Abstract
Apple ber (Zizyphus mauritiana) is an underutilized fruit, rich in vitamin C and polyphenols. The raw juice after mechanical pressing of this fruit is very turbid and needs to be clarified using the pectinase enzyme. The study deals with the optimization of the enzymatic clarification process of apple ber juice using the incubation time (0.5–2.5 h), temperature (30–50 °C), and pectinase concentration (0.2–0.6%) as independent variables with the enhancement in juice clarity and yield as dependent parameters. The experiments were conducted according to a three-level full factorial design (33 runs), and the polynomial models were developed using response surface methodology (RSM). The RSM models were further optimized using numerical optimization. The optimized clarification condition was to incubate the mixture at 41 °C for 2.5 h with 0.50% enzyme. Clarity and yield were greatly enhanced by 44.7% and 43.4%, respectively. The thermal pasteurization (90 °C for 3 min) of the clarified apple ber juice led to a 53.4% loss in vitamin C and a 59.3% loss in antioxidant capacity compared to clarified juice. The clarified juice pasteurized by pulsed light treatment (treated at a total fluence of 2400 J/cm2) preserved 90% of vitamin C from the clarified juice. A team of 30 semi-trained sensory panelists scored its overall acceptability (OA on a 9-point scale) as 6.6, whereas the fresh juice had an OA of 7.3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apple ber (Ziziphus mauritiana), also called Indian jujube, is a tropical and underutilized fruit characterized by a highly lignified and pointed endocarp, a fleshy mesocarp, and a thin pericarp [1, 2]. India produces about 0.580 million tonnes of ber annually [3]. The apple ber juice is an excellent source of vitamin C (ascorbic acid) and polyphenols comparable to orange juice [4]. However, value-added products from apple ber are scarce in the market [5]. Converting whole apple ber into juice allows easy preservation, improves convenience, and can enhance its utilization. However, the juice obtained after the mechanical expression is turbid due to its pectin content [6]. During the ripening of apple ber, most of the pectin gets converted into soluble pectin, which is responsible for gel formation inducing cloudiness or turbidity in the juice [2]. Enzyme polygalacturonase (PG) is a commercially used pectinase enzyme for juice clarification that helps minimize turbidity by hydrolyzing the pectin into simpler forms, like monosaccharides and oligosaccharides [7]. Dagadkhair et al. [8] studied the effect of a crude pectinolytic enzyme from Aspergillus niger on ber pulp and obtained a clear juice after liquefication. Koley et al. [9] studied the effect of the pectinase enzyme on apple ber pulp by varying enzyme concentration from 0.05 to 0.20% keeping constant incubation time (1 h) and temperature (50 °C). However, no study has been carried out on the effect of change in incubation time and temperature on the apple ber pulp clarification process. In addition to the enzyme concentration, time and temperature also significantly affect the clarity of the juice [10].
Response surface methodology (RSM) is one of the most popular and reliable methods for the systematic design of experiments and optimization [11, 12]. Numerous authors have used the RSM technique to optimize the pulp liquefaction or juice clarification processes of various fruits, such as banana, pineapple, guava, and apricot [13,14,15,16,17]. The pectinase enzyme has been employed for the liquefaction of pectin backbone from orange waste, apricot, pineapple, custard apple, and bael [18,19,20,21,22].
After optimizing a food product’s processing condition, its sensory analysis is also crucial for evaluating its acceptability [23, 24]. Pulsed light (PL) treatment is a nonthermal technique that uses high intensity with short pulses of the light spectrum (200–1100 nm) to decontaminate liquid food products, as summarized by Dhar et al. [25]. However, no study has been undertaken on PL treatment of apple ber juice. The study aims to quantify the influence of pectinase enzyme and incubation conditions on apple ber juice clarification and yield. The conditions were optimized using response surface methodology and a numerical optimization technique. When the clarified juice was subjected to pulsed light and thermal treatments, a comparison of the quality attributes of the pasteurized juice was made. Further, the sensory profile and physicochemical analysis were conducted for the optimally clarified and pasteurized juices.
Materials and methods
Raw materials
Apple ber fruits (Zizyphus mauritiana) were grown on a farm located at Pune, Maharashtra, India (18° 40′ 37.42″ N 73° 53′ 47.76″ E) and transported at ambient condition (30 ± 2 °C) to the institute within 24 h of harvesting. All the fruits were at commercial maturity level (12–14 °Brix). The pulp was crushed through a home grinder (Model HL1645, Philips, India) at 6000 rpm for 5 min. The total soluble solids (TSS) and the pH of the homogenate obtained were 13.0 ± 0.2 °Brix and 4.9 ± 0.1, respectively. Pectinase (polygalacturonase) enzyme from Aspergillus niger with a specific activity of 10 U/g (HiMedia Laboratories Pvt. Ltd., India) was used for clarification.
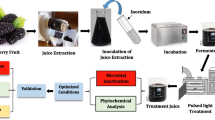
Enzymatic clarification treatment
Pectinase and cellulase are the most frequently employed clarifying enzymes in the fruit juice industry. Pectinase enzymes, including pectinesterase and polygalacturonase, are utilized to break down pectin, a polysaccharide found in plant cell walls like apple ber. They help in reducing turbidity and improve juice clarity. On the other hand, cellulase enzymes assist in the breakdown of cellulose, another polysaccharide presents in plant tissues. Cellulase can help enhance the filtration and separation of solids from the juice, resulting in improved clarity [22]. In general, pectinase tends to be more cost-effective and widely available compared to cellulase [8, 9]. Therefore, the pectinase (polygalacturonase) enzyme was used in this study. For enzymatic clarification, the slurry sample (100 mL) was incubated at a certain temperature in a thermostatic water bath (± 0.5 °C). The temperature for the clarification was decided by the experimental design (Table 1). The mixture was passed through a 110-press filter (Microfilt India, Mumbai, India) having a mesh size of 100 μm, and the clarified apple ber juice thus obtained was used for further analysis.
Experimental design
The clarification process was optimized using response surface methodology (RSM). The experimental design employed was a three-factors-three-levels (33) full factorial design [12]. The three independent variables were incubation time (X1, h), temperature (X2, °C), and enzyme concentration (X3, % w/w); the responses analyzed were Δclarity (Y1, %) and Δyield (Y2, %). The ‘Δ’ refers to the difference between post incubation to no-incubation samples. The real values of the independent variables (Xi) were converted into corresponding dimensionless coded values (xi) using Eq. 1, where Xmax and Xmin are the maximum and minimum values of Xi, respectively [11].
The extreme points of the domain were set by performing some preliminary experiments and from the literature review. The domain (− 1 to + 1) for incubation time (X1, h), temperature (X2, °C), and enzyme concentration (X3, % w/w) were 0.5–2.5 h, 30–50 °C, and 0.2–0.6 g/100 g, respectively. Each of the independent variables was varied at three equidistant levels (xi = − 1, 0, & +1), resulting in a total of 33 (= 27) experimental runs (factorial points). In addition, five experiments were replicated at the center point of the domain (0, 0, 0 in coded form) to visualize the lack of fit.
Optimization using response surface methodology
A quadratic polynomial model (Eq. 2) was developed for each response as a function of independent variables (coded forms).
where Yi (i = 1 or 2) is the actual value of the response, the regression coefficients of the model are represented as βo (constant term), β1, β2, and β3 (coefficients for linear terms), β4, β5, and β6 (coefficients for interaction terms), and β7, β8, and β9 (coefficients for quadratic terms); x1, x2, and x3 are the coded values of time, temperature, and enzyme concentration, respectively. The adequacy of model fitting was adjudged by the coefficient of determination (R2), adjusted R2, F-value, and p-value of the model, along with the non-significant lack of fit (plof). Response surface was generated to visualize the combined or interaction effect between any two parameters on either experimental response.
For optimizing the clarification and juice yield, the desirability of individual responses was calculated as a function of process parameters according to Eq. 3.
where di represents the desirability index of Yi. Li and Ui are the lower and upper limits of the Yi, respectively. Further, a single parameter called the overall desirability index (D) was calculated using Eq. 4.
The r1 and r2 are the relative importance (scale 1–5) of Y1 and Y2, respectively. The numerical optimization technique was targeted to achieve the maximum value of D (within 0–1, 1 being the most desirable) within the domain at any given combination of X1, X2, & X3. The process condition with the maximum D value was adjudged as the optimized condition. The optimized condition thus obtained was further validated by actual experimental conditions.
Physical and chemical analysis of the clarified juice
The pH, total soluble solids, total phenolics, antioxidant capacity, and flavonoid content in the clarified juice were determined following the protocols described by Chakraborty et al. [26]. The yield of juice was estimated as a ratio of the weight of clear juice to the weight of the unclarified (control) juice sample. Total phenolics were expressed as mg of gallic acid equivalent/100 mL; antioxidant capacity was estimated as gallic acid equivalent antioxidant capacity/100 mL. The flavonoid content was expressed as mg quercetin equivalent/100 mL. The total sugar in the juice was quantified using a phenol-sulphuric acid reaction [27], and reducing sugar was quantified using dinitrosalicylic acid (DNSA) reagent [28]. The change in yield (Δyield, % w/w) of the juice was calculated as per Eq. 5.
The clarity of juice was determined as % transmittance at 660 nm, as described by Chang et al. [29]. The relative increase in clarity was denoted as Δclarity (%) and quantified as per Eq. 6.
Vitamin C content in the juice was measured by a spectrophotometric method using 2,6-dichlorophenolindophenol (DCPIP) dye, as described by Chakraborty et al. [26]. Ascorbic acid (AA) was extracted using 2% metaphosphoric acid, and the change in color of samples after adding DCPIP dye was measured at 518 nm. The results were expressed as mg AA/L of the sample.
Kinetic study of pectinase
The pectinase enzyme-substrate reaction’s kinetic parameters were estimated per the protocol described by Dhar and Chakraborty [22]. The substrate-enzyme reaction was conducted for pectinase concentrations (0.01–2.0%) and incubation time (up to 4 h). The pectinase activity (U/mg) was measured as |Δ pectin concentration%| (min·‘mg protein of enzyme’)−1. The Michaelis–Menten equation (Eq. 6) was fitted to describe the change in the initial velocity (V0) of the enzymatic reaction as a function of pectinase concentration [EC] (mg protein of enzyme).
Vm is the maximum initial pectinase velocity, and km is the Michaelis–Menten constant. The linearized form of the Michaelis–Menten (Eq. 6) equation was fitted to the kinetic data. The model is the Lineweaver–Burk model (Eq. 7).
Thermal and pulsed light pasteurization of the clarified juices
The heat treatment was conducted in the thermostatic water bath at the desired temperature (± 0.5 °C). The clarified juice (50 mL) was packed in a polypropylene pouch (film thickness of 80 μm) and dipped into the water bath. The juice, after optimal clarification, was heated at 90 °C up to 5 min (holding period). The come-up time for 90 °C was 147 s, and the cooling-down time was 20 s. The core of the dummy pouch showed the temperature as 90 ± 1 °C throughout the isothermal treatment.
The pulsed light pasteurization treatment was conducted using a benchtop batch mode pulsed light (PL) assembly (Xenon X-1100, MA, USA), as detailed by Vollmer et al. [30]. 50-mL juice was exposed to PL at a fluence rate of 10.0 ± 0.1 W/cm2 (2.9 kV, 1 Hz; ON and OFF time was 400 µs) for 3, 3.5, and 4 min, respectively. The corresponding total fluence values were 1800, 2100, and 2400 J/cm2. Before the treatment, the juice temperature was 20.0 ± 0.5 °C. The maximum temperature rise of the sample surface was 13.7 ± 0.3, 15.8 ± 0.2, and 17.6 ± 0.3 °C when the sample was exposed at 1800, 2100, and 2400 J/cm2, respectively. After the treatment, the sample was cooled in ice condition. The pasteurization condition was recognized as the treatment intensity required to inactivate the microbial population to below the detection limit (> 5 log reduction) and to inactivate the spoilage enzyme (here PME) by > 99%.
Enzyme activity and microbial enumeration
The residual pectin methylesterase (PME) activity in the juice was estimated per the protocol detailed by Chakraborty et al. [31]. The microbial enumeration for yeast and mold (YM) and aerobic mesophiles (AM) was done through a serial dilution with a pour plate technique, described by Chakraborty et al. [32]. The detection limit was 10 colony-forming units (CFU)/mL of the sample.
Sensory analysis of the juice
The unclarified juice (J1), optimally clarified juice (J2), optimally clarified + pulsed light pasteurized juice (J3), and optimally clarified + thermally pasteurized juice (J4) were compared through sensory analysis. A panel of 30 members was made who do not have any smoking habits and are not allergic to apple ber or any fruit juice. They were trained for three weeks on alternate days and for about 1–1.5 h per day. The training of the panel members and the conduction of the sensory analysis was performed according to the methods followed by Chakraborty et al. [33]. All the panel members were familiarized with the type of sample, different sensory attributes, the significance of these attributes, the type of score sheet, rating style, and intensity range of the attributes. The panelists were allowed to analyze juices to obtain good repeatability in their scoring. Five sensory attributes were followed: appearance, aroma, taste, mouthfeel, and consistency. They were instructed to use a hedonic scale (S) for rating, which ranges from ‘1-dislike extremely’ to ‘9-like extremely’. The panelists were also instructed to provide relative importance (RI) score for each sensory attribute on a scale ranging from ‘1-not at all important’ to ‘5-extremely important’ for the juice. They were also asked to taste some plain biscuits and drink water between evaluations to neutralize their palate. All four juice samples (J1, J2, J3, and J4) were served at a temperature of 10–15 °C in transparent glasses with three-digit random codes. The overall acceptability (OA) score was calculated for each sample as per Eq. 9, where five is the number of sensory attributes [24].
Statistical analysis
The experiments were repeated twice at the same condition, and the responses were analyzed in triplicate (total replications = 2 × 3). The one-way analysis of variance (ANOVA) was carried out to check the significant difference between the means at a 5% significance level. It was performed in SPSS software (IBM SPSS Statistics 16). Microsoft Office Excel (Microsoft Corporation, 2016) was used for RSM and numerical optimization.
Results and discussions
The effect of incubation time, temperature, and enzyme concentration (EC) on juice clarity and yield are summarized in Table 1. The change in clarity (Δclarity) of the juice varied from 12.8 to 49.8% transmittance in the entire domain. The minimum clarity in the juice was obtained at the lower limit of the domain (incubation with 0.2% EC at 30 °C for 30 min). The juice was highly transparent (49.8% transmittance as clarity) when the pulp was incubated with 0.6% EC at 40 °C for 2.5 h. As expected, the clarity of the juice increased with an increase in incubation time at any given temperature and enzyme concentration. For instance, at 50 °C, keeping the EC fixed at 0.2% w/w, the clarity increased from 23.0 to 27.9% when the incubation time increased from 0.5 to 2.5 h (Table 1). The extent of clarification also increased with an increase in EC. For example, after incubation for 0.5 h at 40 °C, the clarity was 23.7% and 27.0% for having EC of 0.2% and 0.6% w/w in pulp, respectively (Table 1). Lee et al. [13] reported the same trend in the case of banana juice. On the other hand, for a fixed incubation time and EC, an increase in incubation temperature does not necessarily elevate the clarity of the juice. For instance, while incubating for 2.5 h with 0.6% EC, the corresponding clarity was 31.9, 49.8, and 34.8% transmittance for an incubation temperature of 30, 40, and 50 °C, respectively (Table 1), which indicates that an optimum temperature is required for efficient clarification of apple ber juice.
In the case of change in juice yield (Δyield, Y2 in %), the domain varied from 9.5 to 45.1% (Table 1). The three independent parameters significantly influenced the juice yield. The Δyield was 9.5% while incubated for 0.5 h at 30 °C with 0.2% w/w EC. The sample treated for 2.5 h at 40 °C with 0.6% w/w EC) showed the maximum yield (Δyield = 45.1%). From Table 1, it is clear that an increase in EC led to a more clarified juice. Taking an example of incubation for 2.5 h at 30 °C, the Δyield of the clarified juice was 24.0, 32.1, and 41.9% for 0.2, 0.4, and 0.6% w/w EC, respectively (Table 1). Keeping the incubation temperature and EC fixed, the change in juice yield increased significantly when incubated for longer. For instance, at 40 °C with 0.2% EC, the juice showed 17.8, 27.1, and 33.7% Δyield for the incubation time of 0.5, 1.5, and 2.5 h, respectively (Table 1). On the other hand, the change in yield with an increase in incubation temperature was not uniform for all the cases. For instance, at a fixed incubation time (1.5 h) and EC (0.4% w/w), the Δyield in the juice were 26.0, 33.2, and 28.5% corresponding to incubation temperatures of 30, 40, and 50 °C, respectively.
Response surface method
Response surface methodology (RSM) develops a relationship between the independent and response variables while targeting an optimum condition. The data in the experimental design matrix were fitted into the various polynomial equation, and the quadratic polynomial (Eq. 2) appeared to be the best in terms of the higher R2 (> 0.9) and adjusted R2 (> 0.8) along with an insignificant lack of fit (plof > 0.05). Therefore, a quadratic polynomial model was developed depicting the effect of varying clarification process parameters on the dependent variables, such as change (Δ) in clarity and yield. The summary of the regression coefficients and other model-fitting parameters for both responses have been presented in Table 2. The R2 for the polynomial model developed for the Δclarity (Y1) and Δyield (Y2) was 0.93 and 0.95, with adjusted R2 of 0.90 and 0.93, respectively. It represents a good fit of the data in the model for each response. The model p-value is less than 0.0001 in both cases, with a higher F-value (34 and 505 for Δclarity and Δyield, respectively). An insignificant lack of fit (p-value > 0.1) means that process variables, instead of noise, influence the change in response variables. The equation thus generated showed good fit and reliability, which helps understand the relative influence of process variables (linear terms) and various types of combined effects between the variables (quadratic and interaction terms) occurring during the clarification process.
Linear terms
The linear terms (x1, x2, and x3) in the quadratic model are significant (p < 0.05), contributing to the observed variation in the two responses (Table 2). The coefficients for the linear terms depict that incubation time is the most influencing parameter for both clarity and yield in the juice. EC influences the yield more than the incubation temperature, whereas clarity experiences the opposite trend. All three parameters positively manipulate the juice clarity and yield, viz. incubation time, temperature, and EC. Positive influence refers to an increase in the magnitude of the independent variable that leads to a higher response value. Bashir et al. [19] reported the same trend in the case of apricot juice recovery with the pectinase enzyme. Initially, as the substrate concentration was higher, the clarification rate also became higher; however, after some time, it might become slow with an increase in incubation time due to substrate depletion [34]. Thus, incubation time dramatically influences the activity of the pectinase enzyme, and an optimum time should be reached until saturation of enzyme activity occurs.
Square terms
A few square terms in the polynomial models are also contributing to visualizing the overall behavior between process variables and responses. Scrutiny about the significance of these square terms reveals that the square terms of incubation time and temperature are significantly (p < 0.05) affecting the clarity and yield of the juices (Table 2). In the case of juice clarity, the coefficient for the quadratic term of incubation temperature is negative (− 10.29), which signifies that the juice becomes clear with an increase in temperature. After reaching the optimum temperature, a reverse trend becomes predominant (Table 2). A similar trend has been reported by Kaur et al. [14] during the clarification of guava juice. An initial increase in the clarity might be due to the optimum temperature for efficient enzyme activity. With a further increase in temperature beyond the optimum value, the extent of clarification is compromised due to heat-induced enzyme denaturation [13, 16].
The coefficients of the square terms for incubation time and temperature affecting the Δyield of the juice are − 2.52 and − 5.40, respectively (Table 2). The contribution of temperature appears to be more than the incubation time in this case. A negative coefficient of the square term and a positive linear term of both incubation time and temperature states that the juice yield increases initially with an increase in either of these parameters; however, after reaching optima, a reverse trend becomes significant. The yield is compromised until the incubation temperature of 40 °C, and it is retained effectively. After reaching the optimum temperature, the extent of clarification was compromised because of heat-induced denaturation of the enzyme. In the clarified juice, the movement of molecules increases at a higher temperature; however, beyond a certain limit, enzyme denaturation lowers the yield [17]. At 50 °C, the enzyme activity is less compared to 40 °C. However, the initial conclusion is also true; when we compare 30 °C and 50 °C, the clarity obtained are not significantly different (p > 0.05), so the decrease in yield can be attributed due to its thermal destruction of pectinases [22].
Interaction terms
The only interaction term significantly (p < 0.05) influencing the juice clarification process is the incubation time–temperature (x1x2) term. The combined effect of incubation time and temperature modulates the clarity of the juice in an antagonistic manner (coefficient = − 2.61). At the same time, Δyield was influenced synergistically (coefficient = 1.44) (Table 2). The enzyme activity becomes saturated early at increased enzyme concentration. It might be due to the depleted substrate concentration in which the interaction effect between an incubation time and enzyme concentration was non-significant. A similar interaction effect between time and temperature in the case of clarity was observed during banana juice clarification [13]. While visualizing the interaction between an incubation time and temperature on the responses, the contour plots are generated in x1x2 landscape at a fixed EC value (x3) (Fig. 1). The parabolic nature of the clarity response surface (Fig. 1) depicts that with an increase in both time and temperature, the juice becomes clearer until 40 °C. After that, the juice appears to be hazy as the temperature increases, reflecting the dominant contribution of the square term of incubation temperature. This trend agrees with the literature [16]. The synergistic impact of incubation time and temperature on the yield (Fig. 2) might be due to the optimum pectinase activity at an optimum temperature and time [6]. On the other hand, an increase in both incubation temperature and EC leads to a compromised yield (Fig. 2). This might be due to the high temperature-induced degradation of enzymes in the juice. As discussed, the trend is due to the dominant square term of temperature (Table 2). A similar trend was reported by Gani et al. [6] during pear juice clarification.
Contour plots showing the influence of enzymatic clarification conditions on the change in clarity (%) of the juice; a effect of time and temperature at 0.4% enzyme concentration; b effect of incubation time and enzyme concentration at an incubation temperature of 40 °C; c effect of incubation temperature and enzyme concentration at an incubation time of 1.5 h
Contour plots showing the influence of enzymatic clarification conditions on the yield enhancement (%) of the juice; a effect of time and temperature at 0.4% enzyme concentration; b effect of incubation time and enzyme concentration at an incubation temperature of 40 °C; c effect of incubation temperature and enzyme concentration at an incubation time of 1.5 h
Numerical optimization
Numerical optimization was performed to obtain maximum enhancement in Δclarity and Δyield. The relative importance (ri) was assigned on a 1–5 scale in which 5 refers to the most important. Both Δclarity and Δyield were given the highest priority (ri = 5) (Table 3). Considering the cost of the enzyme, it was also aimed to minimize the enzyme concentration while employing numerical optimization. The numerical optimization suggested that Δclarity with 0.5% EC at 40.7 °C for 2.5 h would give Δclarity of 44.4% and Δyield of 44.1% with overall desirability of 0.92. The validation experiment was carried out at 2.5 h/41 °C/0.5% enzyme concentration, and the observed values were clarity 44.7 ± 1.2% transmittance and Δyield of 43.4 ± 0.5 mg/L (Table 3).
Characteristics of the pectinase
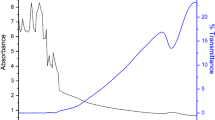
Lineweaver–Burk model fitted the kinetic data of the pectinase enzyme well. The maximum velocity (Vm) is 2.4 ± 0.2 U (|Δ substrate%|/min), and the equilibrium constant (km) is estimated as 15.2 ± 0.4 mg. The adjusted R2 for this model fitting was 0.93 with a mean sum of the square of error (MSE) of 0.052. The initial velocity of the enzymatic action of the pectinase increased with an increase in enzyme concentration. This is in line with Dhar and Chakraborty [22], Ninga et al. [35], and Mutlu et al. [36]. The km of 15.2 mg represents that the initial velocity will reach half of the maximum reaction velocity when 15.2 mg of pectinase is present in the mixture [37]. The pectinase acts on the insoluble pectin of the apple ber pomace and produces soluble low molecular weight fragments, thus increasing the juice yield and clarity [16].
Sensory analysis
A semi-trained panelist evaluated the apple ber juice samples, and their overall acceptability (OA) scores were calculated. The samples under evaluation included an unclarified juice (J1), optimally clarified juice (J2), optimally clarified + pulsed light pasteurized juice (J3), and optimally clarified + thermally pasteurized juice (J4). The OA values for sample J1, J2, J3, and J4 were obtained as 7.3 ± 0.9, 6.8 ± 0.4, 6.4 ± 0.3, and 5.5 ± 0.5, respectively. The OA values of the four samples varied significantly (p < 0.05) from each other. The enzyme clarification process has slightly decreased the acceptability of the juice from the ‘7-like moderately’ to ‘6-like slightly’ category. Reduction in the organoleptic quality can be attributed to the loss of essential bioactive and volatile components by the action of aerobic oxidation and thermal deactivation step of the enzyme treatment process [24]. In terms of the clarified juices, sample J2 was found to be more acceptable than pasteurized juices (J3 and J4) by the panelists. The clarified juice pasteurized by pulsed light treatment at 2400 J/cm2 scored OA of 6.4, whereas the thermally pasteurized one scored OA of 5.5. Having an enzyme concentration of 0.5% in sample J2 may reduce the acceptability of the treated product from 7.3 to 6.8 out of 9. Apart from the thermal pasteurization in J4 may interfere with the natural taste and aroma of the apple ber juice, thus compromising the overall acceptability from 6.8 to 5.5 out of 9. On the other hand, pulsed light treatment preserved the taste and retained the maximum phytochemicals in the juice (J3), thus being accepted by the panelists. A higher acceptance of PL-treated juice over thermal-treated juice has been reported in earlier studies, such as mixed fruit beverages [38].
Quality attributes of the clarified and pasteurized juices
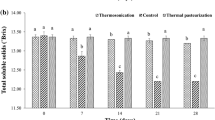
The quality attributes of selected juices samples, such as unclarified juice, optimally clarified juice, and optimally clarified + pasteurized juices, have been summarized in Table 4. It can be observed that compared to the unclarified juice, the clarity improved by 32%, and the yield improved by 11%. After enzyme treatment, the pH of the juice slightly decreased from 4.9 to 4.4, mainly due to the breakdown of pectin into galacturonic acid and simpler pectic substances. Similar results were also observed by Chang et al. [29] during the enzyme treatment of plum juice. It is natural that due to the enzymatic hydrolysis of pectin by the pectinase enzyme, the reducing sugar content increases, and for the current situation, it increased by 3.3%. An increment in the reducing sugar of enzyme-clarified apple juice was also detected by Ázar et al. [28] to a relatively larger extent, which could be due to different target samples. The enzyme treatment of the apple ber fruit negatively affected the bioactive components of the juice. The ascorbic acid, total phenol, antioxidant capacity, and flavonoid content in the clarified juice decreased by 36%, 33%, 49%, and 35%, respectively. The bioactive degradation may also be contributed by aerobic oxidation during the incubation period of enzyme hydrolysis.
On the other hand, a minimal pectin methylesterase (PME) activity was present in the clarified juice. However, thermal pasteurization (90 °C/3 min) and PL treatment (2400 J/cm2) completely inactivate the pectinase and PME in the juice. The thermal and PL pasteurization treatments inactivated aerobic mesophiles (AM) from 5.81 log cfu/mL to below the detection limit. The yeast & mold (YM) count in the clarified juice was 5.5 log cfu/mL, whereas pasteurized juices showed no fungi count. A similar thermal and PL intensity for 5-log cycle reduction in natural microbiota has been reported in the case of pineapple juice [30], pomegranate juice [39], and amla juice [40].
The color parameters of the clarified juice were L* = 1.24 ± 0.03, a* = − 0.44 ± 0.14, and b* = − 2.30 ± 0.05. The highest ΔE* of 3.9 was observed for the thermally treated juice (90 °C/3 min), whereas the ΔE* for PL exposure at 2400 J/cm2 was 1.37. The brownish tint of the thermally treated sample might be due to the nonenzymatic Maillard browning products. This is in line with the reported literature on coconut water [41] and mixed fruit beverages [42]. The pH, acidity, and soluble solids of the juice remain unaffected (p > 0.05) by the thermal and PL pasteurization intensities. The vitamin C content in the clarified juice was 108.3 mg AA/L. It was reduced to 98.5 and 50.4 mg AA/L for PL and thermally pasteurized samples. Similarly, the PL-pasteurized juice retains 33% more TPC, 43% more AOC, and 33% more TFC than the same from thermally pasteurized juice. Higher retention in bioactive compounds in the PL-treated sample than in the thermally treated sample is well represented in the earlier studies [22, 38, 39, 42,43,44]. The microbial reduction and the loss of bioactive compounds can be attributed to the thermal or pulsed light deactivation step. However, bioactive degradation may also be contributed by aerobic oxidation during the treatment.
Conclusions
The study envisages the optimized clarification condition for apple ber juice as incubated for 2.5 h at 40.7 °C while the enzyme concentration is 0.5% w/w. The clarity and yield of the juice were significantly improved after treatment with the pectinase enzyme. The optimally clarified and pasteurized apple ber juice was acceptable to the semi-trained sensory panelists, which may help promote the utilization of underexplored fruits like apple ber. The clarified juice can be thermally pasteurized at 90 °C/3 min. A pulsed light intensity of 2400 J/cm2 could also pasteurize the juice while reducing the microbial count by 5-log cycles. However, the bioactive concentration and organoleptic properties were compromised considerably due to the thermal pasteurization than the pulsed light pasteurization. Further study on the shelf-life of the clarified and pasteurized apple ber juice at different storage conditions is of great interest. Besides, the juice clarification using cellulase and a mixture of pectinase and cellulase may be explored further.
Data availability
The calculation and data sheets supporting the findings of this research are available from the corresponding author upon a reasonable request.
Code availability
Not applicable.
References
Q.H. Gao, C. Wu, J.G. Sen, M. Yu, C.L. Ma, Li, J. Food Sci. 77, 11 (2012)
E. Kovács, S. Siddiqui, Z. Kristóf, M. Tóth-Markus, E. Róth, Acta Aliment. 39, 109 (2010)
National Horticulture Board (NHB), Second Advance Estimates (2021–22) of Area and Production of Horticultural Crops released by the Department of Agriculture and Farmers Welfare, Ministry of Agriculture & Farmers Welfare, Government of India (2020)
H. Krishna, A. Parashar, J. Food Biochem. 37, 571 (2013)
F. Elmas, E. Varhan, M. Koç, J. Food Meas. Charact. 13, 70 (2019)
G. Gani, H.R. Naik, N. Jan, O. Bashir, S.Z. Hussain, A.H. Rather, T. Amin, J. Food Meas. Charact. 15, 743 (2021)
A. Nighojkar, M.K. Patidar, S. Nighojkar, in Processing and Sustainability of Beverages, Vol. 2: The Science of Beverages, ed. by A.M. Grumezescu, A.M. Holban (Woodhead Publishing, United Kingdom, 2019), p. 235
R.A. Dagadkhair, M.S. Pawar, S.A. Wattamwar, S.R. Popale, G.H. Athawale, Food Sci. Res. J. 4, 41 (2013)
T.K. Koley, S. Walia, P. Nath, O.P. Awasthi, C. Kaur, Int. J. Food Sci. Nutr. 62, 276 (2011)
F. Danalache, P. Mata, V.D. Alves, M. Moldão-Martins, in Fruit Juices - Extraction, Composition, Quality and Analysis, ed. by G. Rajauria, B.K. Tiwari (Academic Press, Elsevier Inc., London, 2018), p. 183
V. Borkar, S. Chakraborty, J.S. Gokhale, Appl. Biochem. Biotechnol. 193, 1321 (2021)
S. Chakraborty, C. Shrivastava, J. Food Process. Eng. 42, e13008 (2019)
W.C. Lee, S. Yusof, N.S.A. Hamid, B.S. Baharin, J. Food Eng. 73, 55 (2006)
S. Kaur, B.C. Sarkar, H.K. Sharma, C. Singh, J. Food Process. Eng. 34, 1298 (2009)
S. Kumar, H.K. Sharma, Food Sci. Biotechnol. 21, 959 (2012)
S.T. Sagu, E.J. Nso, S. Karmakar, S. De, Food Chem. 151, 182 (2013)
E.D. Kasapoğlu, S. Kahraman, F. Tornuk, J. Food Meas. Charact. 15, 5277 (2021)
H. Shahram, S. Dinani, M. Amouheydari, J. Food Meas. Charact. 13, 487 (2019)
O. Bashir, S.Z. Hussain, G. Gani, N. Jan, A.H. Rather, M. Reshi, T. Amin, J. Food Meas. Charact. 15, 2645 (2021)
S. Kumar, H.K. Sharma, J. Food Meas. Charact. 9, 414 (2015)
N.S. Bhatkar, R. Dhar, S. Chakraborty, J. Food Process. Preserv. 45, 15256 (2021)
R. Dhar, S. Chakraborty, Food Biosci. 47, 101779 (2022)
H. Chutia, C.L. Mahanta, N. Ojah, A.J. Choudhury, J. Food Meas. Charact. 14, 1926 (2020)
P.P. Bhalerao, S.A. Mahale, R. Dhar, S. Chakraborty, LWT 133, 109907 (2020)
R. Dhar, S. Basak, S. Chakraborty, Compr. Rev. Food Sci. Food Saf. 21, 499 (2022)
S. Chakraborty, P.S. Rao, H.N. Mishra, Innov. Food Sci. Emerg. Technol. 28, 10 (2015)
M. Dubois, K.A. Gilles, J.K. Hamilton, P.T. Rebers, F. Smith, Anal. Chem. 28, 350 (1956)
R.I.L. Ázar, M.L. Morales, G.P. Maitan-Alfenas, D.L. Falkoski, R.F. Alfenas, V.M. Guimarães, Food. Bioprod. Process. 119, 238 (2020)
T.S. Chang, M. Siddiq, N.K. Sinha, J.N. Cash, J. Food Process. Preserv. 19, 89 (1995)
K. Vollmer, S. Chakraborty, P.P. Bhalerao, R. Carle, J. Frank, C.B. Steingass, Food Bioprocess. Technol. 13, 1095 (2020)
S. Chakraborty, P.S. Rao, H.N. Mishra, Innov. Food Sci. Emerg. Technol. 52, 271 (2019)
S. Chakraborty, P.S. Rao, H.N. Mishra, Int. J. Food Microbiol. 211, 117 (2015)
S. Chakraborty, P.S. Rao, H.N. Mishra, J. Food Sci. 80, E1763 (2015)
A. Cornish-Bowden, Fundamentals of Enzyme Kinetics, 4th edn., (Wiley-Blackwell, Weinheim, 2012), pp. 39-59
K.A. Ninga, S. Sengupta, A. Jain, Z.S.C. Desobgo, E.J. Nso, S. De, J. Food Eng. 221, 158 (2018)
M. Mutlu, K. Sarioǧlu, N. Demir, M.T. Ercan, J. Acar, J. Food Eng. 41, 147 (1999)
M. Mohammadi, R.R. Mokarram, R. Shahvalizadeh, K. Sarabandi, L.T. Lim, H. Hamishehkar, Food Biosci. 36, 100625 (2020)
S. Basak, S. Mahale, S. Chakraborty, Innov. Food Sci. Emerg. Technol. 78, 103025 (2022)
B. Bhagat, S. Chakraborty, LWT 159, 113215 (2022)
S. Chakraborty, S. Ghag, P.P. Bhalerao, J.S. Gokhale, J. Food Process. Preserv. 44, e14932 (2020)
S. Donsingha, K. Assatarakul, Food Control 92, 162 (2018)
S. Chakraborty, S. Mahale, R. Dhar, S. Basak, Food Biosci. 45, 101508 (2022)
K. Rybak, A. Wiktor, K. Pobiega, D. Witrowa-Rajchert, M. Nowacka, LWT 149, 111906 (2021)
E.M. Bobasa, S. Srivarathan, A.D.T. Phan, M.E. Netzel, D. Cozzolino, Y. Sultanbawa, J. Food Meas. Charact. 17, 244 (2023)
Funding
The project is funded by the Ministry of Food Processing Industries, New Delhi, India, under Grant File No. Q-11/42/2018-R&D.
Author information
Authors and Affiliations
Contributions
SC: Conceptualization (lead); Investigation (equal); Supervision (lead); Funding acquisition (lead); Writing-review & editing (lead). SM: Data curation (equal); Investigation (equal); Methodology (lead); Formal analysis (equal); Writing-original draft (equal). RD: Writing-original draft (equal); Data curation (equal); Formal analysis (equal).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human and/or animal participants
This research does not include any studies or trials with human participants or animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chakraborty, S., Mahale, S. & Dhar, R. Response surface optimization of the enzymatic clarification process for apple ber juice and pasteurization by thermal and pulsed light treatments. Food Measure 17, 4495–4505 (2023). https://doi.org/10.1007/s11694-023-01976-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01976-8