Abstract
Sheath blight, caused by the fungus Rhizoctonia solani, is a major problem that significantly impacts rice production and can lead to substantial yield losses. The disease has become increasingly problematic in recent years due to the widespread use of high-yielding semi-dwarf rice cultivars, dense planting, and heavy application of nitrogenous fertilizers. The disease has become more challenging to manage due to its diverse host range and the lack of resistant cultivars. Despite utilizing traditional methods, the problem persists without a satisfactory solution. Therefore, modern approaches, including advanced breeding, transgenic methods, genome editing using CRISPR/Cas9 technology, and nanotechnological interventions, are being explored to develop rice plants resistant to sheath blight disease. This review primarily focuses on these recent advancements in combating the sheath blight disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice stands as one of the most vital staple foods, nourishing over half of the global population. As the population elevates in countries where rice is a staple crop, it is anticipated that there will be an increased demand for rice in the future. Despite tremendous advancements in agricultural technology over the past fifty years, a significant proportion of the global population still faces starvation and undernourishment. Hunger and malnutrition are caused by an imbalance between crop production and population for food leading to starvation and malnutrition, especially among children. Despite the effective implementation of many scientific advancements in the cultivation of rice crops, pests and diseases continue to exist, so preventative measures should be taken to minimize yield loss [1].
Sheath blight (ShB), caused by Rhizoctonia solani Kuhn [teleomorph - Thanatephorus cucumeris Frank (Donk)], is one of the most serious diseases of rice worldwide, which poses a significant threat to rice production. Despite the effective control of bacterial blight and rice blast through advanced resistance breeding, managing ShB remains challenging due to the limited understanding of its underlying molecular resistance mechanisms. In the early stages of the disease, water-soaked lesions appear, which later develop into a rattlesnake-like pattern with greyish-white cents and dark brown margins. As the infection becomes severe, the fungus produces sclerotia interwoven mycelial masses coated in hydrophobic layers. These sclerotia, measuring 1 to 3 mm in diameter, initially appear white and gradually turn brown or dark brown, forming on the surface of infected rice. These remains dormant in the soil between crops for many years and regains its their infectivity under favourable conditions [2]. ShB causes yield loss of up to 50% at the field level, whereas artificially inoculated plots show 20–42% yield loss [3].
R. solani is a soil-borne necrotrophic fungal pathogen that infects plants of over 32 taxonomic families. R. solani isolates had fourteen different anastomosis groups (AGs). AGs are a classification system based on hyphal fusion reactions. These groups are crucial for understanding the genetic diversity in this plant pathogenic fungus, indicating its great genetic variability [4]. The first thirteen groups were called AG1–AG13, while the 14th group, AGB1, is a bridging isolate. Bridging isolate is a specific isolate that can undergo hyphal fusion with isolates from different AGs, making it important for understanding the genetic diversity and taxonomic connections within the R. solani species complex. Several anastomosis groups are further subdivided into intraspecific groups (ISGs). R. solani AG1 isolates are classified into three ISGs, viz., IA, IB, and IC, based on host origin, symptoms, cultural characteristics, DNA sequence homology and sclerotia morphology [5]. R. solani AG1-IA is generally accepted to be the cause of ShB disease in rice. Pathogens enter plants by lobate appressoria, infection cushions, or both. R. solani runner hyphae form convoluted hyphal clusters, known as infection cushions. Infection typically occurs through direct cuticular penetration via an infection cushion, while stomatal penetration via lobate appressoria is less common [2].
The pathogen is difficult to manage due to its broad host range, high genetic variability, and lack of natural resistance in available rice germplasm. Finding solutions to combat the pathogen is crucial for reducing rice yield losses and ensuring global food security. This review outlines breeding methodologies, focusing on the molecular mechanisms of defense-related genes, the use of genome editing to target negative regulators, and the roles of nanoparticles in enhancing ShB resistance.
Virulence factors involved in R. solani infection in rice
The pathogen R. solani infiltrates the host through multiple mechanisms, while rice counters with innate and systemic acquired resistance (SAR) immunity. Effector proteins are utilized by pathogens to infect host plants and can cause disease. R. solani is known to produce a variety of effector molecules with different functions that enable successful colonization. Domains of three potential secreted effectors of R. solani AG1-1 A: glycosyltransferase GT family 2, cytochrome C oxidase assembly protein CtaG/ cox11, and peptidase inhibitor I9 have been identified to induce cell death in rice during R. solani invasion [6]. Genomic analysis of virulent Indian strains identified additional effectors, including histone acetyltransferase, MDR transporter, polygalacturonase, and pectin lyase [7]. The polygalacturonase gene significantly contributes to R. solani pathogenesis [8].
R. solani secretes a variety of secondary metabolites, such as host-selective toxins and biologically active molecules, which enhance pathogen virulence by breaking down host physical barriers and disrupting normal physiological functions of the host plant [6]. Biologically active molecules produced by R. solani include oxalic acid (OA), 3-methylthiopropionic acid (MTPA), phenylacetic acid (PAA) and its derivatives [9]. OA produced by necrotrophic pathogens is an essential virulence factor for successful infection. During R. solani infection, OA inhibits the synthesis of various phenolic substances and degrades the cell wall for effective penetration [10].
Fungal plant pathogens typically secrete various types of carbohydrate-active enzymes (CAZymes), for successful invasion, including cell wall-degrading enzymes (CWDEs) viz., cellulases, pectinases, and hemicellulases to breach plant cell wall components. R. solani evades plant immunity by masking its cell wall chitin with α-1,3-glucan. The combined action of these CWDEs allows R. solani to effectively penetrate and spread within the host plant tissues, contributing to the pathogen’s ability to cause disease. Understanding the role of CWDEs is a crucial strategy to combat R. solani infection [11].
Factors influencing ShB pathogenesis
Sheath Blight pathogenesis is influenced by various factors, including hormones, sugars, and nitrogen sources. Hormones play a crucial role in regulating plant responses to pathogens. Sugars and nitrogen sources are essential nutrients utilized by pathogens for their growth and development during host-pathogen interactions [12]. Understanding and effectively managing these factors are critical for controlling and mitigating the spread of ShB disease. Comprehending how hormones, sugars, and nitrogen sources interact with R. solani and the host plant is crucial for reducing disease incidence and enhancing crop yield.
Hormones
Plant hormones such as auxin, ethylene (ET), salicylic acid (SA), jasmonic acid (JA), brassinosteroids (BRs), gibberellin (GA), abscisic acid (ABA), strigolactone (SL), and cytokinin (CTK) intricately regulate rice defense responses against R. solani [12]. Auxin, essential for rice growth, facilitates transport via PIN-FORMED 1a (OsPIN1a) and enhances resistance to ShB when overexpressed, suggesting its role in modulating plant-pathogen interactions [13]. Ethylene, traditionally associated with fruit ripening, also significantly enhances rice resistance to pathogens by activating defense responses such as reactive oxygen species (ROS) and phytoalexin production, mediated by OsACS2 and OsEIL1 [14]. Salicylic acid and jasmonic acid, two distinct defense-related hormones, play differing roles in rice immunity: SA primarily acts against biotrophic, while JA defends against necrotrophic pathogens. Both hormones contribute positively to rice resistance against ShB through pathways involving NPR1-mediated SA signaling and OsWRKY30-mediated JA responses [15]. Beyond SA and JA, BRs, GA, ABA, SL, and CTK also modulate rice’s defense strategies. BRs, typically associated with growth promotion, negatively regulate ShB resistance [16]. GA’s influence on immunity involves complex interactions with JA signaling and developmental processes [17]. ABA acts as a negative regulator of rice immunity, contrasting with CTK’s role in promoting defense responses against pathogens. These hormones collectively illustrate the intricate balance and interplay required for rice to effectively combat ShB, underscoring the complexity of plant-pathogen interactions [12].
Sugar
Sugars produced through photosynthesis are essential for plant growth and serve as vital nutrients utilized by R. solani during the invasion. SWEET proteins, such as OsSWEET11/Os8N3, mediate sugar transport across cell membranes, facilitating pathogen access to extracellular sugars [18]. Modulating sugar levels impacts rice susceptibility to ShB, linking sugar metabolism directly to disease outcomes. Effector protein AOS2 interact with host proteins (e.g., WRKY53, GT1) to activate sugar transporters (e.g.,OsSWEET2a, OsSWEET3a), influencing the rice-ShB interaction and resistance mechanisms [19].
Nitrogen fertilizer
Nitrogen (N) fertilizer has played a crucial role in boosting rice yields since the Green Revolution, particularly with the development of semi-dwarf varieties, such as those carrying the sd1 allele, which enhance the yield but often exhibit poor nitrogen use efficiency (NUE) [20]. While high nitrogen levels support rice growth and yield, they also correlate with increased susceptibility to ShB disease [21]. Recent studies have highlighted specific genes influencing nitrogen’s impact on ShB resistance. For example, OsAMT1;1, a rice ammonium transporter, has been identified as crucial for enhancing ShB resistance by promoting the accumulation of nitrogen metabolites and activating the ethylene signaling pathway [22]. Additionally, OsDEP1, associated with both NUE and ShB resistance, regulates susceptibility to ShB; silenced plants and mutants exhibit enhanced resistance, whereas overexpression aggravates susceptibility [23]. These findings underscore the complex interplay among nitrogen management, yield enhancement, and disease resistance strategies in rice.
Management of ShB disease in rice
Management of ShB disease is challenging because of their wide host range, genetic variability, fungicidal resistance and lack of durable resistance cultivars in rice. The excessive use of fungicides harms beneficial microorganisms and human health. In this context, there is an urgent need for alternative and sustainable management strategies.
Biological and chemical control
Biological control methods offer promising strategies for managing R. solani. Plant growth-promoting actinomycetes, particularly Streptomyces sp. exhibit strong microbial antagonism against R. solani. Bacterial biocontrol agents such as Pseudomonas and Bacillus not only suppress fungal pathogens but also promote plant growth through various mechanisms such as nutrient solubilization and phytohormone synthesis [24]. Timely application of fungicides between panicle differentiation and heading stages is a crucial factor in overcoming resistance development, especially in susceptible varieties [25]. However, the continuous use of single fungicides can lead to resistance by R. solani, necessitating combination formulations like Azoxystrobin + Difenoconazole that will delay resistance development [26]. Despite their efficacy, chemical methods pose environmental risks. Hence, the use of non-chemical methods, which include modern breeding strategies such as QTLs, genome editing, and nanotechnological aspects, to develop viable resistance against ShB is indispensable.
Breeding strategies for ShB resistance
Breeding strategies aimed at enhancing ShB resistance in rice elite cultivars involve several key approaches. ShB resistance may be attributed to two main mechanisms: disease escape and physiological resistance. Disease escape relies heavily on crop architecture, with morphological traits such as plant height, heading date, and stem thickness showing positive correlations with resistance. Physiological resistance, on the other hand, is linked to processes that reduce the efficiency of one or several stages of the pathogen’s infection cycle [27].
Utilizing host plant resistance
Host plant resistance is considered the most sustainable method for managing ShB disease. When a plant recognizes a pathogen, signal transduction pathways collaborate to establish a complex network that triggers defence responses. During pathogen invasion, certain genes, known as disease resistance genes, respond by changing their expression or protein modifications. These include R (Resistance) genes and HPRR (host pattern recognition receptor) genes, which help detect and counteract the pathogen. In rice, disease resistance is either qualitative or quantitative. Qualitative resistance, controlled by a single R gene, is race-specific and offers strong, targeted protection against specific pathogens [28].
Quantitative resistance is controlled by multiple genes or quantitative trait loci (QTL), which confers partial resistance to various pathogens. A plethora of reports elucidates that quantitative resistance can work against different strains and even different species of the pathogen, which offer broad-spectrum resistance from annual to perennial crops under conducive environmental conditions [28]. ShB resistance in rice Indica cultivar was commonly controlled by numerous minor genes, which exhibit broad-spectrum disease resistance comparable with Japonica cultivars. In addition, wild relatives such as Oryza rufipogon, Oryza nivara, Oryza meridionalis, and Oryza barthii have shown resistance traits against ShB [4, 29].
Resistant cultivars against ShB
The use of ShB-resistant rice varieties is the most economical and effective strategy to combat ShB disease. Identifying ShB-resistant germplasm and mapping resistance genes are essential to develop resistant varieties. Still, none of the genotypes with absolute resistance are identified [30]. The list of rice varieties with variable resistance is noted in Table 1.
Marker-assisted breeding
Marker-assisted breeding is pivotal for integrating identified resistance QTLs into popular rice cultivars. By identifying and integrating genes that offer resistance to ShB, breeders can develop rice varieties with reduced vulnerability to the disease. Researchers have employed advanced backcross methods and double haploid (DH) populations to map rice’s QTLs responsible for ShB resistance. They’ve discovered potential genetic markers for breeding resistant varieties, from both wild rice species like O. minuta and O. rufipogon, as well as cultivated varieties through backcrossing with species like O. officinalis [36]. These QTLs, notably located on chromosome 9, contribute to the complex genetic basis of ShB resistance, with studies indicating its polygenic nature influenced by multiple genes. This approach aids in reducing linkage drag during the introgression of genomic regions and facilitates the pyramiding of resistance genes. By utilizing molecular markers, breeders can precisely select rice plants carrying desired ShB resistance genes, thereby accelerating the breeding process [37]. While individual R genes have proven effective against ShB disease in rice, the persistent challenge of rapid pathogen evolution exists. To tackle this issue, integrating genetic diversity of wild rice species and utilizing breeding methods to incorporate advantageous ShB resistance QTLs into japonica cultivars shows significant potential for enhancing resistance. Ultimately, sustainable management strategies for ShB disease in rice can be achieved through the integration of various resistance mechanisms and genetic resources.
QTL mapping
Sheath blight resistance in rice is a quantitative trait controlled by multiple genes, making QTL identification, mapping, validation, and characterization crucial for developing resistant varieties [38]. Using diverse mapping populations and molecular markers, numerous QTLs for ShB resistance have been detected on all 12 rice chromosomes [2]. RIL (Recombinant Inbred Line) and DH mapping populations have largely replaced F2-derived populations due to lower recombination and their ephemeral nature [39]. Despite their potential, wild relatives of cultivated varieties have rarely been used for QTL detection due to crossability barriers [40]. Due to environmental influences, accurate disease phenotyping remains a significant challenge for fine mapping of ShB-resistant loci. Resistance to rice ShB is governed by polygenes [41, 42] although some studies suggest control by major genes in certain varieties [31]. Over the two decades, numerous quantitative trait loci (QTLs) contributing to ShB resistance have been identified. Notable QTLs for sheath blight resistance in rice were mapped on chromosomes 2, 3, 7, 9, 11, and 12, with additional QTLs detected by various studies [36, 41]. However, these QTLs have yet to be exploited in developing ShB-resistant cultivars, and their breeding potential remains unassessed.
Introducing desirable ShB resistance QTLs into Japanese cultivars involves overcoming the predominance of such QTLs in indica rice. Most ShB resistance QTLs identified originate from indica rice, with few from japonica rice. Notably, qShB-7TQ and qShB-9TQ, originating from indica rice Teqing (TQ), have been located on chromosomes 7 and 9, respectively. Research indicates that qShB-9TQ provides significant resistance to ShB, reducing disease ratings by 1.0. (using the qShB-9TQ rating scale). However, Japanese rice varieties lack qShB-9TQ.To address this, breeding strategies involving crossing and backcrossing indica varieties with japonica varieties have been employed. This approach aims to introduce qShB-9TQ into japonica varieties, thereby enhancing their resistance to ShB [38]. Crossing the moderately-resistant CR 1014 with the susceptible Swarna-Sub results in identification of three QTLs (qShB-1.1, qShB-1.2, qShB-1.3) on chromosome 1, with qShB-1.1 consistently showing a high Logarithm of odds (LOD) score. This QTL co-located with qShB1 from Oryza nivara, includes potential candidate genes LOC_Os01g65650 and LOC_Os01g65900. Near-isogenic lines of Swarna-Sub1 carrying qShB-1.1 exhibited a 27.8% reduction in lesion height [43]. Most of the QTLs have been summarized in previous studies [27]. Only a few QTLs discussed in later studies are covered in the following section.
Role of major and minor QTLs
Among the identified QTLs for ShB resistance in rice, qShB9-2 and qSBR11-1 stand out as major contributors, explaining 25% and 14% of the phenotypic variation, respectively [44, 45]. qShB9-2 encompasses candidate genes such as β-1,3-glucanase and OsWAK91 and has been fine-mapped to a 146-Kb region [46]. Similarly, qSBR11-1, derived from the partially resistant line Tetep, spans a 0.85 Mb region on chromosome 11, featuring a tandem array of eleven class III chitinase genes and LOC_Os11g47510, which confer tolerance in susceptible cultivars like Taipei 309 [47]. Despite extensive studies, no novel candidate genes for breeding or genetic engineering have emerged from these QTLs. In contrast, qSB12YSB, originating from rice variety YSBR1, has been mapped to a 289-Kb region on chromosome 12. Through the use of 150 BC4 backcross inbred lines and 34 chromosomal segment substitution lines, researchers identified 18 candidate genes associated with qSB12YSB, including those implicated in secondary metabolite biosynthesis and ROS scavenging systems according to KEGG analysis. Field trials confirmed qSB12YSB’s efficacy, showing significant resistance in commercial rice cultivars NJ9108, NJ5055, and NJ44 under severe ShB conditions, reducing yield losses by up to 13.5% in the Lemont background. These findings underscore qSB12YSB’s potential in rice breeding programs aimed at developing new, resistant varieties to combat ShB [48].
Minor QTLs in rice refer to genetic loci with moderate effects on phenotypic variation and lower LOD scores compared to major QTLs. In ShB resistance studies, these QTLs play a role in fine-tuning resistance traits across varying environmental conditions [49]. Exploring these minor QTLs is crucial for uncovering genes that could significantly enhance ShB resistance in rice breeding programs.
Pyramiding of genes
Pyramiding is a process of combining major and minor resistant genes to enhance and prolong resistance against ShB in rice varieties. Traditional breeding methods have struggled to produce ShB-resistant rice varieties due to the quantitative nature of the trait [38]. Marker-assisted selection has not been used to incorporate ShB resistance QTL (qShB)s into commercial rice varieties despite its widespread use in disease resistance breeding [47]. The introduction of qShB-9TQ and qShB-3TQ into Lemont could potentially reduce ShB loss by 15% [42].
Overexpressing a single defense-related protein may not be highly effective in enhancing resistance. Combining multiple ShB resistance QTLs can increase resistance to ShB. Combinatorial expression of defense genes has shown better results. Examples include combinations like MOD1 and RCH10, Chi11 and thaumatin-like protein, Chi11 and β-1,3-glucanase, DmAMP1 and RsAFP2, Chi11 and ap24, RCH10 and AGLU1, Oxalate oxidase 4 and Chi11, NPR1 and Chi11 [50, 51]. Studies indicate that dual-gene cassettes are more effective for ShB resistance than single-gene cassettes [50]. Additionally, combining glycoside hydrolase genes from Trichoderma atroviride (ech42, nag70, and gluc78) enhances pathogen tolerance [2, 52]. By combining these strategies, breeders can develop rice cultivars with enhanced tolerance to ShB disease in rice.
Omics approach to understanding the ShB pathogenesis
Omics is a comprehensive field of study focused on understanding the relationships among various molecules, particularly interactions between plants and pathogens. Recently, research in this area has provided deeper insights into these interactions through transcriptomic, and metabolomic analyses etc. Whole-genome sequencing on 13 inbred rice lines in this study revealed over 200 candidate genes, encompassing a total of 333 nonsynonymous single nucleotide polymorphisms (SNPs), distinguishing between susceptible and resistant genotypes to ShB [53]. Comparative transcriptome analysis has revealed that alternative splicing of key pathogenic genes in R. solani AG1-1 A plays significant roles during its infection of rice, soybean, and corn plants [54]. Comparative transcriptomics between ShB-susceptible (Lemont) and tolerant (Teqing) rice cultivars identified 4806 differentially expressed genes (DEGs) [55]. Further investigations into the proteome and metabolome of rice lines pre- and post-ShB infection uncovered 38 differentially expressed proteins and 40 differentially accumulated metabolites, underscoring the significance of energy and carbohydrate metabolism in the plant’s response to R. solani [56]. Recently identification of 23 putative candidate rice miRNAs that could potentially be involved in the defense mechanisms against R. solani [57]. Genomic, transcriptomic, proteomic, and metabolomic studies have identified key genes, proteins, metabolites, and miRNAs involved in rice and R. solani host-pathogen interactions.
Biotechnological approaches
Transgenic approaches
In rice, conventional breeding has attained partial resistance to ShB. Genetic engineering techniques give a promising foundation for further improvement to get complete resistance. Transgenic approaches have been adopted by researchers to facilitate the introduction of desired genes, aiming to achieve comprehensive resistance in a relatively condensed timeframe compared to traditional breeding methods. This strategy not only reduces dependability on chemical pesticides but also enhances key agronomic parameters, effectively addressing challenges such as sexual incompatibility. Moreover, transgenic technology has the potential to introduce novel traits from disparate systems into the target organism, amplifying its versatility in crop improvement [58].
In a transgenic strategy, researchers develop resistance against ShB disease in rice by elevating innate immune responses such as pathogenesis-related genes and introducing several foreign genes. Transgenic plants were developed in elite indica cultivars viz., Pusa Basmati, ASD16, ADT38, and IR50 with co-expression of pathogenesis-related (PR) genes such as chitinase (chi11) and thaumatin-like protein (TLP) reveals enhance resistance through synergistic activity against ShB [59]. Subsequently, the conclusive evidence on transgenic rice plants harbouring rice chitinase chi11 gene, belonging to a PR-3, confers ShB resistance. Overexpression of the OsACS2 gene, a crucial ET synthesis enzyme, is controlled by the vigorous pathogen-responsive promoter (PBZ1). Besides enhancing their resistance to R. solani, transgenic lines also contribute to maintaining crop productivity. These findings underscore the potential of manipulating ethylene levels to fortify rice’s defence mechanisms against fungal pathogens [14].
Recently, 352 differentially expressed genes in six diverse rice genotypes resistant to ShB disease caused by R. solani AG1-IA are identified. Among 352 genes, Oschib1, a class III chitinase, was significantly overexpressed and cloned from the resistant variety Tetep. Overexpression of Oschib1 in the susceptible variety Taipei 309 conferred resistance to R. solani, it demonstrates dose-dependent enzyme activity [60]. The list of transgenes utilized to develop resistance is listed in Table 2.
Genome editing through CRISPR technology
Earlier, techniques like EMS (Ethyl methane sulfonate) mutagenesis and T-DNA (Transfer-DNA) insertion induce random mutations in the genome. ZFN and TALEN represent advancements over earlier genome editing methods. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is a natural defense mechanism in bacteria against phages, now widely utilized as a precise genome editing tool, surpassing the limitations of ZFN and TALEN. Among CRISPR/Cas systems, Type II CRISPR/Cas9 is commonly used, consisting of RNA-guided Cas9 endonuclease and single-guide RNA (sgRNA). In plants, the endogenous repair system employs two mechanisms to repair double-strand breaks: NHEJ (non-homologous end-joining), which is error-prone, and HDR (homology-directed repair), which can lead to extensive insertion or fragment replacement [85]. To enhance precision and minimize unintended effects, protein engineering techniques have been employed to modify the Cas9 nuclease, resulting in variants such as Cas9D10A for more accurate editing [86].
The utilization of CRISPR/Cas9 technology will facilitate resistance creation against R. solani infection by targeting the negative regulators. The knocking out of genes like OsMESL, OsEIL2, and OsSLR in rice enhances resistance against R. solani by reducing ROS accumulation and downregulating GA signaling pathways [87,88,89]. Emerging shreds of evidence on CRISPR-Cas9 knockout of the microRNA Osa-miR444b.2 elucidate slower lesion expansion, reduced grain weight (GW), and smaller panicles with increased tillering and plant height. Subsequently, gene expression analysis indicated that Osa-miR444b.2 regulates plant hormone signaling pathways, which play a pivotal role in disease resistance [90]. Several other genes are also employed to develop a resistance against ShB in rice, and they are listed in Table 3.
Role of pathogenesis-related genes
PR proteins play an important role in rice disease resistance response. During R. solani infection, activation of the SAR pathway in rice induces PR genes (PR-3, PR-5, PR-9, PR-10, PR-12 and PR-13) and PAL, enhancing resistance [94]. Subsequently, specific PR-5 genes (TLP-D-34 and OsOSM1) give promising evidence for improving ShB resistance [65, 74]. Moreover, overexpression of ethylene biosynthetic genes such as PR1b and PR5 elevates resistance [14].
Signaling related genes
The nuclear localization of NPR1 (Nonexpressor of pathogenesis related genes 1) is essential for activating the expression of PR genes. BjNPR1 and AtNPR1 show enhanced resistance to ShB in rice by activating the SA-mediated SAR pathway [78, 79]. In addition, transgenic rice lines expressing the ACS2 (1-aminocyclopropane-1-carboxylic acid synthase) gene for ethylene synthesis exhibit increased resistance to R. solani [14].
Antimicrobial peptides
The Glycine and cysteine-rich antimicrobial peptides (AMPs) like thionin (Thi3.1), defensin (PDF1.2) and lipid transfer proteins (LTPs) will defend by forming membrane pores, causing ion leakage and cell death [95]. AMP1 from Dahlia merckii and Allium cepa, AFP2 from Raphanus sativus, puroindoline (pinA and pinB) from wheat, Snakin-1, Stomoxyn ZH1, Purothionin, Cecropin B, D4E1, and Phor21 expressed either through transgenic methods or by intrinsic inhibitory properties, contribute to elevate resistance against R. solani [96].
Host-induced gene silencing
Host-induced gene Silencing (HIGS) leverages RNA interference (RNAi) to boost plant resistance by targeting pathogen genes. It relies on host plants by constitutively expressing dsRNA constructs that transfer siRNA complementary to the virulence factors of the pathogen. Recent studies in rice R. solani pathogen system have successfully targeted key pathogenicity genes like MAP kinase (PMK) and polygalacturonase (PG), significantly reducing disease susceptibility [2]. Moreover, the Grassy tiller 1 (GT1) gene in rice, induced by R. solani, increases susceptibility to ShB by activating SWEET2a and SWEET3a genes. GT1 RNAi plants and mutants for sweet2a and sweet3a show reduced susceptibility to the disease compared to wild-type [97].
Nanotechnological approaches
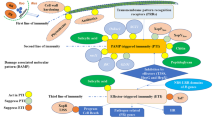
Nanotechnology is the study of understanding the matter at nano dimensions. It involves building, controlling and structuring nanomaterials. So far, we broadly use chemical fungicides to manage ShB disease in rice. These chemicals are expensive and have residual effects. To overcome these things nowadays farmers use nanomaterials to combat ShB disease in rice [98]. Foliar application of lanthanum-based nanomaterials, particularly La10Si6O27 nanorods, suppresses ShB in rice. NR (nanorods) are more advantageous because of their good bioavailability, slower dissolution, and silicon nutrient benefits. Simultaneously, they activate the CAM (Crassulacean acid metabolism) pathway, enhances PAL, leading to the production of SA, lignin, and antioxidants. SA, which upregulates the SAR, lignin acts as a physical barrier, and ROS scavenges through antioxidants. These comprehensively result in ShB resistance in rice [99]. Figure 1 illustrates the mechanisms by which nanomaterials combat ShB disease.
Mechanism of various nanomaterials that develop resistance against ShB in rice plants. (Figure created with www.Biorender.com)
Silver nanoparticles (SNPs) are employed to combat ShB disease in rice, targeting the crucial factor of sclerotia germination. SNPs form an antimicrobial layer around rice plants, and penetrate the fungal cell membrane to eliminate pathogens. This process inhibits sclerotia formation and germination. The eco-friendly nature of SNPs, with fungistatic, bacteriostatic, and plasmonic properties, positions them as environmentally conscious inhibitors against plant pathogens, contrasting with synthetic fungicides [100]. The use of Boro gold also reduces the severity of ShB in rice. Boro gold (SNSp) is a combination of nanosilver particles and peroxy acid. This can be administered through root dipping for 24 h followed by 2–3 times spray when it is needed [101].
Chitosan Nanoparticle (ChNP), partially or fully deacetylated chitin, is a hydrolytic enzyme and a pathogenesis-related (PR) protein. It is a non-toxic, biodegradable biopolymer and a powerful enhancer of plant immunity. Chitin is a primary component of the cell walls of fungi, insects, and crustaceans. Insoluble chitosan hinders agricultural use, but water-soluble chitosan nanoparticles overcome this, exhibiting enhanced efficacy. Shrimp shell waste, sourced for chitosan extraction, is transformed into nanoparticles through ionic gelation with polyanion tripolyphosphate. Increased peroxidase in ChNP-treated plants defends against oxidative stress during pathogen invasion. PAL activates phenylpropanoid pathways, and ß-1,3 glucanase breaks down fungal cell wall components. Chitosan nanoparticles trigger plant resistance by prompting diverse defence responses, including the formation of structural barriers. Chitosan nanoparticles can penetrate plant cells deeply, integrating into the plant’s defence mechanism systemically. They serve as powerful inducers of systemic resistance in rice against ShB disease [102].
Foliar application of selenium nanomaterials (Se0 NMs) significantly reduced ShB severity in rice by 68.8% at 5 mg/L, outperforming Se ions and Thifluzamide. Se0 NMs’ controlled release increased bioavailability and promoted SA and JA-dependent resistance pathways. Additionally, Se0 NMs improved rice yield by 31.1%, enhanced nutritional quality by 6.4–7.2%, raised organic Se content by 44.8%, and decreased arsenic and cadmium levels by 38.7% and 42.1%, respectively. They also increased Se bio-accessibility by 22.0% and reduced As and Cd bio-accessibility by 20.3% and 13.4%, respectively, suggesting a sustainable strategy for better food quality and security [103].
Enzyme-responsive AZOX-AFS-Pec nanoparticles (NPs) use iron-based mesoporous materials and pectin as carriers to combat rice ShB. These NPs showed high AZOX loading capacity and released the fungicide selectively under acidic conditions in the presence of pectinase. They exhibited superior wetting and adhesion on rice blades, enhanced fungicidal activity against ShB, and promoted rice growth by releasing Fe ions. Moreover, the NPs increased SA levels in rice plants, bolstering disease resistance while reducing toxicity to earthworms compared to AZOX suspension [104]. Table 4 lists nanoparticles used to manage ShB.
Applying silver nanoparticles deactivates cellular enzymes that destroy pathogen cell division, enhancing plant resistance. Chitosan nanoparticles activate ß-1,3 glucanase, which breaks down fungal cell walls. Lanthanide nanorods activate the CAM pathway, enhancing PAL activity and producing SA, lignin, and antioxidants. SA upregulates SAR, lignin acts as a physical barrier, and antioxidants scavenge ROS accumulation. Collectively, these effects confer ShB resistance in rice.
Key databases related to rice ShB
KRiShI - A Knowledgebase for Rice Sheath Blight Information, a comprehensive, manually curated platform, integrates dispersed unstructured scientific data on the rice ShB disease into an easy-to-use interface for effective mining, visualisation and search. In addition to offering comprehensive information on host resistance, gene expression, proteins, metabolites, resistance genes, pathways and OMICS studies (http://www.tezu.ernet.in/krishi/ ) [105]. RSIADB - Rice Sheath Blight Information and Analysis Database, is a dedicated resource offering extensive information on rice ShB disease. It includes data on pathogens, host interactions, resistance mechanisms, and related research findings (http://genedenovoweb.ticp.net:81/rsia/index.php) [106].
Conclusion
ShB stands out as an emerging concern among various rice diseases, capable of severely disrupting rice production and yield. Cultural and biological methods represent sustainable approaches for mitigating the severity of ShB disease. Promising bio-agents of Pseudomonas and Bacillus play a significant role in growth promotion and suppression of hyphal invasion [30]. In extending context, the role of systemic fungicides in the management of ShB at the field level is inevitable. Even though, the multifaceted necrotrophic pathogen naturally has a wide host range, making its management is challenging till date. The lack of the R gene in available rice germplasm and wild weeds has not yet been elucidated. However, marker-assisted breeding aids in mapping QTL, offer a promising approach to identifying and utilising genes associated with ShB resistance. It underscores the necessity for continued exploration and innovation in breeding and biotechnological approaches to uncover and utilize potential R genes for enhancing disease resistance. In recent eras, emerging molecular techniques have created opportunities to explore host-pathogen interaction by utilizing CRISPR/Cas-mediated genome editing. It allows precise modifications of the rice genome by enabling scientists to successfully delve into targeting candidate negative regulators viz., OsSWEET, OsMSEL, OsNYC3 and Osa-miR444b.2 etc., associated with pathogenesis [67, 84, 88, 90]. These Transgene-free SDN-1 type of mutants will hold a great potential for widespread commercial adoption and mark a significant advancement in developing resilient cultivars. Despite genetic resistance, growing evidence leads to uncovering the utilization of nanomaterials in and along with fungicides such as SNPs, ChNP and AZOX-AFS-Pec, etc., triggers resistance against ShB [100, 102, 104]. To combat the devastating impact of sheath blight disease and ensure stable and sustainable agricultural practices in the future, there is an increasing focus on integrating conventional, nanotechnological, and novel approaches, including CRISPR-mediated genome editing of susceptible genes.
Data availability
No datasets were generated or analysed during the current study.
References
Shaheen N, Ahmad S, Alghamdi SS, Rehman HM, Javed MA, Tabassum J, Shao G (2023) CRISPR-Cas System, a possible Savior of Rice threatened by Climate Change: an updated review. Rice 16(1):39. https://doi.org/10.1186/s12284-023-00652-1
Molla KA, Karmakar S, Molla J, Bajaj P, Varshney RK, Datta SK, Datta K (2020) Understanding sheath blight resistance in rice: the road behind and the road ahead. Plant Biotechnol J 18(4):895–915. https://doi.org/10.1111/pbi.13312
Khoshkdaman M, Mousanejad S, Elahinia SA, Ebadi AA, Padasht-Dehkaei F (2021) Sheath blight development and yield loss on rice in different epidemiological conditions. J Plant Pathol 103(1):87–96. https://doi.org/10.1007/s42161-020-00653-9
Bashya BM, Rawat K, Singh D, Krishnan SG, Singh AK, Singh NK, Aggarwal R (2021) Screening and identification of new sources of resistance to sheath blight in wild rice accessions. Indian J Genet Plant Breed 77(03):341–347. https://doi.org/10.31742/ijgpb.v77i03.2
Taheri P, Gnanamanickam S, Höfte M (2007) Characterization, genetic structure, and pathogenicity of Rhizoctonia Spp. Associated with Rice Sheath diseases in India. Phytopathology® 97(3):373–383. https://doi.org/10.1094/phyto-97-3-0373
Li D, Li S, Wei S, Sun W (2021) Strategies to Manage Rice Sheath Blight: lessons from interactions between Rice and Rhizoctonia solani. Rice 14(1):21. https://doi.org/10.1186/s12284-021-00466-z
Ghosh S, Mirza N, Kanwar P, Tyagi K, Jha G (2019) Genome analysis provides insight about pathogenesis of Indian strains of Rhizoctonia solani in rice. Funct Integr Genomics 19(5):799–810. https://doi.org/10.1007/s10142-019-00687-y
Rao TB, Chopperla R, Methre R, Punniakotti E, Venkatesh V, Sailaja B, Reddy MR, Yugander A, Laha GS, Madhav MS, Sundaram RM, Ladhalakshmi D, Balachandran SM, Mangrauthia SK (2019) Pectin induced transcriptome of a Rhizoctonia solani strain causing sheath blight disease in rice reveals insights on key genes and RNAi machinery for development of pathogen derived resistance. Plant Mol Biol 100(1):59–71. https://doi.org/10.1007/s11103-019-00843-9
Yamamoto N, Wang Y, Lin R, Liang Y, Liu Y, Zhu J, Wang L, Wang S, Liu H, Deng Q, Li S, Li P, Zheng A (2019) Integrative transcriptome analysis discloses the molecular basis of a heterogeneous fungal phytopathogen complex, Rhizoctonia Solani AG-1 subgroups. Sci Rep 9(1):19626. https://doi.org/10.1038/s41598-019-55734-2
Molla KA, Karmakar S, Chanda PK, Ghosh S, Sarkar SN, Datta SK, Datta K (2013) Rice oxalate oxidase gene driven by green tissue-specific promoter increases tolerance to sheath blight pathogen (Rhizoctonia Solani) in transgenic rice. Mol Plant Pathol 14(9):910–922. https://doi.org/10.1111/mpp.12055
Bhaskar Rao T, Chopperla R, Prathi NB, Balakrishnan M, Prakasam V, Laha GS, Balachandran SM, Mangrauthia SK (2020) A Comprehensive Gene expression Profile of Pectin degradation enzymes reveals the molecular events during cell Wall Degradation and Pathogenesis of Rice Sheath Blight Pathogen Rhizoctonia Solani AG1-IA. J Fungi (Basel) 6(2). https://doi.org/10.3390/jof6020071
Chen J, Xuan Y, Yi J, Xiao G, Yuan DP, Li D (2023) Progress in rice sheath blight resistance research. Front Plant Sci 14. https://doi.org/10.3389/fpls.2023.1141697
Sun Q, Li TY, Li DD, Wang ZY, Li S, Li DP, Han X, Liu JM, Xuan YH (2019) Overexpression of Loose Plant Architecture 1 increases planting density and resistance to sheath blight disease via activation of PIN-FORMED 1a in rice. Plant Biotechnol J 17(5):855–857. https://doi.org/10.1111/pbi.13072
Helliwell EE, Wang Q, Yang Y (2013) Transgenic rice with inducible ethylene production exhibits broad-spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia solani. Plant Biotechnol J 11(1):33–42. https://doi.org/10.1111/pbi.12004
De Vleesschauwer D, Gheysen G, Höfte M (2013) Hormone defense networking in rice: tales from a different world. Trends Plant Sci 18(10):555–565. https://doi.org/10.1016/j.tplants.2013.07.002
Yuan DP, Zhang C, Wang ZY, Zhu XF, Xuan YH (2018) RAVL1 activates brassinosteroids and Ethylene Signaling to modulate response to Sheath Blight Disease in Rice. Phytopathology® 108(9):1104–1113. https://doi.org/10.1094/phyto-03-18-0085-r
Qin X, Liu JH, Zhao WS, Chen XJ, Guo ZJ, Peng YL (2013) Gibberellin 20-Oxidase gene OsGA20ox3 regulates Plant stature and Disease Development in Rice. Mol Plant-Microbe Interact 26(2):227–239. https://doi.org/10.1094/mpmi-05-12-0138-r
Chen L-Q, Cheung LS, Feng L, Tanner W, Frommer WB (2015) Transport of sugars. Annu Rev Biochem 84(84, 2015):865–894. https://doi.org/10.1146/annurev-biochem-060614-033904
Yang S, Fu Y, Zhang Y, Peng Yuan D, Li S, Kumar V, Mei Q, Hu Xuan Y (2023) Rhizoctonia Solani transcriptional activator interacts with rice WRKY53 and grassy tiller 1 to activate SWEET transporters for nutrition. J Adv Res 50:1–12. https://doi.org/10.1016/j.jare.2022.10.001
Liu Q, Wu K, Song W, Zhong N, Wu Y, Fu X (2022) Improving Crop Nitrogen Use Efficiency Toward Sustainable Green Revolution. Annu Rev Plant Biol 73(Volume 73, 2022): 523 – 51. https://doi.org/10.1146/annurev-arplant-070121-015752
Castilla N, Elazegui F, McLaren C, Ynalvez M, Teng P Direct and indirect effects of nitrogen supply and disease source structure on rice sheath blight spread. https://doi.org/10.1094/Phyto-85-959
Wu XX, Yuan DP, Chen H, Kumar V, Kang SM, Jia B, Xuan YH (2022) Ammonium transporter 1 increases rice resistance to sheath blight by promoting nitrogen assimilation and ethylene signalling. Plant Biotechnol J 20(6):1085–1097. https://doi.org/10.1111/pbi.13789
Miao Liu J, Mei Q, Yun Xue C, Yuan Wang Z, Pin Li D, Xin Zhang Y, Hu Xuan Y (2021) Mutation of G-protein γ subunit DEP1 increases planting density and resistance to sheath blight disease in rice. Plant Biotechnol J 19(3):418–420. https://doi.org/10.1111/pbi.13500
Aydın M (2022) Rhizoctonia Solani and its Biological Control. Türkiye Tarımsal Araştırmalar Dergisi 9. https://doi.org/10.19159/tutad.1004550
Uppala S, Zhou XG (2018) Field efficacy of fungicides for management of sheath blight and narrow brown leaf spot of rice. Crop Prot 104. https://doi.org/10.1016/j.cropro.2017.10.017. 72 – 7
Kumaar B (2020) Efficacy of modern combination fungicide molecules against sheath blight of rice. Indian Phytopathol 73:1–5. https://doi.org/10.1007/s42360-020-00273-4
Srinivasachary WL, Savary S (2011) Resistance to rice sheath blight (Rhizoctonia Solani Kühn) [(teleomorph: Thanatephorus Cucumeris (A.B. Frank) Donk.] Disease: current status and perspectives. Euphytica 178(1):1–22. https://doi.org/10.1007/s10681-010-0296-7
Kou Y, Wang S (2012) Toward an understanding of the molecular basis of quantitative disease resistance in rice. J Biotechnol 159(4): 283 – 90. https://doi.org/https://doi.org/https://doi.org/10.1016/j.jbiotec.2011.07.002
Prasad B, Eizenga GC (2008) Rice Sheath Blight Disease Resistance identified in Oryza Spp. Accessions. Plant Dis 92(11):1503–1509. https://doi.org/10.1094/pdis-92-11-1503
Senapati M, Tiwari A, Sharma N, Chandra P, Bashyal BM, Ellur RK, Bhowmick PK, Bollinedi H, Vinod KK, Singh AK, Krishnan SG (2022) Rhizoctonia Solani Kühn Pathophysiology: Status and prospects of Sheath Blight Disease Management in Rice. Front Plant Sci 13. https://doi.org/10.3389/fpls.2022.881116
Pan XB, Rush MC, Sha XY, Xie QJ, Linscombe SD, Stetina SR, Oard JH (1999) Major Gene, Nonallelic Sheath Blight Resistance from the Rice cultivars Jasmine 85 and Teqing. Crop Sci 39(2). https://doi.org/10.2135/cropsci1999.0011183X003900020006x. cropsci1999.0011183X003900020006x
Zuo S-M, Wang Z-B, Chen X-J, Gu F, Zhang Y-F, Chen Z-X, Pan X-B, Pan C-H (2009) Evaluation of resistance of a Novel Rice Line YSBR1 to Sheath Blight. Acta Agron Sinica 35:608–614. https://doi.org/10.1016/S1875-2780(08)60075-9
Sharma A, McClung AM, Pinson SRM, Kepiro JL, Shank AR, Tabien RE, Fjellstrom R (2009) Genetic mapping of Sheath Blight Resistance QTLs within Tropical Japonica Rice cultivars. Crop Sci 49(1):256–264. https://doi.org/10.2135/cropsci2008.03.0124
Chen Z, Feng Z, Kang H, Zhao J, Chen T, Li Q, Gong H, Zhang Y, Chen X, Pan X, Liu W, Wang G, Zuo S (2019) Identification of new resistance loci against Sheath Blight Disease in Rice through genome-wide Association study. Rice Sci 26(1):21–31. https://doi.org/10.1016/j.rsci.2018.12.002
Hossain MK, Islam MR, Sundaram RM, Bhuiyan MAR, Wickneswari R (2023) Introgression of the QTL qSB11-1TT conferring sheath blight resistance in rice (Oryza sativa) into an elite variety, UKMRC 2, and evaluation of its backcross-derived plants. Proc Natl Acad Sci 13. https://doi.org/10.3389/fpls.2022.981345
Xu Q, Yuan X, Yu H, Wang Y, Tang S, Wei X (2011) Mapping quantitative trait loci for sheath blight resistance in rice using double haploid population. Plant Breed 130(3):404–406. https://doi.org/10.1111/j.1439-0523.2010.01806.x
Koshariya A, Kumar I, Pradhan A, Shinde U, Verulkar S, Agrawal T, Kotasthane A (2018) Identification of quantitative trait loci (QTL) associated with sheath blight tolerance in rice. Indian J Genet Plant Breed (The) 78:196. https://doi.org/10.5958/0975-6906.2018.00025.1
Zuo S, Zhang Y, Yin Y, Li G, Zhang G, Wang H, Chen Z, Pan X (2014) Fine-mapping of qSB-9TQ, a gene conferring major quantitative resistance to rice sheath blight. Mol Breed 34(4):2191–2203. https://doi.org/10.1007/s11032-014-0173-5
Schneider K (2005) Mapping Populations and Principles of Genetic Mapping In The Handbook of Plant Genome Mapping. pp 1–21. https://doi.org/10.1002/3527603514.ch1
Eizenga GC, Prasad B, Jackson AK, Jia MH (2013) Identification of rice sheath blight and blast quantitative trait loci in two different O. sativa/O. Nivara advanced backcross populations. Mol Breed 31(4):889–907. https://doi.org/10.1007/s11032-013-9843-y
Sato H, Ideta O, Ando I, Kunihiro Y, Hirabayashi H, Iwano M, Miyasaka A, Nemoto H, Imbe T (2004) Mapping QTLs for sheath blight resistance in the rice line WSS2. Breed Sci 54(3):265–271. https://doi.org/10.1270/jsbbs.54.265
Pinson SRM, Capdevielle FM, Oard JH (2005) Confirming QTLs and finding additional loci conditioning Sheath Blight Resistance in Rice using recombinant inbred lines. Crop Sci 45(2):503–510. https://doi.org/10.2135/cropsci2005.0503
Bal A, Samal P, Chakraborti M, Mukherjee AK, Ray S, Molla KA, Behera L, Samal R, Sarangi S, Sahoo P, Behera M, Lenka S, Azharudheen TPM, Khandual A, Kar MK (2020) Stable quantitative trait locus (QTL) for sheath blight resistance from rice cultivar CR 1014. Euphytica 216(11):182. https://doi.org/10.1007/s10681-020-02702-x
Li Z, Pinson SRM, Marchetti MA, Stansel JW, Park WD (1995) Characterization of quantitative trait loci (QTLs) in cultivated rice contributing to field resistance to sheath blight (Rhizoctonia Solani). Theor Appl Genet 91(2):382–388. https://doi.org/10.1007/BF00220903
Liu Y, Chen L, Fu D, Lou Q, Mei H, Xiong L, Li M, Xu X, Mei X, Luo L (2014) Dissection of additive, epistatic effect and QTL × environment interaction of quantitative trait loci for sheath blight resistance in rice. Hereditas 151(2–3):28–37. https://doi.org/10.1111/hrd2.00026
Zuo SM, Zhu YJ, Yin YJ, Wang H, Zhang YF, Chen ZX, Gu SL, Pan XB (2014) Comparison and confirmation of quantitative trait loci conferring partial resistance to Rice Sheath Blight on chromosome 9. Plant Dis 98(7):957–964. https://doi.org/10.1094/pdis-09-13-0940-re
Zuo S, Yin Y, Pan C, Chen Z, Zhang Y, Gu S, Zhu L, Pan X (2013) Fine mapping of qSB-11LE, the QTL that confers partial resistance to rice sheath blight. Theor Appl Genet 126(5):1257–1272. https://doi.org/10.1007/s00122-013-2051-7
Wang Y, Sun Q, Zhao J, Liu T, Du H, Shan W, Wu K, Xue X, Yang C, Liu J, Chen Z, Hu K, Feng Z, Zuo S (2023) Fine mapping and candidate gene analysis of qSB12YSB, a gene conferring major quantitative resistance to rice sheath blight. Theor Appl Genet 136(12):246. https://doi.org/10.1007/s00122-023-04482-z
Chen J-Y, Guo L, Ma H, Chen Y-Y, Zhang H-W, Ying J-Z, Zhuang J-Y (2014) Fine mapping of qHd1, a minor heading date QTL with pleiotropism for yield traits in rice (Oryza sativa L). Theor Appl Genet 127(11):2515–2524. https://doi.org/10.1007/s00122-014-2395-7
Karmakar S, Molla KA, Das K, Sarkar SN, Datta SK, Datta K (2017) Dual gene expression cassette is superior than single gene cassette for enhancing sheath blight tolerance in transgenic rice. Sci Rep 7(1):7900. https://doi.org/10.1038/s41598-017-08180-x
Karmakar S, Molla KA, Chanda PK, Sarkar SN, Datta SK, Datta K (2016) Green tissue-specific co-expression of chitinase and oxalate oxidase 4 genes in rice for enhanced resistance against sheath blight. Planta 243(1):115–130. https://doi.org/10.1007/s00425-015-2398-x
Liu M, Sun Z-x, Zhu J, Xu T, Harman GE, Lorito M (2004) Enhancing rice resistance to fungal pathogens by transformation with cell wall degrading enzyme genes from Trichoderma Atroviride. J Zhejiang Univ Sci 5(2):133–136. https://doi.org/10.1007/bf02840913
Silva J, Scheffler B, Sanabria Y, De Guzman C, Galam D, Farmer A, Woodward J, May G, Oard J (2012) Identification of candidate genes in rice for resistance to sheath blight disease by whole genome sequencing. Theor Appl Genet 124(1):63–74. https://doi.org/10.1007/s00122-011-1687-4
Xia Y, Fei B, He J, Zhou M, Zhang D, Pan L, Li S, Liang Y, Wang L, Zhu J, Li P, Zheng A (2017) Transcriptome analysis reveals the host selection fitness mechanisms of the Rhizoctonia solani AG1IA pathogen. Sci Rep 7(1):10120. https://doi.org/10.1038/s41598-017-10804-1
Zhang J, Chen L, Fu C, Wang L, Liu H, Cheng Y, Li S, Deng Q, Wang S, Zhu J, Liang Y, Li P, Zheng A (2017) Comparative transcriptome analyses of gene expression changes triggered by Rhizoctonia Solani AG1 IA infection in resistant and susceptible Rice varieties. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.01422
Karmakar S, Datta K, Molla KA, Gayen D, Das K, Sarkar SN, Datta SK (2019) Proteo-metabolomic investigation of transgenic rice unravels metabolic alterations and accumulation of novel proteins potentially involved in defence against Rhizoctonia solani. Sci Rep 9(1):10461. https://doi.org/10.1038/s41598-019-46885-3
Lin R, He L, He J, Qin P, Wang Y, Deng Q, Yang X, Li S, Wang S, Wang W, Liu H, Li P, Zheng A (2016) Comprehensive analysis of microRNA-Seq and target mRNAs of rice sheath blight pathogen provides new insights into pathogenic regulatory mechanisms. DNA Res 23(5):415–425. https://doi.org/10.1093/dnares/dsw024
Bajaj S, Mohanty A (2005) Recent advances in rice biotechnology—towards genetically superior transgenic rice. Plant Biotechnol J 3(3):275–307. https://doi.org/10.1111/j.1467-7652.2005.00130.x
Kalpana K, Maruthasalam S, Rajesh T, Poovannan K, Kumar KK, Kokiladevi E, Raja JAJ, Sudhakar D, Velazhahan R, Samiyappan R, Balasubramanian P (2006) Engineering sheath blight resistance in elite indica rice cultivars using genes encoding defense proteins. Plant Sci 170(2):203–215. https://doi.org/10.1016/j.plantsci.2005.08.002
Prathi NB, Durga Rani CV, Prakasam V, Mohan YC, Mahendranath G, Sri Vidya GK, Neeraja CN, Sundaram RM, Mangrauthia SK (2024) Oschib1 gene encoding a GH18 chitinase confers resistance against sheath blight disease of rice caused by Rhizoctonia Solani AG1-IA. Plant Mol Biol 114(3):41. https://doi.org/10.1007/s11103-024-01442-z
Datta K, Tu J, Oliva N, Ona I, Velazhahan R, Mew TW, Muthukrishnan S, Datta SK (2001) Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Sci 160(3):405–414. https://doi.org/10.1016/S0168-9452(00)00413-1
Kim J-K, Jang I-C, Wu R, Zuo W-N, Boston RS, Lee Y-H, Ahn I-P, Nahm BH (2003) Co-expression of a modified Maize Ribosome-inactivating protein and a Rice Basic Chitinase Gene in Transgenic Rice plants confers enhanced resistance to Sheath Blight. Transgenic Res 12(4):475–484. https://doi.org/10.1023/A:1024276127001
Mao B, Liu X, Hu D, Li D (2014) Co-expression of RCH10 and AGLU1 confers rice resistance to fungal sheath blight Rhizoctonia solani and blast Magnorpathe oryzae and reveals impact on seed germination. World J Microbiol Biotechnol 30(4):1229–1238. https://doi.org/10.1007/s11274-013-1546-3
Shah JM, Raghupathy V, Veluthambi K (2009) Enhanced sheath blight resistance in transgenic rice expressing an endochitinase gene from Trichoderma virens. Biotechnol Lett 31(2):239–244. https://doi.org/10.1007/s10529-008-9856-5
Datta K, Velazhahan R, Oliva N, Ona I, Mew T, Khush GS, Muthukrishnan S, Datta SK (1999) Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor Appl Genet 98(6):1138–1145. https://doi.org/10.1007/s001220051178
Maruthasalam S, Kalpana K, Kumar KK, Loganathan M, Poovannan K, Raja JAJ, Kokiladevi E, Samiyappan R, Sudhakar D, Balasubramanian P (2007) Pyramiding transgenic resistance in elite indica rice cultivars against the sheath blight and bacterial blight. Plant Cell Rep 26(6):791–804. https://doi.org/10.1007/s00299-006-0292-5
Gao Y, Zhang C, Han X, Wang ZY, Ma L, Yuan DP, Wu JN, Zhu XF, Liu JM, Li DP, Hu YB, Xuan YH (2018) Inhibition of OsSWEET11 function in mesophyll cells improves resistance of rice to sheath blight disease. Mol Plant Pathol 19(9):2149–2161. https://doi.org/10.1111/mpp.12689
Kim P, Xue CY, Song HD, Gao Y, Feng L, Li Y, Xuan YH (2021) Tissue-specific activation of DOF11 promotes rice resistance to sheath blight disease and increases grain weight via activation of SWEET14. Plant Biotechnol J 19(3):409–411. https://doi.org/10.1111/pbi.13489
Krishnamurthy K, Balconi C, Sherwood JE, Giroux MJ (2001) Wheat puroindolines enhance fungal Disease Resistance in Transgenic Rice. Mol Plant Microbe Interact 14(10):1255–1260. https://doi.org/10.1094/mpmi.2001.14.10.1255
Patkar RN, Chattoo BB (2006) Transgenic indica Rice expressing ns-LTP-Like protein shows enhanced resistance to both fungal and bacterial pathogens. Mol Breed 17(2):159–171. https://doi.org/10.1007/s11032-005-4736-3
Jha S, Tank HG, Prasad BD, Chattoo BB (2009) Expression of Dm-AMP1 in rice confers resistance to Magnaporthe oryzae and Rhizoctonia solani. Transgenic Res 18(1):59–69. https://doi.org/10.1007/s11248-008-9196-1
Jha S, Chattoo BB (2010) Expression of a plant defensin in rice confers resistance to fungal phytopathogens. Transgenic Res 19(3):373–384. https://doi.org/10.1007/s11248-009-9315-7
Peng X, Hu Y, Tang X, Zhou P, Deng X, Wang H, Guo Z (2012) Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Cell 236(5):1485–1498. https://doi.org/10.1007/s00425-012-1698-7
Xue X, Cao ZX, Zhang XT, Wang Y, Zhang YF, Chen ZX, Pan XB, Zuo SM (2016) Overexpression of OsOSM1 enhances resistance to Rice Sheath Blight. Plant Dis 100(8):1634–1642. https://doi.org/10.1094/pdis-11-15-1372-re
Wang R, Lu L, Pan X, Hu Z, Ling F, Yan Y, Liu Y, Lin Y (2015) Functional analysis of OsPGIP1 in rice sheath blight resistance. Plant Mol Biol 87(1):181–191. https://doi.org/10.1007/s11103-014-0269-7
Zhu G, Liang E, Lan X, Li Q, Qian J, Tao H, Zhang M, Xiao N, Zuo S, Chen J, Gao Y (2019) ZmPGIP3 gene encodes a polygalacturonase-inhibiting protein that enhances resistance to Sheath Blight in Rice. Phytopathology® 109(10):1732–1740. https://doi.org/10.1094/phyto-01-19-0008-r
Liu X, Li J, Noman A, Lou Y (2019) Silencing OsMAPK20-5 has different effects on rice pests in the field. Plant Signal Behav 14(9):e1640562. https://doi.org/10.1080/15592324.2019.1640562
Molla KA, Karmakar S, Chanda PK, Sarkar SN, Datta SK, Datta K (2016) Tissue-specific expression of Arabidopsis NPR1 gene in rice for sheath blight resistance without compromising phenotypic cost. Plant Sci 250:105–114. https://doi.org/10.1016/j.plantsci.2016.06.005
Sadumpati V, Kalambur M, Vudem DR, Kirti PB, Khareedu VR (2013) Transgenic indica rice lines, expressing Brassica juncea Nonexpressor of pathogenesis-related genes 1 (BjNPR1), exhibit enhanced resistance to major pathogens. J Biotechnol 166(3):114–121. https://doi.org/10.1016/j.jbiotec.2013.04.016
Panthapulakkal Narayanan S, Lung S-C, Liao P, Lo C, Chye M-L (2020) The overexpression of OsACBP5 protects transgenic rice against necrotrophic, hemibiotrophic and biotrophic pathogens. Sci Rep 10(1):14918. https://doi.org/10.1038/s41598-020-71851-9
Wang A, Shu X, Jing X, Jiao C, Chen L, Zhang J, Ma L, Jiang Y, Yamamoto N, Li S (2021) Identification of rice (Oryza sativa L.) genes involved in sheath blight resistance via a genome-wide association study. Plant Biotechnol J 19(8):1553–1566. https://doi.org/10.1111%2Fpbi.13569
Lin QJ, Chu J, Kumar V, Yuan DP, Li ZM, Mei Q, Xuan YH (2021) Protein phosphatase 2A Catalytic Subunit PP2A-1 enhances Rice Resistance to Sheath Blight Disease. Front Genome ed 3. https://doi.org/10.3389/fgeed.2021.632136
Parween D, Sahu BB (2022) An Arabidopsis nonhost resistance gene, IMPORTIN ALPHA 2 provides immunity against rice sheath blight pathogen, Rhizoctonia solani. Curr Res Microb Sci 3:100109. https://doi.org/10.1016/j.crmicr.2022.100109
Cao W, Zhang H, Zhou Y, Zhao J, Lu S, Wang X, Chen X, Yuan L, Guan H, Wang G, Shen W, De Vleesschauwer D, Li Z, Shi X, Gu J, Guo M, Feng Z, Chen Z, Zhang Y, Pan X, Liu W, Liang G, Yan C, Hu K, Liu Q, Zuo S (2022) Suppressing chlorophyll degradation by silencing OsNYC3 improves rice resistance to Rhizoctonia Solani, the causal agent of sheath blight. Plant Biotechnol J 20(2):335–349. https://doi.org/10.1111/pbi.13715
Hille F, Charpentier E (2016) CRISPR-Cas: biology, mechanisms and relevance. Philos Trans R Soc Lond B Biol Sci 371(1707):20150496. https://doi.org/10.1098/rstb.2015.0496
Tycko J, Myer VE, Hsu PD (2016) Methods for Optimizing CRISPR-Cas9 Genome Editing Specificity. Mol Cell 63(3): 355 – 70. https://doi.org/10.1016/j.molcel.2016.07.004
Chen J, Wang S, Jiang S, Gan T, Luo X, Shi R, Xuan Y, Xiao G, Chen H (2024) Overexpression of calcineurin B-like interacting protein kinase 31 promotes lodging and sheath blight resistance in Rice. Plants 13(10):1306. https://doi.org/10.3390/plants13101306
Hu B, Zhou Y, Zhou Z, Sun B, Zhou F, Yin C, Ma W, Chen H, Lin Y (2021) Repressed OsMESL expression triggers reactive oxygen species-mediated broad-spectrum disease resistance in rice. Plant Biotechnol J 19(8):1511–1522. https://doi.org/10.1111/pbi.13566
Zhao Y, Zhu X, Shi C-M, Xu G, Zuo S, Shi Y, Cao W, Kang H, Liu W, Wang R, Ning Y, Wang G-L, Wang X (2024) OsEIL2 balances rice immune responses against (hemi)biotrophic and necrotrophic pathogens via the salicylic acid and jasmonic acid synergism. New Phytol 243(1):362–380. https://doi.org/10.1111/nph.19809
Feng T, Zhang Z-Y, Gao P, Feng Z-M, Zuo S-M, Ouyang S-Q (2023) Suppression of Rice Osa-miR444.2 improves the resistance to Sheath Blight in Rice mediating through the Phytohormone Pathway. Int J Mol Sci 24(4):3653
Xie W, Cao W, Lu S, Zhao J, Shi X, Yue X, Wang G, Feng Z, Hu K, Chen Z, Zuo S (2023) Knockout of transcription factor OsERF65 enhances ROS scavenging ability and confers resistance to rice sheath blight. Mol Plant Pathol 24(12):1535–1551. https://doi.org/10.1111/mpp.13391
Wang ST, Guo XF, Yao TS, Xuan YH (2020) Indeterminate domain 3 negatively regulates plant erectness and the resistance of rice to sheath blight by controlling PIN-FORMED gene expressions. Plant Signal Behav 15(11):1809847. https://doi.org/10.1080/15592324.2020.1809847
Wang Y, Wang H, Zhang L, Wang Y, Wei S, Wang L (2024) Mechanism analysis of OsZF8-Mediated regulation of Rice Resistance to Sheath Blight. Int J Mol Sci 25(11). https://doi.org/10.3390/ijms25115787
Sayari M, Babaeizad V, Tajick Ghanbary M, Rahimian H (2014) Expression of the pathogenesis related proteins, NH-1, PAL, and lipoxygenase in the Iranian Tarom and Khazar rice cultivars, in reaction to Rhizoctonia solani – the causal agent of rice sheath blight. J Plant Prot Res 54:36–43. https://doi.org/10.2478/jppr-2014-0006
Nawrot R, Barylski J, Nowicki G, Broniarczyk J, Buchwald W, Goździcka-Józefiak A (2014) Plant antimicrobial peptides. Folia Microbiol 59(3):181–196. https://doi.org/10.1007/s12223-013-0280-4
Elhag O, Zhou D, Song Q, Soomro AA, Cai M, Zheng L, Yu Z, Zhang J (2017) Screening, expression, purification and functional characterization of Novel Antimicrobial peptide genes from Hermetia illucens (L). PLoS ONE 12(1):e0169582. https://doi.org/10.1371/journal.pone.0169582
Yang S, Kumar V, Jia XT, Zheng Ap, Xuan YH (2024) Mutation at grassy tiller 1 increases rice yield production and resistance to sheath blight. Crop Health 2(1):4. https://doi.org/10.1007/s44297-024-00025-0
Panpatte DG, Jhala YK Nanotechnology for agriculture: crop productionprotection., Springer (2019) https://doi.org/10.1007/978-981-32-9374-8
Cao X, Chen X, Liu Y, Wang C, Yue L, Elmer WH, White JC, Wang Z, Xing B (2023) Lanthanum Silicate nanomaterials enhance Sheath Blight Resistance in Rice: mechanisms of action and Soil Health evaluation. ACS Nano 17(16):15821–15835. https://doi.org/10.1021/acsnano.3c03701
Soltani Nejad M, Bonjar GHS, Khatami M, Amini A, Aghighi S (2017) In vitro and in vivo antifungal properties of silver nanoparticles against Rhizoctonia Solani, a common agent of rice sheath blight disease. IET Nanobiotechnol 11(3):236–240. https://doi.org/10.1049/iet-nbt.2015.0121
Thakur M, Tiwari P, Kerketta V (2020) Effect of application of borogold (combination of nano silver particles peroxy acid) on management of sheath blight of Rice. J Pharmacogn Phytochem 9(2):2271–2274
Divya K, Thampi M, Vijayan S, Varghese S, Jisha MS (2020) Induction of defence response in Oryza sativa L. against Rhizoctonia solani (Kuhn) by chitosan nanoparticles. Microb Pathog 149:104525. https://doi.org/10.1016/j.micpath.2020.104525
Chen X, Jiang Y, Wang C, Yue L, Li X, Cao X, White JC, Wang Z, Xing B (2024) Selenium nanomaterials enhance Sheath Blight Resistance and Nutritional Quality of Rice: mechanisms of Action and Human Health Benefit. ACS Nano 18(20):13084–13097. https://doi.org/10.1021/acsnano.4c01835
Meng Z, Wu Q, Wu X, Yang C, Xu W, Lin T, Liang Y, Chen X (2024) Nanoparticles of Fe3O4 loaded with Azoxystrobin and pectin to Enhance Resistance of Rice to Sheath Blight. ACS Appl Nano Mate 7(3):2675–2686. https://doi.org/10.1021/acsanm.3c04801
Das A, Mishra A, Kashyap A, Naika MBN, Barah P (2022) KRiShI: a manually curated knowledgebase on rice sheath blight disease. Funct Integr Genom 22(6):1403–1410. https://doi.org/10.1007/s10142-022-00899-9
Chen L, Ai P, Zhang J, Deng Q, Wang S, Li S, Zhu J, Li P, Zheng A (2016) RSIADB, a collective resource for genome and transcriptome analyses in Rhizoctonia solani AG1 IA. Database 2016. https://doi.org/10.1093/database/baw031
Funding
This work was supported by grants from the Department of Biotechnology, New Delhi, India (BT/PR40456/AGIII/103/1248/2020), and Tamil Nadu Agricultural University, Coimbatore-641003 India.
Author information
Authors and Affiliations
Contributions
The conception and design of the review work was undertaken by DJ, JM, VS and HS. DJ and VP gathered the literature and drafted the manuscript. SD and KKK did the critical revision. AL and KE carried out verification and supervision. DJ and MR prepared figure. VS provided approval for the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human or animal subjects.
Consent for publication
All authors have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jesudoss, D., Ponnurangan, V., Kumar, M.P.R. et al. Advances in breeding, biotechnology, and nanotechnological approaches to combat sheath blight disease in rice. Mol Biol Rep 51, 958 (2024). https://doi.org/10.1007/s11033-024-09889-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09889-5