Abstract
The aim of the study was to optimize the process for enzymatic extraction of pear juice from William Bartlett variety using central composite rotatable design (CCRD) and characterize its physicochemical and antioxidant properties. Pectinase enzyme concentration (0.4–2.4%), incubation temperature (20–70 °C) and incubation time (13–147 min) were evaluated as the independent factors of the optimization and the yield, clarity, TSS, acidity, turbidity, ascorbic acid, color and pectin were analyzed as responses. The results revealed that the enzyme concentration (A) significantly (p < 0.05) affected all the responses and increasing enzyme concentration led to an increase in the juice yield with better physicochemical characteristics. Enzyme concentration (1.90%), incubation temperature (30 °C) and incubation time (120 min) were determined to be the optimum condition to get the desired responses. The optimum conditions were experimentally validated. The derived optimum conditions were used for the production of pear juice which was further characterized for physicochemical and antioxidant properties. At these optimum conditions, pear juice of desirable quality in terms of physicochemical and antioxidant properties were obtained. Conclusively, pectinase enzyme assisted extraction has potential to enhance the yield and quality of pear juice during processing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pear (Pyrus communis L.) belongs to Rosaceae family and is rich in vitamins, minerals, dietary fiber, antioxidants and polyphenols with negligible amounts of sodium and fat contents. It is due to this nutritional value that this fruit is reported to help in the prevention and management of several metabolic disorders such as obesity, diabetes, hypertension, cardiac problems, etc. [1]. India ranks second in the World in pear production with an annual production of around 340,000 Metric Tons [2], with the Union Territory of Jammu and Kashmir producing 89,500 Metric Tons annually [3]. Although several varieties of pear are grown in the Union Territory of Jammu and Kashmir, but William Bartlett var. is being preferred over other varieties due to its characteristic sweet taste, pleasant aroma, and high juice yield [4].
The moisture content of fresh pear is about 80% which makes it highly perishable with a shelf life of only about 5–6 days [5]. Its high perishability affects the quality attributes of fruit after harvest. In order to reduce post-harvest losses and facilitate its value addition, extraction of fresh juice from pear seems to be a beneficial approach [6]. Several traditional methods are available for extraction of juice. One such method is the use of mechanical presses but these result in less recovery of juices with inferior quality, including the loss of some vital constituents. Use of enzymes for juice extraction offers a better choice, since it helps in the retention of vital constituents of fruit [7]. A structural component of pear viz. pectin, pectin–protein complexes and high concentration of pulp hampers juice extraction process [8]. Therefore, it is imperative to remove or breakdown these structural components for ensuring better juice yield. Several beverage industries are using pectinases obtained from various sources to maximize the yield of juice with better organoleptic quality [9]. Pectin is acted upon by pectinase enzyme in the middle lamella of cell wall, thereby improving efficiency of juice extraction with enhanced clarity [10]. Traditionally, use of mechanical presses have been used, apart from using diffusion, extraction, decanter centrifuge, fruit pulper and screw type juice extracter [5]. The juice yield could be increased by combining them with various pretreatments viz. cold, hot and enzymatic extraction [11]. Enzymatic treatments however, give significant increase in juice recovery compared to hot and cold extraction [5]. The use of enzymes has been found to offer a number of advantages over thermal or mechanical processes of several fruit pulps. Pectinases in particular, have been an integral part of modern fruit processing technology involving treatment of fruit masses. Enzymatic treatment facilitates the extraction of juice from pulp thereby increasing the recovery of juice and retaining quality of end product [5].
To the best of our knowledge, no such study has been carried out wherein the pectinases have been used for enzymatic extraction of pear juices. Enzymatically extracted fruit juices retain better organoleptic characteristics than conventionally prepared fruit juices [12]. However, the enzyme-assisted juice clarification and extraction process is quite sensitive and controlled by various factors like enzyme concentration, incubation temperature and incubation time [13]. It is therefore, of vital importance to know the optimum levels of enzyme concentration with optimum temperature and time for incubation during the enzymatic extraction of fruit juices. The present investigation was, therefore, conducted to optimize the process parameters for the enzymatic extraction of pear juice using pectinase enzyme as an aid for extraction. Besides, physicochemical and antioxidant properties of pear juice were evaluated in the study.
Material and methods
Procurement of fruit
About 100 kg fresh pear (William Bartlett variety) were harvested from the orchards of the Division of Fruit Science of our university, SKUAST-Kashmir and immediately taken to cold store maintained at 5 °C, which is located adjacent to the orchard within the university campus. Fully matured fruits, without any visual defects, were selected for the study, which were processed on the next day.
Extraction of pear juice and clarification
Pear were thoroughly cleaned and washed in tap water to remove any debris or dirt. About 10 kg pear was ground into pulp by grinder (Bajaj mixer, India). Addition of water was not necessary, since, pear already had higher moisture content. Potassium metabisulphite (KMS) was added at the concentration of 1% in order to prevent discolouration. Following this, pectinases were added to pulp for the temperature and time combinations as per the experimental design (Table 3). The whole mixture was filtered through a fine mesh cloth to remove any fruit residues and the juice was then stored in glass bottles until required.
Experimental design and process optimization
Central composite rotatable design (CCRD) was used to examine the effects of three independent variables viz. enzyme concentration (A), incubation temperature (B) and incubation time (C) on dependent variables viz. juice yield, juice clarity, total soluble solids, turbidity, titratable acidity, ascorbic acid, instrumental color and pectin content. The experimental ranges along with the respective coded levels of independent variables are given in Table 1. The table indicates that the enzyme concentration ranged from 0.4% to 2.4% with central point as 1.4%. Similarly, incubation temperature and time ranged from 20–70 °C and 13–147 min, respectively with central points as 45 °C and 80 °C. Statistical Software Design Expert 12 (Stat Ease Inc, Minneapolis, MN, USA) was used to develop second order polynomial models for dependent variables to fit the experimental data for each response. Multiple regression models were used for data analysis and statistical significance of each response was tested by analysis of variance (ANOVA). Optimization of pear juice extraction was done by numerical optimization using response surface of desirability function. For numerical optimization, the optimum condition criteria was used to maximize yield, clarity, TSS, ascorbic acid and lightness (L*) while minimizing the turbidity and pectin content.
Determination of product responses
Yield
Juice yield was determined following the procedure described by Akesowan and Choonhahirun [14] given in Eq. (1);
Clarity
The juice clarity was determined by percentage transmittance (%T) at wavelength of 660 nm [15]. Briefly, 10 mL of juice was centrifuged at 360 × g for 10 min to remove pulp and coarse cloud particles. Percent transmittance was determined at 660 nm by spectrophotometer (Systronics, India). The percent transmittance was considered a measure of juice clarity.
Turbidity
Turbidity was determined using portable Turbidimeter (2100 AN – HACH Company). The results were reported as Nephelometric Turbidity Units (NTU).
Total soluble solids
TSS was estimated using refractometer (Atago, Tokyo, Japan) following the procedure given by AOAC [16].
Titratable acidity
Titratable acidity (as citric acid) was determined by the procedure as described by Barrett et al. [17], and was expressed as percentage citric acid.
Ascorbic acid
Ascorbic acid was determined by titrating sample with 2, 6-dichlorophenolindophenol sodium salt solution as reported in AOAC [18]. Approximately, 5 g sample was weighed and volume was made up to 100 mL with 3% metaphosphoric acid and filtered. An aliquot of 10 mL was taken in a titration flask and titrated against 2, 6-dichlorophenol indophenol till light pink colour appeared. Ascorbic acid was then calculated using an Eq. (2);
Dye factor was calculated by titrating 5 mL standard ascorbic acid plus 5 mL (3%) metaphosphoric acid against 2, 6-dichlorophenol indophenol till pink colour appeared and volume used (titre value) was noted.
Pectin
Pectin was determined following the procedures as described by Onwuka [19]. Briefly, 25 g homogenized sample was boiled in 300 mL 0.03 N HCl for 30 min in 1 L beaker and residue was washed with hot water and filtrate was collected. To the residue, 100 mL 0.05 N HCl was added and boiled for 20 min and residue was washed with hot water and filtrate was collected. Again to the residue, 100 mL 0.3 N HCl was added and boiled for 10 min and filtrate was collected. Filtrates were pooled, cooled and volume was made up to 500 mL. 100 mL aliquot was pipetted into 1 L beaker. Then, 250 mL water was added and acid was neutralized with 1 N NaOH using phenolphthalein indicator. Excess of 10 mL 1 N NaOH with constant stirring was added and allowed to stand overnight. After this, 50 mL 1 N acetic acid was added and after 5 min, 25 mL of 1 N calcium chloride was added while stirring. After allowing it to stand for 1–2 min, the solution was filtered through oven dried Whatman filter paper no. 4 grade. The precipitate was washed with boiling distilled water until free from chloride. The filtrate was tested with 1% silver nitrate for chloride. The filter paper containing precipitate was dried at 100 °C, cooled in a dessicator and weighed. The results were expressed in percent calcium pectate on oven dry basis. Pectin was then calculated using an Eq. (3),
Instrumental color
Color values i.e. lightness (L*), redness (a*) and yellowness (b*) were estimated by Hunter Lab Colorimeter (SN3001476, Accuracy Micro-sensors, New York).
Physicochemical analysis of enzyme extracted pear juice
The pear juice was obtained using the optimized process conditions and was then evaluated for the following parameters:
Proximate composition and water activity
Standard AOAC [16] procedures were followed for estimation of moisture, protein, fat, ash and fiber contents. Carbohydrate was estimated by subtracting the sum of the percentages of moisture, protein, fat, fiber and ash contents from 100. Energy value was determined by multiplying the percentages of protein, fat and carbohydrate by 4, 9 and 4, respectively. Water activity (aw) was estimated using water activity meter (Pre-Aqua Lab, Water Activity Analyzer, Decagon Devices Inc., Pullman, Wash., USA).
Total, reducing and non-reducing sugars
Total and reducing sugars were estimated by Lane and Eynon method, as described by Ranganna [20]. Non-reducing sugars were determined by subtracting reducing sugars from total sugars.
TSS/acid ratio and pH
TSS/acid ratio was determined by dividing the °Brix (TSS) by the acid content of juice. pH values were measured using pH-meter (Inolab. WTW Series, Germany) according to AOAC [16] procedures.
Mineral contents
Mineral contents (calcium, magnesium, iron, phosphorus, potassium and sodium) of pear juice were determined according to AOAC [18] procedures.
Anthocyanin
Anthocyanins were determined following the procedures of Ozgen et al. [21] with some modifications. Anthocyanins were extracted by soaking fruit mass (1 g) in 10 mL aqueous methanol (95%) to concentrated HCl in the ratio (97:3 v/v) overnight at 2 to 4 °C in dark. The resulting solution was decanted and centrifuged at 3963 g for 20 min, and anthocyanin absorbance (1 cm light path) was determined from supernatant solution at 530 nm using an ultraviolet/visible spectrophotometer (model 6405; Jenway Spectrophotometers, Dunmow, Essex, UK). Anthocyanins were calculated using an Eq. (4),
Antioxidant activity (DPPH and FRAP) and total phenolic content
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assays elucidated by Chan et al. [22] were followed. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as a standard to calculate standard curve. The ferric reducing antioxidant power (FRAP) assay was carried out following the procedures of Pulido et al. [23]. Total phenolic contents (mg GAE/100 g) were estimated by the Folin-Ciocalteu method [24].
Besides the above parameters, pectin, TSS, titratable acidity, ascorbic acid and juice yield were determined for the enzyme extracted pear juice produced using optimum processing conditions, the procedures of which are already explained in previous sections.
Statistical analysis
For each experimental condition, all of the readings were taken three times and the mean values were reported. Data was analyzed using the general linear model (GLM) procedure of SPSS Statistics (V. 16, Inc., Chicago, IL). The difference between the means was determined using Duncan’s multiple range test (p < 0.05).
Results and discussion
Fit of models
Analysis of variance (ANOVA) for different responses indicating the statistical validity is given in Table 2. The results indicate that the models developed for all the product responses (juice yield, clarity, TSS, turbidity, titratable acidity, ascorbic acid, L*, a*, b* and pectin) were significantly (p < 0.05) influenced by independent variables viz. enzyme concentration (A), incubation temperature (B) and incubation time (C). For all the dependent variables, significant (p < 0.001) models with high coefficient of determination (R = 0.9213–0.999), were developed. The coefficient of determination computed were highly desirable (R2 = 0.92–0.99) for all the selected parameters which ensures the reasonable fit of empirical models with actual data. The predicted and adjusted R2 values for all the product responses were found to be in sound agreement with each other. The coefficient of variation (CV), which depicts the accuracy and reproducibility of models ranged from 0.09 to 8.33%. A highly desirable adequate precision range (13.83–421.136) was observed in all the models, suggesting the adequacy of model discrimination in all the parameters. Models for all the parameters showed non-significant lack of fit thereby depicting that the second order polynomial models correlated well with the measured data. It is imperative to mention here that for each of the dependent variable; only those independent variables have been included in the models, which had significant effect as was depicted from ANOVA results.
Effect of process parameters on product responses
Yield (Y)
Juice yield is an important parameter from economic point of view and ranged between 67.62 ± 6.33% and 90.57 ± 9.01% (Table 3). The regression model for juice yield is shown in Eq. (5);
where A is the enzyme concentration, B is the incubation temperature and C is the incubation time.
The fitted model indicates significant (p < 0.05) linear effect of all the three independent variables on juice yield. The positive coefficients of linear terms of enzyme concentration (A), incubation temperature (B) and incubation time (C) implied that with the increase in A, B and C, juice yield increased (Fig. 1a). With increase in the concentration of enzyme, the rate of pectin and protein hydrolysis increases [13]. The higher molecular weight polysaccharides are hydrolyzed into lower molecular weight components which in turn reduces the viscosity of juice [25]. Similar results were reported by Diwan and Shukla [26] in guava juice. Significant (p < 0.05) increase in juice yield with an increase in incubation temperature (B) and incubation time (C) might be due to the increased enzyme activity at optimum temperature and time. High temperature enhances the molecular motion which results in faster reaction rate. The interactive effect of all the independent variables was significant (p < 0.05) and showed negative effect on juice yield due to loss of enzyme activity beyond optimum temperature. With increased incubation time beyond optimum level, juice yield decreased as represented by negative coefficient of quadratic term of A (Eq. 5). This may be attributed to the loss of substrate for pectinase as the reaction progresses. The findings of Zhang et al. [27] for juice yield are in sound agreement with our results. The significant (p < 0.05) negative quadratic effect of enzyme concentration and incubation temperature on juice yield states that an optimum level of enzyme concentration and incubation temperature increased juice yield beyond which it gets decreased due to the less availability of substrate concentration for enzyme molecules. From the regression Eq. (5), it is evident that the linear effect of enzyme concentration (A) was dominant amongst all the three independent variables.
Clarity (C)
Juice clarity is one of the most important parameters of juice since it affects its market value and overall acceptability [25]. It ranged between 38.80 ± 5.03% and 91.10 ± 9.03% as shown in Table 3. The regression Eq. (6) obtained for juice clarity is given below;
where A is the enzyme concentration.
From the regression Eq. (6), juice clarity significantly (p < 0.05) increased with increase in enzyme concentration (A) (Fig. 1b). It may be due to the degradation of pectin and protein molecules resulting in an enhancement of juice clarity thereby reducing the repulsion between these cloud forming particles, causing them to clump together and hence forming larger aggregates. These settle down faster resulting in production of clarified juice [21]. Similar results have also been obtained by Abdullah et al. [25] in carambola juice.
Total soluble solids (TSS)
TSS of fruit juices reflects the amount of soluble constituents like sugars, carbohydrates and proteins. TSS of enzymatically extracted juice ranged between 13.00 ± 4.00°B and 13.80 ± 4.05°B (Table 3). The regression Eq. (7) obtained for TSS is given below;
where A is the enzyme concentration and C is the incubation time.
Enzyme concentration (A) and incubation time (C) showed a significant (p < 0.05) positive effect on TSS (Eq. 7). TSS increased with increase in enzymatic concentration (A) due to hydrolysis of fruit pulp [28] (Fig. 1c). Our results are in close agreement with the previous results of Joshi et al. [29].
Titratable acidity (TA)
TA of enzymatically extracted juice ranged between 0.33 ± 0.03% and 0.39 ± 0.02% (Table 3). The regression Eq. (8) obtained for TA is given below;
where A is the enzyme concentration and C is the incubation time.
Enzyme concentration (A) and incubation time (C) had positive significant (p < 0.05) linear effects on TA as is observed in Eq. (8) (Fig. 1d). Enzymes act upon pectin, where carboxylic and galactouronic acid monomers are released thereby leading to the development of higher acidity in enzymatically extracted juice [12]. Thus, an increase in TA with increase in enzymatic concentration was found. Similar results were also observed by Akesowanand and Choonhahirun [14] for enzyme-extracted guava juice. Further, for prolonged incubation, released organic acids are accumulated thus supporting our results of significant (p < 0.05) linear effect of incubation time (C) on TA.
Turbidity (T)
Turbidity refers to the haziness in juice which contributes to its cloudy appearance and must be as low as possible for high quality juices [13]. It ranged between 2.89 ± 0.03 NTU and 9.03 ± 3.66 NTU (Table 3). The regression Eq. (9) obtained for juice turbidity is given below;
where A is the enzyme concentration, B is the incubation temperature and C is incubation time.
From regression Eq. (9), it was observed that all the independent variables i.e. enzyme concentration (A), incubation temperature (B) and incubation time (C) significantly (p < 0.05) affected turbidity. The negative coefficients of the linear terms of enzyme concentration (A), incubation time (B) and incubation temperature (C) implied an inverse relation of these variables with turbidity (Fig. 1e). Enzymatic degradation of pectin resulted in the reduction of turbidity in juices [30] in contrast to the clarity, which increases with increase in enzyme concentration (Eq. 3). Hamid et al. [31] have also reported the similar results for enzymatically clarified pomegranate juice by protease and pectinase treatments, wherein they have reported increase in clarity and decrease in turbidity with increase in enzyme concentration. With increase in incubation temperature, the activity of pectin splitting enzyme increases making juice more clear [32]. Higher enzyme concentration with longer incubation time causes breakdown of cloud forming particles followed by their settling due to the reduction in electrostatic repulsion between protein-pectin particles thereby reducing turbidity. The quadratic effects of A, B and C had a negative effect on turbidity since the excessive enzyme concentration with prolonged time and high temperature reduces juice turbidity by exposure of positive nucleus sites to the surrounding negative charges where large protein-pectin clusters are formed [8]. Similar results have also been obtained by Saxena et al. [33] for watermelon juice.
Ascorbic acid (AA)
Vitamin C is a highly bio-available antioxidant present in fruit juices. Its strong free radical scavenging activity makes it a functional component in fruit juices [34]. Ascorbic acid of enzymatically extracted pear juice ranged between 4.12 ± 1.06 mg/100 g and 4.21 ± 1.02 mg/100 g (Table 3). The regression Eq. (10) obtained for ascorbic acid is given as;
where A is the enzyme concentration, B is the incubation temperature and C is the incubation time.
Enzyme concentration (A) had a significant (p < 0.05) negative linear and quadratic effects on ascorbic acid (Fig. 1f). This is due to the oxidation of ascorbic acid during clarification process [30]. A significant (p < 0.05) negative interactive effect of incubation temperature (B) and incubation time (C) is due to partial loss of nutrients during heat treatment. Significant (p < 0.05) negative quadratic effects of incubation temperature (B) is ascribed to thermal degradation of ascorbic acid.
Instrumental color
Color is an influential quality attribute in visual appearance of juices and is directly related to the acceptability of food products. Higher lightness values (L*) are desirable for clarified juice from consumer point of view. L* value ranged between 58.93 ± 7.01 and 59.99 ± 7.02, whereas a* value ranged between – 1.68 ± 0.02 and – 2.32 ± 0.01 and b* value ranged between 57.30 ± 5.02 and 59.22 ± 6.02 (Table 3). The regression Eqs. (11), (12) and (13) obtained for L*, a*, b* value are given below;
where A is the enzyme concentration, B is the incubation temperature and C is the incubation time.
From the regression Eqs. (11), (12) and (13), increase in enzyme concentration (A) increased L* due to enzymatic breakdown of structural tissues and complex polysaccharides thus decreasing viscosity and enhancing lightness of juice (Fig. 1g, h, i). L* value also increased with the increase in incubation time (C) which could be due to hydrolysis of fruit pulp [19]. Similar findings have also been observed for jamun juice [28]. Furthermore, with the increase in enzyme concentration (A) and incubation time (C), a* value decreased whereas, all the three independent variables had a significant (p < 0.05) positive linear effect on b* value. The more the positive value, more is the original color of pear juice. Additionally, the interactive effect of enzyme concentration and incubation temperature (AB) and incubation temperature and incubation time (BC) showed a negative significant (p < 0.05) effect on b* value.
Pectin content (PC)
Pectin is a high molecular weight, negatively charged, and acidic polysaccharide that is mainly found in the middle lamella of higher plants [35]. It ranged between 0.18 ± 0.01% and 0.71 ± 0.03% (Table 3). The regression Eq. (14) obtained for pectin content is given below;
where A is the enzyme concentration, B is the incubation temperature and C is the incubation time.
From the regression Eq. (14), it was observed that all the three independent variables had negative significant (p < 0.05) linear effect on pectin. With an increase in enzyme concentration (A), pectin content decreased (Fig. 1j). Enzymatic treatment resulted in drastic reduction in pectin content and consequently, reduces viscosity [36]. In combination with time and temperature, pectinase degrades pectin, exposing positively-charged proteins. The electrostatic repulsion between cloud particles is thereby reduced so that they aggregate together [37]. Degradation and solublization of insoluble materials from fruit cell wall by enzymatic action resulted in reduction of pectin content [38]. Similar findings have also been reported by Ucan et al. [39] for lemon juice. From the above Eq. (14), a negative interactive effect of incubation temperature (B) and incubation time (C) and their quadratic effect was observed on pectin content. It was observed that with increasing temperature (B) and time (C) for incubation, hydrolysis of complex polysaccharides increases, forming simpler substances and reducing pectin content.
Optimization and model validation
The optimum condition was obtained by employing the desirability function method. The desirability value obtained was 0.81 (Fig. 1k). The optimum conditions obtained for the production of pear juice were enzyme concentration (1.90%), incubation temperature (30 °C) and incubation time (120 min). Predicted response values and the actual values obtained were almost similar with a variation of less than 4% (Table 4).
Physicochemical characteristics of enzymatically extracted pear juice
Proximate composition
The results of physicochemical properties of control and enzymatically extracted juice are presented in Table 5. The moisture content of enzymatically extracted pear juice (84.70 ± 0.20%) was significantly (p < 0.05) higher than control (83.01 ± 0.01%) due to breakage of glycosidic bond of pectin by two different mechanisms viz. hydrolysis, with the introduction of water across the oxygen bridge and the transelimination lysis [40, 41]. The crude protein content of enzymatically extracted pear juice (0.33 ± 0.01%) was significantly (p < 0.05) lower than that in the control (0.41 ± 0.02%). The reduction in protein content is due to the breakdown of pectin–protein interactions by pectinase enzyme [42]. Non-significant (p > 0.05) change was however, observed in crude fat and ash contents in enzymatically extracted pear juice and control. A significantly (p < 0.05) lower crude fiber was observed in enzymatically extracted pear juice (2.18 ± 0.05%) than control (2.31 ± 0.05%) which is due to the solubilization and degradation of insoluble materials like cellulosic and hemicellulosic components [38]. The carbohydrate content of enzymatically extracted pear juice (12.01 ± 0.03%) was significantly (p < 0.05) lower than control (13.69 ± 0.02%). The polysaccharides and colloidal materials present as solid materials in juices form gels and thus accumulate on filter surface during filtration process thereby reducing their respective contents [43]. Significantly (p < 0.05) lower energy value was observed in enzymatically extracted juice (51.90 ± 0.30 kcal/100 g) compared to control (58.70 ± 0.30 kcal/100 g) which is due to the reduction in carbohydrate and protein contents in the clarified juice. A significant (p < 0.05) increase in water activity (aw) was observed after enzymatic treatment (0.98 ± 0.01) compared to control (0.94 ± 0.02) which is due to the increased amount of unbound water by hydrolysis of cellular components after enzyme extraction [41].
Pectin, total, reducing and non-reducing sugars
The results of pectin, total sugars, reducing sugars and non-reducing sugars of control and enzymatically extracted pear juice are presented in Table 5. There was a significant (p < 0.05) decrease in pectin content after enzymatic extraction which is due to the degradation of pectin by pectinase enzyme [37].
Pectin, a major component of pear juice, is thought to play an important role in destabilization of juices. Thus, its removal is essential [44]. Besides, pectin becomes soluble in water and some of the pectin molecules are released into the juice, making it more viscous and thus difficult to squeeze from the fruit during processing [45]. Pectinases breakdown pectin molecules into simpler monomer units, galactouronic acid residues, thus, the decrease in pectin content was expected. Enzymatic treatments significantly (p < 0.05) increased the amount of total sugars in juice (11.43 ± 0.01%) compared to control (10.3 ± 0.10%) which might be due to the conversion of insoluble pectin into soluble sugars by pectinase [46]. A significant (p < 0.05) increase in reducing sugar was observed after enzymatic treatment (9.02 ± 0.05%) compared to control (8.61 ± 0.06%) due to the action of pectinase (polygalacturonases and pectin lyases) on polygalacturonic chains as well as hydrolysis of non-reducing sugars. Similar results were reported by Fang et al. [47] in passion fruit. Non-significant (p > 0.05) change was, however, observed in non-reducing sugar content.
Total soluble solids (TSS), titratable acidity, TSS/acid ratio and pH
The results of TSS, titratable acidity, TSS/acid ratio and pH of control and enzymatically extracted pear juice are presented in Table 5. A significantly (p < 0.05) higher TSS was observed in enzymatically extracted juice(13.92 ± 0.04°B) relative to control (13.30 ± 0.3°B) which is due to the breakdown of intercellular tissues and release of soluble components [28]. The higher TSS values of enzymatically extracted pear juice may be associated to an increase in reducing sugars due to the action of pectinases on polygalacturonic chains as well as hydrolysis of non-reducing sugars by weak acids at higher temperatures [48]. Similar results were observed by Brasil et al. [49] in guava pulp.
A significant (p < 0.05) increase in titratable acidity was observed after enzyme extraction (0.39 ± 0.02%) than control (0.35 ± 0.01%) due to the hydrolysis of proteinacious pectin matrix with the release of carboxylic and galactouronic acid monomers [12]. Acidity of enzymatically extracted pear juice was higher which may be due to the action of pectinase enzyme on cell wall constituents consisting of protopectin and pectin, thereby increasing the acidity of product [50, 51]. The release of galacturonic acid units may have been responsible for the increase of acidity in clarified juice [47].
The results further revealed a significantly (p < 0.05) lower TSS/acid ratio of enzymatically extracted juice (37.08 ± 0.05) than control (38.00 ± 0.02). Higher titratable acidity than TSS of enzymatically extracted juice may have resulted in the decrease in TSS/acid ratio [29]. Similar findings have also been reported in grape juice by Aponso et al. [52]. pH of enzymatically extracted pear juice (3.84 ± 0.03) was significantly (p < 0.05) lower compared to control (4.08 ± 0.02) which is due to de-esterification of highly methylated pectin molecules [50]. Thus, it indicates that the enzymatically extracted pear juice is acidic than control, owing to the higher titratable acidity of enzymatically extracted pear juice.
Mineral contents
The results of mineral contents are presented in Table 5. A significant (p < 0.05) increase in calcium, potassium and sodium contents was observed after enzymatic extraction, however, magnesium, iron and phosphorous contents showed a non-significant (p > 0.05) increase. Enzymatic treatment results in the movement of minerals from cells into the solution. Carcel et al. [53] have also reported the similar results.
Anthocyanin and ascorbic acid
The results of anthocyanin and ascorbic acid of control and enzymatically extracted juice are presented in Table 5. Anthocyanins are mainly located in the skin of the fruit and during pressing, it is important to transfer it into the juice [54]. A significantly (p < 0.05) higher anthocyanin content was observed in enzymatically extracted juice (2.33 ± 0.04 mg/100 g) compared to control (2.23 ± 0.04 mg/100 g) possibly due to the release of pigments from plant cells [29]. Similar findings were also reported in plum and raspberry juice by Rommel et al. [55]. Pectinase treatment increases the release of anthocyanins due to the modified level of individual pigment [5], thus increase in anthocyanin content was observed. The pectolytic enzymes resulted in a stationary higher level of total anthocyanins over time [56]. The higher retention of anthocyanins in enzymatically extracted pear juice may also be due to the fact that its pH value (3.84 ± 0.03) was favourable for anthocyanin stability [57]. Further, the higher anthocyanin content of enzymatically extracted pear juice relative to control indicates the breakdown of plant cell walls with subsequent recovery of anthocyanins. Our results are similar to those reported by Siddiq et al. [58] who reported significantly higher anthocyanin contents of enzymatically extracted blueberry juice relative to the control (juice extracted without the use of pectinase).
A significantly (p < 0.05) lower ascorbic acid was found in enzymatically extracted juice (4.17 ± 0.02 mg/100 g) compared to control (4.69 ± 0.01 mg/100 g). This is due to the thermal degradation and oxidation of ascorbic acid during enzymatic extraction [46]. During clarification, ascorbic acid decreases which is ascribed to oxygen exposure of clarifying aids and processing conditions [43].
Antioxidant activity (DPPH and FRAP) and total phenolic content
Table 5 shows that the antioxidant activity (DPPH and FRAP) was significantly (p < 0.05) lower in enzymatically extracted pear juice (19.30 ± 0.30% and 18.70 ± 0.10 mg Catechin/100 g), than control (20.30 ± 0.10% and 18.91 ± 0.04 mg Catechin/100 g), respectively. This is ascribed to the oxidation of various bioactive antioxidant components [57]. These results are comparable to the previously reported values for açai juice [58] and açai pulp [59]. During heat treatment, various plant materials get oxidized, thus reducing antioxidant activity [60]. The polyphenolic and antioxidant activities are dependent on matrix component interactions and are influenced by processing, temperature and chemical composition [57], further supporting our results. Phenolic compounds (quinones) react with sulfhydryl and amine groups of proteins forming irreversible and insoluble complexes that are separated from juice by decantation, thus explaining the lower content of phenolic compounds in clarified juice [61]. The total phenolic compounds mainly comprise chlorogenic acid and its esters, which are important components of the aroma, taste and color of juices [62]. The oxidation of these compounds due to the action of endogenous enzyme system during clarification step constitutes the most frequent cause of changes in the levels [63]. It is therefore, for this reason that the total phenolic contents of enzymatically extracted pear juice (67.34 ± 0.02 mg GAE/100 g) are significantly (p < 0.05) lower than control (68.55 ± 0.03 mg GAE/100 g) as shown in Table 5. Sidiq et al. [58] have also reported the similar findings for blueberry juice. Phenolic compounds bind to plant cell materials like pectin during their diffusion from the plant cell walls, which affect the extract ability of the phenolic acids [64].
The results also indicate that both the antioxidant activity (DPPH radical scavenging activity and FRAP assay) and total phenolic content of enzymatically extracted juice was lower than that of control (Table 5). This suggests a possible correlation between antioxidant activity (DPPH and FRAP) and the total phenolic content. Kim et al. [65] have also reported a correlation between the DPPH radical scavenging activity and phenolic compounds for colored barley germplasm. Similarly, Shao et al. [66] have also reported a correlation between antioxidant activity and total phenolic content for non-pigmented, red and black rice. However, no such relationship was found between the antioxidant activity and anthocyanin content, which may be attributed to the differences in chemical structure and concentration [67], for which the molecular mechanisms need to be clarified with further studies.
Juice Yield
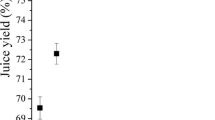
The results of juice yield of control and enzymatically extracted juice are presented in Table 5. A significant (p < 0.05) increase in juice yield (89.61 ± 0.03%) was obtained compared to control (69.80 ± 0.20%) possibly due to the breakage of cellular tissues by pectinase enzyme. The increase in the juice yield is ascribed to the hydrolysis of pectin thus releasing the sap inside the cells of pulp [68].
Conclusion
The response surface methodology (RSM) was proven to be successful for the optimization of the process for enzymatic extraction of pear juice using pectinase. The study revealed the significant effect of all the three independent variables viz. enzyme concentration, incubation temperature and incubation time on physicochemical attributes of juice such as juice yield, clarity, TSS, acidity, turbidity, ascorbic acid, L*, a*, b* and pectin of pear juice. Within the experimental range, enzyme concentration was the most important factor affecting physicochemical properties of juice. The optimum processing conditions for pectinase-assisted extraction of quality pear juice were 1.90% enzyme concentration, 30 °C incubation temperature and 120 min incubation time. Overall, the nutritional profile and yield of pectinase assisted extraction of pear juice were positively affected compared to control. Pear juice could offer a healthy dietary choice for the consumers and may add value to pear fruit. These promising results confirm the potential of using pectinases for the production of high quality juice, both in terms of yield and nutritional value.
References
D. Xie, H.Y. Zhong, J. Mo, Z.H. Li, T. Cui, C.P. Yi, Food 1, 41–48 (2007)
USDA-National Agricultural Statistics Service, Census of Agriculture Data Collection Tops NASS (2018)
GoI, Government, of India Ministry of Agriculture (Cooperation & Farmers Welfare Directorate of Economics & Statistics New Delhi, Farmers Welfare, Department of Agriculture, 2018)
G.J. da Silva, F. Villa, F. Grimaldi, P. S. da Silva, J. F. Welter, Advances in Plant Breeding Strategies: Fruits, https://doi.org/https://doi.org/10.1007/978-3-319-91944-7_4 (2018)
H.P. Sharma, H. Patel, S. Sharma, Trends Post-Harvest Technol. 2, 1–14 (2014)
R. Maiti, A.K. Thakur, A. Gupta, D. Mandal, Res (Trends Biores. Manag, Techn, 2018)
G. Garg, A. Singh, A. Kaur, R. Singh, J. Kaur, R. Mahajan, Biotech 6 (47), (2016), DOI https://doi.org/10.1007/s13205-016-0371-4
D.R. Kashyap, P.K. Vohra, S. Chopra, R. Tewari, Biores. Technol. 77, 215–227 (2001)
T. Tu, K. Meng, Y. Bai, P. Shi, H. Luo, Y. Wang, B. Yao, Food Chem. 141(3), 2974–2981 (2013)
A. Nighojkar, M.K. Patidar, S. Nighojkar, Processing and Sustainability of Beverages, https://doi.org/10.1016/B978-0-12-815259-1.00008-2, (2018)
R. Chadha, B.K. Kumbhar, B.C. Sarkar, J. Food Sci. Technol. 40(1), 35–39 (2003)
G.O. Fetuga, M.A. Idowu, L.O. Sanni, F.O. Bamiro, Nig. Food J. 26, ISSN 0189-7241 (2008). DOI: https://doi.org/10.4314/nifoj.v26i1.47421.
P. Ghosh, R.C. Pradhan, S. Mishra, J. Food Proc. Eng. 40(2), (2016). DOI https://doi.org/10.1007/s13205-016-0561-0.
A. Akesowanand, A. Choonhahirun, J. An, Plant Sci. 23(1), 114–120 (2013)
P. Rai, C. Rai, G.C. Majumdar, S. Dasgupta, S. De, J. Membrane Sci. 283, 116–122 (2006)
AOAC, Official Method of Analysis. 18th ed. Association of Official Analytical Chemists, Maryland, USA (2005)
D.M. Barrett, C. Weakley, J.V. Diaz, M. Watnik, J. Food Sci. 72, C441–C451 (2007)
AOAC, Official Methods of Analysis, 17th Ed Association of Official Analytical Chemists, Arlington, USA (2000)
G.I. Onwuka, Naphthali prints, Nigeria p.97 (2005)
S. Ranganna, McGraw Hill Publication Co. Ltd., New Delhi (1997).
M. Ozgen, C. Durgaç, S. Serçe, C. Kaya, Food Chem. 111, 703–706 (2008)
E.W.C. Chan, Y.Y. Lim, M. Omar, J. Food Chem. 104(4), 1586–1593 (2007)
R. Pulido, L. Bravo, F. Saura-Calisto, J. Ag, Food Chem. 48(8), 3396–3402 (2000)
R. Bobinait, P. Viskelis, P.R. Venskutonis, Food Chem. 132(3), 1495–1501 (2012)
L. A. G. Abdullah, N.M. Sulaiman, M.K. Aroua, M.J. Megat Mohd Noor, J. Food Eng. 81(1), 65–71 (2007)
A. Diwan, S.S. Shukla, J. Food Sci. Technol. 42(3), 245–249 (2006)
H. Zhang, Z. Wang, S.Y. Xu, J. Food Eng. 80(4), 1226–1232 (2007)
I. Norjana, A.A. Noor Aziah, J. Int. Food Res. 18(3), 1117–1122 (2011).
V.K. Joshi, M. Parmar, N. Rana, Ind. J. Nat. Prod. Res. 2(2), 189–197 (2011)
H.N. Sin, S. Yusuf, A.H.N. Sheikh, R.A. Rahman, J. Food Eng. 73(4), 313–319 (2006)
I. Hmid, D. Elothmani, H.A. Hanine, A. Oukabli, J. Bio. Innov. 5(4), 506–515 (2016)
C. Grassin, P. Fauquembergue, Wiley, New York, 1030–1061 (1999)
D. Saxena, L. Sabikhi, S.K. Chakraborty, D. Singh, J. Food Sci. Technol. 49, (2012).
B. Halliwell, Free Radic. Res. 25(5), 439–454 (1996)
D.B. Pedrolli, A.C. Monteiro, E. Gomes, E.C. Carmona, Open Biotechnol. J. 3, 9–18 (2009)
D. Vaidya, M.J. Vaidya, S. Sharma, Ghanhyam, Nat. Prod. Rad. 8(4), 380–385 (2009)
V. Sorrivas, D.B. Genovese, J.E. Lozano, J. Food Proces. Pres. 30, 118–133 (2006)
K. Dorreich,. In XII International congress of fruit juice report of congress pp. 183–197 IFU, Interlaken, 20–24 May (1996)
F. Uçan, A. Akyildiz, E. Agçam, J. Food Process. (2014). https://doi.org/10.1155/2014/215854
R.C. Codner, J. Applied Bacteriol. 84, 147–160 (2001)
H.A. Murad, H.H. Azzaz, J. Microbiol. 6, 246–269 (2011)
E.F. Torres, C. Aguilar, J.C.C. Esquivel, G.V. Gonzalez, Enzyme Technol. 273–296 (2005)
K.S. Youn, J.H. Hong, D.H. Bae, S.J. Kim, S.D. Kim, J. Membr Sci. 228, 179–186 (2004)
S. Croak, M. Corredig, J. Food Hydrocolloids 20(7), 961–965 (2006). https://doi.org/10.1016/j.foodhyd.2005.10.016
F. Danalache, P. Mata, V.D. Alves, M.M. Martins, Enzyme Assist. Extract. Fruit Juices (2018). https://doi.org/10.1016/B978-0-12-802230-6.00010-2
P. Arsad, R. Sukor, W.Z. Wan Ibadullah, N.A. Mustapha, A.S. Meor Hussin, Int. J. Adv. Sci. Eng. 5(5), (2015)
Y.Z. Fang, S. Yang, G.Y. Wu, Nutrition 18, 872–879 (2002)
L.T. Cesar, C. Marília de Freitas, A.M. Geraldo, R. Wilane de Figueiredo, M.R. Alcântara de Miranda, P.H. Machado de Sousa, I.M. Brasil, C.L. Gomes, J Food Sci Technol. https://doi.org/10.1007/s13197-012-0809-6 (2012)
I.M. Brasil, G.A. Maia, R.W. Figueiredo, J. Food Chem. 54, 383–386 (1995)
A.K. Sharma, B.C. Sarkar, H.K. Sharma, Eur. Food Res. Technol. 221, 106–112 (2005)
R. Chadha, B.K. Kumbhar, B.C. Sarkar, J. Food Sci. Technol. 40, 35–39 (2003)
M. Aponso, U. Marapana, R. Manawaduge, E. J. Acad. Essays 4(4), 165–174 (2017)
J. Carcel, J. Benedito, N. San-Juan, E. Sanchez, Aliment. Equipos Tecnol. 17, 135–141 (1998)
M. Mieszczakowska-Frac, J. Markowski, M. Zbreznail, W. Pocharski, LWT Food Sci Technol. 49, 251–252 (2012)
R.E. Rommel, Wrolstad, D.A. Heatherbell, J. Food Sci. 57(2), 385 (1992)
A.A. Joshi, R.B. Kshirsagar, A.R. Sawate, J. Food Proc. Technol. 3(2), 1–3 (2012)
A. Grobelna, S. Kalisz, M. Kieliszek, Agronomy 9, 860 (2019)
M. Siddiq, K.D. Dolan, P. Perkins-Veazie, J.K. Collins, LWT Food Sci. Technol. 92, 127–132 (2018)
R. Lichtenthaler, F. Marx, O.M. Kind, Eur. Food Res. Technol. 216, 166–173 (2003)
A.M. Goula, K.G. Adampoulos, P.C. Chatzitakis, V.A. Nikas, J. Food Eng. 74, 37–46 (2006)
S. Damodaran, K. L. Parkin, O. R. Fennema, Alegre: Artmed (2010)
F.V.B. Wiecheteck, A. Nogueira, J.F. Drilleau, G. Wosiacki, Ciências Agrárias e Engenharias 11(1), 27–34 (2005)
A. Nogueira, L.D. Santos, F.V.B. Wiecheteck, S. Guyot, G. Wosiacki, Ciências Agrárias e Engenharias 9(3), 7–14 (2003)
A.G. Padaychee, M. Netzel, **Food Chem. 135(22), 87–92 (2012)
M.J. Kim, J.N. Hyun, J.A. Kim, J.C. Park, M.Y. Kim, J.G. Kim, S.J. Lee, S.C. Chun, I.M. Chung, J. Agric. Food Chem. 55, 4802–4809 (2007)
Y. Shao, Z. Hu, Y. Yu, R. Mou, Z. Zhu, T. Beta, Food Chem. 239, 733–741 (2018)
M.B. Aziz, F. Garcia, L. Mouls, H. Fulcrand, H. Hajjaj, J Food Meas. Charac. (2019). https://doi.org/10.1007/s11694-019-00126-3
H.P. Sharma, H. Patel, Crit Rev. Food Sci. Nutr. 57(6), 1215–1227 (2016)
Acknowledgement
The authors are grateful to the Division of Fruit Science, Sher-e-Kashmir University of Agricultural Sciences and Technology-Kashmir (SKUAST-K) for providing the raw material (Pear var. William Bartlett).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gani, G., Naik, H.R., Jan, N. et al. Physicochemical and antioxidant properties of pear juice prepared through pectinase enzyme-assisted extraction from William Bartlett variety. Food Measure 15, 743–757 (2021). https://doi.org/10.1007/s11694-020-00676-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00676-x