.25b ± 2.01 mg/dl) < STZ + 50% CF + 50% WF bread + FL (99.19c ± 1.61 mg/dl) < STZ + Acarbose + 100% WF (104.25d + 2.03 mg/dl) < STZ + 20% CF + 80% WF bread + FL (110.66e ± 2.33 mg/dl) < STZ + 10% CF + 90% WF bread + FL (122.43f ± 2.00 mg/dl) < STZ + 100% WF bread + FL (135.43 g ± 0.66 mg/dl) < STZ + 100% WF bread (148.54 h ± 1.08 mg/dl). Similarly, the result of low-density lipoprotein (LDL) is also presented in Table 2. From the result, the value of LDL was significantly (P < 0.05) lower in the plasma of the rats’ group with STZ + 100% CF bread + FL as compared to the acarbose group with STZ + acarbose + 100% WF bread and the normal control group with basal diet + 100% WF bread. The decrease in the value of LDL-cholesterol is sequential in this order; STZ + 100% CF bread + FL (4.78a ± 0.39 mg/dl) < Basal diet + 100% WF bread (26.52b ± 0.33 mg/dl) < STZ + 50% CF + 50% WF bread + FL (35.79c ± 0.52 mg/dl) < STZ + Acarbose + 100%WF bread (36.29c ± 0.75 mg/dl) < STZ + 20% CF + 80% WF bread + FL (69.90d ± 0.88 mg/dl) < STZ + 10% CF + 90% WF bread + FL (92.75e ± 1.31 mg/dl) < STZ + 100% WF bread + FL (117.50f ± 2.56 mg/dl) < STZ + 100% WF bread (136.40g ± 3.02). HDL constitutes a dynamic polydisperse group of particles that are central to lipid metabolism [64, 65]. The inhibition of LDL oxidation is a major antiatherogenic property of HDL. This activity is due, in part, to HDL associated proteins [66]. It is worth noting that 100% CF bread + FL bread has an outstanding hypolipidemic effect on the plasma lipid level of the diabetic rats. The result agrees with the one earlier reported by Ajani, et al. [67], on the hypolipidemic effect and antioxidant properties of cassava wheat composite bread in rats and Alsoodeeri, Alqubbani and Aldossari [68], on cinnamon extract consumption and serum lipid profile in Albino rats.
Histopathological results in Fig. 5 reveal the pancreas micrographs of the normal and HFD/STZ-induced type-diabetic rats fed yellow fleshed cassava/wheat flour composite bread. It was observed from the results that parenchyma (PP) in the Type-2 diabetic control group (CC2) and E groups shows the presence of infiltration of inflammatory cells, red cells with normal serous acinar, and Zymogenic cells containing abundant eosinophilic cytoplasm with a well-defined, conspicuous degenerative changes occurring in the islet of Langerhans (IL). However, the control group CC1 showed intact cytoarchitecture with an intact islet of Langerhans (IL), pancreatic parenchyma (PP), and blood vessel (BV). The rats in the Acarbose group treated with the Acarbose drug (CC3) and the rats fed vitamin-A enriched cassava/wheat flour composite bread (A, B, C, and D) showed mild fibrosis (F) in their pancreatic degenerative changes, insignificant mild fibrosis (F) in their pancreases, which are mildly similar to the control treatment. It is highly commendable to note that yellow -fleshed cassava /wheat flour composite bread has an ameliorative effect on the degenerative changes that occurred in the pancreases of the rats as a result of induction with STZ, which has damaged the β-cell of the pancreas.
Representative light micrographs of Wistar rat Pancreas panoramic view across different groups showing the islet of Langerhans. KEY: Group CC1-Normal Rats fed with Basal diet + 100% WF bread without cocoa powder; Group CC2- Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% WF bread without cocoa powder; Group CC3-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 25 mg/kg/body weight Acarbose + 100% WF bread without cocoa powder; Group A-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% CF bread + cocoa powder; Group B-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 50% CF + 50% WF bread + cocoa powder; Group C-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 20% CF + 80% WF bread + cocoa powder; Group D-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 10% CF + 90% WF bread + cocoa powder; Group E-Diabetic Rats fed with High fat diet + 25 mg/kg/ body weight STZ + 100% WF bread + cocoa powder
Conclusion
The results of this study demonstrated strong antihyperglycemic and strong hypolipidemic effects in rats with Type-2 diabetes induced by HFD/STZ when yellow fleshed cassava flour was added to wheat flour during the composite bread production process. As functional foods, these goods may protect against life-threatening conditions like hyperlipidemia, oxidative stress, and other related issues. It is noteworthy to mention, though, that the combination of 100CF bread and FL exhibited the highest level of HDL cholesterol and antihyperglycemic effect. For the purpose of validating the impact of yellow fleshed cassava/wheat composite bread, more research is required from clinical trials.
Data Availability
The data that support the findings of this study are available upon reasonable request.
References
World Health Organization (WHO) (2017) WHO STEPS surveillance manuals, in The Two STEPwise approach to non-communicable disease risk factor surveillance
J.C. Mbanya, A.A. Motala, E. Sobngwi, F.K. Assah, S.T. Enoru, Lancet 375, 225–2266 (2010)
International Diabetes Federation (IDF), IDF Diabetes Atlas, 6th edn. (Brussels, 2013)
American Diabetes Association, Diab. Care 32(1), 562–567 (2009)
A. Quazi, M. Patwekar, F. Patwekar et al., Evid. Based Compl. Altern. Med. Pg. 7, 1577957 (2022)
M.M. Rahmaa, M.R. Islam, S. Sholag, M.E. Hossain, M.S. Rahaman, F. Islam, M. Ahmed, S. Mitra, M.U. Khandakar, A.M. Idris, Molecule. 27, 1713 (2022)
E.J. Barrett, Z. Liu, M. Khamaisi, G.L. King, R. Klein et al., J. Clin. Endocrin. Metabol. 102(12), 4343–4410 (2017)
J.-S. Yun, S.-H. Ko, J.-H. Kim, K.-W. Moon, Y.-M. Park, K.-D. Yoo, Y.-B. Ahn, Diabetes Metab. J.Metab. J. 37, 262–269 (2013)
M.M. Rahman, M.J. Uddin, A.S.M.A. Reza, A.M. Teraq, T.B. Emran, J. Simal-Gandara, Plants. 10, 729 (2021). https://doi.org/10.3390/plants10040729
I.B. Amor, D. Chandran, R. Amin, T.B. Emran, Ann. Med. Surg. 185, 2230–2231 (2023)
T.B. Emran, M.A. Kaque, P.R. Guntaka, L.P. Singh, M.W. Khan, A. Unnisa, M.H. Nafady, A.A. Kazi, K. Doukani, F. Nainu, Biointerface Res. Appl. Chem. 13, 6 (2023). https://doi.org/10.33263/BRIAAC136.588
M. Roden, G.I. Shulman, Nature 576, 51–60 (2019)
S. Chatterjee, K. Khunti, M.J. Davies, Lancet 389, 2239–2251 (2017)
R. Vieira, S.B. Souto, E. Sanchez-Lopez, A.L. Machado, P. Severino et al., J. Clin. Med.Clin. Med. 8, 1332 (2019). https://doi.org/10.3390/jcm8091332
Z. Bloomgarden, Atlas. J. Diabetes 8, 746–747 (2016)
H. Toru, F. Mutsunori, Y. Zemin, J. Biomed. Res.. Res. 33(1), 1–16 (2019). https://doi.org/10.7555/JBR.31.20160164
M.M. Rahman, M.R. Islam, S. Shohag, M.E. Hossain, M.S. Rahaman, F. Islam, M. Ahmed, S. Mitra, M.U. Khandaker, A.M. Idris, Molecules 27, 1713 (2022)
F. Islam, S. Mitra, M.H. Nafady, M.T. Rahman, V. Tirth, A. Akter, T.B. Emran, A.A. Mohamed, A. Algahtani, S.S. El-khohy, Evid. Based Compl. Altern. Med. Pg 10, 6144733 (2022)
U. Galicia-Garcia, A. Benito-Vicente, S. Jebari, A. Larrea-sebai, H. Siddiqi, C. Martin, Int. J. Mol. Sci.. J Mol. Sci 21(17), 6275 (2020). https://doi.org/10.3390/ijms2176275
A.Y. Cheng, I.G. Fantus, Can. Med. Assoc. J. 172(2), 213–226 (2005)
M.J. Davies, D.A. Dalessio, J. Fradkin, W.N. Kernan, C. Mathieu et al., Diabetes Care 41(12), 2669–2701 (2018)
H.A. Oboh, A.U. Osagie, A.O. Omotosho, Diadetol. Croat. 394(4), 113–138 (2010)
L. Wen, Y. Li, S. Li, X. Hu, Q. Wei, Z. Dong, Front. Med. (2021). https://doi.org/10.3389/fmed.2021.744122
C. Chourpiliadis, S.S. Mohiuddin. Biochemistry, StatPearls; National center for Biotechnology information. National Library of Medicine. (2022)
M.C. Petersen, G.I. Shulman, Physiol. Rev. 98(4), 2133–2223 (2018). https://doi.org/10.1152/physrev.00063.2017
C.F. Lewis, A.C. Carpentier, S. Pereira, M. Hahn, A. Giacca, Cell Metab. Metab. 33(4), 709–720 (2021). https://doi.org/10.1016/j.cmet2021.03.007
A.J. King, Br. J. Pharmacol.Pharmacol 166, 877–894 (2012)
C.P.D. Kottaisamy, D.S. Raj, V.P. Kumar, U. Sankaran, Lab. Anim. Res. 37, 23 (2012). https://doi.org/10.1186/s42826-021-00101-4
K. Hu, H. Huang, H. Li, Y. Wei, C. Yao, Nutrients 15(5), 1096 (2023). https://doi.org/10.3390/nu15051096
N.M. Alzamel, Pakistan J. Med. Health Sci. 16, 1085 (2022). https://doi.org/10.53350/pjmhs221621085
R. Jeetah, A. Bhaw-Luximon, D. Jhurry, J. Biomed. Nanotechnol.Nanotechnol. 10, 1810–1840 (2014). https://doi.org/10.1166/jbn.2014.1884
N. Dewan, G. Chowdhary, S. Pandit, P. Ahmed, D. Dasgupta, J. Pharmacogn. Phytochem. 5, 104–108 (2016)
A.K. Jugran, S. Rawat, H.P. Devkota, I.D. Bhatt, R.S. Rawal, Phyther. Res. 35, 223–245 (2021). https://doi.org/10.1002/ptr.6821
S. Anwar, S. Khan, A. Almatroudi, A.A. Khan, M.A. Alsahli, S.S. Almatroodi, A.H. Rahmani, Mol. Biol. Rep. 48, 787–805 (2021). https://doi.org/10.1007/s11033-020-06084-0
T.E. Eyinla, B. Maziya-Dixon, O.E. Alamu, R.A. Sanusi, Foods 8(5), 177 (2019). https://doi.org/10.3390/foods8050177
International Institute of Tropical Agriculture (IITA). (2017). Available online: http://www.iita.org/news-item/nigeria-releases-cassava-higher-pro-vitamin-fight-micronutient-deficiency. Accessed 27 Jan 2017
R.M. Omodamiro, E. Oti, H.A. Etudaiye, C. Egesi, B. Olasanmi, U.J. Ukpabi, Adv. Appl. Sci. Res. 3, 2566–2572 (2012)
E. Alos, J. MariaRodrigo, L. Zacarias, Carotenoids Nat. 79, 311–343 (2016)
N. Arlappa, N. Balakrishna, A. Laxmaiah, P. Raghu, V. Vikas Rao et al., Ann. Human Biol. 38(2), 131–136 (2016)
S.O. Oluwajoba, O. Malomo, O.A.B. Ogunmoyela, O.E.O. Dudu, A. Odeyemi, J. Microb. Biotech. Food Sci. 2(1), 42–68 (2012)
O.A. Olaoye, A.A. Onilude, Afr. J. Food Agric. Nutr. Devlop. 8(2), 192–203 (2012)
J.A. Ayo, V.A. Ayo, C. Popoola, M. Omosebi, C. Joseph, Afr. J. Food Sci. Technol. 5(1), 21–28 (2014)
S.A. Adefegha, G. Oboh, O.M. Adefegha, A.A. Boligon, M.L. Athayde, J. Sci. Food Agric. 94(13), 2726–2737 (2014)
H. Ali, P.J. Houghton, A. Soumyanath, J. Ethnopharmacol.Ethnopharmacol. 107, 449–455 (2006)
Y.M. Kim, Y.K. Jeong, M.H. Wang, E.T. Lee, H.I. Rhee, Nutrition 21, 756–761 (2005)
W.T. Friedewald, R.I. Levy, D.S. Fredrickson, Clin. Chem.. Chem. 18, 499–502 (1972)
M.F. Lopes-Virella, P. Stone, S. Ellis, J.A. Colwell, Clin. Chem.. Chem. 23, 265–270 (1977)
J.D. Bancroff, M. Gamble, Theory and practice of histological techniques, 4th edn. (Churchill Livingstone, 1996)
World Health Organization (WHO), Global Report on Diabetes, 88, (2016)
Diagnosis and classification of Diabetes Mellitus. Diabetes Care. 36(Supplement1), S67–S74 (2013)
Diagnosis and classification of Diabetes Mellitus. Diabetes Care. 36(Supplement_1), S62–S69 (2010)
Y. Tan, S.K.C. Chang, Y. Zhang, Food Chem. 214, 259–268 (2017)
E.J. Rossi, L. Sim, D.A. Kuntz, D. Hahn, B.D. Johnston et al., FEBS J. J. 273(12), 2673–2683 (2006)
R.A. Ajani, G. Oboh, S.A. Adefegha, A.A. Akindahunsi, K.E. Nwokocha, J. Food Proc. Preserv. 44(9), e14673 (2020)
R.A. Ajani, G. Oboh, S.A. Adefegha, A.A. Akindahunsi, Trop. J. Natural Product Res. 6(2), 227–235 (2022)
G. Oboh, A.J. Akinyemi, A.O. Ademiluyi, S.A. Adefegha, J. Food Nutr. Res. 49, 14–20 (2010)
G. Guiberti, A. Gallo, C. Cerioli, P. Fortunati, F. Masoero, Food Chem. Chem. 175, 43–49 (2015). https://doi.org/10.1016/j.foodchem.2014.11.127
M. Kaun, K.S. Sandi, Carbohydr. Polym.. Polym. 43, 263–268 (2010)
V. Mlotha, A.M. Nwangwela, W. Kasapila, E.V.P. Siyane, K. Masamba, Food Sci. Nutr. Sci. Nutr. 4, 322–328 (2015). https://doi.org/10.1002/fsn3.293
S.A. Sheweita, S. Mashaly, A.A. Newairy, H.M. Abdou, S.A. Eweda, Oxidative Medicine and Cellular longetivity. (2016) https://doi.org/10.1155/2016/5264064
T.B. Emran, M. Dutta, M.N. Uddin, A. Nath, M.Z. Uddin, Jahangirnagar Univ. J. Bio. Sci. 4(1), 51–59 (2015)
M. Prasathkumar, S. Anisha, A. Khusro, M.M. Essa, S.B. Chiadambaram, M.W. Qoronfleh, S. Sadhasivam, M.U.K. Sahibzaba, S. Alghamdi, M. Almehmadi, O. Abdulaziz, M.U. Khandaker, M.R.L. Faraque, T.B. Emran, Arabia J. Chem. 15, 104112 (2022)
M.S. Shahin, M.S. Talukder, A.M.K. Uddin, M.N. Hazan, S.A.J. Sayem G. Mostafa-Hedead et al. Evid. Based Compl. Altern. Med. Pg 15, (2023). https://doi.org/10.1155/2023/6359818
A. Jahangiri, Curr. Endocrinol. Diabetes Obes 17, 156–160 (2010)
E. Eren, N. Yilmaz, O. Aydin, Open Biochem. J.Biochem. J. 6, 78–93 (2012)
M. Aviram, M. Rosenblat, C.L. Bisgaier, R.S. Newton, S.L. Primoparmo, B.N.J. La-Du, J. Clin. Invest.Clin. Invest. 101, 1581–1590 (1998)
R.A. Ajani, S.A. Adefegha, G. Oboh, J. Food Meas. Charact. (2022). https://doi.org/10.1007/s11694-022-01600-1
F.N. Alsoodeeri, N.M. Alqubbani, J. Aldossari, Lipids (2020). https://doi.org/10.1155/2020/84699830
Acknowledgements
The authors wish to acknowledge the Functional Food and Nutraceutical Research Laboratory Unit of the Department of Biochemistry, Federal University of Technology, Akure, Ondo State, Nigeria for granting the opportunity to carry out this research in their Laboratory.
Funding
The authors wish to acknowledge the Tertiary Education Trust Fund for providing the grant for the project (Grant Number: TETFUND/ES/DR&D-CE/NRF2021/pmSETI/AFS/00405/01/VOL.1 for the accomplishments of this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ajani, R.A., Adefegha, S.A., Adekunle, A.I. et al. Antihyperglycemic and hypolipidemic effect of yellow-fleshed cassava/wheat flour composite bread in high fat/streptozotocin induced diabetic rats. Food Measure 18, 5971–5981 (2024). https://doi.org/10.1007/s11694-024-02622-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02622-7