Abstract
Cancer pain is most commonly classified as nociceptive (somatic or visceral) or neuropathic. Different types of pain or pain syndromes are present in all phases of cancer (early and metastatic) and are inadequately treated in 56% to 82.3% of patients. Percutaneous neurolysis and neuromodulation are feasible and reproducible, efficient (70–80% success rate) and safe (≈ 0.5% mean complication rate) palliative therapies for pain reduction in oncologic patients with refractory pain. Percutaneous neurolysis can be performed either by injection of a chemical agent (phenol or alcohol) or by application of continuous radiofrequency or cryoablation. During chemical neurolysis nerve damage is achieved by means of Wallerian degeneration. A thorough knowledge of neural anatomy and pain transmission pathways is fundamental to appropriate patient and technique selection. Imaging guidance and strict asepsis are prerequisites. The purpose of this article is to describe the basic concepts of percutaneous neurolysis in oncologic patients. Controversies concerning techniques and products will be addressed. Finally, the necessity for an individually tailored approach for the selection of the different techniques and targets will be emphasized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is one of the most distressing symptoms of cancer, often having the greatest impact on patients’ quality of life. The prevalence of pain is estimated at 51–66% of cancer patients, the majority of these patients remaining undertreated [1, 2]. The goal of treatment is to minimize patients’ pain without rendering them incapacitated due to drug effects, or causing complications which contribute further to their morbidity in their remaining, often shortened lives [3, 4].
Neurolysis has a role to play in the management of pain arising from direct invasion of pain-sensitive structures, and insult as a result of radiotherapy, surgery or chemotherapy. Patient selection is key and the most commonly utilized criteria are: advanced, progressive cancer and a life expectancy of 6–12 months.
The purpose of this article is to describe the basic concepts of percutaneous neurolysis in oncologic patients. Controversies concerning techniques and products will be addressed. The necessity for an individually tailored approach for the selection of the different techniques and targets will be emphasised.
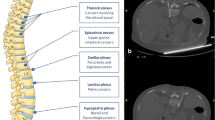
Anatophysiology of the Sympathetic Plexus
The ‘sympathetic axis’ is a pair of ganglionated chains running from the base of the skull to the tip of the coccyx which transmits nociceptive visceral pain from sympathetically mediated organs. These ganglia are organized in cervical (3), thoracic (12), lumbar (4), and sacral (4) ganglia groups. Sympathetically-mediated pain may also have a neuropathic component secondary to direct neural injury e.g. brachial and lumbosacral plexopathy. Most of the various plexi and ganglia described are readily accessible to percutaneous interruption, the most common indication being the control of pain arising from malignancies of the abdomino-pelvic viscera.
Types of Agents/Energy Used
Percutaneous neurolysis can be effected using chemical or thermal means. The two most commonly used agents in chemical neurolysis are phenol (injected at a concentration of 3%–20%) and absolute alcohol (95%–100%) which result in Wallerian degeneration, protein denaturation and coagulative necrosis of neural tissue [5].
Ethanol acts through immediate endoneural lipoprotein and mucoprotein precipitation which causes cholesterol, phospholipid, and cerebroside extraction from the neurilemma; phenol, on the other hand diffuses into axonal and perineural blood vessels causing denaturation of proteins [5]. The most frequently used neurolytic injectate consists of a 6:3:1 ratio of absolute ethanol (95%–100%), bupivacaine, and contrast material [6].
When compared to phenol, which has an immediate local anaesthetic effect, ethanol may cause severe transient pain; phenol is more viscous than absolute alcohol and is therefore more difficult to mix with contrast medium; additionally, the volume of phenol used needs to be decreased by half to prevent local irritation [6].
Alcohol is preferred to phenol because of longer lasting pain palliation, but no evidence is available comparing alcohol to phenol in this setting and the choice is usually based on clinical judgment and local practice. It is important to minimize the volumes of contrast medium and local anaesthetic used so as to avoid dilution of the neurolytic agent and preserve its efficacy.
Thermal neurolysis can be effected by means of heat [radiofrequency (RFA) and microwave (MWA)] or cold [cryoablation (CWA)]. When a tumour engulfs the neural target(s), these methods effect tumour ablation resulting in additional pain alleviation [7].
For radiofrequency neurolysis, a 22 gauge Radiofrequency electrode with a 5 mm active tip is inserted to the target and thermocoagulation performed at 80 degrees for 60 s; the number of heat lesions required to coagulate the target nerve depending on the size of the tip of the electrode. Despite some documented advantages over radiofrequency with regards to tumour ablation, Microwave is rarely used to effect neurolysis, as the volume of ablation and the heat sink effect are not major issues in neurolysis [8,9,10,11].
The main advantage of cryoablation is the visibility of the ablation zone on peri-procedural imaging, facilitating a predictable and reproducible ablation shape. In addition, given its relative analgesic effect, the procedure itself is not particularly painful [7].
In the setting of neurolysis, the cryoablation protocol is still not well established as, in order to obtain only neurolysis, a single freezing cycle may be sufficient provided temperatures of − 140 degrees centigrade are reached effecting irreversible loss of axon continuity, followed by a 2 min thawing cycle allowing the needles to become ‘unstuck’ and safely removed. A bilateral approach, using 1 or 2 small cryoprobes at each side of the targeted plexus seems sufficient. However, due to the high cost of cryoprobes the technique is rarely used for neural ablation. When tumor ablation is needed, the more classical protocol is used. In most centres the procedure is performed under conscious sedation allowing real time neurologic control when ablation is done in the vicinity of sensitive structures. Because of the relative cost of the technique, cryoablation is reserved for patients for whom the risk–benefit ratio of chemical neurolysis appears excessive due to potential unpredictable diffusion of alcohol, or where there is bulky tumor involvement indicating formal ablation.
For all of these techniques, there are a number of relative contraindications, specifically those increasing the risk of significant haemorrhage in the event of inadvertent vascular puncture namely, coagulopathy, thrombocytopenia, or anticoagulant therapy. Coagulation parameters should be normalised and anticoagulants discontinued pre-procedure, and particular care is required when the anatomy is pathologic or variant e.g. disrupted by tumor. Location specific contraindications will be described in their relative sections below.
Anatomic Locations/Neurolysis Targets
Stellate Ganglion
The principal ganglion measures approximately 0.5–2.0 cm and lies near the anterior aspect of the head of the first rib. The goal of treatment is to deliver the drug/device as close to the ganglion without damaging surrounding structures (lung, carotid and vertebral arteries, brachial plexus).
Stellate ganglion neurolysis is indicated in the management of nociceptive and neuropathic pain secondary to malignancies affecting the upper limb, thoracic viscera and head and neck, including iatrogenic plexopathy and postmastectomy pain [12,13,14,15,16,17,18].
Other symptoms, such as hot flashes in breast cancer patients also respond favourably [19].
A 22–25G spinal needle is directed to the head of the first rib and withdrawn 0.25 cm (Fig. 1). Chemical neurolysis is typically effected using the recipe above with the addition of 80 mg of Methylprednisolone to render the after-effects of the procedure less painful and to dampen post-neurolysis inflammation which may be very uncomfortable. A successful blockade is evinced by an increase in the ipsilateral hand skin temperature and a Horner’s syndrome comprising ptosis, pupillary miosis and facial anhydrosis [20, 21].
Computed tomography-guided Stellate ganglion chemical neurolysis in 2 different patients: A contrast medium (white arrow) dispersion anterior to the head of the first rib (black arrow). B trans-thyroidal approach for stellate ganglion neurolysis—final position of the needle (thick white arrow) is anterior to the head of the first rib (thin white arrow)
The procedure has been described under ultrasound guidance; however, the majority of the cases and series described are in patients with benign disease [14, 22,23,24].
Radiofrequency neurolysis has been performed under both CT and ultrasound guidance for both benign and malignant conditions [25,26,27]. A single trial comparing conventional radiofrequency and pulsed mode RF in the treatment of post-mastectomy neuropathic pain has been performed, demonstrating superiority of the conventional mode at early and delayed (6 month) follow-up. No significant difference was demonstrated in the patients’ quality of life or functional capacity [28]. In refractory Type 1 chronic regional pain syndrome (CRPS), CT-guided radiofrequency neurolysis has been shown to be more effective than chemical neurolysis [29,30,31]; however, to our knowledge, this comparison has not been performed in the oncologic population.
In addition to the general relative contraindications described above, contralateral pneumothorax or major pulmonary dysfunction contraindicates the procedure, as pneumothorax would result in severe respiratory compromise. Similarly, in the presence of recognized unilateral vocal cord paralysis, the expected cord paralysis on the treated side would result in complete airway obstruction. Cardiac compromise contraindicates the procedure as the sudden change in sympathetic tone may be too great a challenge for a compensated heart [32, 33].
A degree of brachial plexus infiltration is expected, leading to transient arm anaesthesia/weakness; however, more serious and potentially fatal complications have been described, including pneumothorax and tracheal and oesophageal perforation. Bleeding severe enough to result in leading to significant haematoma and airway compromise has been described [34, 35].
Other complications that may occur, common to all neurolytic procedures include: dysaesthesia, neuritis or neuroma formation, failure to relieve pain and early return of pain (secondary to CNS plasticity, axonal regrowth or tumour progression). In the event of an early return of pain, neurolysis may be repeated.
Thoracic Plexus
The sympathetic chain in the thorax runs alongside the spine. At T2, the ganglion lies on the 2nd rib neck, between T3 and T6 levels, the ganglia lie on the rib heads. Between T7 and T10 levels, the ganglia are located at the level of the costovertebral joints, whilst at T11–T12 levels the ganglia are located anterolateral to the vertebral bodies.
Neurolysis of the thoracic plexus is indicated in upper limb and thoracic pain of sympathetic origin caused by paravertebral gutter neoplasms with vertebral association and in patients with phantom limb pain [36]. Relative contraindications are similar to other neurolytic techniques.
The procedure is most commonly performed under CT guidance; the needles advanced under sequential scanning and the correct (extravascular) final position verified with injection of 1-3 ml of contrast (Fig. 2). After a positive diagnostic block with local anaesthetic, one proceeds with the neurolytic technique of choice [37]. Bed rest and observation are necessary post-procedure.
Celiac Plexus–Splanchnic Nerves
Celiac plexus (CPN) and splanchnic neurolysis (SNN) are the most commonly performed sympathetic neurolytic procedures. The celiac plexus is the largest visceral plexus and is retroperitoneal but antecrural, at the anterolateral aortic surface next to the celiac axis and the superior mesenteric artery origins (at the level of the T12-L1 vertebrae); the greater (T5–T9), the lesser (T10–T11) and the least (T12) splachnic nerves consist of presynaptic sympathetic fibers traversing over the anterolateral vertebral aspect. Both of these techniques are effective in minimising afferent pain transmission from abdominal viscera. Although initiated over a century ago by Max Kappis (1914) and routinely used to treat pain arising from upper abdominal cancers [38] and metastases, the literature remains controversial regarding the indications, technical considerations, results and even complications of this technique [39].
CPN or SNN are indicated for visceral (versus somatic or neuropathic) pain in patients with imaging confirmation of visceral (mainly pancreatic, gastric, biliary, colon or adrenal) and/or sympathetic chain involvement. CPN and SNN are recommended for chronic pain, refractory to opioids or to decrease their use because of adverse side-effects, [38, 40, 41].
Earlier use, at the initial timing of pain appearance in a patient’s disease course has been evaluated with promising results [41,42,43]. Tumoural vascular involvement, intra-abdominal infection and bowel obstruction are considered specific contra-indications.
Neurolysis is always performed using image guidance, either percutaneously or under endoscopic ultrasound (EUS). When the percutaneous route is preferred, CT is the most frequent image guidance used to reach the plexus. There is a growing interest in the use of Cone-beam CT with fusion and guidance software. MRI-guidance has been described [44]. Fluoroscopy is not recommended as it lacks adequate anatomic precision.
The posterior paravertebral ante-crural approach requires either bilateral needle placement in the peri-aortic fat on both sides of the celiac trunk origin or a trans-aortic path with the 22G Chiba bevelled needle tip placed just between the aorta and the celiac trunk (Fig. 3). A posterior trans-intervertebral disk approach can be used for patients at an increased risk of haemorrhage, when the anatomy is distorted by tumour or the presence of an aortic aneurysm or when the paravertebral approach is not feasible due to obstruction by ribs, transverse processes or large osteophytes [6]. The use of an anterior approach using a single injection site (theoretically facilitating better diffusion of the neurolytic agent) is limited because of the risk of gut and visceral organ injury and the difficulty performing SNN concurrently.
The intervention is usually performed under local anaesthesia and conscious sedation. Patients should be advised of the potential risks and that their pain will be alleviated but not eradicated, with a concomitant reduced opioid requirement. Careful planning and prior hydration with 0.5–1 L of saline (in order to alleviate potentially severe post-treatment orthostatic hypotension) are mandatory pre-procedure. Having placed the needles, contrast is injected to exclude an intravascular location and to predict the pattern of diffusion. Bupivacaine is injected as a diagnostic test and to decrease the pain related to neurolysis. Then, chemical neurolysis is performed with the recipe as described above. Prior to removing the needles, they should be purged with 1 ml of saline, and the stylet reinserted to avoid agent spillage in the needle tract.
SNN is usually performed during the same procedure or to palliate unsatisfactory diffusion of the neurolytic agent during CPN (Fig. 4), [45, 46]. When the bilateral posterior approach is used, the same needles are pulled back to a retrocrural location and the steps repeated as for CPN [47] the main difference being in the volume of neurolytic agent needed: 5–10 mls of alcohol at both sides is sufficient. When combining both techniques, a total of 30 mls of absolute alcohol can be used (20 ml for CPN and 10 mls for SNN). Specifically for SNN, thermal ablation (RFA, MWA, or CWA) looks promising as an alternative to chemical neurolysis [27, 48].
Endoscopic Ultrasound guided Coeliac Plexus Neurolysis (EUS-CPN) is increasingly performed; however, no comparative study has shown an advantage over the percutaneous approach [49]. Good visualisation of the target, real time imaging and the opportunity to perform CPN very early in the disease course (potentially at the time of diagnosis) are the advantages of this technique [6], the principle disadvantages being limited availability and the difficulty targeting the celiac plexus in cases of massive tumor infiltration.
Whichever technique is used, most series of CPN and SNN report a pain decrease lasting several months in 70%–90% of patients [50], and a significant reduction in opioid medication and opioid-related side effects [51,52,53]. This has translated in some studies to quality of life improvement and in some, an increased overall survival [54].
The complication rate is below 2% [46]; the most common being transient post-neurolysis hypotension, and diarrhoea [55], the most feared being neurological injury [46, 50].
Lumbar Plexus
The lumbar sympathetic chain consists of multiple interconnected autonomic ganglia lying anterolateral to the lumbar second, third and fourth vertebral bodies and supplies the lower abdominal viscera, perineum and lower limbs. Lumbar Sympathectomy (LS) is indicated in the palliation of pain related to lower abdominal visceral and perineal cancers [14, 15, 56, 57]. There are no absolute contraindications to LS; however, the relative contraindications apply, as above.
The procedure has been most commonly described under fluoroscopic guidance [13, 58]. The patient is placed prone on the procedure table and the chain targeted on each side using osseus landmarks at L2, L3 and L4 via a posterior approach with ipsilateral oblique angulation of the C-arm (Fig. 5). At each level, a 22G 15–20 cm needle is advanced inferior to the transverse process to hit the anterolateral vertebral wall and then “stepped-off” anteriorly. Craniocaudal spread of injected contrast confirms an extravascular and extramuscular needle tip location prior to neurolytic injection.
Lumbar plexus neurolysis: A Schematic showing the location of the lumbar plexus anterior to the anterolateral margin of the lumbar vertebra with the patient in the prone position. B Computed tomography—guided lumbar plexus thermal neurolysis RF electrode (thick white arrow) is advanced via a posterolateral approach to the target anterolateral to the L3 vertebral body. C, D Oblique and postero-anterior fluoroscopy view confirming needle-tip (thick white arrow) placement level with the anterior margin of the L4 vertebral body
Imaging techniques with multiplanar reconstruction capabilities (CT or CBCT) permit direct visualization of the needle tip, conferring greater confidence prior to definitive ablation [59, 60]. In one study [61], where fluoroscopic guidance alone was used, psoas muscle injection occurred in 21.3% (46/216), and intravascular injection occurred in 12.5% (27/216). Of the latter, 5% showed contrast spread in the typical craniocaudal pattern in addition to intravascular injection and only 7% showed intravascular spread only. The aspiration test was shown to be poorly sensitive for an intravascular location of the needle tip (40.7%), static radiography post-injection performing better, but still relatively poorly at 70% [62].
A diagnostic block with Bupivacaine is performed and subsequently, 5 ml of absolute Alcohol are injected via each needle. The needle contents should be cleared by flushing with a small volume of saline and replacing the stylet prior to removal. A successful ablation is evinced by flushing, erythema and increased skin temperature on the treated side.
In patients with benign disease, single-level injections at L2 are performed as they have the lowest incidence of psoas muscle injection and genitofemoral nerve effects. When single-level and multiple-level needle techniques were compared, unsurprisingly, a more complete block was achieved with the multiple needle technique [62], thus this is the preferred approach in the oncologic population.
CT-guided Radiofrequency neurolysis has been performed with good results [27]. Magnetic Resonance Imaging was demonstrated as feasible in 91 out of a cohort of 100 patients using an open 0.2 MR system [63].
Bed rest is required for at least 12 h post-procedure as there is a risk of profound orthostatic hypotension from decreased sympathetic tone, occurring in 10%–52% of patients. To reduce this risk, it is advised not to perform bilateral injections at the same setting.
40–50% of patients are expected to improve with reduced pain and opioid requirements. In addition to visceral pain palliation, LS has had promising results in managing other cancer-related symptoms such as tenesmoid rectal pain which can be particularly distressing for patients and difficult to manage, but in a limited series, 83% of treated patients received sustained complete relief of their tenesmus, having failed a trial of standard approaches [57].
A more recently described benefit of LS is that of a reduction in limb girth, tightness and heaviness in patients with gynaecology cancer-related lower limb lymphoedema unresponsive to conservative measures [64].
Complications of LS are not infrequent, and are presumed to be reduced by the use of CBCT or MDCT. Such complications can be severe, including intravascular, aortic wall, muscle and organ injection, renal collecting system injuries and somatic nerve damage. Lateral femoral cutaneous and genitofemoral nerve neuritis are most frequently described, at L3-4 and L4-5, occurring typically with a psoas muscle needle-tip location. Transient and permanent lesions have been described [65].
Ohno and Ohshita described a transdiscal technique at L2-3 and L3-4 to reduce the risk of genitofemoral nerve neuritis by avoiding a transpsoas path, with no neuritis occurring in 14 patients [66, 67].
Haynsworth and Noe demonstrated a lesser incidence of post-sympathetic neuralgia with radiofrequency denervation compared to phenol; however, the phenol effect was longer lasting, 89% with sympathetic blockade after 8 weeks post phenol compared to 12% with radiofrequency [68].
There are numerous case-reports of pelvicalyceal and ureteric injuries, particularly in relation to the use of alcohol and phenol delivered under fluoroscopic guidance, some of which were thought to result from inadvertent needle puncture of the renal tract, others from dissemination of correctly deposited chemical [69,70,71].
Superior Hypogastric Plexus
The superior hypogastric plexus lies anteriorly to the L5-S1 intervertebral disc. Neurolysis is indicated in patients with pain secondary to pelvic cancer related to uterus, ovaries, prostate and rectum [72, 73]. The relative contraindications are similar to other neurolytic techniques.
The procedure is most commonly performed under fluoroscopic or CT guidance; approaches include a posterolateral or trans-discal route. After a positive diagnostic block with local anaesthetic, one may proceed to the neurolytic technique (either by alcohol injection or by thermal energy application). Bed rest and observation are required post-procedure.
Conclusion
Percutaneous neurolytic procedures are low cost, of short duration and hospitalisation with low complication rates (< 2%), decreasing pain and improving quality of life in oncologic patients with refractory pain. Sympathetic chain neurolysis is an effective but often clinically neglected route for pain management and should be considered for these patients whose pain is all too often undertreated.
References
ESMO Guidelines Working Group. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2014;25(Supplement 3):iii124–37.
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag. 2016;51(6):1070–90.
Filippiadis D, Tutton S, Kelekis A. Pain management: the rising role of interventional oncology. Diagn Interv Imaging. 2017;98(9):627–34.
Tam A, Ahrar K. Palliative interventions for pain in cancer patients. Semin Interv Radiol. 2007;24:419–29.
Koyyalagunta D, Engle MP, Yu J, Feng L, Novy DM. The Effectiveness of alcohol versus phenol based splanchnic nerve neurolysis for the treatment of intra-abdominal cancer pain. Pain Physician. 2016;19(4):281–92.
Kambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. RadioGraphics. 2011;31(6):1599–621.
Yarmohammadi H, Nakamoto DA, Azar N, Hayek SM, Haaga JR. Percutaneous computed tomography guided cryoablation of the celiac plexus as an alternative treatment for intractable pain caused by pancreatic cancer. J Cancer Res Ther. 2011;7(4):481–3.
Kurup AN, Callstrom MR. Image-guided percutaneous ablation of bone and soft tissue tumors. Semin Intervent Radiol. 2010;27(3):276–84.
Filippiadis DK, Tutton S, Mazioti A, Kelekis A. Percutaneous image-guided ablation of bone and soft tissue tumours: a review of available techniques and protective measures. Insights Imaging. 2014;5(3):339–46.
Rhim H, Goldberg SN, Dodd GD 3rd, Solbiati L, Lim HK, Tonolini M, Cho OK. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. RadioGraphics. 2001;21:S17–35.
Amabile C, Ahmed M, Solbiati L, Meloni MF, Solbiati M, Cassarino S, Tosoratti N, Nissenbaum Y, Ierace T, Goldberg SN. Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia. 2017;33(1):34–42.
Warfield CA, Crews DA. Use of stellate ganglion blocks in the treatment of intractable limb pain in lung cancer. Clin J Pain. 1987;3(1):13–5.
Koyyalagunta D, Burton AW. The role of chemical neurolysis in cancer pain. Curr Pain Headache Rep. 2010;14(4):261–7.
Ghai A, Kaushik T, Kumar R, Wadhera S. Chemical ablation of stellate ganglion for head and neck cancer pain. Acta Anaesthesiol Belg. 2016;67(1):6–8.
Arter OE, Racz GB. Pain management of the oncologic patient. Semin Surg Oncol. 1990;6(3):162–72.
Okuda Y, Kitajima T, Asai T. Stellate ganglion block, cervical sympathetic block and cervicothoracic sympathetic block. Eur J Anaesthesiol. 1999;16(4):272–3.
Fathers E, Thrush D, Huson SM, Norman A. Radiation-induced brachial plexopathy in women treated for carcinoma of the breast. Clin Rehabil. 2002;16(2):160–5.
Abbas DN, Abd el Ghafar EM, Ibrahim WA, Omran AF. Fluoroscopic stellate ganglion block for postmastectomy pain: a comparison of the classic anterior approach and the oblique approach. Clin J Pain. 2011;27(3):207–13.
Pachman DR, Barton D, Carns PE, Novotny PJ, Wolf S, Linquist B, Kohli S, Smith DR, Loprinzi CL. Pilot evaluation of a stellate ganglion block for the treatment of hot flashes. Support Cancer Care. 2011;19(7):941–7.
Erickson SJ, Hogan QH. CT-guided injection of the stellate ganglion: description of technique and efficacy of sympathetic blockade. Radiology. 1993;188(3):707–9.
Gangi A, Dietemann JL, Schultz A, Mortazavi R, Jeung MY, Roy C. Interventional radiologic procedures with CT guidance in cancer pain management. RadioGraphics. 1996;16(6):1289–304.
Gofeld M, Bhatia A, Abbas S, Ganapathy S, Johnson M. Development and validation of a new technique for ultrasound-guided stellate ganglion block. Reg Anesth Pain Med. 2009;34(5):475–9.
Narouze S. Ultrasound-guided stellate ganglion block: safety and efficacy. Curr Pain Headache Rep. 2014;18(6):424.
Narouze SN. Ultrasound-guided cervical spine injections: ultrasound “prevents” whereas contrast fluoroscopy “detects” intravascular injections. Reg Anesth Pain Med. 2012;37(2):127–30.
Kim ED, Yoo WJ, Kim YN, Park HJ. Ultrasound-guided pulsed radiofrequency treatment of the cervical sympathetic chain for complex regional pain syndrome: a retrospective observational study. Medicine. 2017;96(1):e5856.
Golovac S. Radiofrequency neurolysis. Neuroimaging Clin N Am. 2010;20(2):203–14.
Zavridis P, Tsitskari M, Mazioti A, Filippiadis D. Percutaneous, computed tomography guided neurolysis using continuous radiofrequency for pain reduction in oncologic patients. J Cancer Metastasis Treat. 2017. https://doi.org/10.20517/2394-4722.2017.04.
Abbas DN, Reyad RM. Thermal versus super voltage pulsed radiofrequency of stellate ganglion in post-mastectomy neuropathic pain syndrome: a prospective randomized trial. Pain Physician. 2018;21(4):351–62.
Kastler B, Michalakis D, Clair CH, Allal R, Delabrousse E, Peireira PH, Boulahdour Z, Litzler JF, Monnier F, Fergane B. Stellate ganglion radiofrequency neurolysis under CT guidance. Preliminary study. JBR BTR. 2001;84(5):191–4.
Kastler A, Aubry S, Sailley N, Michalakis D, Siliman G, Gory G, Lajoie JL, Kastler B. CT-guided stellate ganglion blockade vs. radiofrequency neurolysis in the management of refractory type I complex regional pain syndrome of the upper limb. Eur Radiol. 2013;23(5):1316–22.
Forouzanfar T, van Kleef M, Weber WE. Radiofrequency lesions of the stellate ganglion in chronic pain syndromes: retrospective analysis of clinical efficacy in 86 patients. Clin J Pain. 2000;16(2):164–8.
Kimura T, Nishiwaki K, Yokota S, Komatsu T, Shimada Y. Severe hypertension after stellate ganglion block. Br J Anaesth. 2005;94(6):840–2.
Schlack W, Schafer S, Thamer V. Left stellate ganglion block impairs left ventricular function. Anesth Analg. 1994;79(6):1082–8.
Mishio M, Matsumoto T, Okuda Y, Kitajima T. Delayed severe airway obstruction due to hematoma following stellate ganglion block. Reg Anesth Pain Med. 1998;23(5):516–9.
Okuda Y, Urabe K, Kitajima T. Retropharyngeal or cervicomediastinal haematomas following stellate ganglion block. Eur J Anaesthesiol. 2003;20(9):757–9.
Kastler B, Manzoni P, Laborie L, Hussein HH. Other sympatholysis. In: Kastler B, editor. Interventional radiology in pain treatment. Berlin: Springer; 2005. p. 105–7.
Wilkinson HA. Percutaneous radiofrequency upper thoracic sympathectomy: a new technique. Neurosurgery. 1984;15:811–4.
Drewes AM, Campbell CM, Ceyhan GO, Delhaye M, Garg PK, van Goor H, Laquente B, Morlion B, Olesen SS, Singh VK, Sjøgren P, Szigethy E, Windsor JA, Salvetti MG, Talukdar R. Pain in pancreatic ductal adenocarcinoma: a multidisciplinary, International guideline for optimized management. Pancreatology. 2018;18(4):446–57.
Nagels W, Pease N, Bekkering G, Cools F, Dobbels P. Celiac plexus neurolysis for abdominal cancer pain: a systematic review. Pain Med. 2013;14(8):1140–63.
Kawamata M, Ishitani K, Ishikawa K, Sasaki H, Ota K, Omote K, Namiki A. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain. 1996;64(3):597–602.
Amr YM, Makharita MY. Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic cancer: one-year follow-up. Clin J Pain. 2013;29(9):807–13.
de Oliveira R, dos Reis MP, Prado WA. The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cancer pain. Pain. 2004;110(1–2):400–8.
Erdek MA, Halpert DE, Fernandez M, Cohen SP. Assessment of celiac plexus block and neurolysis outcomes and technique in the management of refractory visceral cancer pain. Pain Med. 2010;11(1):92–100.
Hol PK, Kvarstein G, Viken O, Smedby O, Tonnessen TI. MRI-guided celiac plexus block. J Magn Reson Imaging. 2000;12(4):562–4.
Marra V, Debernardi F, Frigerio A, Menna S, Musso L, Di Virgilio MR. Neurolytic block of the celiac plexus and splanchnic nerves with computed tomography. The experience in 150 cases and an optimization of the technic. Radiol Med. 1999;98(3):183–8.
Wang PJ, Shang MY, Qian Z, Shao CW, Wang JH, Zhao XH. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging. 2006;31(6):710–8.
Fujita Y. CT-guided neurolytic splanchnic nerve block with alcohol. Pain. 1993;55(3):363–6.
Amr SA, Reyad RM, Othman AH, Mohamad MF, Mostafa MM, Alieldin NH, Hamed FA. Comparison between radiofrequency ablation and chemical neurolysis of thoracic splanchnic nerves for the management of abdominal cancer pain, randomized trial. Eur J Pain. 2018;2(10):1782–90.
Wyse JM, Battat R, Sun S, et al. Practice guidelines for endoscopic ultrasound-guided celiac plexus neurolysis. Endosc Ultrasound. 2017;6(6):369–75.
Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995;80(2):290–5.
Polati E, Finco G, Gottin L, Bassi C, Pederzoli P, Ischia S. Prospective randomized double-blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. Br J Surg. 1998;85(2):199–201.
Ischia S, Ischia A, Polati E, Finco G. Three posterior percutaneous celiac plexus block techniques. A prospective randomized study in 61 patients with pancreatic cancer pain. Anesthesiology. 1992;76(4):534–40.
Wyse JM, Chen YI, Sahai AV. Celiac plexus neurolysis in the management of unresectable pancreatic cancer: when and how? World J Gastroenterol. 2014;20(9):2186–92.
Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, Mantilla CB, Warner DO. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291(9):1092–9.
Bahn BM, Erdek MA. Celiac plexus block and neurolysis for pancreatic cancer. Curr Pain Headache Rep. 2013;17(2):310.
Kosugi S, Hashiguchi S, Nishimura D, Seki H, Suzuki T, Katori N, Morisaki H. Neurolysis targeting both the aorticorenal ganglia and lumbar sympathetic plexus for kidney tumor-related pain. Pain Med. 2015;16(1):202–3.
Bristow A, Foster JM. Lumbar sympathectomy in the management of rectal tenesmoid pain. Ann R Coll Surg Engl. 1988;70(1):38–9.
Zechlinski JJ, Hieb RA. Lumbar sympathetic neurolysis: how to and when to use? Tech Vasc Interv Radiol. 2016;19(2):163–8.
Dondelinger R, Kurdziel JC. Percutaneous phenol neurolysis of the lumbar sympathetic chain with computed tomography control. Ann Radiol. 1984;27(4):376–9.
Tay VK, Fitridge R, Tie ML. Computed tomography fluoroscopy-guided chemical lumbar sympathectomy: simple, safe and effective. Australas Radiol. 2002;46:163–6.
Hong JH, Kim AR, Lee MY, Kim YC, Oh MJ. A prospective evaluation of psoas muscle and intravascular injection in lumbar sympathetic ganglion block. Anesth Analg. 2010;111(3):802–7.
Hong JH, Oh MJ. Comparison of multilevel with single level injection during lumbar sympathetic ganglion block: efficacy of sympatholysis and incidence of psoas muscle injection. Korean J Pain. 2010;23(2):131–6.
König CW, Schott UG, Pereira PL, Trübenbach J, Schneider W, Claussen CD, Duda SH. MR-guided lumbar sympathicolysis. Eur Radiol. 2002;12(6):1388–93.
Woo JH, Park HS, Kim SC, Kim YH. The effect of lumbar sympathetic ganglion block on gynecologic cancer-related lymphedema. Pain Physician. 2013;16(4):345–52.
Pennekamp W, Krumova EK, Feigl GP, Frombach E, Nicolas V, Schwarzer A, Maier C. Permanent lesion of the lateral femoral cutaneous nerve after low-volume ethanol 96% application on the lumbar sympathetic chain. Pain Physician. 2013;16(4):391–7.
Ohno K, Oshita S. Transdiscal lumbar sympathetic block: a new technique for a chemical sympathectomy. Anesth Analg. 1997;85(6):1312–6.
Feigl GC, Dreu M, Ulz H, Breschan C, Maier C, Likar R. Susceptibility of the genitofemoral and lateral femoral cutaneous nerves to complications from lumbar sympathetic blocks: is there a morphological reason? Br J Anaesth. 2014;112(6):1098–104.
Haynsworth RF Jr, Noe CE. Percutaneous lumbar sympathectomy: a comparison of radiofrequency denervation versus phenol neurolysis. Anesthesiology. 1991;74(3):459–63.
Wijeyaratne SM, Seneviratne LN, Umashankar K, Perera ND. Minimal access is not maximal safety: pelviureteric necrosis following percutaneous chemical lumbar sympathectomy. BMJ Case Rep. 2010. https://doi.org/10.1136/bcr.12.2009.2538.
Antao B, Rowlands TE, Singh NP, McCleary AJ. Pelviureteric junction disruption as a complication of chemical lumbar sympathectomy. Cardiovasc Surg. 2003;11:42–4.
Trigaux JP, Decoene B, Van Beers B. Focal necrosis of the ureter following CT-guided chemical sympathectomy. Cardiovasc Intervent Radiol. 1992;15:180.
Schmidt AP, Schmidt SR, Ribeiro SM. Is superior hypogastric plexus block effective for treatment of chronic pelvic pain? Rev Bras Anestesiol. 2005;55:669–79.
Nabil D, Eisa AA. Evaluation of posteromedial transdiscal superior hypogastric block after failure of the classic approach. Clin J Pain. 2010;26:694–7.
Acknowledgements
Lorie Marchinkow, Vancouver General Hospital for the schematic in Fig. 5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Filippiadis, D.K., Tselikas, L., Tsitskari, M. et al. Percutaneous Neurolysis for Pain Management in Oncological Patients. Cardiovasc Intervent Radiol 42, 791–799 (2019). https://doi.org/10.1007/s00270-019-02185-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02185-x