Abstract
Purpose
To evaluate technical success, complications, and effective dose in patients undergoing CT fluoroscopy-guided iliosacral screw placement for the fixation of unstable posterior pelvic ring injuries.
Materials and methods
Our retrospective analysis includes all consecutive patients with vertical sacral fractures and/or injury of the iliosacral joint treated with CT fluoroscopy-guided screw placement in our department from 11/2005 to 03/2013. Interventions were carried out under general anesthesia and CT fluoroscopy (10–20 mAs; 120 kV; 16- or 128-row scanner, Siemens Healthcare, Erlangen, Germany). Technical outcome, major and minor complications, and effective patient dose were analyzed.
Results
We treated 99 consecutive patients (mean age 53.1 ± 21.7 years, 50 male, 49 female) with posterior pelvic ring instability with CT fluoroscopy-guided screw placement. Intervention was technically successful in all patients (n = 99). No major and one minor local complication occurred (1 %, secondary screw dislocation). General complications included three cases of death (3 %) due to pulmonary embolism (n = 1), hemorrhagic shock (n = 1), or cardiac event (n = 1) during a follow-up period of 30 days. General complications were not related to the intervention. Mean effective patient radiation dose per intervention was 12.28 mSv ± 7.25 mSv. Mean procedural time was 72.1 ± 37.4 min.
Conclusions
CT fluoroscopy-guided screw placement for the treatment of posterior pelvic ring instabilities can be performed with high technical success and a low complication rate. This method provides excellent intrainterventional visualization of iliac and sacral bones, as well as the sacral neuroforamina for precise screw placement by applying an acceptable effective patient dose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pelvic fractures represent approximately 3 % of all skeletal injuries [1, 2]. Of these, 17–30 % are unstable fractures of the posterior pelvic ring including a possible disunion of the iliosacral joint [3, 4]. Unstable pelvic fractures of the posterior pelvic ring are potentially life-threatening injuries, which require early and effective fixation.
In most centers, percutaneous placement of iliosacral screws is currently the most established treatment for fixation of posterior pelvic bones [5–7]. This method was initially performed under c-arm fluoroscopic guidance in the operating theatre. However, c-arm fluoroscopic guidance may constitute important disadvantages: As it can be difficult to sufficiently visualize the posterior pelvic anatomic structures, there is a considerable risk of screw malpositioning into the sacral neuroforamina or extra vertebrally. This may cause severe complications such as injury of the sacral nerves or the venous plexus leading to paralysis or hemorrhage. Under c-arm fluoroscopic guidance, the incidence of malpositioning of the inserted screws has been reported to be up to 15 % [6, 8, 9].
Therefore, in selected centers, CT-guided screw placement has been introduced into clinical practice for the treatment of patients with unstable posterior pelvic fractures [7, 10, 11]. CT guidance offers an accurate visualization of the anatomical posterior pelvic structures, as well as of the inserted guide wires and screws, thus avoiding malpositioning and injury of critical anatomical structures. Excellent results regarding the safety, accuracy, and cost-effectiveness using CT-guided screw placement have been reported [7, 10, 12, 13]. These published clinical studies reported on screw placement under sequential CT-guidance without the use of CT fluoroscopy.
In contrast to sequential CT guidance, CT fluoroscopy-guided interventions can be performed faster and with lower patient radiation dose [14–16]. To the best of our knowledge, there was only one comparatively small technical report about CT fluoroscopy guidance in the placement of iliosacral screws [17]. This technical report showed an excellent technical outcome in six patients [17]. No study reported on the procedure-related effective patient radiation dose.
The purpose of this study was to evaluate technical success, complications, and effective dose in patients undergoing CT fluoroscopy-guided iliosacral screw placement for the fixation of unstable posterior pelvic ring injuries.
Materials and methods
Patients
This is a retrospective single-center clinical study. We included all consecutive patients with vertical fractures of the sacrum and/or injury of the iliosacral joint who underwent CT fluoroscopy-guided screw placement in our department from 11/2005 to 03/2013. Our institutional ethics board did not demand approval of this retrospective study regarding the review of clinical patient charts and images. The principles of the Declaration of Helsinki were followed.
The local trauma department referred patients to our department after high- or low-energy trauma, or due to osteoporosis, or due to metastases. Subsequently, a multidisciplinary team consisting of an interventional radiologist, a trauma surgeon, and an anesthesiologist confirmed the indication for CT fluoroscopy-guided screw placement. The following inclusion criteria were applied:
-
The instability of the posterior pelvic ring was caused by a sacral fracture or an injury of the iliosacral joint.
-
The fracture was classified as AO type B with rotational instability or AO type C with rotational and vertical instability.
-
The indication for one screw on the affected side was given in cases of dislocation of the iliosacral joint. The indication for two screws on the affected side was given in cases of vertical sacral fractures.
Procedures
All procedures were performed under general anesthesia in collaboration of one board-certified interventional radiologist with at least 5 years of experience in CT-guided interventions and one board-certified trauma surgeon with also at least 5 years of experience in trauma surgery. Interventions were carried out on a 16- or 128-row scanner with CT fluoroscopy mode (Siemens Somatom Sensation 16, Definition AS or Somatom Definition Edge, Siemens Healthcare Sector, Erlangen, Germany). Patients were positioned in a stable right or left lateral position, depending on the side of the planned screw insertion, using a vacuum mattress. First, a preinterventional non-enhanced planning CT scan (3-mm axial images) was performed to determine the anatomical approach for an exact placement of K-wires and screws. By means of the axial images, the suitable slice positions for cortical entry of the K-wires were planned with particular respect to a screw placement that would be perpendicular to the S1 and S2 pedicles [13]. In addition, the length of the surgical screws was calculated from the outer cortex of the iliac bone to the intended depth of penetration.
After skin disinfection and sterile draping including the gantry, the calculated entry points were marked with a 22-gauge needle under CT fluoroscopy, and small skin incisions were made, which were deepened with a clamp to facilitate passing of wires and screws. The K-wires were introduced into the iliac bone by the interventional radiologist with the help of a lightweight hammer under intermittent single-shot CT fluoroscopic acquisitions (CARE Vision CT; Siemens Healthcare Sector, Erlangen, Germany). After correct K-wire positioning into the first and second sacral vertebral body, predrilling for screw insertion was performed in high bone quality. Afterwards, the planned hollow screws (ACE DePuy, Warsaw, IN, USA) were inserted by the trauma surgeon via the K-wires with the use of a surgical drill using the compression effect by the screw for final injury reposition. Subsequently, the K-wires were removed. A final unenhanced CT scan of the pelvic region was performed to evaluate the correct fracture repositioning and screw position (see an example for the intervention in Fig. 1). If there was an indication for bilateral screw placement, the patient was positioned on the opposite side and the procedure was repeated on the respective side. In cases of severely osteoporotic pelvic bones, an additional sacroplasty had to be performed. After successful screw placement, the interventional radiologist inserted a ten-gauge vertebroplasty cannula (Optimed Medical Devices, Ettlingen, Germany) with a length of 15 cm into the hollow screws, monitored by CT fluoroscopy acquisitions. Thereafter, up to 5 ml of PMMA (Biomet Osteopal V, Biomet GmbH, Berlin, Germany) were injected through each cannula into the fractured sacral region under short continuous CT fluoroscopic acquisitions. After cement application, a control CT scan was obtained to control the PMMA distribution (see Fig. 2). After final skin disinfection, the surgeon performed the wound closure using one to two stitches per screw, followed by sterile wound dressing. The duration of the procedure was determined as the period between the first CT acquisition and the last CT control.
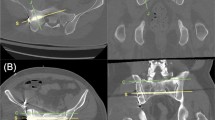
CT fluoroscopy-guided screw placement in a 68-year-old male patient with bilateral vertical fractures of the sacrum after a fall. a CT shows bilateral vertical fracture of the sacrum (arrows indicating fracture lines). b Intrainterventional CT fluoroscopy image (patient in right lateral decubitus position) shows insertion of K-wire. c Final screw position. d VRT (volume rendering technique) image shows final position of four screws inserted into S1 and S2
Screw placement and sacroplasty in an 89-year-old male patient. The fracture of the osteoporotic sacral bone was caused by a fall. a CT shows bilateral sacral fracture (arrows indicating fracture lines). b CT fluoroscopy image shows cement injection and partially inserted screw in S1; after cement injection, the screw is inserted to its final position. In this case, the vertebroplasty cannula was inserted next to the screw with a surgical hammer, with the needle tip next to the fracture region. c Final position of the screw and injected cement in S1. d VRT image of inserted screws and cement. There is a small leakage of PMMA in a sacral vein in S2
Technical results
Technical success was defined as primarily successful procedure with stabilization of the posterior pelvic ring.
Effective patient dose
Effective patient dose was calculated for each procedure. Therefore, we summarized the effective dose of the preinterventional planning CT, the sum of all intrainterventional CT fluoroscopic acquisitions, and the post-interventional control CT. Effective patient dose for planning CT scan and post-interventional control CT scan were calculated by the following formula: E = DLP * t (effective dose = dose-length product * tissue weighting factor). The tissue weighting factor for the pelvic region was defined as t = 0.0129 [18]. The sum of all CT fluoroscopic acquisitions was evaluated for effective dose by the adjusted formula for CT fluoroscopy: E = DLP * k (k = 0.018) [19].
Complications
To assess per interventional complications, an experienced radiologist evaluated each CT scan and all CT fluoroscopic acquisitions in each procedure. CT scans were evaluated for position of the screws, bleeding, material failure, and affection of the neuroforamina and sacral canal. Additionally, the respective surgery records were evaluated. For the assessment of post-interventional morbidity and mortality, we evaluated patient files and post-interventional imaging up to 30 post-interventional days.
Statistical analysis
For data collection and statistical analysis, the software SPSS Version 17.0 (SPSS Inc., Chicago, IL, USA) was used.
Results
Patient characteristics
A total of 99 patients (50 male, 49 female) with rupture of the iliosacral joint and/or sacral fractures underwent percutaneous CT fluoroscopy-guided iliosacral screw placement in our department between November 2005 and March 2013. The mean age was 53.1 ± 21.7 years (range, 17–89 years). Most sacral fractures and iliosacral injuries were caused by high-energy trauma (n = 57), which were car accidents (n = 44) or falls from a great height (n = 13). There were also cases with low-energy trauma (n = 37), which were falls by stumbling (n = 28) and sports accidents (n = 9). Furthermore, there were fractures due to osteoporosis in four patients and due to a sacral metastasis from renal cell cancer in one patient. Fractures were classified as AO type B (n = 74) with horizontal instability and vertical stability of the pelvis, or as AO type C (n = 25) with horizontal and vertical instability. Most fractures (n = 75) were limited to the sacrum without injury of the iliosacral joint. The patient characteristics are summarized in Table 1.
Intervention characteristics
Due to severity of pelvic injury, there were patients (n = 30) who had to undergo pelvic surgery before percutaneous CT fluoroscopy-guided screw placement. Previous pelvic surgery included external fixation (n = 21), plate fixation of the symphysis (n = 8), or both (n = 1). The mean interventional procedure time was 72.1 ± 37.4 min (range, 30–235 min). A total of 222 screws were placed in 99 patients, corresponding to a mean of 2.2 screws per patient (see Tables 2 and 3: Intervention characteristics).
Mean effective patient dose was 12.3 ± 7.3 mSv, including effective dose of the preinterventional planning scan, intrainterventional CT-fluoroscopy, and post-interventional control scan. Mean dose length product was 860 ± 512 mGy*cm, consisting of planning and control CT scan (mean DLP including both was 626 ± 452 mGy*cm) and the fluoroscopic CT images during procedure (mean DLP was 234 ± 239 mGy*cm).
Cement injection was necessary in nine cases with fracture and osteoporotic bone. There was one complication related to the cement injection, which was a small leakage of PMMA in a sacral vein without any consequence or further clinical sequelae.
Technical outcome/complications
The intervention was primarily technically successful in 99 patients (100 %). There was no need of a secondary operative or interventional revision as the screws could be positioned correctly in all cases.
There was one local complication (1 %), which was a slight dislocation of a screw. The dislocation was seen in the post-interventional control scan, which showed the marginal perforation of the cortical bone of the neural foramen S2 (see Fig. 3). There was no affection of the sacral nerves and the patient showed no clinical symptoms. There was no local complication such as screw malpositioning, material failure, harm of the sacral canal or inter vertebral foramina, or bleeding in the remaining 98 cases (99 %).
Case with perforation of the cortical bone of the neuroforamen S2 in a 40-year-old female patient. The polytrauma patient suffered from multiple severe injuries after a car accident. a Bilateral sacral fracture indicated by arrows. b CT fluoroscopy-guided insertion of a screw into S2 on the left side. The patient is positioned in a right lateral decubitus position during the insertion. c CT fluoroscopy image shows modified patient position before insertion of a screw on the right pelvic side. d CT control image shows perforated cortical bone of the second sacral neuroforamen on the left side (star) (no clinical symptoms, no need of revision)
Post-procedural morbidity/mortality
During the 30-day post-interventional period, three cases of death (3.0 %) occurred, with no evidence for a relation to the screw placement procedure.
A 55-year-old woman died 14 days after the procedure due to a hemorrhagic shock after thoracic and pelvic hematoma. A 33-year-old man had a fulminant pulmonary embolism associated with cardiac arrest 12 days postoperatively. An 89-year-old man died 7 days after the intervention due to an acute coronary syndrome with heart and acute renal failure.
The post-interventional 30-day morbidity (without relation to the procedure) was 8.1 %. The cases of ARDS/ALI (5.1 %), SIRS (1.0 %) and severe decubitus ulcer (1.0 %) were caused during the stay in the intensive care unit. In one case, a patient suffered post-interventional from a non-ST-elevation myocardial infarction (NSTEMI).
Discussion
In this study, we report an excellent technical outcome of CT fluoroscopy-guided screw placement for the treatment of unstable fractures of the posterior pelvic ring with all procedures being technically successful, and only one local complication (marginal screw dislocation) without consequences for the patient. Mean radiation dose was 12.28 mSv, and mean procedure time was 72 min.
To achieve a correct screw placement in posterior pelvic fractures, various authors have used c-arm fluoroscopy, which can be performed solely by surgeons in an operating room [6, 9, 20, 21]. Due to poor visualization of the posterior pelvic anatomy, c-arm fluoroscopy-guided interventions are characterized by a significant technical disadvantage: The reported rate of screw malpositioning ranges from 2–15 % [9, 21] and is accompanied by neurological sequelae in 0.5–7.7 % of the cases [6, 8]. In addition, there is a report of a damage of the superior gluteal artery [22]. In contrast, CT-guided placement of iliosacral screws, which was first described by Ebraheim et al. in 1987 [23], is reported to be a feasible, safe, and appropriate technique [7, 10, 12, 13, 17]. The two largest studies on conventional CT-guidance were published by Ziran et al. in 2003 and by Sciulli et al. in 2007. Ziran et al. reported on no displaced screws and no cases of infection or non-union in 66 patients, and only one case of screw breakage at the final follow-up, which did not require treatment [10]. The study by Sciulli et al. included 26 patients: There were two cases of screw dislocations in obese patients, which could be successfully corrected in a second session without any consequences for the patients [7]. In three smaller case series, Blake-Toker et al., Chmelova et al., and Iguchi et al. reported no local complications of CT-guided pelvic screw placements [12, 13, 17]. Our results are in line with the previous reports with only one minor complication, which consisted of a perforated cortical bone of the second sacral neuroforamen with no clinical symptoms, no need of a revision, and therefore no consequences for the patient. All cases of post-procedural morbidity and mortality were most likely related to already-existing comorbidities or severe injuries caused by polytrauma and not related to the procedure. We cannot prove definitely that the deaths that did occur were not related to our procedure. Although, all these patients suffered from the consequences of multiple trauma and had to undergo several trauma operations as well as our procedure. Therefore, we think that these comorbidities overweigh the morbidity caused by our minimally invasive procedure.
However, none of the published studies reported on the effective patient radiation dose during CT- or CT fluoroscopy-guided iliosacral screw placement. Computed tomography imaging makes up the largest part of radiation exposure of the population due to medical reasons and—particularly in interventional radiology—it can be associated with a high level of radiation dose for the patient as well as for the personnel [24–26]. Therefore, we think it is important to report the mean effective dose for the patients, which was 12.28 mSv ± 7.25 mSv in our study. Due to the mostly severe injuries of the patients, we think that this is an acceptable patient dose. Furthermore, as part of the learning curve of the treating interventionalist and technical advancement of CT technology, the dose might be further reduced in the future.
In our study, all screws were inserted with the use of CT fluoroscopy guidance in cooperation of an interventional radiologist and a trauma surgeon in a sterile setting in a CT-interventional suite. We believe that this interdisciplinary approach accounts for a significantly better procedural outcome than if it would be performed by one specialist alone, with the interventional radiologist having profound skills in CT-guidance and cross-sectional imaging anatomy, and the trauma surgeon having skills and knowledge about fracture reduction and treatment as well as about the different osteosynthetic materials such as K-wires and screws.
In comparison to c-arm fluoroscopy, the major advantage of CT fluoroscopy-guided screw placement is the direct visualization of the sacral neuroforamina and canal; however, a potential limitation is particularly given in obese patients with only a limited access within the gantry during K-wire insertion. Nevertheless, in our hospital, none of these patients had to be excluded from CT fluoroscopy-guided screw placement due to obesity, most probably because our CT scanners have a gantry diameter of up to 78 cm (Siemens Somatom Definition AS and Edge).
With sequential CT, reconstruction and display of the images may be time-consuming. In contrast, CT fluoroscopy is able to provide rapid reconstruction and in-room display of the images leading to real-time visualization during interventional procedures, which facilitates a shorter needle placement time. Since the introduction of CT fluoroscopy in the 1990s, several advantages such as a lower mean patient dose, a shorter procedure time, and an increased procedure efficacy have been reported, when compared to sequential CT guidance [14, 15, 27, 28]. However, radiation dose to the interventionalist and the other staff still remains a concern [29]. Modifications and advances of the CT fluoroscopy technique can reduce the fluoroscopic time, and effective patient and operator dose. These include the preferential use of an intermittent quick-check technique instead of real-time CT fluoroscopy [29], low-milliampere CT fluoroscopy (depending on the weight of the patient), the use of radiation-protective devices such as needle holders and special gloves, as well as an angular beam modulation, which particularly contributes to a substantially lower radiation dose for the interventionalist [30]. So far, the only publication of CT fluoroscopy guidance in pelvic screw placement was a technical report of Iguchi et al. about six patients [17]. Although the authors did not present information about radiation dose or cases with concomitant cement injection and only one case of a bilateral fracture, we confirm their results regarding an excellent technical outcome under CT fluoroscopic guidance.
Based on our results, we think that CT fluoroscopy guidance might be the preferred method for insertion of iliosacral screws in selected patients with unstable injuries of the posterior pelvic ring.
However, this study does have several limitations: The analysis has a retrospective design and included only patients from a single institution. Further, our study was not designed to compare the effectiveness of different guidance modalities such as c-arm fluoroscopy, sequential CT, and CT fluoroscopy guidance. In the future, it might be interesting to perform a prospective, multicenter trial to compare the different above-mentioned imaging modalities regarding technical outcome, complications, and radiation exposure.
In conclusion, CT fluoroscopy-guided screw placement for posterior pelvic ring instabilities can be performed with a high technical success and a low complication rate. By applying an acceptable effective patient dose, this method provides an excellent visualization of the sacral nerves and the sacral canal for precise screw placement.
References
Giannoudis PV, Grotz MR, Tzioupis C, Dinopoulos H, Wells GE, Bouamra O, et al. Prevalence of pelvic fractures, associated injuries, and mortality: the United Kingdom perspective. J Trauma. 2007;63(4):875–83.
Grotz MR, Allami MK, Harwood P, Pape HC, Krettek C, Giannoudis PV. Open pelvic fractures: epidemiology, current concepts of management and outcome. Injury. 2005;36(1):1–13.
Denis F, Davis S, Comfort T. Sacral fractures: an important problem. Retrospective analysis of 236 cases. Clin Orthop Relat Res. 1988;227:67–81.
Dudda M, Hoffmann M, Schildhauer TA. Sacrum fractures and lumbopelvic instabilities in pelvic ring injuries: classification and biomechanical aspects. Unfallchirurg. 2013;116(11):972–8.
Routt Jr ML, Nork SE, Mills WJ. Percutaneous fixation of pelvic ring disruptions. Clin Orthop Relat Res. 2000;375:15–29.
van den Bosch EW, van Zwienen CM, van Vugt AB. Fluoroscopic positioning of sacroiliac screws in 88 patients. J Trauma. 2002;53(1):44–8.
Sciulli RL, Daffner RH, Altman DT, Altman GT, Sewecke JJ. CT-guided iliosacral screw placement: technique and clinical experience. AJR Am J Roentgenol. 2007;188(2):W181–92.
Zwingmann J, Hauschild O, Bode G, Sudkamp NP, Schmal H. Malposition and revision rates of different imaging modalities for percutaneous iliosacral screw fixation following pelvic fractures: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2013;133(9):1257–65.
Templeman D, Schmidt A, Freese J, Weisman I. Proximity of iliosacral screws to neurovascular structures after internal fixation. Clin Orthop Relat Res. 1996;329:194–8.
Ziran BH, Smith WR, Towers J, Morgan SJ. Iliosacral screw fixation of the posterior pelvic ring using local anaesthesia and computerised tomography. J Bone Jt Surg Br Vol. 2003;85(3):411–8.
Trumm CG, Rubenbauer B, Piltz S, Reiser MF, Hoffmann RT. Screw placement and osteoplasty under computed tomographic-fluoroscopic guidance in a case of advanced metastatic destruction of the iliosacral joint. Cardiovasc Intervent Radiol. 2011;34 Suppl 2:S288–93.
Chmelova J, Sir M, Jecminek V. CT-guided percutaneous fixation of pelvic fractures. Case reports. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslovakia. 2005;149(1):177–81.
Blake-Toker AM, Hawkins L, Nadalo L, Howard D, Arazoza A, Koonsman M, et al. CT-guided percutaneous fixation of sacroiliac fractures in trauma patients. J Trauma. 2001;51(6):1117–21.
Carlson SK, Bender CE, Classic KL, Zink FE, Quam JP, Ward EM, et al. Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology. 2001;219(2):515–20.
Paprottka PM, Helmberger T, Reiser MF, Trumm CG. Computed tomography guidance: fluoroscopy and more. Radiologe. 2013;53(11):974–85.
Sarti M, Brehmer WP, Gay SB. Low-dose techniques in CT-guided interventions. Rev Publ Radiol Soc N Am Inc. 2012;32(4):1109–19. discussion 19–20.
Iguchi T, Ogawa K, Doi T, Miyasho K, Munetomo K, Hiraki T, et al. Computed tomography fluoroscopy-guided placement of iliosacral screws in patients with unstable posterior pelvic fractures. Skeletal Radiol. 2010;39(7):701–5.
The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Annals of the ICRP. 2007; 37 (2–4):1–332.
Joemai RM, Zweers D, Obermann WR, Geleijns J. Assessment of patient and occupational dose in established and new applications of MDCT fluoroscopy. AJR Am J Roentgenol. 2009;192(4):881–6.
Routt Jr ML, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207–14.
Hinsche AF, Giannoudis PV, Smith RM. Fluoroscopy-based multiplanar image guidance for insertion of sacroiliac screws. Clin Orthop Relat Res. 2002;395:135–44.
Altman DT, Jones CB, Routt Jr ML. Superior gluteal artery injury during iliosacral screw placement. J Orthop Trauma. 1999;13(3):220–7.
Ebraheim NA, Rusin JJ, Coombs RJ, Jackson WT, Holiday B. Percutaneous computed-tomography-stabilization of pelvic fractures: preliminary report. J Orthop Trauma. 1987;1(3):197–204.
Galanski M, Nagel HD, Stamm G. CT radiation exposure risk in Germany. Fortschr Geb. 2001;173(10):R1–66.
Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84.
Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT. CT fluoroscopy-guided interventional procedures: techniques and radiation dose to radiologists. Radiology. 2001;220(1):161–7.
Daly B, Templeton PA. Real-time CT fluoroscopy: evolution of an interventional tool. Radiology. 1999;211(2):309–15.
Katada K, Kato R, Anno H, Ogura Y, Koga S, Ida Y, et al. Guidance with real-time CT fluoroscopy: early clinical experience. Radiology. 1996;200(3):851–6.
Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF. CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology. 1999;212(3):673–81.
Hohl C, Suess C, Wildberger JE, Honnef D, Das M, Muhlenbruch G, et al. Dose reduction during CT fluoroscopy: phantom study of angular beam modulation. Radiology. 2008;246(2):519–25.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Frederik F. Strobl and Sophia M. Haeussler contributed equally to this work and share first authorship.
Rights and permissions
About this article
Cite this article
Strobl, F.F., Haeussler, S.M., Paprottka, P.M. et al. Technical and clinical outcome of percutaneous CT fluoroscopy-guided screw placement in unstable injuries of the posterior pelvic ring. Skeletal Radiol 43, 1093–1100 (2014). https://doi.org/10.1007/s00256-014-1890-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-014-1890-x