Abstract
Purpose of Review
To describe several effective imaging-guided, minimally invasive treatments to relieve cancer-associated pain in oncologic patients. Clinical applications, technical considerations, and current controversies are addressed.
Recent Findings
The great variability in tumor subtype, location, and growth rates dictate the necessity for a tailored treatment approach. While opioids and radiotherapy may provide adequate relief for some patients, alternative minimally invasive procedures may augment theses more traditional treatments or even provide superior palliative relief. Recent image-guided percutaneous techniques applied to reduce cancer-associated pain and minimize opioid dependence include neurolysis, ablation, high intensity focused ultrasound, and bone consolidation. Each technique treats cancer pain in a unique method.
Summary
Minimally invasive interventional radiology techniques can provide effective and lasting pain palliation for cancer patients through both indirect and direct effects. Selection among treatments techniques should be based upon an individually tailored approach, to include consideration of all treatment modalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer-associated pain can be a source of significant emotional and physical burden that can affect quality of life and daily activities. While it is estimated that more than half of oncologic patients develop pain regardless of cancer type and stage, prevalence of cancer-associated pain is greater in patients with advanced disease stages and with certain tumor types including head and neck, lung, and breast cancers [1, 2]. Pain may be a result of direct tissue damage (nociceptive) or a result of dysfunction or damage to the nervous system (neuropathic). Despite the burden on quality of life, the majority of symptomatic patients (56% to 82.3%) remain undertreated [1], which may in part be related to access limitations for effective treatment options.
The traditional therapies for cancer pain can be generalized as conservative analgesic therapy [provided according to the World Health Organization (WHO) scale of analgesics], surgery, and radiation therapy. Each categorical treatment possesses strengths and weaknesses. Opioid administration requires the least amount of technical expertise and is effective for generalized pain relief. Limitations to opioid treatment include financial and public health concerns related to refractory pain relief requiring frequent medication usage and potential opioid dependence, systemic side effects that affect daily activities and mentation, and absence of treatment for the underlying pain stimulus [3]. Surgical resection of a tumor-causing pain may provide a more tailored approach to painful stimulus; however, cancer patients may present with contraindications to resection that include age-related co-morbidities, advanced tumor stage precluding major interventions, and potential for surgery to delay systemic chemotherapy or immunotherapy. Radiation therapy provides a minimally invasive localized treatment option that may be very appealing for patients and providers; however, limitations include radiation dose constraints due to concern for iatrogenic induced sarcoma and blood born cancers, skin and muscular damage resulting in new pain, and overall moderate pain response that can be variable depending on tumor type, size, and location, and subtype [4].
Over the last few decades, a new, broad category of palliative care therapies has been introduced into the cancer pain management armamentarium. These minimally invasive techniques are made possible by advancements in imaging techniques, the development of low-profile technologies, and are utilized by interventional radiology practitioners who are skilled at performing the gamut of image-guided interventions. Each technique applies highly advanced imaging (e.g., computed tomography, magnetic resonance imaging) to guide treatment to directly or indirectly target the tumor that results in the painful stimulus. Each technique described herein (e.g., neurolysis, ablation, high intensity focused ultrasound, and bone consolidation) treats cancer pain in a unique method by destroying tumor stimuli, limiting stimuli from regional nerves, or stabilizing osseous structures to overcome mechanical pain [5, 6]. Limitations for these techniques include poor patient access, due to high-level specialization and procedural skill required, a limited overall awareness of these treatment strategies, and an unclear understanding by oncologists with regards to the how effective these therapies can be in treating cancer pain.
The purpose of this article is to describe several imaging-guided, minimally invasive techniques that are performed by interventional radiologists for pain relief in symptomatic oncologic patients. Clinical applications, technical considerations, and current controversies are reviewed.
Percutaneous Neurolysis
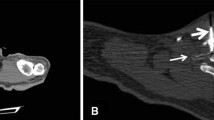
Percutaneous neurolysis (PN) is a pain alleviation technique that aims to interrupt the pain signal transmission by the autonomous nervous system, specifically the sympathetic plexus [7•]. In cancer patients, the primary indication is to control visceral pain that is refractory to opioid therapy to improve quality of life and decrease opioid dependence [8]. The treatments can be applied for symptoms related to primary and metastatic tumor extension through visceral organs, or for pain that has been associated as a side effect of systemic therapies [9,10,11]. The most common location for treatment is the celiac plexus and splanchnic nerves [12], with current guidelines to support the procedure for tumor invasion into the bowel and visceral organs including the pancreas [13]. The palliative pain relief is achieved through the targeted degeneration of the sympathetic nerve fibers and ganglia via percutaneous thermal ablation or percutaneous injection of a neurolytic chemical agent (Fig. 1). Both chemical and thermal ablation techniques are performed by the image-guided advancement of small caliber needles to the sympathetic ganglia.
a Potential levels of percutaneous neurolysis for cancer pain alleviation. b, c A 69-year-old female patient with pancreatic cancer treated with splanchnic nerve neurolysis for pain reduction; radiofrequency electrodes were bilaterally placed under computed tomography anterolateraly to T11 vertebral body
Chemical Neurolysis
Chemical neurolysis is performed through the image-guided advancement of a 21 or 22 Gauge needle to the sympathetic chain. Overall, consistent results can be expected regardless of needle approach, which include anterior trans-abdominal versus posterior paravertebral, and unilateral versus bilateral blocks. Once the needle is in place, gentle syringe aspiration and small volume contrast injection can confirm an extravascular needle tip position. An injection of anesthetic can further validate needle position, as well as decrease peri-procedural pain. Immediate chemical neurolysis is then achieved by injection of a small volume of absolute alcohol. Injection volume is typically between 10 and 20 mL [10]. After subsidence of immediate procedural pain from chemical neurolysis, a significant pain reduction can be expected in 70–90% of patients [11].
Thermal Neurolysis
Thermal ablation has been recently advocated as an effective alternative to chemical neurolysis. The low-profile ablation needle is advanced under image guidance to the sympathetic ganglion, and the tip is then activated to apply a predetermined zone of cell destruction by either heat-based technology (radiofrequency or microwave energy) or the creation of a therapeutic ice ball (cryoablation). These technologies may be particularly appealing over chemical neurolysis when tumor bulk prevents diffusion of liquid chemical agents, or in cases refractory to chemical neurolysis. A randomized clinical trial comparing bilateral splanchnic nerve radiofrequency ablation at T10-T11 levels versus bilateral ethanol neurolysis at a single T11 level favored thermal ablation, which provided more immediate results in a higher proportion of patients with greater durability [14].
Technical Considerations
Regardless of whether chemical or thermal ablation neurolysis is applied, the cornerstone of effective neurolysis is image guidance. During pre-procedure work up, a careful evaluation of cross-sectional imaging will identify visceral tumor involvement and exclude procedural contraindications or negative predictive factors such as bowel obstruction and vascular invasion. During the procedure, the proceduralist relies on high-quality imaging to precisely guide the treatment needle to the targeted location. Historically, neurolysis has been performed under fluoroscopic guidance; however, the availability and access to CT and fluoroscopic cone beam CT have advanced cross-sectional imaging as the gold standard for both chemical neurolysis and ablation [10]. Cross-sectional imaging provides greater contrast, spatial, and anatomical resolution than standard fluoroscopy to improve needle placement and ensure optimal clinical outcome.
Successful application of neurolysis requires timely diagnosis and a conscientious approach to pain management. Early diagnosis and referral for treatment are important to improve palliative efficacy, which is less beneficial in end-stage disease [15]. Patients should be carefully evaluated for baseline pain. Depending on clinical assessment, general anesthesia may be arranged during the procedure to ensure patient comfort throughout the procedure and immediate post-procedure recovery, as neurolysis can temporarily exacerbate pain during the process of nerve degeneration. Lastly, appropriate expectations for treatments, potential for retreatment, and possible complications of diarrhea and hypotension should be discussed with the patient and their family [2, 16].
Bone Consolidation
Tumor destruction of bone can result in structural instability, fractures, or erosion of muscle attachments. Image guidance provides the opportunity to provide effective pain and stabilization via minimally invasive techniques that include vertebral augmentation, cementoplasty, and fixation by internally cemented screws. Vertebral augmentation and cementoplasty reinforce weakened or fractured bone by the injection of bone cement (typically polymethyl methacrylate—PMMA), which provides resistance to axial compressive forces. Fixation by internally cemented screws describes the placement of metallic screws or rods with concomitant injection of bone cement. The addition of metallic hardware provides resistance to torque and tension biomechanical stresses to compliment the axial compression resistance provided by the cement.
Vertebral Augmentation
Vertebral augmentation methods include vertebroplasty and kyphoplasty as well as percutaneous insertion of spine implants [17,18,19]. Both vertebroplasty and kyphoplasty require the careful passage of small caliber needles via a posterior approach into the fractured vertebral body (Fig. 2). In vertebroplasty, the palliative effect is achieved by cement injection that fills the weakened vertebral body to reinforce the bone and prevent further loss of vertebral body height. In kyphoplasty, an additional step is performed before the cement injection to attempt restoration of vertebral body height before cement injection. The most common techniques for vertebral body height restoration require advancement of an inflatable balloon catheter or other expandable device through the access needle with subsequent device deployment to elevate the collapsed superior vertebral body endplate. The application of vertebroplasty versus kyphoplasty often depends on multiple factors including operator experience, degree of vertebral body compression, and tumor extension into the posterior vertebral body. Regardless of the vertebral augmentation method applied, studies have demonstrated significant pain reduction, decreased analgesic use, and improvement in disability scores [20, 21]. The treatments are indicated for vertebral body fractures caused by either cancer-related osteopenia or pathologic fractures [22].
A 60-year-old male patient with bronchogenic carcinoma and spinal metastatic disease was treated with percutaneous vertebroplasty post laminectomy and radiotherapy due to persistent pain. a Magnetic resonance imaging, STIR sequence depicting vertebral edema at T7, T8, T9 levels; T8 vertebral body is illustrated with significant height reduction (white arrow). b Sagittal fluoroscopy view post cement injection in T7, T8, T9 levels. c Computed tomography sagittal reconstruction 24 h post vertebroplasty
Cementoplasty
Cementoplasty applies the same principles of vertebral augmentation to stabilize extra-spinal osseous structures including the bony pelvis, ribs, and sternum [23, 24]. The technique is also known as percutaneous osteoplasty. The physical qualities of the bone cement are thought to provide pain relief through durable resistance to compression forces, adhesion of microfractures, and possibly exothermic destruction of nociceptive pain fibers that occurs during cement polymerization [25]. In addition to pain relief, cementoplasty can be applied as a preventative measure for impending pathological fractures [24, 26]. Similar to vertebral augmentation, the procedure can be scheduled in the outpatient setting. Durable pain palliation is expected in 1–2 days, once healing from the needle manipulation subsides [27, 28]. While cementoplasty can provide pain relief in osseous structures that bear axial weight, the procedure is less effective in bones subjected to torque stresses [29•].
Fixation by Internal Cemented Screw
Percutaneous fixation by internal cement screws provides an additional level of osseous reinforcement for metastatic bone disease that has extensively eroded a weight bearing bone. Advanced image guidance with navigation software and real-time tracking enables the operator to advance metallic screws or rods into the bone through 1–2-cm incisions. The hardware is subsequently anchored into the bone by cement (PMMA) consolidation [30, 31]. The addition of the metallic hardware confers a resistance to torque and tension stresses that complement the bone cement resistance to compression forces [32,33,34,35,36]. The most common utilization of FICS techniques is for pathologic fractures of the pelvis and femoral neck (Fig. 3) [37,38,39,40,41]. Effective FICS can be performed as a pain palliative procedure or as a preventative measure for an impending pathologic fracture [42].
Percutaneous Ablation
Imaging guided, percutaneous ablation either with high (radiofrequency, microwave, or laser) or with low (cryoablation) temperature techniques focuses on tumor destruction to significantly reduce pain up to 68–100% [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. While thermal ablation is an effective stand-alone treatment, the treatment can also be combined with radiation therapy to improve the results [48, 49]. The pain palliative effect from thermal ablation results from necrosis of the interface between tumor and the pain-sensitive periosteum, decompression of tumor volume to relieve surrounding nerves and tissues, and reduction of tumor-induced cytokines to alleviate inflammation and nerve stimulation [42, 44]. Percutaneous ablation modalities include radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation (CA), and laser ablation (LA) [43]. All modalities have proven pain palliative effects. Selection of the specific ablation technique takes into consideration operator experience and tumor histology, location, and size.
Percutaneous thermal ablation is performed by advancing small profile needle probes into the tumor and applying the thermal energy (Fig. 4). Each ablation modality provides treatment in a pre-defined zone of ablation. The procedures can be performed under local anesthetic or general anesthesia, depending on the modality, treatment location, and the patient’s positional comfort. For tumor locations in close proximity to a critical structure such as a nerve or hollow organ, several thermoprotective techniques have been used to minimize complications. Active and passive thermoprotective techniques include thermal insulation with liquid or gas agent, as well as intraprocedural temperature and neurophysiological monitoring [45•, 46]. Percutaneous ablation of bone and musculoskeletal lesions is as safe technique with a 2.5% rate of major complications more commonly associated with age greater than 70 years and use of more than three cryoprobes [57]. Ablation techniques in weight-bearing bones can be combined with bone consolidation techniques in order to provide structural support and avoid subsequent pathological fractures [47].
A 56-year-old female sarcoma patient with a large sized mass infiltrating thoracic wall and pain resistant to medication and radiotherapy. a Computed tomography axial scan illustrating the microwave antenna inside the mass close to the mass-thoracic wall interface. b Computed tomography axial scan post intra-venous contrast medium injection illustrating the necrotic zone close to the mass-thoracic wall interface. There was significant self-reported pain reduction (> 70%) 1 week post ablation lasting for 9 months of follow-up
Numerous studies in the literature report the efficacy and safety of percutaneous ablation performed for curative intent in oligometastatic patients [58,59,60,61]. McMenomy et al. report post cryoablation a significant overall survival benefit that is governed by factors including size and number of metastases, length of disease-free interval, treatment adequacy of primary tumor, and presence of multiple metastatic sites [58]. Cazzato et al. report similar local progression-free survival rates among all the different tumor histologies and lesion size > 2 cm as a predictive factor for local tumor progression in a series of oligometastatic patients who underwent either radiofrequency or cryoablation [59]. Similarly, in a patient cohort undergoing radiofrequency or cryoablation sessions, Deschamps et al. report that good prognostic factors for local success include small-size (< 2 cm), lack of cortical erosion, and oligometastatic and/or metachronous diseases [60]. Recent studies suggest percutaneous ablation as an attractive alternative offering potential for complete remission in oligometastatic sarcoma patients [61].
As far as pain palliation is concerned, NCCN guidelines propose percutaneous ablation for adult cancer pain in cases of no oncologic emergency (e.g., pathologic fracture or epidural disease) of adequate pharmacologic therapy and/or radiation therapy contraindication or not desired by the patient [62]. Dupuy et al. applied radiofrequency ablation in previously radiated lesions with persistent pain; the authors report statistically significant pain reduction effect at 1 and 3 months of follow-up despite the fact that prior radiotherapy was not associated with significant pain reduction and mood improvement [63]. In a series of studies, Di Staso et al. reported that combining percutaneous ablation (radiofrequency or cryoablation) and EBRT may act synergistically with no increase in the adverse event rate [49, 64]. Specifically, combined therapies resulted in significantly higher pain reduction effects earlier in the follow-up period, lasting for a longer duration of time and being associated with a more significant decrease in analgesics uptake [49, 64].
High-Intensity-Focused Ultrasound
HIFU (high-intensity-focused ultrasound) is a noninvasive palliative option for the precise ablation of targeted lesions [65]. The technique is based on producing and focusing high-intensity ultrasound waves to deliver mechanical (thermal) energy on a location with no skin incisions or punctures (Fig. 5). The improvement of symptoms may be immediate, and the procedure can be used in conjunction with chemotherapy and radiotherapy, to augment palliative effects. Procedural anesthesia is selected based on patient condition and positional comfort, and treatment can be performed in either the inpatient or outpatient setting [66•].
Bone metastasis of a rib treated by MRgHIFU. a Sagittal view of the system with the HIFU transducer (white arrow), a gel pad (arrowhead), and the patient lying in lateral-oblique decubitus. The cross is the focus of the ultrasound beam, and the circle in the rib (lesion) is the targeted area of the selected sonication (area for energy delivery ≥ ablation). b A pre-treatment CT is registered and fused showing the area of sonication in axial view. c Magnitude and d temperature map: images acquired during the sonication; at the end of the sonication, the area of expected ablation of current sonication is shown in green; the cumulative treated area including all previous sonications is shown in blue; in the box on the right, a graph shows the evolution of temperature at the target during sonication
HIFU acts upon different sources of cancer-related pain with a collective mechanism of action from both thermal and mechanical effects that include cavitation, microstreaming, and radiation force. The thermal effect is likely responsible for the greatest treatment effect, with neurolytic damage of nociceptive fibers to denervate the treatment area. The cavitation and acoustic pressure on tumors may also cause malfunction of membrane proteins, which alters ion interchange and membrane function, triggering variations on their electric transmission, with pain palliation achieved through neuromodulation [67, 68]. Lastly, fibrosis induced by ablation and shrinkage of the tumor mass reduces the regional mass effect and the degree of compression of adjacent nerve structures.
HIFU treatments are performed under ultrasound (USgHIFU) or MR (MRgHIFU) guidance [66]. Ultrasound guidance is less expensive and more widely available. During USgHIFU, the ablated area appears as hyperechoic spots; however, real-time monitoring of tissue temperature is not possible [67]. In comparison, MRI guidance offers greater anatomical resolution for pre-treatment planning, treatment monitoring, and assessment of post-treatment effects.
HIFU has proven effective for cancer-related pain in solid tissue organs and muscular metastases. Studies in symptomatic patients with pancreatic adenocarcinoma who underwent HIFU report significant (up to 81%) and long-lasting (up to 17 months) pain reduction effects [68,69,70,71,72]. Case reports of significant pain reduction post HIFU session include other neoplastic substrates such as pancreatic neuroendocrine tumors, hepatocellular carcinoma, abdominal lymph node metastases from gastric cancer, renal malignancies, and recurrent cervical cancer [73,74,75,76,77,78,79].
For bone metastases, HIFU achieves pain palliation through periosteal neurolysis by cortical heating [80,81,82], reduction of tumor size by coagulative necrosis, inhibition of osteoclast mediated osteolysis and skeletal remodeling, and accelerated sclerosis of osteolytic lesions [80,81,82,83]. Bone cancers can present a treatment challenge due to several factors. The relative low content of water in cortical bone and high-fat content of bone marrow preclude reliable temperature measurements and necessitate temperature extrapolation from the immediately adjacent soft tissue [83]. Furthermore, the difference in acoustic properties of bone and surrounding tissue results in heat dispersion that makes ablation of a subcortical lesion challenging and also consequently rises the safety profile [83]. HIFU can cause necrosis in the surrounding soft tissues or loss of functionality of joints due to transmission of heat and bone damage or necrosis leading to fractures [66, 84]. Lastly, bone HIFU treatment can increase the fracture risk [82, 83], similar to percutaneous needle-guided ablation technologies. An understanding of these limitations ensures appropriate application of HIFU for specific bone tumors to optimize treatment outcome and minimize complication risks.
Conclusion
Imaging-guided percutaneous techniques may act either indirectly or directly to provide significant pain alleviation and life quality improvement. Palliative treatment options include neurolysis, thermal ablation, bone consolidation, and high-intensity-focused ultrasound. Technical advantages of these procedures include the minimally invasive nature that can performed in the outpatient setting or with a short hospitalization, low complication rates, little to no interruption of systemic chemotherapy agents, and ability to combine with other palliative treatment options. Advanced imaging and timely referral for pain palliation can optimize selection of the most appropriate technique in a patient-tailored approach to maximize efficacy of palliative pain relief.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
ESMO Guidelines working group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25(Supplement 3):iii124–37. https://doi.org/10.1093/annonc/mdu103.
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–1090.e9. https://doi.org/10.1016/j.jpainsymman.2015.12.340.
Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. BMJ. 2016;352:i20. https://doi.org/10.1136/bmj.i20.
Dennis K, Makhani L, Zeng L, Lam H, Chow E. Single fraction conventional external beam radiation therapy for bone metastases: a systematic review of randomised controlled trials. Radiother Oncol. 2013;106(1):5–14. https://doi.org/10.1016/j.radonc.2012.12.009.
Filippiadis DK, Cornelis FH, Kelekis A. Interventional oncologic procedures for pain palliation. Presse Med. 2019;48(7–8 Pt 2):e251–6. https://doi.org/10.1016/j.lpm.2019.06.006.
Kelekis A, Cornelis FH, Tutton S, Filippiadis D. Metastatic osseous pain control: bone ablation and cementoplasty. Semin Intervent Radiol. 2017;34(4):328–36. https://doi.org/10.1055/s-0037-1608747.
• Filippiadis DK, Tselikas L, Tsitskari M, Kelekis A, de Baere T, Ryan AG. Percutaneous neurolysis for pain management in oncological patients. Cardiovasc Intervent Radiol. 2019;42(6):791–9. https://doi.org/10.1007/s00270-019-02185-xRecent multi-centered review providing details as well as tips and tricks for all kinds of percutaneous neurolysis techniques.
Edelstein MR, Gabriel RT, Elbich JD, Wolfe LG, Sydnor MK. Pain outcomes in patients undergoing CT-guided celiac plexus neurolysis for intractable abdominal visceral pain. Am J Hosp Palliat Care. 2017;34(2):111–4. https://doi.org/10.1177/1049909115604670.
Gangi A, Buy X, Garnon J, Tsoumakidou G, Moser T, Bierry G, et al. Pain management in oncology. J Radiol. 2011;92(9):801–13. https://doi.org/10.1016/j.jradio.2011.07.014.
Kambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics. 2011;31(6):1599–621. https://doi.org/10.1148/rg.316115526.
Nagels W, Pease N, Bekkering G, Cools F, Dobbels P. Celiac plexus neurolysis for abdominal cancer pain: a systematic review. Pain Med. 2013;14(8):1140–63. https://doi.org/10.1111/pme.12176.
Mercadante S, Klepstad P, Kurita GP, Sjogren P, Giarratano A. European palliative care research C. sympathetic blocks for visceral cancer pain management: a systematic review and EAPC recommendations. Crit Rev Oncol Hematol. 2015;96(3):577–83. https://doi.org/10.1016/j.critrevonc.2015.07.014.
Drewes AM, Campbell CM, Ceyhan GO, Delhaye M, Garg PK, van Goor H, et al. Pain in pancreatic ductal adenocarcinoma: a multidisciplinary, international guideline for optimized management. Pancreatology. 2018;18(4):446–57. https://doi.org/10.1016/j.pan.2018.04.008.
Amr SA, Reyad RM, Othman AH, Mohamad MF, Mostafa MM, Alieldin NH, et al. Comparison between radiofrequency ablation and chemical neurolysis of thoracic splanchnic nerves for the management of abdominal cancer pain, randomized trial. Eur J Pain. 2018;22(10):1782–90. https://doi.org/10.1002/ejp.1274.
Amr YM, Makharita MY. Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic cancer: one-year follow-up. Clin J Pain. 2013;29(9):807–13. https://doi.org/10.1097/AJP.0b013e3182757673.
Koker IH, Aralasmak A, Unver N, Asil T, Senturk H. Spinal cord ischemia after endoscopic ultrasound guided celiac plexus neurolysis: case report and review of the literature. Scand J Gastroenterol. 2017;52(10):1158–61. https://doi.org/10.1080/00365521.2017.1335771.
Filippiadis DK, Marcia S, Masala S, Deschamps F, Kelekis A. Percutaneous vertebroplasty and kyphoplasty: current status, new developments and old controversies. Cardiovasc Intervent Radiol. 2017;40(12):1815–23. https://doi.org/10.1007/s00270-017-1779-x.
Santiago FR, Chinchilla AS, Alvarez LG, et al. Comparative review of vertebroplasty and kyphoplasty. World J Radiol. 2014;6(6):329–43. https://doi.org/10.4329/wjr.v6.i6.329.
Berenson J, Pflugmacher R, Jarzem P, Zonder J, Schechtman K, Tillman JB, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicenter, randomized controlled trial. Lancet Oncol. 2011;12:225–35. https://doi.org/10.1016/S1470-2045(11)70008-0.
Anselmetti GC, Marcia S, Saba L, Muto M, Bonaldi G, Carpeggiani P, et al. Percutaneous vertebroplasty: multi-centric results from EVEREST experience in large cohort of patients. Eur J Radiol. 2012;81(12):4083–6. https://doi.org/10.1016/j.ejrad.2012.07.005.
Pron G, Holubowich C, Kaulback K. Vertebral augmentation involving vertebroplasty or kyphoplasty for cancer-related vertebral compression fractures: a systematic review. Ontario Health Technol Assess Ser. 2016;16(11):1–202.
Gangi A, Guth S, Imbert JP, Marin H, Dietemann JL. Percutaneous vertebroplasty: indications, technique, and results. Radiographics. 2003;23(2):e10.
Anselmetti GC, Manca A, Ortega C, Grignani G, Debernardi F, Regge D. Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients. Cardiovasc Interv Radiol. 2008;31:1165–73. https://doi.org/10.1007/s00270-008-9396-3.
Deschamps F, De Baere T. Cementoplasty of bone metastases. Diagn Intervent Imaging. 2012;93(9):685–9. https://doi.org/10.1016/j.diii.2012.06.009.
Wang Z, Zhen Y, Wu C, et al. CT fluoroscopy-guided percutaneous osteoplasty for the treatment of osteolytic lung cancer bone metastases to the spine and pelvis. J Vasc Interv Radiol. 2012;23(9):1135–42. https://doi.org/10.1016/j.jvir.2012.06.007.
Deschamps F, Farouil G, Hakime, et al. Percutaneous stabilization of impending pathological fracture of the proximal femur. Cardiovasc Interv Radiol. 2012;35:1428–32. https://doi.org/10.1007/s00270-011-0330-8.
Anselmetti GC. Osteoplasty: percutaneous bone cement injection beyond the spine. Semin Intervent Radiol. 2010;27(2):199–208. https://doi.org/10.1055/s-0030-1253518.
Sun G, Jin P, Xun-wei L, et al. Cementoplasty for managing painful bone metastases outside the spine. Eur Radiol. 2014;24:731–7. https://doi.org/10.1007/s00330-013-3071-z.
• Yevich S, Tselikas L, Gravel G, et al. Percutaneous cement injection for the palliative treatment of osseous metastases: a technical review. Semin Intervent Radiol. 2018;35(4):268–80. https://doi.org/10.1055/s-0038-1673418Recent paper reviewing challenges as well as potential complications and providing technical pearls to refine consolidative techniques.
Deschamps F, de Baere T, Hakime A, Pearson E, Farouil G, Teriitehau C, et al. Percutaneous osteosynthesis in the pelvis in cancer patients. Eur Radiol. 2016;26:1631–9. https://doi.org/10.1007/s00330-015-3971-1.
Cazzato RL, Koch G, Buy X, Ramamurthy N, Tsoumakidou G, Caudrelier J, et al. Percutaneous image-guided screw fixation of bone lesions in cancer patients: double-centre analysis of outcomes including local evolution of the treated focus. Cardiovasc Interv Radiol. 2016;39:1455–63. https://doi.org/10.1007/s00270-016-1389-z.
Collinge CA, Crist BD. Combined percutaneous iliosacral screw fixation with sacroplasty using resorbable calcium phosphate cement for osteoporotic pelvic fractures requiring surgery. J Orthop Trauma. 2016;30(6):e217–22. https://doi.org/10.1097/BOT.0000000000000520.
Tian QH, He CJ, Wu CG, Li YD, Gu YF, Wang T, et al. Comparison of percutaneous cementoplasty with and without interventional internal fixation for impending malignant pathological fracture of the proximal femur. Cardiovasc Intervent Radiol. 2016;39(1):81–9. https://doi.org/10.1007/s00270-015-1133-0.
Kelekis A, Lovblad KO, Mehdizade A, Somon T, Yilmaz H, Wetzel SG, et al. Pelvic osteoplasty in osteolytic metastases: technical approach under fluoroscopic guidance and early clinical results. J Vasc Interv Radiol JVIR. 2005;16(1):81–8. https://doi.org/10.1097/01.RVI.0000141717.84515.92.
Strobl FF, Haeussler SM, Paprottka PM, Hoffmann RT, Pieske O, Reiser MF, et al. Technical and clinical outcome of percutaneous CT fluoroscopy-guided screw placement in unstable injuries of the posterior pelvic ring. Skelet Radiol. 2014;43(8):1093–100. https://doi.org/10.1007/s00256-014-1890-x.
Amoretti N, Huwart L, Hauger O, Marcy PY, Nouri Y, Ibba C, et al. Percutaneous screw fixation of acetabular roof fractures by radiologists under CT and fluoroscopy guidance. AJR Am J Roentgenol. 2013;200(2):447–50. https://doi.org/10.2214/AJR.11.7968.
Pusceddu C, Fancellu A, Ballicu N, Fele RM, Sotgia B, Melis L. CT-guided percutaneous screw fixation plus cementoplasty in the treatment of painful bone metastases with fractures or a high risk of pathological fracture. Skelet Radiol. 2017;46(4):539–45. https://doi.org/10.1007/s00256-017-2584-y.
Routt ML, Simonian PT, Grujic L. The retrograde medullary superior pubic ramus screw for the treatment of anterior pelvic ring disruptions: a new technique. J Orthop Trauma. 1995;9(1):35–44. https://doi.org/10.1097/00005131-199502000-00006.
Fischer S, Vogl TJ, Marzi I, Zangos S, Wichmann JL, Scholtz JE, et al. Percutaneous cannulated screw fixation of sacral fractures and sacroiliac joint disruptions with CT-controlled guidewires performed by interventionalists: single center experience in treating posterior pelvic instability. Eur J Radiol. 2015;84(2):290–4. https://doi.org/10.1016/j.ejrad.2014.11.017.
Roux C, Tselikas L, Yevich S, Sandes Solha R, Hakime A, Teriitehau C, et al. Fluoroscopy and cone-beam CT-guided fixation by internal cemented screw for pathologic pelvic fractures. Radiology. 2019;290(2):418–25. https://doi.org/10.1148/radiol.2018181105.
Deschamps F, Yevich S, Gravel G, Roux C, Hakime A, de Baère T, et al. Percutaneous fixation by internal cemented screw for the treatment of unstable osseous disease in cancer patients. Semin Intervent Radiol. 2018;35(4):238–47. https://doi.org/10.1055/s-0038-1673359.
Callstrom MR, Charboneau JW, Goetz MP, Rubin J, Wong GY, Sloan JA, et al. Painful metastases involving bone: feasibility of percutaneous CT and US-guided radio-frequency ablation. Radiology. 2002;224(1):87–97.
Auloge P, Cazzato RL, Koch G, Caudrelier J, De Marini P, Garnon J, et al. Percutaneous tumor ablation. Presse Med. 2019. https://doi.org/10.1016/j.lpm.2019.10.011.
Kurup AN, Callstrom MR. Image-guided percutaneous ablation of bone and soft tissue tumors. Semin Intervent Radiol. 2010;27(3):276–84.
• Garnon J, Cazzato RL, Caudrelier J, Nouri-Neuville M, Rao P, Boatta E, et al. Adjunctive thermoprotection during percutaneous thermal ablation procedures: review of current techniques. Cardiovasc Intervent Radiol. 2019;42(3):344–57. https://doi.org/10.1007/s00270-018-2089-7Recent review paper describing all available thermoprotection techniques applied during percutaneous ablation.
Filippiadis DK, Tutton S, Mazioti A, Kelekis A. Percutaneous image-guided ablation of bone and soft tissue tumours: a review of available techniques and protective measures. Insights Imaging. 2014;5(3):339–46. https://doi.org/10.1007/s13244-014-0332-6.
Gangi A, Tsoumakidou G, Buy X, Quoix E. Quality improvement guidelines for bone tumour management. Cardiovasc Intervent Radiol. 2010;33(4):706–13. https://doi.org/10.1007/s00270-009-9738-9.
Di Staso M, Zugaro L, Gravina GL, Bonfili P, Marampon F, Di Nicola L, et al. Can radiotherapy be combined with radiofrequency ablation in the management of symptomatic osteolytic skeletal metastasis? Clin Oncol (R Coll Radiol). 2011;23(1):65–6. https://doi.org/10.1016/j.clon.2010.11.002.
Di Staso M, Zugaro L, Gravina GL, Bonfili P, Marampon F, Di Nicola L, et al. A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol. 2011;21(9):2004–10. https://doi.org/10.1007/s00330-011-2133-3.
Vaswani D, Wallace AN, Eiswirth PS, Madaelil TP, Chang RO, Tomasian A, et al. Radiographic local tumor control and pain palliation of sarcoma metastases within the musculoskeletal system with percutaneous thermal ablation. Cardiovasc Intervent Radiol. 2018;41(8):1223–32. https://doi.org/10.1007/s00270-018-1932-.
Ma Y, Wallace AN, Waqar SN, Morgensztern D, Madaelil TP, Tomasian A, et al. Percutaneous image-guided ablation in the treatment of osseous metastases from non-small cell lung cancer. Cardiovasc Intervent Radiol. 2018;41(5):726–33. https://doi.org/10.1007/s00270-017-1843-6.
Cazzato RL, Garnon J, Caudrelier J, Rao PP, Koch G, Gangi A. Low-power bipolar radiofrequency ablation and vertebral augmentation for the palliative treatment of spinal malignancies. Int J Hyperth. 2018;34(8):1282–8. https://doi.org/10.1080/02656736.2017.1422557.
Gallusser N, Goetti P, Becce F, Vauclair F, Rüdiger HA, Bize PE, et al. Percutaneous image-guided cryoablation of painful bone metastases: a single institution experience. Orthop Traumatol Surg Res. 2019;105(2):369–74. https://doi.org/10.1016/j.otsr.2019.01.001.
Coupal TM, Pennycooke K, Mallinson PI, Ouellette HA, Clarkson PW, Hawley P, et al. The hopeless case? Palliative cryoablation and cementoplasty procedures for palliation of large pelvic bone metastases. Pain Physician. 2017;20(7):E1053–61.
Deib G, Deldar B, Hui F, Barr JS, Khan MA. Percutaneous microwave ablation and cementoplasty: clinical utility in the treatment of painful extraspinal osseous metastatic disease and myeloma. AJR Am J Roentgenol. 2019;1–8. https://doi.org/10.2214/AJR.18.20386.
Pusceddu C, Sotgia B, Fele RM, Ballicu N, Melis L. Combined microwave ablation and cementoplasty in patients with painful bone metastases at high risk of fracture. Cardiovasc Intervent Radiol. 2016;39(1):74–80. https://doi.org/10.1007/s00270-015-1151-y.
Auloge P, Cazzato RL, Rousseau C, Caudrelier J, Koch G, Rao P, et al. Complications of percutaneous bone tumor cryoablation: a 10-year experience. Radiology. 2019;291(2):521–8. https://doi.org/10.1148/radiol.2019181262.
Mc Menomy BP, Kurup AN, Johnson GB, et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. JVIR. 2013;24:207.
Cazzato RL, Auloge P, De Marini P, et al. Percutaneous image-guided ablation of bone metastases: local tumor control in oligometastatic patients. Int J Hyperth. 2018;35(1):1–7.
Deschamps F, Farouil G, Ternes N, et al. Thermal ablation techniques: a curative treatment of bone metastases in selected patients? Eur Radiol. 2014.
Vaswani D, Wallace AN, Elswirth PS, et al. Radiographic local tumor control and pain palliation of sarcoma metastases within the musculoskeletal system with percutaneous thermal ablation. CVIR. 2018.
National Comprehensive Cancer Network. Adult cancer pain v.2. 2016. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 21 Nov 2016.
Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K, et al. Percutaneous radiofrequency ablation of painful osseous metastases. Cancer. 2010;116(4):989–97.
Di Staso M, Gravina GL, Zugaro L, Bonfili P, Gregori L, Franzese P, et al. Treatment of solitary painful osseous metastases with radiotherapy, cryoablation or combined therapy: propensity matching analysis in 175 patients. PLoS One. 2015;10(6):e0129021.
Carrafiello G, Laganà D, Pellegrino C, Fontana F, Mangini M, Nicotera P, et al. Percutaneous imaging-guided ablation therapies in the treatment of symptomatic bone metastases: preliminary experience. Radiol Med. 2009;114(4):608–25.
• Bazzocchi A, Napoli A, Sacconi B, Battista G, Guglielmi G, Catalano C, et al. MRI-guided focused ultrasound surgery in musculoskeletal diseases: the hot topics. Br J Radiol. 2016;89:20150358. https://doi.org/10.1259/bjr.20150358Recent review paper upon treatment of MSK lesions by means of MR-guided HIFU.
Napoli A, Anzidei M, Marincola BC, Brachetti G, Noce V, Boni F, et al. MR imaging -guided ultrasound for treatment of bone metastasis. Radiographics. 2013;33:1555–68. https://doi.org/10.1148/rg.336125162.
Brown MR, Farquhar-Smith P, Williams JE, ter Haar G, de Souza NM. The use of high-intensity focused ultrasound as a novel treatment for painful conditions: a description and narrative review of the literature. Br J Anaesth. 2015;115(4):520–30. https://doi.org/10.1093/bja/aev302.
Dababou S, Marrocchio C, Rosenberg J, Bitton R, Pauly KB, Napoli A, et al. A meta-analysis of palliative treatment of pancreatic cancer with high intensity focused ultrasound. J Ther Ultrasound. 2017;5(1):9. https://doi.org/10.1186/s40349-017-0080-4.
Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236(3):1034–40. https://doi.org/10.1148/radiol.2362041105.
Anzidei M, Marincola BC, Bezzi M, Brachetti G, Nudo F, Cortesi E, et al. Magnetic resonanceguided high-intensity focused ultrasound treatment of locally advanced pancreatic adenocarcinoma: preliminary experience for pain palliation and local tumor control. Investig Radiol. 2014;49(12):759–65. https://doi.org/10.1097/RLI.0000000000000080.
Xiong LL, He CJ, Yao SS, et al. The preliminary clinical results of the treatment for advanced pancreatic carcinoma by high intensity focused ultrasound. Chin J Gen Surg. 2001;16(6):345–7.
Chen Q, Zhu X, Chen Q, Wang K, Meng Z. Unresectable giant pancreatic neuroendocrine tumor effectively treated by high-intensity focused ultrasound: a case report and review of the literature. Pancreatology. 2013;13(6):634–8. https://doi.org/10.1016/j.pan.2013.10.001.
Orsi F, Zhang L, Arnone P, Orgera G, Bonomo G, Vigna PD, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol. 2010;195(3):W245–52. https://doi.org/10.2214/AJR.09.3321.
Orgera G, Monfardini L, Della Vigna P, Zhang L, Bonomo G, Arnone P, et al. High-intensity focused ultrasound (HIFU) in patients with solid malignancies: evaluation of feasibility, local tumour response and clinical results. Radiol Med (Torino). 2011;116(5):734–48. https://doi.org/10.1007/s11547-011-0634-4.
Xu G, Luo G, He L, et al. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol. 2011;37(12):1993–9. https://doi.org/10.1016/j.ultrasmedbio.2011.08.011.
Zhang M, Liu L, Wang J, Lu K, Shu Y, Wang R, et al. Effects of high-intensity focused ultrasound for treatment of abdominal lymph node metastasis from gastric cancer. J Ultrasound Med. 2015;34(3):435–40. https://doi.org/10.7863/ultra.34.3.435.
Wu F, Wang ZB, Chen WZ, Bai J, Zhu H, Qiao TY. Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy. J Urol. 2003;170(6 Pt 1):2237–40. https://doi.org/10.1097/01.ju.0000097123.34790.70.
Machtinger R, Inbar Y, Ben-Baruch G, Korach J, Rabinovici J. MRgFUS for pain relief as palliative treatment in recurrent cervical carcinoma: a case report. Gynecol Oncol. 2008;108(1):241–3. https://doi.org/10.1016/j.ygyno.2007.08.079.
Huisman M, Ter Haar G, Napoli A, Hananel A, Ghanouni P, Lovey G, et al. International consensus on use of focused ultrasound for painful bone metastases: current status and future directions. Int J Hypertermia. 2015;31:251–9. https://doi.org/10.3109/02656736.2014.995237.
Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases: preliminary clinical experience. Ann Oncol. 2007;18(1):163–7. https://doi.org/10.1093/annonc/mdl335.
Yeo SY, Elevelt A, Donato K, et al. Bone metastasis treatment using magnetic resonance-guided high intensity focused ultrasound. Bone. 2015;81:513–23. https://doi.org/10.1016/j.bone.2015.08.025.
Rodrigues DB, Stauffer PR, Vrba D, Hurwitz MD. Focused ultrasound for treatment of bone tumours. Int J Hyperth. 2015;31(3):260–71. https://doi.org/10.3109/02656736.2015.1006690.
Errani C, Bazzocchi A, Spinnato P, Facchini G, Campanacci L, Rossi G, et al. What’s new in management of bone metastases? Eur J Orthop Surg Traumatol. 2019;29(7):1367–75. https://doi.org/10.1007/s00590-019-02446-y Review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dimitrios K. Filippiadis, Lambros Tselikas, Alberto Bazzocchi, Evegnia Efthymiou, Alexis Kelekis, Steven Yevich declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Interventional Oncology
Rights and permissions
About this article
Cite this article
Filippiadis, D.K., Tselikas, L., Bazzocchi, A. et al. Percutaneous Management of Cancer Pain. Curr Oncol Rep 22, 43 (2020). https://doi.org/10.1007/s11912-020-00906-x
Published:
DOI: https://doi.org/10.1007/s11912-020-00906-x