Abstract
Objective
To evaluate the feasibility and effectiveness of computed tomography (CT)-guided percutaneous screw fixation plus cementoplasty (PSFPC), for either treatment of painful metastatic fractures or prevention of pathological fractures, in patients who are not candidates for surgical stabilization.
Materials and methods
Twenty-seven patients with 34 metastatic bone lesions underwent CT-guided PSFPC. Bone metastases were located in the vertebral column, femur, and pelvis. The primary end point was the evaluation of feasibility and complications of the procedure, in addition to the length of hospital stay. Pain severity was estimated before treatment and 1 and 6 months after the procedure using the visual analog scale (VAS). Functional outcome was assessed by improved patient walking ability.
Results
All sessions were completed and well tolerated. There were no complications related to either incorrect positioning of the screws during bone fixation or leakage of cement. All patients were able to walk within 6 h after the procedure and the average length of hospital stay was 2 days. The mean VAS score decreased from 7.1 (range, 4–9) before treatment to 1.6 (range, 0–6), 1 month after treatment, and to 1.4 (range 0–6) 6 months after treatment. Neither loosening of the screws nor additional bone fractures occurred during a median follow-up of 6 months.
Conclusions
Our results suggest that PSFPC might be a safe and effective procedure that allows the stabilization of the fracture and the prevention of pathological fractures with significant pain relief and good recovery of walking ability, although further studies are required to confirm this preliminary experience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone metastases are a common occurrence in advanced malignancies of various organ sites, and represent a cause of severe morbidity. Osteolytic metastases can be responsible for pain and pathological fractures. Avoidance of pathological fractures of bones subjected to load should be achieved by a multidisciplinary approach. The main goals of treatment are the palliation of pain and improvement in the quality of life by the prevention of complications. Management options for skeletal metastases vary in keeping with bone type, anatomical location, and loading condition. Local treatments, principally surgery and radiation, in addition to systemic treatments such as chemotherapy, hormonal therapy, bisphosphonates, and analgesics, are commonly used in different clinical scenarios [1]. Surgery is the standard of care to improve mechanical stability by means of prosthesis insertion or osteosynthesis. Prophylactic stabilization surgery has improved the quality of life of patients by decreasing inability and pain levels related to pathological fractures, and has also reduced morbidity rates compared with surgery performed for pathological fractures. However, prophylactic stabilization surgery is associated with a non-negligible surgical morbidity and mortality [2–4]. Many patients with advanced metastatic disease are considered bad surgical candidates owing to general conditions, short life expectancy, and poor quality of life. In recent years, there has been a surge in the development of minimally invasive approaches, which represent an attractive curative alternative to surgical stabilization surgery [5–7].
Percutaneous osteosynthesis by screws and cementoplasty (PSFPC) is a well-established method for the treatment of skeletal metastases with or without fractures, especially in elderly patients with comorbidities [8–11]. In this technique, devices are inserted under imaging guidance into the metastatic tissue [12]. The aims of this treatment are to stabilize pathological fractures in patients unfit for open surgery, strengthen metastatic bones at a high risk of fracture, achieve pain relief and improve functional outcomes such as walking ability. The aim of this study is to evaluate the feasibility and effectiveness of CT-guided PSFPC in patients who have painful bone metastases located in the vertebrae, pelvis and femur, with or without pathological fractures.
Materials and methods
Informed consent was obtained from all individual participants included in the study. All cases were discussed in multidisciplinary meetings, including interventional radiologists, medical oncologists, radiotherapists and orthopedic surgeons. Twenty-seven patients with 34 bone metastases underwent PSFPC under CT-fluoroscopic guidance. Ten patients were men and 17 women; mean age was 57 years. All patients had previously undergone standard treatments for skeletal metastases: 10 had received radiotherapy, 4 radiotherapy in combination with chemotherapy, and 13 chemotherapy alone. All patients complained of pain refractory to conventional medical management, such as opioid drugs or a combination of opioids and nonsteroidal anti-inflammatory drugs. In each patient, the severity of pain was assessed through a validated pain assessment tool, the visual analog scale (VAS), in which pain levels are measured on a continuous scale ranging from 0 to 10 [13]. Preoperative contrast-enhanced CT and/or spine magnetic resonance imaging (MRI) scans were obtained in all patients to assess the number, location, size, and radiological aspects of the lesions. In 20 out of 27 patients (74%), there was a solitary lesion, whereas the remaining 7 patients (26%) had two or more lesions. Thirty bone lesions were of osteolytic nature, presenting bone destruction with or without a soft-tissue mass and were at a high risk of fracture; 4 lesions had mixed lytic-osteoblastic appearance. Twenty out of 34 lesions (59%) had an associated pathological fracture. The skeletal distribution of metastases and histopathology diagnosis of the primary tumor are summarized in Table 1.
Inclusion criteria for PSFPC treatment were as follows: multiple fractures of the vertebral body; osteolytic lesions involving pedicles; fractures of the pelvis and femur unsuitable for surgical treatment; and life expectancy estimated longer than 2 months. In patients with two or more bone metastatic lesions, treatment was restricted to those that were symptomatic. As for prophylactic PSFPC treatment, metastases located in load-bearing bones (vertebrae, femoral head and neck, and pelvis), lesions causing disruption of the cortical tissue more than 3 cm with tumor tissue arising from the bone, and very large lesions of osteolytic aspect, were considered as lesions at a high risk of fracture. Nonsteroidal anti-inflammatory drugs and opioids were usually discontinued 1 week after treatment, and resumed only in cases of worsening of painful symptoms. Pain levels, as assessed by VAS score, were monitored at 1 week, 1 month and 6 months after treatment. As for functional outcomes, a qualitative scale for the assessment of patients’ walking ability was used that rates this ability as worse, unchanged, or improved. CT scans were acquired 3 and 6 months after the procedure to identify signs of loosening of the screws or new fractures in the treated site.
Treatment technique
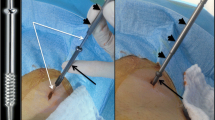
A board-certified interventional radiologist (CP) with 12 years of experience with osteoplasty techniques performed all the treatments. PSFPC was carried out under CT guidance with 5-mm collimation at 80–140 mA (SOMATOM Sensation; Siemens, Forchheim, Germany) with patients in a prone, lateral or supine position depending on the site of the metastasis. To obtain precise needle placement inside the bone, increase operator comfort, and reduce the rate of complications, dual imaging guidance with CT and fluoroscopy was used. Antibiotic preoperative (a single dose of cefazolin 2 g) was administered intravenously 20–30 min before treatment. PSFPC was performed under local anesthesia (using a subcutaneous injection of 2% lidocaine hydrochloride), conscious sedation (achieved with intravenous fentanyl 0.1 mg/2 mL diluted 1:10 with saline solution), plus a precise local anesthesia in the cortical bone using levobupivacaine 5.0 mg/mL in preparation for the successive screw insertion by means of a fine needle. Cannulated screws (2B1®, Milan, Italy) with lateral holes were used. Those devices allowed selective injection of the cement through the screw, avoiding undesired leakage. We used two different kinds of screw depending on the different locations of the metastasis to be treated: screws of 4.5 mm in diameter and 4–6 cm in length for the vertebral approach, and screws of 9.5 mm in diameter and 8–11 cm in length for the extraspinal metastases. In the vertebral approach the pedicle was cannulated percutaneously by using a biopsy bone needle. After correct positioning of the vertebroplasty cannula a k-wire was inserted into the cannula and subsequently the vertebroplasty cannula was removed. After a small incision in the skin we inserted the screw using the k-wire as a guide to push the screw into the final position. To obtain a better augmentation of the vertebral body and to avoid loosening of the screws we completed the treatment with injection of the cement through the screw. In patients who presented a very extensive destruction of vertebral body or in cases of bilateral involvement of both pedicles, we performed the treatment with insertion of two screws in the same session. When extravertebral metastases were treated, the procedure was different because before inserting the screw, a bone canal was created using a cannulated drill of an adequate size. The k-wire previously inserted allowed us to correctly create the bone canal using the drill. After the bone canal was created the screw was inserted, and, similar to the vertebral procedures, the treatment was completed with polymethyl methacrylate (PMMA) injection through the lateral holes of the screw. The use of combined CT and fluoroscopic guidance allows us to avoid complications during screw insertion, especially when treating bone vertebral metastases with severe collapse.
Statistical analysis
For the purposes of this study, continuous variables were shown as mean ± standard deviation (SD). Differences between the average VAS scores at baseline and 1 and 6 months after the procedure were evaluated by means of Student’s t test or Fisher’s exact test as appropriate. A p value less than 0.05 was taken as significant. Statistical analysis was performed using OpenStat software.
Results
In all cases, we observed the technical success of the PSFPC technique, defined as the ability to achieve correct screw insertion and fixation without significant leakage of cement. Minimal leakage of cement occurred in 5 patients out of 27 patients (19%). The average duration of the procedure was 40 min for each single lesion treatment; the quantity of PMMA was within the range of 2.5 to 15 cm3. Usually, a minimal volume of cement is determined before the treatment, depending on the area to be filled, but the amount of cement may vary during injection of the PMMA based on the fluoroscopic imaging. Post-procedural CT scans (without contrast enhancement) did not demonstrate any major complications, such as hemorrhage, incorrect screw position or cement leakage (Figs. 1 and 2). Only 2 patients out of 27 (7%) complained of moderate pain during postoperative day 1. All patients were able to walk within 6 h of the procedure and the average length of the hospital stay was 2 days. In all patients, a pre- and post-procedural evaluation of walking ability was determined. Based on this qualitative scale, functional outcome conditions were considered worse, unchanged or improved. One and 6 months after the treatment, walking ability was improved in all cases (100%). Mean VAS score of pain evaluation on the day before treatment was 7.1 ± 1.1 (range, 4–9). One month after treatment the median VAS score of pain was 1.6 ± 2.0 (range, 0–6) with a mean reduction of 78% (7.1 ± 1.1 vs 1.6 ± 2.0; p < 0.000; Fig. 1). At the 1-month evaluation 16 out of 27 patients (59%) were symptomatic and among these, only 7 (26%) had already undergone analgesic therapy. At the 6-month evaluation, the median VAS score for pain was 1.4 ± 1.8 (range 0–6) with a mean reduction of 80% (7.1 ± 1.1 vs 1.4 ± 1.8; p < 0.000) compared with baseline evaluation. The 11 patients who were asymptomatic 1 month after treatment were still asymptomatic at 6 months. During follow-up, all patients had an improvement in both VAS score and walking ability, but only 5 patients at 6-month evaluation had a VAS score ≥4 (Fig. 3). These patients resumed pain treatment with nonsteroidal anti-inflammatory drugs, having already been treated with radiotherapy and/or radio-chemotherapy. During follow-up, no infectious complications were observed. None of the patients died of disease progression during the considered follow-up.
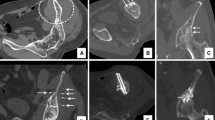
a Axial CT imaging shows multiple fractures in the vertebral body of T11 in patients with multiple bone metastases from breast cancer. b Sagittal CT imaging shows a severe collapse of the vertebral body of T11 (arrow).c Axial CT imaging of T11 shows the screws inserted through the pedicles within the vertebral body. d Axial CT imaging of T11 shows vertebral augmentation after screw positioning and PMMA injection. e Sagittal CT imaging of T11 shows the correct final position of the screws within the vertebral body
a Axial CT scan of the pelvis shows large osteolytic metastasis of the left ilium from breast cancer with multiple pathological fractures (arrows). b Axial CT scan of the pelvis acquired after screw fixation and cementoplasty. c The multiplanar reconstruction CT scan shows the correct positioning of the screw in the left iliac metastasis. The digitations of the cement from the screw to the bone hamper the movement of the screw
Contrast-enhanced CT scans performed 3 and 6 months after the procedure showed no loosening of the screws or new fractures in the treated site.
Discussion
Metastatic bone disease is a common clinical occurrence in patients with advanced cancer and can cause different symptoms and discomfort. In particular, pain symptoms originating from bone metastases are often difficult to treat because of the common unresponsiveness to standard treatments [14]. Pathological fractures of osteolytic lesions cause significant pain and loss of function. The decision to perform an orthopedic treatment for fracture fixation or for the prevention of pathological fractures is a difficult one, because of the general condition of the patient, and the short life expectancy. The results of this study suggest that PSFPC might represent a good alternative for patients who are not candidates for standard surgical stabilization, although it is possible for the mechanical consolidation obtained with PSFPC to be strengthened with regard to orthopedic prosthesis or osteosynthetic devices inserted during open surgery. This technique, initially developed by orthopedic surgeons to stabilize nondisplaced bone fractures during open surgery, is now performed percutaneously by means of interventional radiology techniques using cannulated screws. A limited number of reports using percutaneous osteosynthesis plus cementoplasty have demonstrated promising results in the treatment of femur fractures [15]. Our study included patients with different sites of intervention (vertebrae, pelvis, and femur). The level of consolidation obtained in our series seems sufficient, in patients with advanced cancer disease or those with a poor general condition, because we did not experience any postoperative fractures, despite a high-risk population. The mean reduction of the VAS score observed after treatment may be due to an exothermic reaction of the cement, which has a direct cytotoxic effect on the tumor cells. Moreover, cement may act on the interosseous sensory nerve fibers and together with the screws, contribute to the immediate stability of fractures and microfractures [15]. Our approach is similar to that described by Cazzato et al. [16], who reported on percutaneous image-guided screw fixation of bone lesions in cancer patients. However, our approach was different because cementoplasty was performed after insertion of the screw in all patients. This could explain the absence of screw dislocation. Indeed, the injection of PMMA, performed through the lateral holes from inside to outside of the screw, allows the direct attachment of the screw to the bone, even if an osteolysis is present, preventing the rotation and sliding of the screw after cement solidification. Furthermore, the robustness of the titanium screw prevents, once inserted, further bone fracture without the need for surgical stabilization. We recognize that our study has some limitations, such as the small sample size, retrospective design, and short duration of follow-up. However, we believe that this is a promising new approach for the minimally invasive treatment of bone metastases.
Conclusions
Percutaneous screw fixation plus cementoplasty is a safe and feasible technique for pain palliation and for the consolidation of osteolytic metastases in patients with bone metastases. These results may introduce a new method of palliative treatment in patients with painful bone metastatic lesions with fractures or those at a high risk of fracture in the femur and other skeletal locations.
References
Janjan N. Bone metastases: approaches to management. Semin Oncol. 2001;28:28–34.
Ward WG, Holsenbeck S, Dorey FJ, et al. Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res. 2003;415(Suppl):S230–44.
Popken F, Schmidt J, Oegur H, et al. Treatment outcome after surgical management of osseous breast carcinoma metastases. Preventive stabilization vs. management after pathological fracture. Unfallchirurg. 2002;105(4):338–43.
Ristevski B, Jenkinson RJ, Stephen DJ, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52(4):302–8.
Anselmetti GC, Manca A, et al. Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients. Cardiovasc Intervent Radiol. 2008;31:1165–73.
Botton E. Cementoplasty for painful bone metastases: a series of 42 cases. Med Oncol. 2012;29:1378–83.
Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical followup. Radiology. 1996;200:525–30.
Schep NW, Heintjes RJ, Martens EP, et al. Retrospective analysis of factors influencing the operative result after Percutaneous osteosynthesis of intracapsular femoral neck fractures. Injury. 2004;35(10):1003–9.
Phan K, Rao PJ, Mobbs RJ. Percutaneous versus open pedicle screw fixation for treatment of thoracolumbar fractures: systematic review and meta-analysis of comparative studies. Clin Neurol Neurosurg. 2015;135:85–92.
Trumm CG, Rubenbauer B, Piltz S, Reiser MF, Hoffmann RT. Screw placement and osteoplasty under computed tomographic-fluoroscopic guidance in a case of advanced metastatic destruction of the iliosacral joint. Cardiovasc Intervent Radiol. 2011;34[Suppl 2]:S288–93.
Tan JW, Shen BH, DU W, Liu JQ, Lu SQ. Bone cement enhanced pedicle screw fixation combined with vertebroplasty for elderly patients with malignant spinal tumors. Chin Med J (Engl). 2013;126(13):2495–8.
Clarençon F, Jean B, Pham HP, et al. Value of percutaneous radiofrequency ablation with or without percutaneous vertebroplasty for pain relief and functional recovery in painful bone metastases. Skeletal Radiol. 2013;42:25–36.
Chapman CR, Case KI, Dubner R, et al. Pain measurement: an update. Pain. 1985;22:1–31.
Deschamps F, Farouil G, Hakime A, Teriitehau C, Barah A, de Baere T. Percutaneous stabilization of impending pathological fracture of the proximal femur. Cardiovasc Intervent Radiol. 2012;35(6):1428–32.
Weill A, Chiras J, Simon JM, Rose M, Sola-Martinez T, Enkaoua E. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241–7.
Cazzato RL, Koch G, Buy X, et al. Percutaneous image guided screw fixation of bone lesions in cancer patients: double centre analysis of outcomes including local evolution on the treated focus. Cardiovasc Intervent Radiol. 2016;39(10):1455–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Pusceddu, C., Fancellu, A., Ballicu, N. et al. CT-guided percutaneous screw fixation plus cementoplasty in the treatment of painful bone metastases with fractures or a high risk of pathological fracture. Skeletal Radiol 46, 539–545 (2017). https://doi.org/10.1007/s00256-017-2584-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2584-y