Abstract
In this study a new wheat bread was designed whose sugars were replaced with S. rebaudiana Bertoni aqueous extract. The impact of the S. rebaudiana Bertoni aqueous extract on nutritional and sensory quality, its ability to reduce sugar intake and its antioxidant properties were investigated. Functional bread with 50 % of sugars replaced with S. rebaudiana extract was compared with traditional wheat bread. The extract demonstrated alpha amylase (IC50 = 198.40 μg/mL) glucosidase (596.77 μg/mL) inhibition. The radical scavenging activity exhibited an IC50 value of 335.94 mg/mL. In comparison with the control, the bread with stevia extract was softer and had lower microbial growth during the shelf-life study. The sensory test showed that the substitution of 50 % stevia extract was more acceptable when comparing with all quality characteristics. Regarding the nutritional contribution, the content of dietary fiber and digestible carbohydrates in the bread with stevia extract was higher and lower respectively, so caloric intake was significantly reduced. The results showed that the biological properties of S. rebaudiana extract were retained after the bread making process and that the proposed bread is suitable as functional food in human nutrition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a chronic disease caused by inherited and/or acquired deficiency in insulin secretion and/or by decreased responsiveness of the organs to secreted insulin (insulin resistance). One practical therapeutic approach to the management of diabetes is by controlling postprandial hyperglycemia. This can be achieved by the inhibition of carbohydrate hydrolysing enzymes such as α-amylase and α-glucosidase present in the gastrointestinal tract. It is well known that during the disease state of diabetes mellitus, α-glucosidase and α-amylase can be detrimental, due to the biochemical defect causing blood glucose levels to be elevated (Chen and Kang 2013). The inhibition of α-glucosidase and α-amylase can significantly reduce the postprandial increase of blood glucose and therefore can be an important strategy in the management of blood glucose level in Type 2 diabetic and borderline patients. The α-glucosidase inhibitor, acarbose, improves sensitivity to insulin and decreases postprandial hyperglycemia. However, the most frequent side effects observed with acarbose therapy are gastrointestinal symptoms (Chen and Kang 2013).
Oxidative stress is caused by an imbalance between the production of reactive oxygen and a biological system’s ability to readily detoxify the reactive intermediates or to easily repair the resulting damage. All forms of life maintain a reducing environment within their cells. This reducing environment is preserved by enzymes that maintain the reduced state through a constant input of metabolic energy. Disturbances in this normal redox state can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids and DNA. In humans, oxidative stress is involved in many diseases, such as diabetes mellitus (Sharma et al. 2012). Apart from hyperglycemia, increased oxidative stress is also reported to play a major role in the parthenogenesis of this disease. Oxidative stress occurs due to the glucose autoxidation and glycation of proteins, which thereby depletes the antioxidant defense system and thus promotes free radical generation (Sigh et al. 2013). Recently, much attention has been focused on dietary natural antioxidants capable of inhibiting reactive oxygen radical mediated oxidative stress (Ghanta et al. 2007).
Currently, there is renewed interest in plant-based medicines and functional foods modulating physiological effects in the prevention and cure of diabetes. S. rebaudiana Bertoni is one of the 950 genera of the Asteraceae family, native to the Amambay region in the north-east of Paraguay. It also occurs in the neighboring countries Brazil and Argentina. Today, its cultivation has spread to other regions of the world, including Canada and some parts of Asia and Europe (Lemus-Mondaca et al. 2012). S. rebaudiana is known as “calorie free bio sweetener of high quality” (Preethi et al. 2011). A recent scientific trial shows that this herb has many health benefits. Stevia contains phytochemical compounds that helps to reduce blood sugar, cholesterol and blood pressure. Stevia is used as a flavor enhancer, taste enhancer and for its antibacterial effect (Mishra 2011). It has thermal stability at high temperatures and can be widely used in jams, sauces, confections, beverages as well as dental products (Mishra 2011). Fifty grams of stevia leaf can replace 1000 g of sugar and the sweetness of stevioside is non-fermenting and it does not display browning while cooking (Mishra 2011).
Functional foods represent one of the most interesting areas of research and innovation in the food industry (Annunziata and Vecchio 2011). Recently, the consumer’s awareness of the need to eat superior quality and healthy foods known as functional foods, that is, foods that contain ingredients that provide additional health benefits beyond the basic nutritional requirements, has increased. Therefore, the tendency is to produce specialty breads made from whole-grain flour and other functional ingredients known as functional breads (Alam et al. 2013). Consumption of such functional breads not only improves the nutritional status of the general population but also helps those suffering from degenerative diseases associated with today’s changing lifestyles. Whole-wheat flour has been shown to be a rich source of functional ingredients, such as fiber, phytochemicals, minerals, essential amino acids, which are located in the bran, and of fat-soluble vitamins, which are contained in the germ of the whole-wheat grain (Jideani and Onwubali 2009).
Based on these considerations the objective of this study was to formulate functional breads with sugars replaced with S. rebaudiana Bertoni (var. Morita II) extracts and to evaluate the nutritional intake and the remaining biological activity.
Materials and methods
Raw material and chemicals
S. rebaudiana Bertoni variety Morita II was obtained from plots in the Yucatan State in Mexico. The crop was managed according to the production technology described by Ramírez et al. (2011). Samples were obtained from the first cut of the plot at an age of three months; samples consisted of branches of leaves and stems. All chemicals were reagent grade or better and purchased from Sigma Chemical Co. (St Louis, MO, USA).
Drying treatment and extract preparation
S. rebaudiana leaves were subjected to convection drying at 60 °C for 24 h. The vegetal material was distributed in aluminum trays and drying was stopped when here was no significant weight loss; this is when the vegetal material reached equilibrium in moist content. The leaves were milled in Wiley equipment (Model 4 Willey mill, Thomas Scientific, USA) to obtain particles of 1.0 mm in size. Samples were stored at room temperature in polyethylene bags until extract preparation. The aqueous extract of stevia was obtained according to Woelwer et al. (2010) with some modifications. Uniform particle-sized powder (500 mg) was suspended in 5 mL of water that had been warmed up in a water bath at 100 °C (Cole-Parmer, USA) for 30 min and subsequently centrifuged for 10 min at 2000×g at 10 °C (Z 300 K, Hermle Labortechnik, Germany). The supernatant was decanted and the solid residue was again added to 5 mL of water and the procedure was repeated two more times. The aqueous phase obtained from three extractions was decanted into a 25 mL volumetric flask and then filtered with membrane 0.45 μm.
Antidiabetic and antioxidant potential of stevia aqueous extract
In vitro alpha amylase inhibitory assay
The assay was carried out following the standard protocol with slight modifications (Dineshkumar et al. 2010). Starch (2 mg) was suspended in a tube containing 0.2 mL of 0.5 M Tris–HCl buffer (pH 6.9) with 0.01 M calcium chloride (substrate). The tube was boiled for 5 min and then preincubated at 37 °C for 5 min. Stevia aqueous extract (10 mg/mL) was dissolved with 1 mL of 0.1 % of dimethyl sulfoxide in order to obtain concentrations of 50, 100, 200, 400, 600, 800 and 1000 μg/mL. Then 0.2 mL of stevia aqueous extract of a particular concentration was put in the tube containing the substrate solution. Then, 0.1 mL of porcine pancreatic amylase in Tris–HCl buffer (2units/mL) was added to the tube containing stevia aqueous extract and starch. The process was carried out at 37 °C for 10 min. The reaction was stopped by adding 0.5 mL of 50 % acetic acid to each tube. The reaction mixture was then centrifuged at 2000×g for 5 min at 4 °C. The absorbance of the resulting supernatant was measured at 595 nm using a spectrophotometer. The assay was performed in triplicate. The concentration of the stevia aqueous extract required to inhibit 50 % of α amylase activity under the conditions was defined as the IC50 value. The α-amylase inhibitory activity was calculated as follows:
where, AC+, Ac−, As, Ab are defined as the absorbance of 100 % enzyme activity (only solvent with enzyme), 0 % enzyme activity (only solvent without enzyme), a test sample (with enzyme) and a blank (a test sample without enzyme), respectively.
In vitro alpha glucosidase inhibitory assay
The in vitro α glucosidase inhibitory assay was performed according to Dineshkumar et al. (2010). The α glucosidase (2 U/mL) was premixed with 20 μl of stevia aqueous extract at various concentrations (50, 100, 200, 400, 600, 800 and 1000 μg/mL) and incubated for 5 min at 37 ° C. Then 1 mM para-nitrophenyl gluco-pyranoside (pNPG) (20 μl) in 50 mM of phosphate buffer (pH 6.8) was added to initiate the reaction. The mixture was further incubated at 37 °C for 20 min. The reaction was terminated by the addition of 50 μL of 1 mM sodium carbonate and the final volume was made up to 150 μL. The α-glucosidase activity was determined spectrophotometrically at 405 nm on a Biorad Microplate reader by measuring the quantity of paranitrophenol released from pNPG. The assay was performed in triplicate. The concentration of the stevia extract required to inhibit 50 % of α glucosidase activity under the conditions was defined as the IC50 value.
Determination of total phenolic content
The phenolic compounds were determined using the Folin-Ciocalteu method, based on the reduction of phosphor-wolframate-phosphomolybdate complex by phenolics to a blue reaction product (Singleton and Rossi 1965). A volume of 0.2 mL from the extract was placed in a spectrophotometer plastic cell and 0.2 mL of Folin-Ciocalteu reagent were added and homogenized. Then 2 mL of distilled water were added and the cell was kept in the dark at room temperature for 1 h. Absorbance was measured at 765 nm. The data was calculated by a comparison between a standard curve (0–500 μg/mL of gallic acid) and the absorbance of each sample. The analysis was performed on three replicates. The total amount of phenolic compounds was determined in micrograms of gallic acid equivalents/mg of sample.
Determination of total flavonoid content
The flavonoid content was determined using the aluminum chloride method (Dewanto et al. 2002). Briefly, 0.5 mL from the ethanol extract was placed in a plastic cell for spectrophotometer, then 1.5 mL of ethanol 95 %, 0.1 mL of aluminum chloride (AlCl3) 10 %, 0.1 mL of potassium acetate 1.0 M and 2.8 mL of distilled water were added and the cell was kept in the dark at room temperature for 30 min. Absorbance was measured at 415 nm. The data was calculated by a comparison between a standard curve (0–100 μg/mL of quercetin) and the absorbance of each sample. The analysis was performed on three replicates. The total amount of flavonoid compounds was determined in micrograms of quercetin equivalents/mg of sample.
Determination of scavenging effect on DPPH radical
The scavenging effect on α,α-diphenyl- β-picrylhydrazyl (DPPH) free radical was measured as described by Brand-Williams et al. (1995). With some modifications, a sample solution (1.5 mL) with 20 mg was added to 1.5 mL of 0.1 mM DPPH in 95 % ethanol. The mixture was shaken and left for 30 min at room temperature. Absorbance was measured at 517 nm. Measurement was performed at least in triplicate. The percentage of the DPPH free radical was calculated using the following equation: DPPH scavenging effect (%) = (A0- A1)/ A0) × 100 where A0 was the absorbance of the control and A1 was the absorbance in the presence of the stevia extract. The IC50 (concentration providing 50 % inhibition) values were calculated using the dose inhibition curve in linear range by plotting the extract concentration versus the corresponding scavenging effect. Samples were tested in a range of concentrations from 0.5 to 3.0 mg/mL.
Bread containing stevia aqueous extract
Bread was prepared following a standard formulation (Czuchajowska et al. 1989) with partial replacement of sugar with stevia extract at three levels: 25, 50 and 75 %. The ingredients were mixed in a spiral mixer for 10 min. The dough was divided into 500 g pieces, which were stretched and rolled into a cylindrical shape for placement into molds. The baking was performed in a static oven for 45 min at 180 °C. After baking, the breads were placed on a clean grill until they reached room temperature. Finally, the breads were packed in polyethylene bags until their characterization.
Proximate composition
Proximate composition of bread partially replaced with stevia aqueous extract was determined using official AOAC procedures (AOAC 1997): nitrogen (method 954.01); fat (920.39); ash (923.03); fiber (962.09) and moisture (925.09). Protein content was calculated as nitrogen × 6.25, and carbohydrate content was estimated as nitrogen-free extract (NFE).
Texture changes in bread
Testing of bread texture and hardening were carried out according to the methods proposed by the AACC 74-09. The samples consisted of bread strips of 6 cm wide, 8 cm long and 2.5 cm ± 1 mm thick. The samples were subjected to a uniaxial compression between two parallel plates in a universal test machine Instron, using a test tube with a diameter of 34 mm, a load cell of 500 N with an extension range of 8 mm, an endpoint of 6 mm and 25 mm test speed test/min (AACC 2000).
Microbiological analysis and water activity
Breads were stored in bags at room temperature and microbiological analysis pursuant to the Mexican standard NOM-247-SSA1-2008 was performed. The parameters evaluated were: total aerobic bacteria counts, yeast and mold counts, coliforms, and water activity.
Sensory evaluation
Products with a 25, 50 and 75 % incorporation level of stevia aqueous extract underwent a sensory evaluation using a discrimination test of multiple comparisons with three descriptor points regarding taste. The best-qualified product was tested with an affective test of a satisfaction grade using a structured hedonic scale with seven descriptive points, which is pointed out like feature. A panel of 80 untrained judges carried out a sensory evaluation. A 7-point category scale evaluated the sensory properties of bread samples. (7 = I like it very much, 6 = I like it, 5 = I like it a little, 4 = I neither like nor dislike it, 3 = I dislike it a little, 2 = I dislike it, 1 = I dislike it a lot) (Torricella et al. 1989).
Nutritional contribution
Total (TDF), insoluble (IDF) and soluble (SDF) dietary fiber
This parameter was determined by the bread with the highest score in the affective test with the gravimetric enzymatic method proposed by Prosky et al. (1998). Briefly, 1 g of sample was weighed into each of four flasks, and 50 mL of 0.05 N phosphate buffer at pH 6 were added to each. The flasks were then placed in a Galena bath at 100 °C, with 0.1 mL thermostable α-amylase enzyme (Sigma A-3306) added to each and then they were agitated at 60 rpm for 15 min. After cooling, pH was adjusted to 7.5. The flasks were returned to the bath at 60 °C, 0.1 mL protease (Sigma P-3910) was added to each and then they were agitated at 60 rpm for 30 min. After cooling, pH was adjusted to 4.0. The flasks were again placed in the bath at 60 °C, with 0.3 mL amyloglucosidase (Sigma A-9913) being added and then they were agitated for 30 min. Finally, 95 g/kg ethanol, preheated to 60 °C, were added at a 1:4 (v/v) ratio. In a vacuum, the flask content was filtered into crucibles containing celite (Sigma C-8656). The residue remaining in the flask was washed three times with 20 mL of 780 g/kg ethanol, twice with 10 mL of 950 g/kg ethanol and twice with 10 mL acetone. Crucible content was dried at 105 °C. Protein (N × 6.25) was determined for the residue in two crucibles and the residue in the remaining two was burned at 550 °C for 4 h.
Insoluble dietary fiber (IDF) was determined following the method of Prosky et al. (1998). This is similar to that for total dietary fiber (TDF), except that addition of 95 % alcohol at 1:4 is omitted.
Soluble dietary fiber (SDF) was calculated according to Prosky et al. (1998), by the difference between TDF and insoluble dietary fiber: SDF = TDF - IDF.
Total and digestible carbohydrates were calculated by the difference:
-
Total carbohydrate: 100 – (moisture + crude fiber + protein + fat + ash).
-
Digestible carbohydrate: 100 – (moisture + protein + fat + ash + FDT).
-
Energy was calculated by the Atwater system using 4.0 kcal/g for carbohydrates.
Biological potential of bread containing stevia aqueous extract
For antidiabetic and antioxidant activity analyses, the bread with the highest score in the affective test was sliced, milled and processed according to Dineshkumar et al. (2010) and Shimada et al. (1992) respectively, to determine in vitro alpha amylase and alpha glucosidase inhibitory assay as well as scavenging effect on DPPH radical.
Statistical analysis
All results were analyzed using descriptive statistics with central tendency and dispersion measures. One-way ANOVAs were run to evaluate the physical characteristics, nutritional contribution, sensorial acceptance, and biological capacities of the products. An LSD multiple-range test was used to determine differences between treatments. All analyses were done according to Montgomery (Montgomery 2004) and processed with the Statgraphics Plus version 5.1 software.
Results and discussion
In vitro alpha amylase and alpha glucosidase inhibitory assays
In the present study the leaf extract of S. rebaudiana was found to possess both favorable alpha amylase and alpha glucosidase inhibitory effects on starch and paranitrophenyl gluco-pyranoside breakdown in vitro. There was a dose dependent increase in percentage inhibitory activity against both enzymes. In the concentration range investigated, the extract demonstrated alpha amylase and alpha glucosidase inhibition increased linearly with concentration (50–1000 μg/mL). The maximum values of inhibition were achieved at a concentration of 1000 μg/mL (Fig. 1). The IC50 value for alpha amylase inhibition was 198.40 μg/mL. This value is higher than reported for extracts of Cinnamomum zeylanicum (130.55 μg/mL), Artocarpus altilis (118.88 μg/mL), Piper betel (84.63 μg/mL) and Artocarpus heterophyllus (70.58 μg/mL) (Nair et al. 2012). The IC50 value for alpha glucosidase inhibition was 596.77 μg/mL. Nair et al. (2012) reported IC50 values of 129.85, 76.90, 140.01 and 96.56 μg/mL for extracts of A. altilis, A. heterophyllus, C. zeylanicum and Piper betel respectively. The IC50 value obtained for S. rebaudina was higher. Generally, more S. rebaudina extract is required to inhibit both enzymes. The S. rebaudiana extract produced a slightly weak alpha glucosidase enzyme inhibition when compared with alpha amylase. Both values were found to be seven times higher and twenty times higher, respectively, than the IC50 value for acarbose (29.59 μg/mL).
The purpose of an antidiabetic therapy in insulin-dependent patients (Type 1 diabetes) and insulin-nondependent patients (Type 2 diabetes) is to achieve normoglycemia and reduce insulin resistance with the intention of improving metabolic control and prevent future diabetic patient complications (Önal et al. 2005). An effective strategy for the management of Type 2 diabetes is the inhibition of the two enzymes, alpha amylase and alpha glucosidase (Krentz and Bailey 2005), in order to slow the absorption of carbohydrates, thus moderating the postprandial rise of blood sugar and minimizing the effects of diet on hyperglycemia (Bischoff 1994). Excessive inhibition of alpha amylase due to alpha glucosidase inhibitors such as acarbose carries certain side effects (bloating, flatulence, meteorism and diarrhea) as a result of an abnormal bacterial fermentation of undigested carbohydrates in the colon. According to Kwon et al. (2007), alpha amylase and alpha glucosidase inhibitors derived from plants have mild inhibitory activity against alpha amylase and strong inhibitory activity against alpha glucosidase, which indicates that they can actually be used in therapy for postprandial hyperglycemia with reduced side effects. This research demonstrates that the aqueous extracts of S. rebaudiana have significant inhibitory activity against these enzymes and may be promising candidates for the treatment of both types of diabetes.
Determination of total phenolic content, total flavonoid content, and scavenging effect on DPPH radical
The total phenolic content of S. rebaudiana aqueous extract was determined using the Folin-Ciocalteu method and reported as gallic acid equivalents by reference to standard curve. In the present study, the phenolic content of the extract was 28.4 mg Gallic acid equivalents/g. The total flavonoid content of S. rebaudiana extracts was determined using the aluminum chloride method, and the total amount of flavonoid compounds was 36.7 mg Quercetin equivalents/g. Plants extracts may essentially contain bioactive compounds with ability to inhibit enzymes, act as antioxidants, antimicrobial, anti-inflammatory, etc. Bioactive components could be phenols or flavonoids as literature shows a clear link between polyphenols and bioactivity of herbal extracts (John et al. 2011). Kähkönen et al. (1999) evaluated the antioxidative activity of a total of 92 phenolic extracts from edible and nonedible plant materials (berries, fruits, vegetables, herbs, cereals, tree materials, plant sprouts, and seeds). Among edible plant materials, remarkable high antioxidant activity and high total phenolic content (Gallic acid equivalents > 20 mg/g) were found in berries, especially aronia and crowberry. Apple extracts (two varieties) showed also strong antioxidant activity even though the total phenolic contents were low (Gallic acid equivalents < 12.1 mg/g). S. rebaudiana extract had a higher total phenolic content so its antioxidant activity could be greater than extracts reported by Kähkönen et al. (1999). Figure 2 shows the free radical scavenging activity of the S. rebaudiana extract at different concentrations. The radical scavenging activity of S. rebaudiana extract increased with increasing concentrations (25–625 mg/mL). The IC50 value was found to be 335.94 μg/mL. This behavior is similar to that reported by Criado et al. (2013) which evaluated the effect of different concentrations of S. rebaudiana Bertoni (stevia) on polyphenoloxidase and peroxidase activities, antioxidant capacity and other bioactive compounds of a mixture of fruits, during 10 days of incubation at 10 and 37 °C, was studied. According to these authors, samples with a high stevia concentration showed the highest total phenolic content and antioxidant capacity.
The phenolic compounds present in herbs and spices have been reported to show natural antioxidant activity (Rao et al. 2014). The predominant mode of antioxidant activity of phenolic compounds is believed to be a radical scavenging via hydrogen donation. Other radical-quenching mechanisms are through electron donation and singlet oxygen quenching. Substituents on the aromatic ring affect the stabilization and therefore affect the radical-quenching ability of these compounds (Reyes-Carmona et al. 2005). Meanwhile the protective effects of flavonoids in biological systems are ascribed to their capacity to transfer electrons to free radicals, chelate metal catalysts, activate antioxidant enzymes, reduce alpha-tocopherol radicals and inhibit oxidases (Hirano et al. 2001). These results indicate that S. rebaudiana extract exhibited the ability to quench the DPPH radical, which indicates that extract was a good antioxidant with radical scavenging activity.
Proximate composition
Control and functional breads with sucrose partially replaced with stevia aqueous extract were analyzed for proximate composition and the data are presented in Table 1. Control contained 25.15 % moisture, 10.51 % protein, 2.03 % crude fiber, 16.29 % crude fat, 7.19 % ash and 63.98 % nitrogen free extract (NFE). Breads partially replaced with stevia aqueous extract were richer in protein and crude fat contents compared to control. The carbohydrate content and energy values were highest in control (63.98 % and 255.92 Kcal) and lowest in bread sucrose partially replaced with 75 % of stevia extract (54.92 % and 219.68 Kcal), respectively. The low carbohydrate and energy values were as a result of the replaced sucrose content of the composite breads. Bread produced with 0 % of stevia extract had the highest caloric value while that produced with 75 % of stevia extract had the least caloric value. The breads containing energy values in the range of 219.68 to 255.92 Kcal, and therefore fit the (FAO / WHO 1994) recommended minimum energy content of 399 Kcal / 100 g.
Texture changes in bread
Textural results of the bread with different levels of stevia extract are presented in Fig. 3. The firmness of the bread was measured using a universal test machine, Instron. The data show the force needed for deformation of the bread slices. The lower the value in force needed to deform the slices, the softer the bread. The results of the study showthat the addition of 75 % stevia extract increased the texture value and reduced the softness of bread significantly. The added substances interact in different ways with the gluten and in many cases affect in a negative manner the structure of the gluten network (Ognean et al. 2006). In contrast, the addition of 50 % stevia extract reduced the texture value and increased the softness of the bread significantly.
In fermented breads, sugar affects the volume of dough by speeding up the fermentation process, this gives the bread a more porous structure and a softer crumb. However, in non-fermented breads such as biscuits, large quantities of sugar can be added, because this improves the keeping quality of the products as well as sweetening them. This explains why the substitution of 25 and 50 % of sugar does not adversely affect the softness of the bread. According to Schleißinger et al. (2013) fiber components could interact with water and increase firmness of bread. In the present study bread with 75 % of sugar replacement exhibited the higher water content and the higher texture.
Microbiological analysis
The results of the microbial analysis of the bread samples are presented in Table 2. The microbial analysis carried out during the six days of the shelf-life study indicates that the total aerobic bacteria counts in the breads partially replaced with stevia extract range from 1.0 × 102 to 2.7 × 102 cfu/g. While the highest aerobic bacteria counts (3.9 × 102 cfu/g) were observed in the control bread sample, on the sixth day of storage. The same behavior was observed for total mold and yeasts counts. There were no observable coliforms in the samples during the six days of the shelf study.
During the six days of the study, it was observed that the values of water activity were reduced, however they can still support the growth of microorganisms. The lower microbial growth in partially replaced breads with stevia extract may be due to reduced sugar content. Stevia extract, unlike sugars, are not readily nutritionally utilized by microbes. According to Preethi et al. (2011) Stevia rebaudiana Bertoni extract has the ability to inhibit the growth of certain bacteria. In this sense, Belda-Galbis et al. (2014) used the modified Gompertz equation to assess the leaf crude extract of S. rebaudiana Bertoni antilisterial kinetics. According to these authors, leaf crude extract of stevia could be a bacterial growth control. Due to the high content of various phytoconstituents such as phenols, phenolic acids and flavonoids, stevia extracts have showed significant antimicrobial, anti-hypertensive, anti-tumour, anti-inflammatory, hepatoprotective and immunomodulatory activities both in vitro and in animal studies (Gupta et al. 2013).
Sensory evaluation
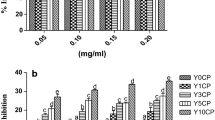
Results of the sensory evaluation of bread samples containing different levels of the stevia extract substitution as compared to the control are shown in Fig. 4. The first evaluation consists of a multiple comparisons test with three descriptor points concerning the taste (sweetness). In general it was observed that as the level the stevia extract replacement increased, judges rated the product as less sweet than the control (Fig. 4a). The stevia extract not only contains glycosides but also chlorophyll pigments, secondary metabolites and plant residues. These other components could contribute to reducing the sweetness of the bread.
According to the ratings, the bread with 50 % of sugar replacement was selected for the general acceptability test. The bread with 50 % replacement was well accepted by the judges since most of the ratings (80.40 %) were above the average level of acceptance with 51.25 % of scores for Like a little, 26.25 % for Like and 2.5 % for Like very much.
Nutritional contribution
Nutrient composition of the selected bread showed that composite bread formulated using stevia extract in place of 50 %) showed significantly higher total dietary, insoluble and soluble fiber than the control bread (Table 3). The control bread contained significantly higher levels of total and digestible carbohydrate. The caloric values of the breads were calculated. A reduction for the caloric value was obtained for bread which had stevia extract added. This reduction is due to the reduced sugar content in the bread with stevia extract.
Consumption of bread supplemented with stevia extract would have less impact on the glycemic index given its lower content of total and digestible carbohydrates, and its high content of dietary fiber. A low glycemic index provides multiple health benefits for the organism, such as losing weight, increasing the organism’s sensitivity to insulin, reducing blood cholesterol levels, reducing the risk of heart disease, reducing hunger, and refueling carbohydrate stores after exercise.
Biological potential of bread containing stevia aqueous extract
In vitro alpha amylase and alpha glucosidase inhibitory activity
With the aim of evaluate the effect of stevia extract in the reduction of glucose absorption, enzymatic assays were performed. As a monosaccharide, glucose can be readily absorbed from the gastrointestinal tract into the bloodstream after the hydrolysis of glycosidic bonds in digestible carbohydrate foods containing starch, by α-amylase and α-glucosidase enzymes. Inhibition of these enzymes could reduce the high postprandial blood glucose peaks in diabetics (Cirillo et al. 2011). These enzymes have been recognized as therapeutic targets for the modulation of postprandial hyperglycemia. Postprandial hyperglycemia is the earliest metabolic abnormality to occur in Type 2 diabetes mellitus. Indeed, literature studies, suggested that α-Amylase and α-Glucosidase inhibitors could lengthen the duration time of carbohydrate absorption and flatten the concentration of the blood glucose curve over time (Puoci et al. 2013). The inhibitory activity of bread with stevia extract substitution (50 %) was evaluated according to the literature tests by employing, in separate experiments, the bread samples as substrates of the enzymatic reactions. The results show the efficiency of the bread in reducing polysaccharide digestion and, thus, glucose absorption. Furthermore, the inhibitory activity towards amylase (IC50 = 521.75 μg/mL) was found to be lower than that against glucosidase (IC50 = 202.74 μg/mL).
Scavenging effect on DPPH radical
In order to evaluate the free radical scavenging properties of bread samples, their reactivity towards a stable free radical, such as DPPH, was evaluated. The scavenger ability of the bread sample was evaluated in terms of radical reduction, and data are expressed as IC50 (μg/mL). The bread with stevia extract was found to have scavenging properties towards the selected radical (IC50 = 51.98 mg/mL). The addition of phenols-rich materials with wheat bread is an effective technique to improve the antioxidant potential of the final product (Swieca et al. 2013). It has been found that the antioxidant potential in bakery products is strongly dependent on manufacturing conditions and recipes (Han and Koh 2011). According to Dziki et al. (2014) antioxidant activity and free phenolic acid levels were reduced by mixing, but recovered after fermentation and baking. This would explain the observed reduction in the antioxidant capacity of the bread.
Conclusions
In this work S. rebaudiana Bertoni aqueous extract was incorporated into the preparation of functional breads with improved biological properties. Three different bread compositions were characterized for nutritional contribution, quality and sensory properties, and tested by specific in vitro assays to determine the antioxidant activity, while the ability to reduce the sugar intake was highlighted by performing enzymatic assays using α-amylase and α-glucosidase. The results showed that the biological properties of S. rebaudiana Bertoni aqueous extract were retained after the bread making process and that the proposed bread is suitable as functional food in human nutrition.
References
Alam J, Talukder MU, Rahman MN, Prodhan UK, Huq AKO (2013) Evaluation of the nutritional and sensory quality of functional breads prepared from whole wheat and soybean flour. Annals Food Sci Technol 14(2):171–175
American Association of Cereal Chemistis-AACC (2000) Approved methods of analysis of the AACC. 10. ed. St. Paul
Annunziata A, Vecchio R (2011) Functional foods development in the European market: a concumer perspective. J Func Foods 3:223–228
Association of Official Analytical Chemists-AOAC (1997) Official Methods of Analysis, AOAC, Arlington, VA, USA. Secs. 920.39, 923.03, 925.09, 954.01, 962.09, 992.16
Belda-Galbis CM, Pina-Pérez MC, Espinosa J, Marco-Celdrán A, Martínez A, Rodrigo D (2014) Use of the modified Gompertz equation to assess the Stevia rebaudiana Bertoni antilisterial kinetics. Food Microbiol 38:56–61
Bischoff H (1994) Pharmacology of alpha-glucosidase inhibition. Eur J Clin Invest 24:3–10
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technology 28:25–30
Chen L, Kang YH (2013) In vitro inhibitory effect of oriental melon (Cucumis melo L. var. mauka Makino) seed on key enzyme linked to type 2 diabetes. J Func Foods 5:981–986
Cirillo G, Puoci F, Iemma F, Curcio M, Parisi OI, Spizzirri UG, Altimari I, Picci N (2011) Starch-quercetin conjugate by radical grafting: synthesis and biological characterization. Pharm Develop Technol 17(4):466–476
Criado MN, Barba FJ, Frígola A, Rodrigo D (2013) Effect of Stevia rebaudiana on oxidative enzyme activity and its correlation with antioxidant capacity and bioactive compounds. Food Bioprocess Technol 7(5):1518–1525
Czuchajowska Z, Pomeranz Y, Jeffers HC (1989) Water activity and moisture content of dough and bread. Cereal Chem 66:128–132
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Dineshkumar B, Mitra A, Manjunatha M (2010) Studies on the anti-diabetic and hypolipidemic potentials of mangiferin (Xanthone glucoside) in streptozotocin-induced type 1 and type 2 diabetic model rats. Int J Advan Pharm Sci 1:75–85
Dziki D, Rozylo R, Gawlik-Dziki U, Swieca M (2014) Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci Technol 40:48–61
FAO⁄WHO⁄UNU Expert Consultation (1994) Food Nutrients Requirements, Report of a Joint FAO⁄WHO⁄UNU Expert Consultation. World Health Organization Technical Report Series. 724. Geneva: WHO
Ghanta S, Banerjee A, Poddar A, Chattopadhyay S (2007) Oxidative DNA damage preventive activity and antioxidant potential of Stevia rebaudiana (Bertoni), a natural Sweetener. J Agric Food Chem 55:10962–10967
Gupta E, Purwar S, Sandaram S, Gai GK (2013) Nutritional and therapeutic values of Stevia rebaudiana: a review. J Med Plants Res 7:3343–3353
Han H-M, Koh B-K (2011) Antioxidant activity of hard wheat flour, dough and bread prepared using various processes with the addition of different phenolic acids. J Sci Food Agric 91:604–608
Hirano R, Sasamoto W, Matsumoto A, Itakura H, Igarashi O, Kondo K (2001) Antioxidant ability of various flavonoids against DPPH radicals and LDL oxidation. J Nut Sci Vitaminol 47:357–362
Jideani V, Onwubali F (2009) Optimisation of wheat-sprouted soybean flour bread using response surface methodology. Afr J Biotechnol 8:6364–6373
John KMM, Rajesh J, Mandal AKA, Natarajan S (2011) Antioxidant and antimicrobial activity of individual catechin molecules: a comparative study between gallated and epimerized catechin molecules. Eur J Exp Biol 1(3):145–153
Kähkönen MP, Hopia AI, Vuorela HJ, Rauha J-P, Pihlaja K, Kujala TS, Heinonen M (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 47:3954–3962
Krentz A, Bailey C (2005) Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 65:385–411
Kwon Y, Apostolidis E, Shetty K (2007) Evaluation of pepper (Capsicum annuum) for management of diabetes and hypertension. J Food Biochem 31:370–385
Lemus-Mondaca R, Vega-Gálvez A, Zura-Bravo L, Ah-Hen K (2012) Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: a comprehensive review on the bichemical, nutritional and functional aspects. Food Chem 132:1121–1132
Mishra N (2011) An Analysis of antidiabetic activity of Stevia rebaudiana extract on diabetic patient. J Nat Sci Res 1(3):1–9
Montgomery D (2004) Diseño y análisis de experimentos. Limusa-Wiley, México
Nair SS, Kavrekar V, Mishra A (2012) In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur J Exp Biol 3(1):128–132
NORMA Oficial Mexicana NOM-247-SSA1-2008, Productos y servicios. Cereales y sus productos. Cereales, harinas de cereales, sémolas o semolinas. Alimentos a base de: cereales, semillas comestibles, de harinas, sémolas o semolinas o sus mezclas. Productos de panificación. Disposiciones y especificaciones sanitarias y nutrimentales. Métodos de prueba
Ognean M, Darie N, Ognean CF (2006) Studies about obtaining low calorie and high fiber content bakery product using wheat bran. Acta Universitatis Cibiniensis Seria F Chemia 9(1):55–66
Önal S, Timmur S, Okuttucu B, Zihnioglu F (2005) Inhibition of alpha-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep Biochem Biotechnol 35:29–36
Preethi D, Sridhar TM, Josthna P, Naidu CV (2011) Studies on antibacterial activity, phytochemical analysis of Stevia rebaudiana (Bert.). An important calorie free biosweetner. J Ecobiotechnol 3(7):5–10
Prosky L, Asp N, Schweitzer T, Debris S, Furda I (1998) Determination of insoluble, soluble and total dietary fiber in food and food products: interlaboratory study. J AOAC 71:1017–1023
Puoci F, Malanchin R, Piangiolino C, Restuccia D, Curcio M, Parisi OI, Cirillo G, Picci N (2013) Maca flour: a powerful ingredient for functionally enhanced bread. Int Food Res J 20(3):1293–1300
Ramírez JG, Avilés BW, Moguel OY, Góngora GS, May LC (2011) Estevia (Stevia rebaudiana, Bertoni), un cultivo con potencial productivo en México. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Centro de Investigación Regional sureste
Rao GN, Rao PP, Balaswamy K, Satyanarayana A (2014) Antioxidant activity of Stevia (Stevia rebaudiana L.) leaf powder and a commercial stevioside powder. J Food Pharm Sci 2:32–38
Reyes-Carmona J, Yousef GG, Martinez-Peniche RA, Lila MA (2005) Antioxidant capacity of fruit extracts of blackberry (Rubus sp.) produced in different climatic regions. J Food Sci 70:497–503
Schleißinger M, Meyer AL, Afsar N, György Nagy Á, Dieker V, Schmitt JJ (2013) Impact of dietary fibers on moisture and crumb firmness of brown bread. Advan J Food Sci Technol 5(10):1281–1284
Sharma R, Yadav R, Manivannan E (2012) Study of effect of Stevia rebaudiana bertoni on oxidative stress in type-2 diabetic rat models. Biomed Aging Pathol 2:126–131
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autioxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Sigh S, Garg V, Yadav D (2013) Antihyperglucemic and antioxidative ability of Stevia rebaudiana (Bertoni) leaves in diabetes induced mice. Int J Pharm Sci 5(2):297–302
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158
Swieca M, Gawlik-Dziki U, Dziki D, Baraniak B, Czyz J (2013) The influence of protein-flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem 141:451–458
Torricella MR, Zamora UE, Pulido AH (1989) Evaluación sensorial aplicada al desarrollo de la calidad en la industria alimentaria. Instituto de Investigaciones para la Industria Alimenticia. La Habana, Cuba
Woelwer RU, Lankes C, Wawrzun A, Wüst M (2010) Improved HPLC method for evaluation of the major steviol glycosides in leaves of Stevia rebaudiana. Eur Food Res Technol 231:581–588
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz-Ruiz, J.C., Moguel-Ordoñez, Y.B., Matus-Basto, A.J. et al. Antidiabetic and antioxidant activity of Stevia rebaudiana extracts (Var. Morita) and their incorporation into a potential functional bread. J Food Sci Technol 52, 7894–7903 (2015). https://doi.org/10.1007/s13197-015-1883-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1883-3