Abstract
Purpose
To compare the efficacy of percutaneous cementoplasty (PCP) with and without interventional internal fixation (IIF) on malignant impending pathological fracture of proximal femur.
Methods
A total of 40 patients with malignant impending pathological fracture of proximal femur were selected for PCP and IIF (n = 19, group A) or PCP alone (n = 21, group B) in this non-randomized prospective study. Bone puncture needles were inserted into the proximal femur, followed by sequential installation of the modified trocar inner needles through the puncture needle sheath. Then, 15–45 ml cement was injected into the femur lesion.
Results
The overall excellent and good pain relief rate during follow-ups were significantly higher in group A than that in group B (89 vs. 57 %, P = 0.034). The average change of VAS, ODI, KPS, and EFES in group A were significantly higher than those in group B at 1-, 3-, 6-month, 1-year (P < 0.05). Meanwhile, The stability of the treated femur was significantly higher in group A than that in group B (P < 0.05).
Conclusion
PCP and IIF were not only a safe and effective procedure, but resulted in greater pain relief, bone consolidation, and also reduced the risk of fracture than the currently recommended approach of PCP done on malignant proximal femoral tumor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous cementoplasty has been used for painful spinal and extraspinal bone metastases, especially when previous treatment strategies, such as hormonal therapy or chemotherapy or radiotherapy, have resulted in inadequate pain control and when the risk of fracture is low. Several studies have shown that this technique is safe and successful in providing immediate pain relief, long-lasting pain relief, rapid rehabilitation, and a resultant better quality of life [1–3]. However, its use is controversial for osteolytic metastases of the proximal femur because several reports in the literature concluded that there was a risk of fracture despite cementoplasty because it does not provide adequate mechanical stability [4–6], and additionally such procedure may complicate future intramedullary nailing by obliteration of the medullary canal [7]. To overcome these disadvantages, we accept an innovative method—interventional internal fixation (IIF), that is to say, we used PCP combined with IIF for minimizing the risk of fracture or proximal femur stabilization during one session. Here, we present the results of a non-randomized prospective study of the interventional treatment for malignant impending pathological fracture of the proximal femur with PCP and IIF, with the goal of comparing the efficacies of PCP and IIF with that of PCP alone.
Materials and Methods
Patients
The Institutional Ethics Committee of our hospital approved this study. Informed consent was obtained from all individual participants included in the study. From Oct–May 2010 to January 2013, patients with proximal femoral metastatic tumor and impending pathologic fracture of the head or trochanteric region of the femur were recruited and prospectively allocated to PCP and IIF (Group A) or PCP alone (Group B) treatment according to their wishes.
All patients who were referred for the treatment were asked by a practitioner to complete a short questionnaire about the presence and severity and duration of pain, the etiology of the femoral disease, and so on. Patients were eligible for enrolment if they met the following criteria: (1) patients with metastasis of the proximal femur with a high risk of pathological fracture, defined by a Mirels’ score over 8, who were not candidates for standard surgical stabilization because of poor performance status or refused surgery; (2) cortical involvement <30 mm and no history of a fracture of the lesser trochanter; (3) age ≥65 or life expectancy ≤5 years; and (4) patients who had one clinical and plain film, CT or MRI follow-up at least 3 months after the initial treatment. Patients were excluded if any of the following was present: (1) untreatable coagulopathy, (2) allergy to polymethyl methacrylate (PMMA), or (3) severe cardiopulmonary comorbidity. There were no statistical significance in baseline characteristics between the two groups, such as age, gender, primary site of the cancer, Mirels’ score, and so on (Table 1).
PCP and IIF Procedures
Diagrams of the technique steps are shown in Figs. 1 and 2. The patient was placed in a lateral position on an operating table. With patient’s informed consent, interventional internal fixation was performed using modifications of the inner needle trocar: they were essentially bone puncture needles after removal of the tails and pinpoints, and are referred to as modified trocar needles in the present report (13 G, Cook, Bloomington, IN, USA) or (11G, Dragon-Crown Co., Jinan, China). After administration of local anesthetic (2 % lidocaine), a bone puncture needle parallel to the axis of the femoral neck was inserted into the femur until the tip reached the bone cortex or the needle was completely inserted into the femur body. Then the modified trocar needle, which was made beforehand according to the extent of the lesion, was implanted into the proximal femur through the bone puncture needle sheath and advanced in by the inner needle of the bone puncture needle with the help of a hammer. In general, the first modified trocar needle was inserted through the centerline of femoral neck. If necessary, another one or two modified trocar needles were placed parallel to the first one. Simultaneously, one or more modified trocar needles parallel to the vertical axis of thighbone were often implanted into the thighbone. Then the bone puncture needles were retreated and re-inserted into the proximal femur from a different route. The whole procedure was performed under real-time fluoroscopic guidance in order to avoid modified trocar needles being inserted into intra-articular. Finally, cementoplasty was performed under fluoroscopic guidance. A maximal amount of polymethyl methacrylate (PMMA) (Osteo-Firm, COOK, Bloomington, IN, USA) as possible was injected into the femur through the bone puncture needle. Injection was ceased when substantial resistance was met or when the cement reached the cortex edge of the femur. Immediately after the procedure, standard anteroposterior and lateral radiographs were obtained.
Diagrams show the steps of PCP and IIF. A Malignant tumor within the proximal femur. B Two bone needles are inserted into the proximal femur, respectively, parallel to the axis of the femoral neck and the vertical axis of thighbone under fluoroscopic monitoring. C Modified trocar needles, which were made beforehand according to the extent of the lesion, were implanted into the proximal femur through the bone puncture needle sheath and advanced in by the inner needle of the bone puncture needle with the help of a hammer. D The modified trocar needles were completely implanted in the femoral neck and thighbone. E The bone puncture needles were retreated and re-inserted into the proximal femur from a different route and cementoplasty was performed under fluoroscopic guidance. F PCP and IIF procedure was completed with PMMA left in the proximal femur
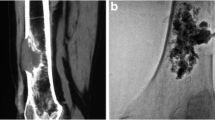
Rhabdomyosarcoma of left proximal femur in a 19-year-old male patient with left leg pain and disability prior to the procedure. A, B, C Malignant tumor in left proximal femur is depicted on coronal T2WI MR, coronal reconstruction CT, and X-ray images. D Two bone punctures were inserted into the proximal femur, respectively, parallel to the axis of the femoral neck and the vertical axis of thighbone. E Modified trocar needles were implanted into the proximal femur through the bone puncture needle sheath. F A total of four modified trocar needles were completely implanted in the proximal femur, of which each two were parallel to the axis of the femoral neck and the vertical axis of thighbone. G Cementoplasty was performed under fluoroscopic guidance. H PCP and IIF procedure was completed and malignant tumor was eliminated with PMMA left in the left proximal femur strengthening the bone
PCP Procedure
The patient was placed in a lateral position on an operating table. After local anesthesia, one or two bone puncture needles (13 G, Cook, Bloomington, IN) were placed in the proximal femur from the anatomical axis or the mechanical axis of the femur. After removal of the inner needle, commercially available polymethyl methacrylate (PMMA) (Osteo-Firm, COOK Medical, Bloomington, IN, USA) was carefully injected into the proximal femur. In general, a total of 15–40 ml PMMA was injected into the proximal femur. Post-procedural fluoroscopic evaluation was also obtained to show optimal filling of the lesion with no evidence of PMMA extravasation.
After the procedure, CT of the treated femur was done with 2-mm slices to identify the distribution of cement in the lesion, cement leakage outside the femoral body, or other possible local complications.
Clinical Outcome Evaluation
Technical success and complications were recorded for all patients. The patients were clinically examined by two of the authors, who gathered initial and follow-up data before and at 1 day, 1 week, and 1-, 3-, 6-, and 12-month after the procedures. Endpoints were loss of stability, worsening of pain at the operated site, and pathological fracture. In cases where clinical examination was not possible, the patient or his or her family was contacted by telephone every 3 months. Imaging follow-up consisted of lateral and anteroposterior femoral radiographs at 1-, 6-month, and 1 year after the procedure. All patients underwent CT 3 days after PCP to check the cement distribution or extravasations. CT and/or MRI was performed in the same manner as before the procedures at 3 and every 6 months afterwards in all patients.
The pain relief as measured by a visual analog scale (VAS) score ranging from 0 (no pain) to 10 (worst pain ever) and categorized into four types: Excellent result (0–2), good result (2.5–4.5), fair, and poor. The functional status of patients for walking, standing, and sleeping was measured by the Oswestry disability index (ODI) questionnaire. The functional outcomes were measured on a 100-point Karnofsky performance scale (KPS) to assess changes in quality of life. The Enneking’s function evaluation system (EFES) was used to evaluate the functional status of lower extremity after such time-salvaging procedures. For each femur, we evaluated the filling quality as “good” (more than 2/3 of the proximal femur volume), “mild” (1/3–2/3), or “insufficient” (less than 1/3), and also the filling volume in milliliters. Any potential complications following PCP and IIF, such as wound infections, nerve injuries, cement leakage, and pulmonary embolism were recorded.
Statistical and Quantitative Assessments
Descriptive data were presented as the mean ± SD. Dichotomous and categorical data were reported as numbers and percentages. Comparisons of the variables between the two groups were performed by applying the Mann–Whitney test, χ 2 test or the Fisher’s exact test as appropriate. All statistical analyses were performed using SPSS version 13.0 (SPSS, Chicago, IL, USA).
Results
Patient Population
Forty patients with malignant impending pathological fracture of the proximal femur and symptoms of uncontrollable pain were enrolled in this study. Initially, the study population consisted of 22 patients in the PCP and IIF group (group A) and 27 patients in the PCP group (group B). Of these, 9 patients did not meet the inclusion criteria, with follow-ups of 1 month or less in three patients and loss to follow-up in eight patients (three in group A and six in group B). So, a final total of 19 and 21 patients were included in group A and group B, respectively. Baseline characteristics of these 40 patients with impending pathological fracture of the proximal femur and symptoms of unbreakable pain are summarized in Table 1. The patients included 18 men and 22 women with a mean age of 60.28 ± 11.11 years (range 19–78 years). Tumor types were lung (n = 9); breast (n = 5); liver (n = 2); prostate (n = 2); and rhabdomyosarcoma (n = 1) in IIF and PCP group, while lung (n = 11); breast (n = 6); liver (n = 2); and prostate, pancreas (each n = 1) in PCP group. The mean Mirel's score was 10.95 ± 1.39 (range 8–12) in group A and 10.76 ± 1.45 (range 8–12) in group B.
Safety
The technical and initial clinical outcomes of the two groups are shown in Table 1. In group A, IIF and PCP were technically successful and well tolerated in all patients. Ten patients resulted in excellent pain relief (52.63 %), seven patients resulted in good pain relief (36.84 %), and two patient resulted in no improvement (10.53 %) at the time of discharge. CT showed cement leakage in 3 (15.79 %) of the 19 patients with PCP and IIF. Leakages were into the puncture path (n = 1), parafemoral space (n = 1), or veins (n = 1). One patient experienced phlebothrombosis at the operative side at 15 days following operation, and then a filter was placed via femoral vein to the inferior vena cava and anticoagulation therapy was taken synchronously. The patient was well recovered 1 month later. The mean number of modified trocar needles installed per patient was 3.42 ± 1.02 (range 2–6) and the mean duration of the procedure was 80.00 ± 7.51 min (range 70–95 min).
In group B, PCP was also technically successful and well tolerated in all patients. Seven patients resulted in excellent pain relief (33.33 %), 11 patients resulted in good pain relief (52.38 %), and three patients resulted in no improvement (14.29 %) at the time of discharge. CT showed cement leakage in 4 (19.05 %) of the 21 patients with PCP. Leakages were into the joint space (n = 1), puncture path (n = 1), parafemoral space (n = 1), or veins (n = 1). Additionally, five patients experienced pathological fracture on the proximal femur and had to accept surgery. The mean duration of the procedure was 69.67 ± 10.18 min (range 55–90 min) (Table 2).
Clinical Evaluation
Clinical follow-up data [mean 9 ± 4 months (95 % CI 1, 17 months), range 6–18 months] were available for 19 patients in group A. In 17 patients with excellent or good pain relief at the time of discharge, two patients experienced worsening of pain at 4-, 5-month follow-up due to new adjacent metastatic pelvic lesions demonstrated at magnetic resonance imaging; the pain was resolved by PCP alone. Good pain relief was achieved in one of the two patients with no change. Median VAS score had declined to 3 (P 25–P 75 range 3–4) at 1 week, 2 (P 25–P 75 range 2–3) at 1 month, 2 (P 25–P 75 range 1–3) at 3 months, 2 (P 25–P 75 range 1–3) at 6 months, and 1 (P 25–P 75 range 0–3) at 1 year from 7 (P 25–P 75 range 6–8) before treatment (Fig. 3).
The overall clinical assessment during follow-ups exhibited excellent pain relief (n = 13) and good pain relief (n = 4) in 17 patients with a pain relief rate of 89 % (95 % CI 81, 99 %). 14 patients continued with follow-up health care after the procedures and were alive with improvement of pain at the time of this report; five patients died of the underlying diseases unrelated to the procedures.
In group B, clinical follow-ups were obtained for 21 patients in group B (mean 8 ± 5 months; 95 % CI 0, 17 months; range 3–18 months). In 18 patients with excellent or good pain relief at the time of discharge, three patients experienced worsening of pain at 3, 4, and 6-month follow-up due to new adjacent metastatic pelvic lesions; these pains were resolved in two patients by PCP alone. Meanwhile, five of the 18 patients with pain relief at initial follow-up experienced pathological fractures of the proximal femur in group B at 3-, 4-,and 6-month follow-up and had to accept further surgery. Pain relief was not achieved in the three patients with no change. Median VAS score had declined to 3 (P 25–P 75 range 2–5) at 1 week, 2 (P 25–P 75 range 2–5) at 1 month, 3 (P 25–P 75 range 2–4.5) at 3 months, 2.5 (P 25–P 75 range 1–4.75) at 6 months, and 2 (P 25–P 75 range 1–3) at 1 year from 6 (P 25–P 75 range 4–8) before treatment (Fig. 3).
The overall clinical assessment during follow-ups exhibited excellent pain relief (n = 8) and good pain relief (n = 4) in 12 patients with a pain relief rate of 57 % (95 % CI 45, 67 %). 13 patients continued with follow-up health care after the procedures and were alive with improvement of pain at the time of this report; 8 patients died of the underlying diseases unrelated to the procedures.
The overall excellent and good pain relief rate during follow-ups was significantly higher in group A than that in group B (89 vs. 57 % P = 0.034). The average VAS score, ODI score at 1-,3-, 6-month, 1-year in group A were significantly lower than that in group B (P < 0.05), while the average KPS score and EFES score at 1-,3-, 6-month, 1-year in group A were obviously higher than that in group B (P < 0.05). There were no significant differences in the average VAS score, ODI score, KPS, and EFES score preoperatively, and 1 week postoperatively between the two groups. There were no significant differences in patient survivals between the two groups (P = 0.383 > 0.05, log-rank test).
Cement Filling and Stability
The mean cement filling volume was significantly higher in group A (31.21 ± 6.30 ml; range 21–45 ml) in 19 treated femur with PCP and IIF than that in 21 treated femur with PCP in group B (24.67 ± 5.59 ml; range 15–40 ml) (P < 0.05). And the cement filling quality of group A was dramatically better than that of group B (P < 0.05). In addition, no patient experienced malignant femoral fractures in group A during follow-ups, while, pathological fractures of the treated proximal femur was observed in five patients in group B (P < 0.05, Fisher’s exact test). The stability of the treated femur in group A was significantly higher than that in the treated femur in group B (P = 0.034, Fisher’s exact test).
Discussion
Metastatic bone disease of the proximal femur and more specifically of the trochanteric area represents a significant clinical problem for both the oncologist and the orthopedic surgeon. Not only is metastatic involvement of this area common [8, 9], but it may also negatively affect the patient’s remaining quality of life with pain and poor mobility. Moreover, osteolytic metastasis can be responsible for pathologic fractures. Ideally, an impending pathologic fracture of the head or trochanteric region of the femur should be treated before the fracture occurs. Surgery is the standard of care for the improvement of mechanical stability with a prosthesis or osteosynthesis. Compared with surgery that is performed once a pathologic fracture has occurred [10], prophylactic surgical stabilization improves the patient’s quality of life by decreasing the stress and pain of a pathologic fracture and decreasing intraoperative and postoperative complications. However, prophylactic surgical stabilization is associated with surgical morbidity and mortality, especially in patients with metastatic disease [11, 12] Painful bone metastases of the proximal femur with a low risk of fracture can be treated conservatively using systemic treatments, such as hormonal therapy or chemotherapy and specific treatments, such as radiotherapy or treatment with bisphosphonates. Consequently, the decision to perform surgical stabilization in patients with cancer must consider not only the risk of fracture but also the patient’s performance status and the prognosis of the cancer—namely, the patient’s life expectancy.
Over the past decade, PCP has gained wide clinical acceptance as an effective treatment option for patients with intractable pain related to osteoporotic fractures and vertebral metastases of the spine [13–17]. However, the use of PCP in the malignant extraspinal lesions has come late. This minimally invasive technique has been extended for the treatment of painful metastatic lesions involving superior and inferior pubic rami, ischial tuberosity, acetabular area, sacrum, mandible, maxilla, and the humerus and considered as a safe and successful technique in providing immediate pain relief, long-lasting pain relief, rapid rehabilitation, and a resultant better quality of life [18–22].
As one of the long weight-bearing bones, the femur is vulnerable to pathological fracture, especially when metastatic tumor occurs on the proximal femur. A pragmatic approach to bone metastases of the proximal femur is to surgically stabilize all patients with metastases with a high risk of fracture. According to Mirel's scoring system, prophylactic fixation is highly indicated for a lesion with an overall score of 8 or greater [23, 24]. However, this could lead to surgical overtreatment in patients whose life expectancy is limited, and it results in postoperative morbidity and mortality that can be considerable in this population. Because of the relatively high mortality and morbidity of surgery in patients with cancer and bone metastases, percutaneous cementoplasty is probably an option, especially for patients with inadequate pain control after radiotherapy.
Although the procedure was simple and rapid, clinical use of PCP in malignant proximal femur has been limited to a few reports in the literature [4–7], as a result of the increased risk of pathological fracture because it does not provide adequate mechanical stability. A new study concluded that PCP can be considered for patients with metastases of the proximal femur only under certain conditions: cortical involvement less than 30 mm and no history of a fracture of the lesser trochanter [25]. Therefore, interventional radiologists need a new approach for these patients.
Combined PCP and IIF, developed from PCP and percutaneous coaxial switching technology and vividly described as reinforced concrete, involves percutaneous extirpation of the femoral metastatic tumor and internal fixation of the proximal femur. Bone puncture needles were inserted into the proximal femur, respectively, parallel to the axis of the femoral neck and the vertical axis of thighbone under fluoroscopy, then modified trocar inner needles were then installed into the proximal femur through the bone puncture needle sheath from which the PMMA cement is subsequently injected.
Compared with PCP alone, this procedure can make much better distribution of the bone cement and support much enough consolidation of the proximal femur, which provides both mechanical stability as well as durability. Compared with conventional surgery, this procedure has showed more advantages. First, blood loss and surgical trauma could be negligible, which can decrease the risk of infection and hypervascular metastases and avoid damage to the muscles around the femur. Second, it can be performed under conscious sedation with very low level of pain. Third, the short period of bed rest required associated with a short hospital stay can certainly decrease the risk of thromboembolic complications compared with conventional surgery. Last but not least, this treatment could be associated with other diagnoses and treatment during the same procedure, for example needle biopsy, thermal ablation, and so on.
In this study, we aimed to compare the clinical safety and results of two regimens in patients with malignant impending pathological fracture of the proximal femur. Distribution and amount of bone cement was significantly better following PCP and IIF compared with PCP alone, which provides much mechanical stability as well as durability and reduced the risk of pathologic fracture, and the significant improvement in follow-up clinical results seemed to be predominantly attributable to the internal fixation that increased the cement flew path by IIF, thereby allowing more PMMA to be injected into the proximal femur and almost complete elimination of the malignant tissue. Thus, the principle of the method described here is similar to that underlying reinforced concrete: the modified trocar needles enhance the longitudinal force and the PMMA stabilizes the modified trocar needles in the destructive femoral lesions. To some extent, the lower fracture rate in group A compared to group B was a result of both injecting more bone cement and implanting the internal fixation. In a previous study on the use of PCP only for impending fracture of the proximal femur in patients with cancer [25], even much more bone cement was injected into the target bone, the 1-year pathologic fracture rate was as high as 40.6 %. We also observed no differences in the overall mortality, morbidity, complications, or survival between the groups, although there have been relatively some deaths in both groups. Meanwhile, we observed significant differences in the VAS score, ODI score, KPS, EFES, and the cement filling stability in group A than those in group B during the follow-up. These results suggest that combined PCP and IIF can be considered a viable and effective treatment for patients with malignant impending pathological fracture of the proximal femur. To the best of our knowledge, this is the first study directly comparing these two techniques for treatment of malignant impending pathological fracture of the proximal femur. Although, the number of size was small, the promising results indicate that combined PCP and IIF achieved better pain relief as well as improvement in bone consolidation than PCP alone.
There are some limitations in our study. First, the number of patients treated was relatively small, their life span was short, and death due to rapid progression of the disease might have masked both benefits and risks of the procedure; thus, expanded clinical trials are required to determine mid-term outcomes. Second, these procedures are time-consuming and relatively expensive. Third, patients in whom IIF is indicated are relatively few in number compared with the majority of cancer patients where PCP alone is sufficient. Finally, the treatments of primary cancer during follow-up or just very closely before the procedure in this study were heterogeneous, which could influence the comparison of the treatment effect between the two groups to some extent. However, as we have described, our results can serve as a scaffold for the design of future clinical trials.
Conclusions
In this non-randomized prospective trial, treatment of malignant impending pathological fracture of the proximal femur using combined PCP and IIF resulted in greater pain relief and bone consolidation and also reduced the risk of pathologic fracture than the currently recommended approach of PCP alone. These initial results are promising, but longer follow-up and expanded clinical trials are needed.
References
Weill A, Chiras J, Simon JM, Rose M, Sola-Martinez T, Enkaoua E. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241–7.
Yamada K, Matsumoto Y, Kita M, Yamamoto K, Kobayashi T, Takanaka T. Long-term pain relief effects in four patients undergoing percutaneous vertebroplasty for metastatic vertebral tumor. J Anesth. 2004;18:292–5.
Anselmetti GC, Manca A, Ortega C, Grignani G, Debernardi F, Regge D. Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients. Cardiovasc Intervent Radiol. 2008;6:1165–73.
Anselmetti GC. Osteoplasty: percutaneous bone cement injection beyond the spine. Semin Intervent Radiol. 2010;2:199–208.
Chang SW, Murphy KP. Percutaneous CT-guided cementoplasty for stabilization of a femoral neck lesion. J Vasc Interv Radiol. 2005;6:889–90.
Basile A, Giuliano G, Scuderi V, Motta S, Crisafi R, Coppolino F, Mundo E, Banna G, Di Raimondo F, Patti MT. Cementoplasty in the management of painful extraspinal bone metastases: our experience. Radiol Med. 2008;7:1018–28.
Dayer R, Peter R. Percutaneous cementoplasty complicating the treatment of a pathologic subtrochanteric fracture: a case report. Injury. 2008;7:801–4.
Habermann ET, Lopez RA. Metastatic disease of bone and treatment of pathological fractures. Orthop Clin North Am. 1989;20:469–86.
Sim FH. Diagnosis and management of metastatic bone disease. New York: Raven Press; 1988.
Ward WG, Holsenbeck S, Dorey FJ, Spang J, Howe D. Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res. 2003;415S:230–44.
Popken F, Schmidt J, Oegur H, Göhring UJ, König DP, Braatz F, Hackenbroch MH. Treatment outcome after surgical management of osseous breast carcinoma metastases. Preventive stabilization vs. management after pathological fracture. Unfallchirurg. 2002;4:338–43.
Ristevski B, Jenkinson RJ, Stephen DJ, Finkelstein J, Schemitsch EH, McKee MD, Kreder HJ. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;4:302–8.
Evans AJ, Jensen ME, Kip KE, DeNardo AJ, Lawler GJ, Negin GA, Remley KB, Boutin SM, Dunnagan SA. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology. 2003;226:366–72.
Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31:1983–2001.
Zoarski GH, Snow P, Olan WJ, Stallmeyer MJ, Dick BW, Hebel JR, De Deyne M. Percutaneous vertebroplasty for osteoporotic compression fractures: quantitative prospective evaluation of long-term outcomes. J Vasc Interv Radiol. 2002;13:139–48.
Taylor JW, Schiff D. Metastatic epidural spinal cord compression. Semin Neurol. 2010;30:245–53.
Shimony JS, Gilula LA, Zeller AJ, Brown DB. Percutaneous vertebroplasty for malignant compression fractures with epidural involvement. Radiology. 2004;232:846–53.
Toyota N, Naito A, Kakizawa H, Hieda M, Hirai N, Tachikake T, Kimura T, Fukuda H. Ito K (2005) Radiofrequency ablation therapy combined with cementoplasty for painful bone metastases: initial experience. Cardiovasc Intervent Radiol. 2005;28:578–83.
Wang WG, Wu CC, Gu YF, Li MH. Percutaneous osteoplasty for the management of a femoral head metastasis: a case report. Korean J Radiol. 2009;10:641–4.
Giovanni Carlo Anselmetti. Percutaneous Bone Cement Injection beyond the Spine. Semin Intervent Radiol. 2010;27:199–208.
Sun G, Jin P, Li M, Lu Y, Ding J, Liu X, Li F. Percutaneous cementoplasty for painful osteolytic humeral metastases: initial experience with an innovative technique. Skeletal Radiol. 2011;40:1345–8.
Clarençon F, Jean B, Pham HP, Cormier E, Bensimon G, Rose M, Maksud P, Chiras J. Value of percutaneous radiofrequency ablation with or without percutaneous vertebroplasty for pain relief and functional recovery in painful bone metastases. Skeletal Radiol. 2013;42:25–36.
Mirels H. Metastatic disease in long bones. A proposed scoring sys-tem for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256–64.
Jawad MU, Scully SP. Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;10:2825–7.
Deschamps F, Farouil G, Hakime A, Barah A, Guiu B, Teriitehau C, Auperin A, deBaere T. Cementoplasty of metastases of the proximal femur: is it a safe palliative option? J Vasc Interv Radiol. 2012;23:1311–6.
Acknowledgments
Supported by Shanghai Municipal Commission of Health Family Programme (Contract Number: 201440269).
Conflict of interest
Authors Qing-Hua Tian, Cheng-Jian He, Chun-Gen Wu, Yong-Dong Li, Yi-Feng Gu, Tao Wang, Quan-Ping Xiao, and Ming-Hua Li have no conflict of interests to declare.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qing-Hua Tian and Cheng-Jian He contributed equally to this work and share first authorship.
Rights and permissions
About this article
Cite this article
Tian, QH., He, CJ., Wu, CG. et al. Comparison of Percutaneous Cementoplasty with and Without Interventional Internal Fixation for Impending Malignant Pathological Fracture of the Proximal Femur. Cardiovasc Intervent Radiol 39, 81–89 (2016). https://doi.org/10.1007/s00270-015-1133-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-015-1133-0