Abstract
Objectives
To determine whether Radiofrequency Ablation (RFA) followed by Radiotherapy (RT) (RFA-RT) produces better palliation in terms of pain than RT alone in patients with osteolytic bone metastases.
Methods
Patients with solitary bone metastases and a pain score of least 5 or more on the VAS scale were selected. Fifteen patients were treated with RFA-RT (20 Gy delivered in 5 fractions of 4 Gy over 1 week) and were compared with a matched group (30 subjects) treated by RT.
Results
A complete response in terms of pain relief at 12 weeks was documented in 16.6% (5/30) and 53.3% (8/15) of the subjects treated by RT or RFA-RT, respectively (p = 0.027). The overall response rate at 12 weeks was 93.3% (14 patients) in the group treated by RFA-RT and 59.9% (18 patients) in the group treated by RT (p = 0.048). Although recurrent pain was documented more frequently after RT (26.6%) than after RFA-RT (6.7%) the difference did not reach statistical significance. The morbidity related to RT did not significantly differ when this treatment was associated with RFA.
Conclusions
Our results suggest that RFA-RT is safe and more effective than RT. The findings described here should serve as a framework around which to design future clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The true incidence of bone metastases is the subject of much debate, and is not fully known. The probability of bone metastasis originating from a primary site can be assessed only by knowing the prevalence of the primary tumour and its predilection for bone [1]. Once metastases occur, patient survival is low with a median survival time of months after chemotherapy or RT [1]. Bone metastases are a major clinical concern and cause severe pain, bone fractures, spinal cord compression, hypercalcaemia with a significant degradation of quality of life [2]. For all these reasons the pain relief is an important clinical challenge and represents the primary goal of any therapy aiming to manage skeletal metastases [2].
Despite its limitations, external beam radiation therapy (RT) is the standard of care for localised pain caused by bone metastases [1]. However, pain relief after RT is temporary with response rate in terms of complete (15–18%) and partial response (48–50%). These results are far from being optimal and many weeks may be required before pain relief occurs [3]. Finally, because of limitations in normal tissue tolerance, recurrent pain at a previously irradiated site usually cannot be treated with additional RT.
Radiofrequency ablation (RFA) has proved to be a useful therapeutic option for the management of bone tumours. Its efficacy lies not only in relieving pain in a shorter time but also in controlling the tumour [4–6]. This technique was initially developed for patients with malignant primary or metastatic liver tumours [7]. Considering that the life expectancy of most patients with bone metastases is limited the aim of any treatment must be to provide the earliest possible pain relief. RFA of tumour lesions less than 3 cm in diameter results in considerable necrosis but this phenomenon decreases drastically for diameters greater than 3 cm. Even among tumours initially deemed radiographically to show complete necrosis, local progression may be detected on follow-up and recurrent pain may occur. Such limitations in RFA and RT indicate the need to investigate integrated therapies in order to achieve higher and persistent levels of pain relief. We hypothesise that RT and RFA may work synergistically by balancing each other’s shortcomings. RT is dependent on oxygen for cytotoxicity and is thought to be deficient in killing centrally located tumour cells that are often hypoxic. Conversely, RFA is dependent on the conduction of heat which dissipates with distance from the electrode tip, resulting in decreased efficacy around the tumour edge.
Literature reveals that few data exist on the association between RFA and RT for the management of bone lesions [8]. We have recently reported that the combined treatment using RFA followed by RT is technically feasible and well tolerated with a satisfactory profile of adverse events [8]. However a comparison between this combined approach and radiotherapy has never been carried out. Thus, the primary objective of this feasibility study was to determine whether the combined treatment using RFA with RT produces better palliation of skeletal metastases in terms of complete and overall response than RT alone in patients with painful bone metastases.
Materials and methods
Study population
Patients with radiologically and histologically confirmed solitary painful osteolytic bone metastases were selected. The worst pain intensity was assessed using the validated visual analogue scale (VAS). The choice of this parameter (worst pain) was because of its greatest correlation with functional interference [9]. The pain score of least 5 or more on a scale of 1–10 (or a score of less than five with the use of narcotic medications) with pain localised to the site of the bone metastases and the Karnofsky performance status (KPS) score greater than 70 were other eligibility criteria. Patients receiving systemic therapy were included in the final analysis. Patients with (1) two or more separate sites of painful bone metastases, (2) a painful area previously treated with radiation therapy or palliative surgery, (3) abnormal fracture of the treatment site, (4) radiographic evidence of spinal cord or cauda equina compression and (5) cardiac pacemaker were not treated with the combination of RFA-RT. From October 2008 to June 2009, 15 patients were treated by RFA-RT after providing informed consent The combined treatment was as follows: RFA followed by RT 6 days later. The group of patients undergoing the combined procedure was retrospectively compared with a group of subjects (30 subjects) treated from August 2003 to December 2006 with RT and matched for age, KPS, primary tumours and VAS scale. Required information before treatments included history and physical examination, Karnofsky performance status and completed VAS scale. Analgesic consumption was recorded and all narcotic analgesics were converted to an oral morphine-equivalent dose. Non-narcotic analgesics were classified as 0 for an oral morphine-equivalent dose. This retrospective study was approved by the local Ethical Committee
Radiation therapy
Computed tomography (CT)-based simulations were routinely performed. A three-dimensional conformal technique was used. The nominal prescribed dose was 20 Gy delivered in 5 fractions of 4 Gy over 1 week using 6 MV photons for both fields. Planning target volume (PTV) was defined as the tumour volume with a surrounding margin varied for the different bone localisations. For long bones the PTV included the radiographic abnormality with a margin of at least 2 cm proximally and distally [10]. For bone lesions localised at the spinal cord, the PTV included one vertebra above and below the involved vertebra(s) [10]. The total dose was prescribed to the isocenter, with the 95% isodose surrounding the PTVs.
Radiofrequency ablation
Procedures were carried out with patients under conscious sedation. This condition was achieved by alfentanil, midazolam and continuous infusion of propofol. During the procedure, all patients received oxygen and heart rate, blood pressure, electrocardiographic trace, oxygen saturation and respiratory rate monitoring. Blood pressure was checked every 4 min. Local anaesthesia (1% carbocaine) was applied to the skin at the access site. Following sterile preparation a LeVeen needle electrode (Boston Scientific Corporation, Natick, MA, USA) was introduced under CT guidance into the metastases. After unfolding the electrode tines into the metastases, the needle was connected with a radiofrequency generator (RF 3,000; Boston Scientific Corporation, Natick, MA, USA). The procedure was conducted according to the protocols supplied by the equipment manufacturers (Boston Scientific Corporation, Natick, MA, USA). Briefly, the developed energy was increased 10 W every 3 min up to 90 W until tissue impedance increased and further current flow was prevented (roll-off). A target intratumoural temperature higher than 60°C was considered as an indicator of adequate thermocoagulation. A single ablation was performed for lesions measuring less than 3 cm in the longest diameter. For larger lesions (3–7 cm) a cluster RFA electrode technique was used (3 needles spaced 5 mm apart). At the end of each procedure, contrasted-enhanced CT was performed to ensure that the extent of ablation was confined to target tissue and that there was no substantial damage in the tissue surrounding the target.
Follow-up
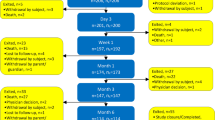
Patients were assessed at baseline, every week for the first month and thereafter every month (at 8, 12, 16, 20 and 24 weeks). Each follow-up visit included a full physical examination, a visual analogue pain score questionnaire (VAS) and a medication level questionnaire, providing data on direct and indirect changes in pain levels.
Complications
Major complications related to RFA were assessed accordingly with the guidelines for imaging-guided tumour ablation [11]. All other complications were considered minor. Radiation related toxicities were documented according to the RTOG scales.
Study endpoint and response criteria
The primary study endpoints were to measure the percentage of patients who achieved a (1) complete (CR) and (2) overall response (OR) at 12 weeks after treatment. Response criteria, with the exception of OR, were defined according to the endpoint criteria defined by the International Bone Metastases Working Party guidelines on palliative radiotherapy endpoints for future clinical trials [10]. CR was defined as pain score of zero at the treated site with no concomitant increase in analgesic intake. OR was defined as combination of CR and PR. Secondary endpoints included (1) the measure of the percentage of patients who achieved a partial response (PR) and overall rate of narcotic analgesic at 12 weeks after treatments.
PR was defined as any of the following: (1) pain reduction of two or more at the treated site on a 0–10 scale without analgesic increase; (2) analgesic reduction of 25% or more from baseline without an increase in pain.
Statistical methods
The primary null hypothesis of this feasibility study was that, for patients with painful solitary bone metastasis, pain relief achieved following RFA-RT should be higher than that achieved following RT. The current study was powered to determine an increase of 20% or greater in the complete response at 12 weeks after RFA-RT. Literature data indicate that when the response outcomes were redefined in accordance with the international consensus criteria about 11–21% of intention-to-treat patients achieved complete responses after RT [12]. Thus we set the rate of complete response after RT at 14% (P0 = 14%). Using a one-sided test and a 5% type I error with a number of matched controls per case of 2:1, 15 subjects in the experimental group (RFA-RT) and 30 in the control group (RT) would provide greater than 80% power to detect an increase of 20% (P1 = 34%) in the complete response. All tests were two-sided except where specified and were determined by Monte Carlo significance. An alpha value threshold of 0.05 was used. An intention-to-treat (ITT) strategy was used for the analysis of primary endpoints. A per-protocol analysis was used for the analysis of toxicity. Continuous variables were not normally distributed (Shapiro-Wilk test) and were presented as medians and confidence intervals at 95% (CI95%). The Mann–Whitney U test was used to test a significant difference between two groups. Dichotomous variables were summarised by absolute and/or relative frequencies. Chi-squared test or Fisher’s exact test were used to test a significant difference between two groups. For multiple comparisons the alpha value threshold was adjusted by using the Bonferroni correction. The odds that a patient treated with RFA-RT will achieve complete or overall response as a function of time before a patient treated by RT alone have been determined by the use of the Cox proportional hazard model. All statistical analyses were performed using the SPSS® statistical analysis software package, version 10.0.
Results
A total of 45 patients with histologically and radiologically confirmed bone metastases were included in the study. Table 1 lists the clinical and demographic characteristics of treated patients. A significant difference between the two groups was documented with regard to the sex of patients and the median size of bone lesions. In the RT group many more subjects were male whereas the median size of skeletal metastases was significantly greater in the groups treated using RFA with RT (Table 1). After Bonferroni correction no difference in metastases locations was documented between the two groups (Table 1). The other pre-treatment variables were balanced between the two groups. The overall number of patients available for the follow-up was 42 (93.3) at 8 weeks, 38 (84.4%) at 12 weeks, and 30 (66.6%) at 24 weeks. The main contributors to the loss of follow-up were death and hospitalisation.
Pain outcome
At baseline the median value of the pain score was 6.5 (95% CI 5.9 to 7.2) and 6.3 (95% CI 5.6 to 8) in the RT and RFA-RT group, respectively (p = 0.36) (Table 1). A complete response at 12 weeks was documented in 16.6% (5/30) and 53.3% (8/15) of the subjects treated by RT or RFA-RT, respectively (p = 0.027) (Table 2). Partial response was documented in 40.0% of 15 patients treated with RFA-RT and in 43.3% of 30 patients treated by RT. The overall response rate at 12 weeks was 93.3% (14 patients) in the group treated with RFA-RT and 59.9% (18 patients) in the group treated with RT alone (p = 0.048). The Cox proportional hazard model indicated that patients treated with RFA in association with RT achieved complete (HR = 7.0; CI 95% 1.96 to 24.8) and overall response (HR = 10.11; CI 95% 3.71 to 27.55) before patients treated with RT alone (Fig. 1).
The analysis of the interval to response indicated that subjects treated by RTFA-RT achieved an overall response faster than patients treated by RT alone. The interval to response after RT was of 9 weeks (CI95% 7.0 to 12.0). In the patients treated by RTFA-RT the interval to response was significantly faster (3 weeks [CI95% 1.6 to 6.4]) (p < 0.0001). Twenty-seven patients (90%) in the RT-RFA group and 12 (80%) in the RT group received oral narcotic analgesic before treatments. No significant difference between the two groups was measured at baseline (p = 0.642) (Table 1). At 12 weeks 23.3% of patients (7/30) in the RT group and 60.0% of patients (9/15) in the RT-RFA group did not require narcotic medications (p = 0.036) (Table 3).
Re-treatment rate and abnormal fractures
Re-treatment was proposed when: (1) no pain relief or pain progression with initial treatment was experienced or when (2) a pain progression occurred after a complete or partial response. The subjects treated by RFA-RT experienced a lower rate of recurrent pain and its onset was moved forward in time. At 12 weeks 26.7% (8/30) in the RT group versus 6.7% (1/15) in the RFA-RT group underwent re-treatment. Although a trend towards an increased rate of recurrent pain was documented in the RT group, this difference did not reach statistical significance (p = 0.236).
Finally, the incidence of abnormal fractures within the treatment field was 10% (3/30) and 6.6% (1/15) in the RT and RFA-RT groups, respectively with no significant difference (p = 0.853).
Morbidity
Treatment safety was monitored by recording the incidence of any major or minor complication after treatments. Patients tolerated the combined treatment well with a very low incidence of adverse events related to RFA. There were no major complications except a transient nerve injury (1/15; 6.7%) and the occurrence of infection at the access site (1/15; 6.7%). In the patient with nerve injury transient leg paralysis occurred 2 days after RFA-RT with improvement within 15 days after steroid administration. Neither a minor complication nor death occurred in relation to combined treatment. The morbidity related to RT did not significantly differ when this treatment was associated with RFA (Table 4).
Discussion
Bone metastases are the most important source of morbidity in cancer [1] and consolidated evidence suggests that radiotherapy may be the standard of care for the management of this condition. However, its efficacy is documented in only a few treated patients [12]. When the International Consensus on Pain Radiotherapy Endpoints criteria are employed, 31–68% and 11–21% of intention-to-treat patients achieved overall and complete response at 3 months of follow-up [12].
Radiofrequency ablation is currently considered to be the procedure of choice in the treatment of osteoid osteomas [7]. A number of reports have been published on RFA as palliative treatment for bone metastases documenting good effectiveness in terms of pain relief and complications [4–6]. According to these studies RFA can provide palliation for patients with painful bone metastases configuring this treatment as an alternative to RT [4–6].
However, despite their effectiveness in reducing pain from bone lesions, RT and RFA have some limitations. These treatments may be time-limited and do not always permit sustained improvement of clinical condition in treated patients [12]. Additionally, a number of studies on RFA suffer from lack of uniformity in their response criteria [4–6] which makes the comparisons of results very complex. Thus, there is a need for studies aimed at comparing the results of different treatments with more standard criteria and to address the role and the timing of other local and systemic treatment in association with RT in the management of bone metastases.
Our feasibility study goes in this direction and tries to achieve this goal by using the response criteria defined during the International Consensus on Palliative Radiotherapy endpoints for future clinical trials in bone metastases [10]. The analysis of our data suggests that the combined treatment using RFA followed by RT is well tolerated and more effective than RT alone. The incidence of adverse events related to RFA is low and the association with RT worsen morbidity related to radiation. A significant improvement in complete and overall response was documented after RFA-RT treatment. The CR rate at 12 weeks was 16.5% (5 of 30) and 53.3% (8 of 15) for RT and RFA-RT, respectively. In terms of overall response, the crude rate significantly differed between the two groups. This was 93.3% (14 of 15 patients) in the group treated by RFA-RT and 59.9% (18 of 30 patients) in the group treated by RT alone. The Cox Proportional Hazard Model indicated that patients treated by RFA in association with RT achieved complete (HR = 7.0; CI 95% 1.96 to 24.8) and overall response (HR = 10.11; CI 95% 3.71 to 27.55) before patients treated by RT alone. On the contrary, no significant difference was documented in terms of PR between the two groups. PR was achieved in 43.3% of patients treated by RT and in 40.0% of those treated with RFA-RT. The significant improvement in the CR and OR after RFA-RT group was associated with a significant decrease in the percentage of patients requiring narcotic analgesics. The interval to response was faster after RFA-RT ranging from 1.69 to 6.4 weeks. In patients treated with RT the interval to response ranged from 7.0 to 12.0 weeks. The number of subjects with stable pain or progression was significantly largest in the population treated with RT; also the re-treatment rate was higher after radiation. The crude rate of stable pain or progression was 40.0% at 12 weeks and 60.0% at 24 weeks after RT. In the RFA-RT group only 1 patient experienced progression after the treatment with no further increase at 24 weeks.
In some measure, our study suffers from considerable limitations such as the relatively low number of patients, the imbalance between the two groups in terms of locations and size of the treated metastases and the retrospective design. The generalisation and the applicability of our results to the general population with osteolytic bone metastases must be demonstrated. Finally, based on the aforementioned biases, we are aware that the methodology of our study is far from being the best way of carrying out a comparative assessment of the two techniques. Therefore, although the results illustrated here suggest that RFA followed by RT can be safe and can significantly reduce the level of pain experienced by cancer patients with bone metastases limiting the need for strong narcotic pain management, our findings should be interpreted cautiously and should serve as a framework around which to design future clinical trials.
References
Chow E, Harris K, Fan G et al (2007) Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 25:1423–1436
Janjan N, Lutz ST, Bedwinek JM et al (2009) Therapeutic guidelines for the treatment of bone metastasis: a report from the American College of Radiology Appropriateness Criteria Expert Panel on Radiation Oncology. J Palliat Med 12:417–426
Lo SS, Sahagal A, Hartsell WF et al (2009) The treatment of bone metastasis with highly conformal radiation therapy: a brave new world or a costly mistake. Clin Oncol 21:662–664
Callstrom MR, Charboneau JW, Goetz MP et al (2002) Painful metastases involving bone: feasibility of percutaneous CT and US guided radiofrequency ablation. Radiology 224:87–97
Goetz MP, Callstrom MR, Charboneau JW (2004) Percutaneous image-guided radiofrequency ablation of painful metastasis involving bone: a multicenter study. J Clin Oncol 22:300–306
Dupuy DE, Liu D, Hartfeil D et al (2010) Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer 116:989–997
Widmann G, Bodner G, Bale R (2009) Tumour ablation: technical aspects. Cancer Imaging 9:63–67
Di Staso M, Zugaro L, Gravina GL, Bonfili P, Marampon F, Di Nicola L, Conchiglia A, Franzese P, Gallucci M, Masciocchi C, Tombolini V (2011) Can Radiotherapy be Combined with Radiofrequency Ablation in the Management of Symptomatic Osteolytic Skeletal Metastasis? Clin Oncol 23:65–66
Harris W, Li K, Flynn C et al (2007) Worst, average or current pain in the Brief Pain Inventory: which should be used to calculate the response to palliative radiotherapy in patients with bone metastases. Clin Oncol 19:523–527
Chow E, Wu JS, Hoskin P et al (2002) International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol 64:275–280
Goldberg SN, Grassi CJ, Cardella JF et al (2005) Society of interventional radiology technology assessment committee; international working group on image-guided tumor ablation. Imaged-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 235:728–739
Lutz S, Hoskin P, Chow E (2009) Update on palliative radiotherapy endpoints for bone metastases trials. Clin Oncol 21:659–661
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Di Staso, L. Zugaro and G. L. Gravina have Equal Contribution
Rights and permissions
About this article
Cite this article
Di Staso, M., Zugaro, L., Gravina, G.L. et al. A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol 21, 2004–2010 (2011). https://doi.org/10.1007/s00330-011-2133-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2133-3