Abstract
A 30-day experiment was carried out to know responses of different weaning approaches to the growth and survival of Anabas testudineus larvae. A total of 10800 larvae (Avg. weight 0.016 ± 0.03 mg; 3DPH) were randomly distributed in nine treatments (triplicates), including two controls. The strategies are as follows: C1 (Control I): feeding with live food (LF) for 30 days and C2 (Control II): feeding with microparticulate diet (MPD) for 30 days; T1: LF for 5 days and MPD for next 25 days; T2: LF for 10 days and MPD for next 20 days; T3: LF for 15 days and MPD for next 15 day; T4: LF for 20 days and MPD for next 10 days; T5: LF for 25 days and MPD for next 5 days; T6: LF for 5 days, then 25% LF replacement by MPD for next 5 days, 50% LF replacement by MPD for next 5 days, 75% LF replacement by MPD for next 5 days, and 100% LF replacement by MPD for last 10 days; and T7: LF for 10 days, then 25% LF replacement by MPD for next 5 days, 50% LF replacement by MPD for next 5 days, 75% LF replacement by MPD for next 5 days, and 100% LF replacement by MPD for last 5 days. Significantly (p < 0.05) higher WG and SGR were recorded in T2 (213.17 ± 0.32, 23.98 ± 0.02) followed by T6, whereas the lowest was found in C2. Significantly higher (p < 0.05) percentage survival was manifested in the T7 (31.83 ± 0.22), followed by T2 (24.75 ± 0.13), and the lowest survival was observed in the C2. The digestive enzyme activities were found to be non-significant (p > 0.05) between different treatment groups. The alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and malate dehydrogenase (MDH) were reported to be significantly higher (p < 0.05) in C2 (68.52 ± 0.08, 19.55 ± 0.10, 21.79 ± 0.04, and 0.044 ± 0.01) followed by T1; however, their reduced level was observed in C1. The activity of superoxide dismutase (SOD), catalase (CAT), glucose, and cortisol levels was observed significantly (p < 0.05) higher in C2 and lower in C1 and T2. As per the finding, it can be recommended that the appropriate weaning time for A. testudineus larvae is from 13 DPH onwards, in which larvae can be fed an initial ten days LF afterward MPD and the best weaning strategy can be adopted as in the T7 group for higher survival percentage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Species diversification is one of the important strategies in aquaculture to increase fish production. The selection of high-demand species is important to getting a higher return. Anabas testudineus, also known as koi or climbing perch, is one of the promising fish found in several countries, including Indian subcontinent (Tan and Lim 2004). It is well known for its superior taste and flavor and is a good source of iron and copper (Sarma et al. 2010; Bhuyan and Hussain 2018). It is highly resistant to unfavorable environments due to its air-breathing ability, which makes it a preferred species for aquaculture (Van Trieu and Long 2001). After fertilization, eggs will hatch in approximately 24h. The newly hatched larvae are 2–3-mm long and begin free swimming within two days (Wei et al. 2021). During larval development, the yolk sac is completely absorbed within 72h, indicating the first external feeding should be begun (Basak et al. 2014). The process of transforming the notochord into vertebrae through segmentation, as well as the development of the alimentary canal, takes place within a time frame of 144 to 192h. The final expansion of paired and median fins, mouth, and scale formation is completed by 336h after hatching. Pectoral, pelvic, caudal, and dorsal fins with fin rays fully form during this stage (Basak et al. 2014). The predominant digestive enzymes found in A. testudineus larvae are proteases and amylases (Chandan et al. 2023). Protease activity is higher than amylase activity, reflecting the carnivorous feeding habits (Singh et al. 2023). Protease activity within the larval gut is significantly higher in the hindgut region than in the foregut (Mondal et al. 2008).
Despite its cultural potential, species is not widely cultivated due to a lack of seed availability (Kohinoor et al. 2012; Jayasankar 2018). In this context, successful larval culture for this species is one of the challenging tasks as the larval survival rate is very poor (Hamre et al. 2013; Singha et al. 2015, Singh et al. 2019; Alang et al. 2020), probably due to a lack of suitable live food (Rao 2003) and weaning diet and weaning strategy (Kolkovski 2001; Hauville et al. 2014; Jayasankar 2018).
Formulated microparticulate diet (MPD) is advantageous over live food because of the balanced nutrient supply, bulk preparation, easy storage, and lower cost of production (Holt 2011; Thompson et al. 2019). However, larvae are always very much fond of live prey like Rotifer, Moina, and Artemia nauplii, which are preferable live foods for koi larvae (Bell et al. 2003; Hamre et al. 2013). But the production cost of live food is high, and it is not nutritionally balanced (Cahu et al. 1998; Callan et al. 2003; Ma et al. 2015; Jamali et al. 2018) and can act as a vector for pathogens (Bonaldo et al. 2011). On the other hand, koi larvae are reluctant to accept artificial feed alone (Conceição et al. 2010; Rønnestad et al. 2013; Chen et al. 2020). Therefore, a suitable weaning strategy is required to be standardized for the large-scale culture of koi larvae with optimum survival rate (Kolkovski 2008), and the formulated feed should be nutritionally balanced with higher nutrient digestibility and well accepted by the larvae (Giri et al. 2002; Radhakrishnan et al. 2020).
Some research has been conducted on larval rearing of A. testudineus for higher seed production (Zalina et al. 2011; Singh et al. 2019) but failed to establish a suitable weaning strategy for successful larval culture (Ahilan and Thangarani 2020; Lazo et al. 2007). However, the majority of researchers suggest that koi fish larvae have a distinct and well-defined digestive physiology, and this information could be helpful in developing suitable MPD and optimal weaning protocol for higher survival and production of larvae (Lazo et al. 2000; Thompson 2013).
Weaning is a transition from one feed to another as well as a period of adjustment in which both types of feeds are supplied for an extended period, with a gradual reduction of live food and a gradual increase in prepared diet (Chèvre et al. 2011; Williot et al. 2011). This is especially required for carnivorous fish larvae, which have high protein and lipid requirements for both energy and body development (Andersen et al. 2016). A relatively successful weaning strategy involves mix feeding with live food and a formulated diet for some period of time (Ljubobratović et al. 2015; Hung et al. 2022); however, co-feeding also plays a significant role in optimizing nutrient intake in the fish larval stage. Co-feeding results in significantly higher (p < 0.05) growth compared to just live feeds or microdiets alone during weaning (Djellata et al. 2021). Sudden weaning with a direct shift from live food to an artificial diet has poor success in larval survival (Herath and Atapaththu 2013). Live feeds provide exogenous enzymes and dietary compounds that stimulate larval digestive activity (Pradhan et al. 2014; Khoa et al. 2020). Co-feeding takes advantage of both live feed and artificial diet, enhancing digestion and growth (Djellata et al. 2021).
Recent advancements in MPD composition have substantially shortened the early weaning period, enabling finfish culture to begin feeding on a specific larval diet as soon as the mouth opens (Cahu and Infante 2001). Weaning is most important stage in larval rearing because larvae need soluble proteins and fatty acids, which can be provided through a prepared diet (Rønnestad et al. 2013). A pre-digested protein source called fish protein hydrolysate (FPH) may be added as a dietary source of protein in the MPD. FPH has a simple form of proteins that includes peptides and amino acids, which serve as feed stimulants and attractants, which improve the diets flavor and acceptance (Chotikachinda et al. 2013; Siddaiah et al. 2022). In order to enhance larval growth performance as well as survival by incorporating FPH has been observed in some species, such as Atlantic salmon, Salmo salar (Berge and Storebakken 1996), Rainbow trout, Onchorhynchus mykiss (Dabrowski et al. 2003), and Clarias magur (Kumar et al. 2022). The FPH might aid in the digestion and absorption of artificial food (Zambonino-Infante et al. 1997; Carvalho et al. 1997; Cahu et al. 1999). Both abruptly weaned and gradually weaned larvae showed improved development and survival when FPH was added to a formulated diet (Kotzamanis et al. 2007; Srichanun et al. 2014). Anguas-Ve'lez et al. (2000) also noted that larvae fed with FPH had the highest overall rate of survival. Several papers discuss the advantages of an FPH-enriched larval diet, which raises the percentage of survival (Cahu et al. 1999; Gisbert et al. 2009; Kumar et al. 2022).
Considering above backgrounds, the current study was conducted to develop a suitable weaning strategy for A. testudineus larvae and its effects on survival, growth, stress, and physio-metabolic and other enzyme responses.
Materials and methods
Experimental animal
The experiment was conducted at ICAR-Central Institute of Fisheries Education, Kolkata Center, West Bengal (India). A. testudineus larvae were produced through induced breeding by administering WOVA-FH™ (Biostadt India Limited, Kolkata) into females (23–38g; n = 3) at 0.4 mlkg−1 and male (21–34g; n = 3) at the rate of 0.2 mlkg−1 (Singh et al. 2019). After hormonal injection, male and female brooders were transferred into the fiber-reinforced plastic (FRP) tank at a 1:1 ratio. After 8–12h of latency period, eggs were released and temperature was maintained at 27 ± 2 °C with mild aeration. Newly hatched larvae stocked in FRP container for the 3-day post hatch (DPH) period.
Microparticulate diet (MPD) preparation
The proximate analysis of ingredients and diet is mentioned in Table 1. The diet was prepared and as protein sources fish meal, groundnut oil cake, and FPH, whereas wheat flour was a carbohydrate source used. Butylated hydroxyl toluene was used as a preservative (antioxidant), and sodium alginate was used as a binder. A vitamin-mineral premix, vitamin C, and betaine hydrochloride were used as additives. Ground all the ingredients as suggested in the formulation, and mix them uniformly. Except for additives and oil, all other ingredients are mixed and the dough is made by mixing the water, then kept in a thick plastic bag and transferred for 25–30 minutes for steam cooking. After that, the mixture was crushed to allow it to cool. The other ingredients, namely, oil, BHT, soy lecithin, sodium alginate, betaine hydrochloride, mineral-vitamin mix, and FPH, were mixed properly. To make pellets, the mixture was placed in the pelletizer (SB-Panchal, Mumbai), then made in uniform spheroid size by using a spheronizer (die size: 200µm) and dried using a mechanical dryer (UNIFLUID NANO, India). The dried feed (size approximately 200–250µm) was kept in a closed container, marked properly, and kept in a cool place.
Experimental design, setup, and management
The ten thousand eight hundred 3 DPH larvae (2.7 ± 0.6 mm (length) and 0.016 ± 0.03 mg (wet body weight)) of A. testudineus were stocked in nine experimental groups in triplicates (Fig. 1) such as C1 (Control I): feeding with live food (LF) for 30 days, C2 (Control II): feeding with microparticulate diet (MPD) for 30 days; T1: LF for 5 days and MPD for next 25 days, T2: LF for 10 days and MPD for next 20 days, T3: LF for 15 days and MPD for next 15 day, T4: LF for 20 days and MPD for next 10 days, T5: LF for 25 days and MPD for next 5 days, T6: LF for 5 days, then 25% LF replacement by MPD for next 5 days, 50% LF replacement by MPD for next 5 days, 75% LF replacement by MPD for next 5 days, and 100% LF replacement by MPD for last 10 days and T7: LF for 10 days, then 25% LF replacement by MPD for next 5 days, 50% LF replacement by MPD for next 5 days, 75% LF replacement by MPD for next 5 days, and 100% LF replacement by MPD for last 5 day. Accordingly, 27 glass aquaria (45 × 30 × 30 cm3; 40 l capacity and 20 l water volume; stocking density 20 larvae l−1) were used. All aquaria were provided with continuous aeration facilities, and 30–35% of daily water was exchanged with freshwater. The temperature, dissolved oxygen (DO), and pH levels of water were continued at 27.7–29.6 °C, 6–8 mg/l, and 6.9–7.6, respectively, throughout the experimental period. The larvae were fed with LF (Brachionus calyciflorus) at the rate of 15–20 individual ml−1 of water while MPD (Fig. 1) was fed five times per day based on visual inspection, approximately amounting to 10% of body weight respectively. Before the feeding at 8:00 a.m., feces were siphoned out every morning. The daily mortality of larvae was meticulously observed and recorded in every experimental aquarium over the entire duration of the experiment.
Growth performance and survival of larvae
At last of the trial, all larvae were collected from every experimental tank, anesthetized with ice, and rinsed in distilled water (DW), and average final body weight (FBW) was measured using an electronic balance. The weight gain (WG) and specific growth rate (SGR) were estimated as
The survival percentage was calculated by
Proximate composition of diet and carcass
The proximate composition of larvae and diet was examined according to the standard method of AOAC (AOAC 1995) on a dry matter basis. Moisture was estimated using a hot air oven (HTLP 28L, Memmert Type HTLP-013, IKON INSTRUMENTS, India), crude protein (CP) [total nitrogen percent (TN %) × 6.25] was estimated using the MicroKjeldahl Semi-automate unit. The quantification of Ether extract (EE) or lipids was accomplished through the solvent extraction method, employing diethyl ether as the solvent (boiling point 55 ± 5 °C), and crude fiber (CF) content was determined using FibroTRON (FRB-8, Tulin Equipment, India), and total ash (TA) was estimated by muffle furnace at 550 °C for four hours. Nitrogen-free extract (NFE) of diet and total carbohydrate (TC) of the whole body calculated as follows:
In samples, CP, lipid, TA, and TC contents were expressed on a wet-weight basis.
Assays of enzymes
Tissue collection and homogenate preparation
On completion of the experiment, 15–20 larvae from each replicate tank were pooled before the first feeding time, then anesthetized using ice, and instantly stored at − 80 °C for biochemical examination. Each larva underwent a distilled water rinse and eliminated excess water using tissue paper before dissection. Whole fish larvae were dissected and anterior, and posterior sections were excised as described by Comabella et al. (2006) on the dry ice packet. Then, the larvae were homogenized while maintaining a cold temperature. A tissue homogenate comprising 0.05-M Tris hydrochloric acid and two percent sucrose was made. Centrifuging (REMI, India) at 5000 rpm for 10 min at 4 °C. The upper layer was collected in Eppendorf tubes and preserved (− 80 °C) until analysis.
Total protein estimation from tissue homogenate
The estimated concentration of total protein of tissue homogenate was obtained by following Lowry et al. (1951). The obtained results are used for enzymatic calculation.
Digestive enzymes
Acid protease
Anson method (1938) measured samples of acid protease (pepsin) activity. Hemoglobin substrate (0.25%) was mixed with sodium acetate buffer-HCl (0.1 M, pH 3). Sample homogenate (25 μl) was added and incubated for 30 min. Trichloroacetic acid (TCA, 12%) was added to break the reaction. After centrifugation, supernatant absorbance was measured at 280 nm (Singh et al. 2023).
Alkaline protease
Alkaline protease activity was assessed by following a modified version of the Drapeau (1974) method. Tissue homogenate was incubated with a one percent casein solution in 0.05-M Tris phosphate buffer (pH 7.8) for 5 minutes. Then solution was then filtered after the addition of 10% TCA. Enzyme activity was quantified based on the acid-soluble fragment release, equal to Δ0.001A280 per minute at 37 °C and pH 7.8.
Amylase
Activity of amylase was measured using the DNS technique with a starch solution, phosphate buffer and tissue homogenate (Rick and Stegbauer 1974). After incubation at 37 °C, absorbance was read at 540 nm following DNS addition. The activity was stated as maltose out from starch per minute at 37 °C (Singh et al. 2023).
Lipase
Activity of lipase was assessed via Cherry and Crandell’s titrimetric method (1932), involving hydrolysis of triglycerides in olive oil emulsion. Fatty acids released were quantified by titration with standard NaOH. The assay comprised 1.5-ml emulsion, 1.5-ml (pH 8.0) Tris-HCl buffer, and 1.0-ml crude enzyme, incubated for 24 h. at 37 °C. Reaction stopped with 3-ml 95% ethyl alcohol, followed by titration with 0.01N NaOH and phenolphthalein indicator (Singh et al. 2023).
Protein metabolic enzymes
ALT (alanine aminotransferase) and AST (aspartate aminotransferase) activity was assessed in tissue homogenates using Wootton’s method (1964). In AST, a substrate comprising 0.2M D, L-aspartic acid and 2-mM α-ketoglutarate in 0.05M (pH 7.4) phosphate buffer was added (0.5ml) to experimental and control tubes. Tissue homogenate (0.1 ml) initiated the reaction. After 60 minutes at 37 °C, 0.5ml of 1mM 2,4 dinitrophenyl hydrazine ended the reaction. In control, samples were added after DNPH and kept at room temperature, shaken occasionally. After twenty minutes, 5ml of NaOH was mixed and OD was recorded at 540 nm in contrast to blank (Washington and Hoosier 2012). ALT (alanine aminotransferase) activity was determined (Wootton 1964) using 0.2M D, L-alanine. Enzyme activity was expressed in nanomole pyruvate formed/min/mg protein at 37 °C.
Carbohydrate metabolic enzymes
Lactate dehydrogenase (LDH) and Malate dehydrogenase (MDH) activities in tissue homogenates were measured using established methods (Wróblewski and Ladue 1955; Ochoa 1955). For LDH, a reaction mixes with phosphate buffer, NADH, tissue homogenate, sodium pyruvate, and checked OD at 340 nm for 3 min. MDH activity was assessed similarly but with oxaloacetate. The activity was expressed in units/mg protein/min at 25 °C, with 1 unit equating Δ 0.01 OD/min at 37 °C (Singh et al. 2023).
Oxidative stress enzymes
Superoxide dismutase (SOD)
Superoxide dismutase (SOD) activity was assessed by following Misra and Fridovich (1972) method. Samples were mixed with a buffer containing EDTA and epinephrine and the variation in OD at 480 nm for three minutes. SOD activity is determined by the protein amount needed for 50% inhibition of epinephrine oxidation (Karmakar et al. 2022).
Catalase (CAT)
Catalase activity was measured using Takahara et al. (1960) method. Tissue homogenate and H2O2 in phosphate buffer were reacted; OD was checked at 240 nm in 15-second intervals for 3 minutes, with distilled water as the blank. The activity is shown as nanomoles of H2O2 decomposed/min/milligram of protein at 37 °C (Singh et al. 2023).
Glucose
Glucose estimation of larval tissue was done using an ELISA-based commercially available ERBA Glucose assay kit (Catalogue No. BLT00002, Cayman Chemical). The activity was expressed in mg/dl.
Cortisol
The cortisol levels in the sample were studied by using a Cortisol EIA kit (Catalogue No. 500360, Krishgen) as per the protocol given in the kit. OD was observed by using an ELISA reader at 412 nm. The activity is expressed in ng/ml.
Statistical analysis
The data study was done using a one-way analysis of variance (ANOVA) in SPSS version 22.0 Software. The normality assumption (using Shapiro-Wilk test) and homoscedasticity (Levene’s test) were made. The results were presented in the format of mean ± standard error. A one-way ANOVA and Duncan’s test were employed to make comparisons between the mean values of the test concentrations and the control group. A significant change was observed at a 5% probability level (p < 0.05).
Results
Growth and survival
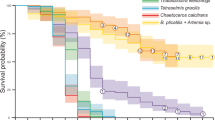
Different weaning strategies significantly (p < 0.05) affected the growth and survival of A. testudineus larvae reared under the experimental duration of 30 days (Tables 2 and 3). The highest WG and SGR were observed in T2 (213.17 ± 0.32 mg; 23.98 ± 0.02), followed by T6 (197.64 ± 0.29 mg; 23.73 ± 0.03, respectively), while the lowest WG and SGR (98.69 ± 0.16 mg; 21.42 ± 0.06, respectively) were found in C2. In the initial first 15 days of culture (3 DPH to 18 DPH), the highest survival was manifested by T7 (32.83 ± 0.60%) followed by T2 (27.83 ± 0.96%). The lowest survival was observed in C2 (10.00 ± 0.14%). At the end of the next 15 days (18 DPH to 33 DPH) of culture, the larvae of T3 showed the highest survival, but insignificant results were found in the remaining treatments. Overall, at the end of the whole experimental duration (3 DPH to 33 DPH), the maximum survival was observed in T7 (31.83 ± 0.22%), followed by T2 (24.75 ± 0.13%), and the lowest survival was found in the C2 (9.20 ± 0.08%).
Carcass proximate composition
The proximate analysis of whole larvae varied significantly (p < 0.05) among the experimental groups (Table 4). The maximum crude protein content was found in T2 and T6, followed by T3 and T7, and the least crude protein was obtained in the C2 group. The higher moisture content was obtained in T2 and T6, and the lower levels were found in C2 and T5. On the other hand, elevated total lipid levels were recorded in C2, T5, and T7 and decreased in T2 and T6.
Effect of weaning strategy on digestive enzymes
The acid and alkaline digestive proteases, amylase, and lipase activities in A. testudineus larvae varied non-significantly (p > 0.05) between the treatments (Table 5).
Effect of weaning strategy on metabolic enzymes
The protein metabolic enzyme activities (AST and ALT) and carbohydrate metabolic enzymes (LDH and MDH) differed significantly (p < 0.05) (Table 6). The highest AST and ALT activities were observed in C2 (19.55 ± 0.10 and 68.52 ± 0.08, respectively), whereas the lowest values were observed in C1 (8.25 ± 0.04 and 59.80 ± 0.08, respectively). Similarly, the highest LDH and MDH activities were observed in C2 (21.79 ± 0.04 and 0.044 ± 0.01), followed by T1 and T3 and lower activity was found in C1 (10.76 ± 0.14; 0.022 ± 0.03) followed by the T2 group.
Effect of weaning strategy on oxidative stress or antioxidant enzymes
The antioxidant enzymes viz., SOD (50% inhibition of epinephrine auto-oxidation/mg protein/min) and CAT (nanomoles H2O2 decomposed / mg protein/min) significantly varied (p < 0.05) between treatments (Fig. 2). Significantly (p < 0.05) higher SOD value was obtained in C2 followed by T1 and T3, whereas the least activity was found in C1 and T2. Similarly, the CAT value was significantly (p < 0.05) highest in C2, followed by T1, and a lower value was found in C1, followed by T2 and T6.
Tissue glucose and cortisol level
The glucose (mg/dl) and cortisol (ng/ml) levels significantly differed (p < 0.05) among treatments (Fig. 3). The significantly lowest (p < 0.05) glucose levels found in larvae of C1, T2, T6, and T7, and the highest level was found in the C2 followed by T1. Significantly (p < 0.05) lower cortisol levels found in T2 followed by C1 and reported higher in C2 followed by T1, although the larvae of T3 and T4 exhibited comparable cortisol levels (p > 0.05).
Discussion
The primary objective of this investigation was an optimization of the weaning strategy with a formulated microparticulate diet (MPD) and live food (LF) for a culture of A. testudines larvae. At onset of exogenous feeding, the larval digestive system is functional but simple, lacking a true stomach and pyloric caeca (Kolkovski 2001). Digestive enzyme activity is low or undetectable at an early stage (Mozanzadeh et al. 2021). Major changes in digestive physiology occur during metamorphosis from larvae to adults in the gut. However, significant functional changes happen during the larval phase ((Kolkovski 2001). Assessing enzyme activity levels in relation to growth and survival data obtained from co-feeding provides insights into the most favorable weaning period (Yufera et al. 2012). In some fish species, gradual replacement of live food with MPD has shown to be an effective weaning approach (Hamre et al. 2013; Biswas et al. 2022). It is well known that adopting larvae on MPD needs a strategy or protocol to adjust them to significant morphological, physiological and behavioral changes (Rao 2003). In contrast, the developmental stage was a significantly non-dependent factor in determining larval development and dietary status (Andrade et al. 2012). In the current investigation, different weaning strategies have shown a substantial impact on the growing performance and survivability of A. testudines. Variations in larval size are inevitable and can be corrected over the rising phase; however, weaning is critical to larval growth and survival during the rearing phase (Ali et al. 2003; Curnow et al. 2006; Valente et al. 2013; Hung et al. 2022).
Growth performance and survival
Several studies showed a good effect of live prey on the growth performance of various species of aquaculture (Conceição et al. 2010; Singh et al. 2019; Chen et al. 2020). A lack suitable weaning diet during initial larval rearing and cannibalism are the main constraints for successful larviculture of A. testudineus (Morioka et al. 2009; Singha et al. 2015; Singh et al. 2019). In existing investigation, significantly (p < 0.05) higher WG and SGR of the larvae were found in T2, which might be due to the better acceptability of live food at the early stage. The larvae lack a well-developed digestive system at their initial development stage to digest a formulated diet; hence, live food could be considered a better one as it probably could supply exogenous digestive enzymes for synergistic effects with endogenous enzymes (Singh et al. 2019). The findings corroborated the observations in larvae of African catfish, Clarias gariepinus (Verreth and Tongeren 1989; Verreth et al. 1992) and Redtail catfish, Mystus nemurus (Kamarudin et al. 2011), where feeding fish of appropriate weaning age with artificial diet could result in better weight gain and SGR. Mir et al. (2018) also reported that Clarias magur larvae could exhibit higher SGR and survival after weaning with an artificial diet. Kolkovski et al. (1997) also demonstrated that the co-feeding of European seabass larvae with formulated feed and live food increased growth and nutrient metabolism. In the current study, poor growth of larvae in C2 might be due to feeding of larvae with artificial feed from the first day of an experiment, which probably could result in lower feed intake due to low palatability with poor nutrient digestibility at early stage of larvae. As MPD lacks the endogenous enzyme, which results in low feed digestion, it consequently results in lower growth of larvae (Cahu and Infante 2001). Similar findings were observed in Sand bass Paralabrax maculatofatofasciatus (Civera-Cerecedo et al. 2008) and Senegalese sole (Engrola et al. 2007), where larvae exhibited poor growth in C1, probably due to limited availability of dietary nutrients resulting mobilization of nutrient from body reserve for energy production and satiation leading to poor growth of larvae. Therefore, an inadequate weaning strategy may cause poor survival as well as growth of larvae (Ma et al. 2012).
The main challenge in A. testudineus larviculture is poor survival, lack of proper weaning strategy, and cannibalism among larvae (Singha et al. 2015). Previous outcomes of A. testudineus larval rearing showed only 4–16% survival solely feeding on formulated diet (Trieu and Long 2001) and 16–28% feeding on live food (Morioka et al. 2009; Singh et al. 2019) during a 15-day period. Furthermore, cannibalistic behavior can be developed and it leads to heterogeneous growth, leading to reduced survivability of larvae (Thanh et al. 1999). However, Dicentrarchus labrax larvae showed low cannibalism after the feeding of the artificial feed after the appropriate age (Infante and Cahu 1994a, b). Therefore, a weaning strategy was optimized in the present study to enhance larval survivability and growth. In comparison to control groups (C1 and C2), higher survival reported in T7 and T6 probably could prove that instead of continuous feeding with live food certain co-feeding patterns may enhance larval survivability, as reported by Brown et al. (1997). Lower survival in larvae of C1 might be due to a deficiency of certain essential nutrients in live food (Segner and Witt 1990; Mejri et al. 2021; Grayson and Dabrowski 2022). Moreover, the lowest survival in C2 also suggests that feeding artificial feed alone can’t sustain larval survival, probably owing to lower feed intake and nutrient digestibility and, eventually, starvation (Salhi and Bessonart 2012).
Proximate composition of carcass
Food availability, fish growth and anabolic or catabolic response are all aspects that affect the chemical composition of the fish body (Abdel-Tawwab et al. 2006). A significant difference (p < 0.05) in carcass proximate analysis found in present study and contradicts the findings of earlier findings may be due to variations in feeding regimens, fish stage, type, nutrient digestibility, and research environment. However, according to Jafari et al. (2011), there was a considerable change in the whole-body composition of Rutilus frisii kutum larvae on different feeding regimens.
Effect of weaning strategy on digestive enzymes
There were no significant (p > 0.05) changes detected in digestive enzyme response between the treatments. In fish larvae, acid protease activity normally emerges after the development of a functioning stomach (Cahu and Infante 2001; Kolkovski 2001). The current research found insignificant acid protease activity, representing that stomach and acid protease action were functional at the end of the experiment. Accordingly, functional intestinal track and pepsin activity were found in the sea bass, Lates calcarifer larvae at 17 days (Walford and Lam 1993) and in red porgy Pagrus pagrus at 30 days (Darias et al. 2005). In larvae, protein assimilations began through alkaline proteolytic enzymes at the initial phase (Darias et al. 2007). The alkali protease activity in larvae was also not affected significantly by MPD or live food (Grendell and Rothman 1981; Tseng et al. 1982; Infante and Cahu 1994a, b). The evolution from the early to the juvenile stage is assumed to have ended when the gut and proteolytic enzyme secretion appear (Govoni et al. 1986). Similarly, amylase and lipase activities were non-significant (p > 0.05) in the present work; most digestive activity, such as acid protease, alkaline protease, amylase, and lipase, shows comparable patterns (Ribeiro et al. 1999). A similar pattern has also been reported in European sea bass, gilthead seabream, red drum, and black spot sea bream (Moyano et al. 1996; Ribeiro et al. 2008). It indicates that once their digestive systems are completely formed and the larvae have acclimated to the formulated diet, larvae are very flexible to various dietary regimes (Pittman et al. 2013; Rønnestad et al. 2013).
Effect of weaning strategy on protein metabolism
The protein metabolic activities are indicators of nutritional status, growth condition, and stress of animals, including fish (Pan et al. 2003; Haridas et al. 2017). Increased AST and ALT activities may be explained in two ways in relation to growth. Accordingly, increased activities of these enzymes with improved growth indicate that apparently produced non-essential amino acids participate in cellular metabolism in the body, leading to growth, in contrast with increased enzyme activity with reduced growth indicates oxidation for energy generation may restrict growth and at the same time releasing ammonia in the environment might create stress in fish. Thus, the highest AST and ALT values in the C2 group with reduced growth may be because less food ingestion and nutrient utilization and leads to energy satiation met through oxidation of body protein-derived amino acids leading to growth retardation. Similarly, higher AST and ALT values follow dietary stress due to low acceptability of feed with reduced feed intake in rats (Stadler et al. 2005). The higher AST and ALT levels with higher growth in larvae of certain weaning groups than C1 might be due to the participation of synthesized amino acids leading to better development of larvae. Moreover, the live food-fed group probably had a deficiency of some essential amino acids, leading to lower growth of larvae (Grayson and Dabrowski 2022).
Effect of weaning strategy on carbohydrate metabolic enzymes
The LDH and MDH activities also followed a similar pattern and were found significantly increasing in C2; this might be due to anaerobic oxidation leading to large amounts of lactate being released since lactate is the preferred substrate for gluconeogenesis to cope with stress and produce glucose and therefore LDH activity is raised (Chatterjee et al. 2006). However, the C1 group has low LDH and MDH, indicating that the live food-fed group had low stress due to better acceptability of live food in this group. Also, MDH is essential for gluconeogenesis as well as lipogenesis, which catalyzes the reversible malate oxidation to oxaloacetate in the cytosol (Panepucci et al. 2000). The increased MDH activity in fish suggests that there is a rising incidence of Kreb’s cycle as a result of rising energy demand to overcome stress (Kumar et al. 2011). However, lower LDH and MDH activities in T2 and T6 suggest they adopted a weaning protocol. Kumar et al. (2015) showed that MDH activity was increased in L. rohita fingerlings in response to temperature shock than control, implying a higher energy requirement during the stress condition.
Effect of weaning strategy on oxidative stress enzymes
The SOD catabolizes the dismutation of superoxide radicals to oxygen and hydrogen peroxide and CAT converts hydrogen peroxide to oxygen and water (Morales et al. 2004; Pérez-Jiménez et al. 2007). These enzymatic pathways reduce stress and have the potential to balance proper oxidation-reduction equilibrium, ameliorate reactive oxygen species (ROS) equilibrium, and save cells from oxidation (Abdel-Tawwab and Wafeek 2017; Hoseinifar et al. 2020). SOD and CAT activities were significantly (p < 0.05) varied between the experimental groups, with C2 indicating the highest SOD and CAT might be due to the higher stress in the larvae that were fed with MPD. As a CAT, SOD speeds up the dismutation of superoxide anion (Kehrer et al. 2010). The C1 had considerably decreased SOD and CAT compared to others due to the exclusive live food fed in this group, demonstrating relatively reduced stress. Lim et al. (2002) also reported that the live food-fed fish group had much less stress than commercial diet-fed fish.
Glucose and cortisol
Glucose and cortisol are another two major stress indicators in fish, and their elevated levels indicate stress in fish. As a useful tool for evaluating the health status of fish larvae, measuring glucose levels is also a good biomarker for research on secondary stress reactions (Wedemeyer 1981). The primary glucocorticoid released by interregnal muscle in the kidney of teleost fish is cortisol (Iwama et al. 1999). The value of glucose and cortisol found to rise in fish is an indicator of various stressors (Luz et al. 2008; Fiúza et al. 2015). Elevated levels of cortisol and glucose levels were observed in both C2 and T1 groups, indicating that the larvae fed with an artificial diet for 30 days and 25 days encountered starvation stress. Similarly, feeding a microdiet to Chinese longsnout catfish larvae significantly increases body glucose content (Liu et al. 2012). A considerable increase in cortisol levels in the C2 group fed with MPD indicates they were under nutritional stress as nutritional requirements have not yet fulfilled, which is also indicates reduced growth in this group (Yu et al. 2019).
Conclusion
The initial phase of larval rearing is a major aspect impeding the growth and survival of Anabas testudineus farming because live food production is both costly and nutritionally deficient and it also needs specialized production facilities. As a result, optimizing the weaning protocol to minimize dependency on live food is of both technical and economic importance. The research outcomes suggest that different weaning approaches substantially influenced growth and survival. Also, proximate composition and enzymatic activities associated with digestion, protein, and carbohydrate metabolism exhibited significant differences among the treatments. Furthermore, antioxidant enzyme activities also displayed variations with the control group. The glucose and cortisol levels demonstrated substantial differences among the experimental groups. Major findings of the experiment reveal A. testudineus larvae can be weaned after 13 DPH on formulated feed. The present study also concludes that feeding A. testudineus larvae with LF for 10 days, then 25% LF replacement by MPD for next 5 days, 50% LF replacement by MPD for next 5 days, 75% LF replacement by MPD for next 5 days, and 100% LF replacement by MPD for last 5 days can be the best weaning strategy as far as survival (31%) is concerned. The implementation of this weaning protocol may ensure seed availability for a widespread culture of A. testudineus. Additional research is advised to explore the comprehensive understanding of larval nutrition, like molecular level examination is essential for precise dietary interventions, along with the implementation of innovative methodologies for investigating the nutritional requirements of A. testudineus larvae.
Data availability
The necessary data has been included in the article as a table and figures in the main article.
References
Abdel-Tawwab M, Khattab YA, Ahmad MH, Shalaby AM (2006) Compensatory growth, feed utilization, whole-body composition, and hematological changes in starved juvenile Nile tilapia, Oreochromis niloticus. J Appl Aquac 18:17–36. https://doi.org/10.1300/j028v18n03_02
Abdel-Tawwab M, Wafeek M (2017) Fluctuations in water temperature affected waterborne cadmium toxicity: hematology, anaerobic glucose pathway, and oxidative stress status of Nile tilapia, Oreochromis niloticus. Aquaculture 477:106–111. https://doi.org/10.1016/j.aquaculture.2017.05.007
Ahilan B, Thangarani AJ (2020) Food and feeding of larval stages and adults of important cultivable brackishwater and marine fishes, In: Fish nutrition and its relevance to human health. CRC Press, pp 79–142. https://doi.org/10.1201/9781003107583-3
Alang AN, Hassan Z, Christianus A, Zulperi Z (2020) Effect of thyroxine hormone towards growth and survival of climbing perch (Anabas testudineus, Bloch) larvae. J Environ Biol 41:1230–1238. https://doi.org/10.22438/jeb/41/5(si)/ms_15
Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish 4:147–190. https://doi.org/10.1046/j.1467-2979.2003.00120.x
Andersen SM, Waagbø R, Espe M (2016) Functional amino acids in fish health and welfare. Front Biosci -Elite 8:143–169. https://doi.org/10.2741/757
Andrade CA, Nascimento F, Conceição LE, Linares F, Lacuisse M, Dinis MT (2012) Red porgy, Pagrus pagrus, larvae performance and nutritional condition in response to different weaning regimes. J World Aquac Soc 43:321–334. https://doi.org/10.1111/j.1749-7345.2012.00574.x
Anguas-Vélez BH, Civera-Cerecedo R, Contreras-Olguin M, Rueda-Jasso RA, Guillaume J (2000) Preliminary study on the timing of weaning of spotted sand bass, Paralabrax maculatofasciatus, larvae with a prepared diet: effects on growth and survival. J Appl Aquac 10:1–15. https://doi.org/10.1300/j028v10n04_01
Anson ML (1938) The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J Gen Physiol 22:79. https://doi.org/10.1085/jgp.22.1.79
AOAC (1995) Official methods of analysis of the association of official analytical chemistry. Arlington, VA, USA., AOAC. https://doi.org/10.1016/0003-2670(91)87088-o
Basak SK, Basak B, Gupta N, Haque MM, Amin R (2014) Embryonic and larval development of silver barb (Barbodes gonionotus) in a mobile hatchery under laboratory condition. Eur J Sci Res 3:258–270. https://doi.org/10.9790/2380-07418190
Bell JG, McEvoy LA, Estevez A, Shields RJ, Sargent JR (2003) Optimising lipid nutrition in first-feeding flatfish larvae. Aquaculture 227:211–220. https://doi.org/10.1016/s0044-8486(03)00504-0
Berge GM, Storebakken T (1996) Fish protein hydrolyzate in starter diets for Atlantic salmon (Salmo salar) fry. Aquaculture 145:205–212. https://doi.org/10.1016/s0044-8486(96)01355-5
Bhuyan S, Hussain SM (2018) Breeding and seed rearing of Climbing perch (Anabas testudineus, Bloch) using farmer friendly innovative technology at farmer’s field: a case study. IJBS 5:101–105. https://doi.org/10.30954/2347%E2%80%939655.02.2018.3
Biswas P, Jena AK, Singh SK (2022) Conservation aquaculture of Ompok bimaculatus (Butter catfish), a near threatened catfish in India. Aquac Fish 8:1–17. https://doi.org/10.1016/j.aaf.2022.04.007
Bonaldo A, Parma L, Badiani A, Serratore P, Gatta PP (2011) Very early weaning of common sole (Solea solea L.) larvae by means of different feeding regimes and three commercial microdiets: influence on performances, metamorphosis development and tank hygiene. Aquaculture 321:237–244. https://doi.org/10.1016/j.aquaculture.2011.09.007
Brown JA, Wiseman D, Kean P (1997) The use of behavioural observations in the larviculture of cold-water marine fish. Aquaculture 155:297–306. https://doi.org/10.1016/s0044-8486(97)00130-0
Cahu C, Infante JZ (2001) Substitution of live food by formulated diets in marine fish larvae. Aquaculture 200:161–180. https://doi.org/10.1016/s0044-8486(01)00699-8
Cahu C, Infante JZ, Escaffre A-M, Bergot P, Kaushik S (1998) Preliminary results on sea bass (Dicentrarchus labrax) larvae rearing with compound diet from first feeding. Comparison with carp (Cyprinus carpio) larvae. Aquaculture 169:1–7. https://doi.org/10.1016/s0044-8486(98)00316-0
Cahu CL, Infante JZ, Quazuguel P, Le Gall MM (1999) Protein hydrolysate vs. fish meal in compound diets for 10-day old sea bass Dicentrarchus labrax larvae. Aquaculture 171:109–119. https://doi.org/10.1016/s0044-8486(98)00428-1
Callan C, Jordaan A, Kling LJ (2003) Reducing Artemia use in the culture of Atlantic cod (Gadus morhua). Aquaculture 219:585–595. https://doi.org/10.1016/s0044-8486(03)00011-5
Carvalho AP, Escaffre A-M, Oliva Teles A, Bergot P (1997) First feeding of common carp larvae on diets with high levels of protein hydrolysates. Aquac Int 5:361–367. https://doi.org/10.1023/a:1018368208323
Chandan NK, Sahu NP, Krishna G, Kumar R, Nandi S, Kumari R, Udit UK, Siddaiah GM, Mohapatra BC and Mohanta KN (2023) Fatty acid utilization and digestive enzyme activity during early larval development of Anabas testudineus. Biol Forum – Int J 15:425–431
Chatterjee N, Pal AK, Das T, Mohammed MS, Sarma K, Venkateshwarlu G, Mukherjee SC (2006) Secondary stress responses in Indian major carps Labeo rohita (Hamilton), Catla catla (Hamilton) and Cirrhinus mrigala (Hamilton) fry to increasing packing densities. Aquac Res 37:472–476. https://doi.org/10.1111/j.1365-2109.2006.01469.x
Chen JY, Zeng C, Jerry DR, Cobcroft JM (2020) Recent advances of marine ornamental fish larviculture: broodstock reproduction, live prey and feeding regimes, and comparison between demersal and pelagic spawners. Rev Aquac 12:1518–1541. https://doi.org/10.1111/raq.12394
Cherry IS, Crandall Jr LA (1932) The specificity of pancreatic lipase: its appearance in the blood after pancreatic injury. Am J Physiol -Leg Content 100:266–273. https://doi.org/10.1152/ajplegacy.1932.100.2.266
Chèvre P, Saint-Sevin J, Mercier D, Jacobs L, Williot P (2011) Recent progress in larval rearing of the European sturgeon, Acipenser sturio. Biol Conserv Eur Sturgeon Acipenser SturioL 1758:49–453. https://doi.org/10.1007/978-3-642-20611-5_33
Chotikachinda R, Tantikitti C, Benjakul S, Rustad T, Kumarnsit E (2013) Production of protein hydrolysates from skipjack tuna (Katsuwonus pelamis) viscera as feeding attractants for Asian seabass (Lates calcarifer). Aquac Nutr 19:773–784. https://doi.org/10.1111/anu.12024
Civera-Cerecedo R, Alvarez-González CA, García-Gómez RE, Carrasco-Chávez V, Ortiz-Galindo JL, Rosales-Velázquez MO, Grayeb-Del Álamo T, Moyano-López FJ (2008) Effect of microparticulate diets on growth and survival of spotted sand bass larvae, Paralabrax maculatofasciatus, at two early weaning times. J World Aquac Soc 39:22–36. https://doi.org/10.1111/j.1749-7345.2007.00132.x
Comabella Y, Mendoza R, Aguilera C, Carrillo O, Hurtado A, García-Galano T (2006) Digestive enzyme activity during early larval development of the Cuban gar Atractosteus tristoechus. Fish Physiol Biochem 32:147–157. https://doi.org/10.1007/s10695-006-0007-4
Conceição LE, Yúfera M, Makridis P, Morais S, Dinis MT (2010) Live feeds for early stages of fish rearing. Aquac Res 41:613–640. https://doi.org/10.1111/j.1365-2109.2009.02242.x./
Curnow J, King J, Bosmans J, Kolkovski S (2006) The effect of reduced Artemia and rotifer use facilitated by a new microdiet in the rearing of barramundi Lates calcarifer (BLOCH) larvae. Aquaculture 257:204–213. https://doi.org/10.1016/j.aquaculture.2006.02.073
Dabrowski K, Lee K-J, Rinchard J (2003) The smallest vertebrate, teleost fish, can utilize synthetic dipeptide-based diets. J Nutr 133:4225–4229. https://doi.org/10.1093/jn/133.12.4225
Darias MJ, Murray HM, Gallant JW, Douglas SE, Yúfera M, Martínez-Rodríguez G (2007) Ontogeny of pepsinogen and gastric proton pump expression in red porgy (Pagrus pagrus): determination of stomach functionality. Aquaculture 270:369–378. https://doi.org/10.1016/j.aquaculture.2007.04.045
Darias MJ, Murray HM, Martínez-Rodríguez G, Cárdenas S, Yúfera M (2005) Gene expression of pepsinogen during the larval development of red porgy (Pagrus pagrus). Aquaculture 248:245–252. https://doi.org/10.1016/j.aquaculture.2005.04.044
Djellata A, Sarih S, Hernández-Cruz CM, Martínez-Rodríguez G, Gilannejad N, Roo J (2021) The effect of different co-feeding protocols on greater amberjack (Seriola dumerili, Risso 1810) larvae. Aquac Nutr 27:1761–1776. https://doi.org/10.1111/anu.13313
Drapeau G (1974) Protease from Staphylococcus aureus, In: Method of enzymology 45b. L. Acad Press NY. https://doi.org/10.1016/s0076-6879(76)45041-3
Engrola S, Conceição LE, Dias L, Pereira R, Ribeiro L, Dinis MT (2007) Improving weaning strategies for Senegalese sole: effects of body weight and digestive capacity. Aquac Res 38:696–707. https://doi.org/10.1111/j.1365-2109.2007.01701.x
Fiúza LS, Aragão NM, Ribeiro Junior HP, de Moraes MG, Rocha ÍRCB, Lustosa Neto AD, de Sousa RR, Madrid RMM, de Oliveira EG, Costa FHF (2015) Effects of salinity on the growth, survival, haematological parameters and osmoregulation of tambaqui Colossoma macropomum juveniles. Aquac Res 46:1–9. https://doi.org/10.1111/are.12224
Giri SS, Sahoo SK, Sahu BB, Sahu AK, Mohanty SN, Mukhopadhyay PK, Ayyappan S (2002) Larval survival and growth in Wallago attu (Bloch and Schneider): effects of light, photoperiod and feeding regimes. Aquaculture 213:151–161. https://doi.org/10.1016/s0044-8486(02)00012-1
Gisbert E, Giménez G, Fernández I, Kotzamanis Y, Estévez A (2009) Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture 287:381–387. https://doi.org/10.1016/j.aquaculture.2008.10.039
Govoni JJ, Boehlert GW, Watanabe Y (1986) The physiology of digestion in fish larvae. Environ Biol Fishes 16:59–77. https://doi.org/10.1007/bf00005160
Grayson JD, Dabrowski K (2022) Utilization of live-food enrichment with polyunsaturated fatty acids (PUFA) for the intensive culture of Yellow perch Larvae. North Am J Aquac 84:131–148. https://doi.org/10.1002/naaq.10227
Grendell JH, Rothman SS (1981) Digestive end products mobilize secretory proteins from subcellular stores in the pancreas. Am J Physiol-Gastrointest Liver Physiol 241:G67–G73. https://doi.org/10.1152/ajpgi.1981.241.1.g67
Hamre K, Yúfera M, Rønnestad I, Boglione C, Conceição LE, Izquierdo M (2013) Fish larval nutrition and feed formulation: knowledge gaps and bottlenecks for advances in larval rearing. Rev Aquac 5:S26–S58. https://doi.org/10.1111/j.1753-5131.2012.01086.x
Haridas H, Verma AK, Rathore G, Prakash C, Sawant PB, Babitha Rani AM (2017) Enhanced growth and immuno-physiological response of Genetically Improved Farmed Tilapia in indoor biofloc units at different stocking densities. Aquac Res 48:4346–4355. https://doi.org/10.1111/are.13256
Hauville MR, Zambonino-Infante JL, Bell G, Migaud H, Main KL (2014) Impacts of three different microdiets on Florida Pompano, Trachinotus carolinus, weaning success, growth, fatty acid incorporation and enzyme activity. Aquaculture 422:268–276. https://doi.org/10.1016/j.aquaculture.2013.12.006
Herath SS, Atapaththu KSS (2013) Sudden weaning of angel fish pterophyllum scalare (Lichtenstein) (Pisces; Cichlidae) larvae from brine shrimp (Artemia sp) nauplii to formulated larval feed. SpringerPlus 2:1–7. https://doi.org/10.1186/2193-1801-2-102
Holt GJ (2011) Larval fish nutrition. John Wiley & Sons.https://doi.org/10.1002/9780470959862
Hoseinifar SH, Yousefi S, Van Doan H, Ashouri G, Gioacchini G, Maradonna F, Carnevali O (2020) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquac 29:198–217. https://doi.org/10.1080/23308249.2020.1795616
Hung T-C, Ellison L, Stevenson T, Sandford M, Schultz AA, Eads AR (2022) Early weaning in endangered delta smelt: effect of weaning time on growth and survival. North Am J Aquac 84:249–260. https://doi.org/10.1002/naaq.10230
Infante JL, Cahu C (1994a) Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 12:399–408. https://doi.org/10.1007/bf00004304
Infante JZ, Cahu CL (1994b) Influence of diet on pepsin and some pancreatic enzymes in sea bass (Dicentrarchus labrax) larvae. Comp Biochem Physiol A Physiol 109:209–212. https://doi.org/10.1016/0300-9629(94)90122-8
Iwama GK, Vijayan MM, Forsyth RB, Ackerman PA (1999) Heat shock proteins and physiological stress in fish. Am Zool 39:901–909. https://doi.org/10.1093/icb/39.6.901
Jafari M, Kamarudin MH, Saad CR, Arshad A, Oryan S, Guilani MHT (2011) Effect of different diets on growth, survival and body composition of Rutilus frisii kutum larvae. J Fish Aquat Sci 6:662–668. https://doi.org/10.3923/jfas.2011.662.668
Jamali H, Ahmadifard N, Noori F, Agh N, Gisbert E (2018) Improving co-feeding strategies for Neotropical green terror cichlid (Aequidens rivulatus) larvae with lecithin-enriched Artemia franciscana nauplii: effects on survival, growth performance and body composition. Aquac Res 49:3909–3918. https://doi.org/10.1111/are.13861
Jayasankar P (2018) Present status of freshwater aquaculture in India-a review. Indian J Fish 65:157–165. https://doi.org/10.21077/ijf.2018.65.4.81300-20
Kamarudin MS, Otoi S, Saad CR (2011) Changes in growth, survival and digestive enzyme activities of Asian redtail catfish, Mystus nemurus, larvae fed on different diets. Afr J Biotechnol 10:4484–4493. https://doi.org/10.5897/AJB09.1895
Kandathil Radhakrishnan D, AkbarAli I, Schmidt BV, John EM, Sivanpillai S, Thazhakot Vasunambesan S (2020) Improvement of nutritional quality of live feed for aquaculture: an overview. Aquac Res 51:1–17. https://doi.org/10.1111/are.14357
Karmakar A, Das AK, Ghosh N, Sil PC (2022) Superoxide dismutase. In Antioxidants effects in health. Elsevier 1:139–166. https://doi.org/10.1016/B978-0-12-819096-8.00027-6
Kehrer JP, Robertson JD, Smith CV (2010) Free radicals and reactive oxygen species.https://doi.org/10.1016/b978-0-08-046884-6.00114-7
Khoa TND, Waqalevu V, Honda A, Shiozaki K, Kotani T (2020) Comparative study on early digestive enzyme activity and expression in red sea bream (Pagrus major) fed on live feed and micro-diet. Aquaculture 519:734721. https://doi.org/10.1016/j.aquaculture.2019.734721
Kohinoor AHM, Islam MS, Jahan DA, Khan MM, Hussain MG (2012) Growth and production performances of crossbred climbing perch koi, Anabas testudineus in Bangladesh. Int J Agric Res Innov Technol 2:19–25. https://doi.org/10.3329/ijarit.v2i1.13990
Kolkovski S (2001) Digestive enzymes in fish larvae and juveniles implications and applications to formulated diets. Aquaculture 200:181–201. https://doi.org/10.1016/s0044-8486(01)00700-1
Kolkovski S (2008) Advances in marine fish larvae diets. In: Cruz-Suárez E, Ricque D, Tapia M, Nieto MG, Villarreal LDA, Lazo JP, Viana MT (eds) Avances en Nutrición Acuícola IX. Universidad Autónoma de Nuevo León, Mexico, Nuevo León, pp 20–45
Kolkovski S, Koven W, Tandler A (1997) The mode of action of Artemia in enhancing utilization of microdiet by gilthead seabream Sparus aurata larvae. Aquaculture 155:193–205. https://doi.org/10.1016/s0044-8486(97)00117-8
Kotzamanis YP, Gisbert E, Gatesoupe FJ, Infante JZ, Cahu C (2007) Effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae. Comp Biochem Physiol A Mol Integr Physiol 147:205–214. https://doi.org/10.1016/j.cbpa.2006.12.037
Kumar P, Pal AK, Sahu NP, Jha AK, Priya P (2015) Biochemical and physiological stress responses to heat shock and their recovery in Labeo rohita fingerlings. Proc Natl Acad Sci India Sect B Biol Sci 85:485–490. https://doi.org/10.1007/s40011-014-0357-0
Kumar S, Jahageerdar S, Shamna N, Chanu TI (2022) Fish protein hydrolysate in larval diet can augment the survival rate, growth performance, and enzyme activities of Indian walking catfish, Clarias magur larvae. Anim Nutr Feed Technol 22:525–542. https://doi.org/10.5958/0974-181x.2022.00042.7
Kumar S, Sahu NP, Pal AK, Subramanian S, Priyadarshi H, Kumar V (2011) High dietary protein combats the stress of Labeo rohita fingerlings exposed to heat shock. Fish Physiol Biochem 37:1005–1019. https://doi.org/10.1007/s10695-011-9504-1
Lazo JP, Dinis MT, Holt GJ, Faulk C, Arnold CR (2000) Co-feeding microparticulate diets with algae: toward eliminating the need of zooplankton at first feeding in larval red drum (Sciaenops ocellatus). Aquaculture 188:339–351. https://doi.org/10.1016/s0044-8486(00)00339-2
Lazo JP, Mendoza R, Holt GJ, Aguilera C, Arnold CR (2007) Characterization of digestive enzymes during larval development of red drum (Sciaenops ocellatus). Aquaculture 265:194–205. https://doi.org/10.1016/j.aquaculture.2007.01.043
Le Thanh H, Tam BM, Cacot P, Lazard J (1999) Larval rearing of the Mekong catfish, Pangasius bocourti (Pangasiidae, Siluroidei): substitution of Artemia nauplii with live and artificial feed. https://doi.org/10.1016/s0990-7440(00)88473-9
Lim LC, Dhert P, Chew WY, Dermaux V, Nelis H, Sorgeloos P (2002) Enhancement of stress resistance of the guppy Poecilia reticulata through feeding with vitamin C supplement. J World Aquac Soc 33:32–40. https://doi.org/10.1111/j.1749-7345.2002.tb00475.x
Liu B, Zhu X, Lei W, Yang Y, Han D, Jin J, Xie S (2012) Effects of different weaning strategies on survival and growth in Chinese longsnout catfish (Leiocassis longirostris Günther) larvae. Aquaculture 364:13–18. https://doi.org/10.1016/j.aquaculture.2012.04.051
Ljubobratović U, Kucska B, Feledi T, Poleksić V, Marković Z, Lenhardt M, Peteri A, Kumar S, Rónyai A (2015) Effect of weaning strategies on growth and survival of pikeperch, Sander lucioperca, larvae. Turk J Fish Aquat Sci 15:327–333. https://doi.org/10.4194/1303-2712-v15_2_15
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) of these enzymes than MQ. Biol Chem 193:265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
Luz RK, Martínez-Álvarez RM, De Pedro N, Delgado MJ (2008) Growth, food intake regulation and metabolic adaptations in goldfish (Carassius auratus) exposed to different salinities. Aquaculture 276:171–178. https://doi.org/10.1016/j.aquaculture.2008.01.042
Ma Z, Zheng P, Guo H, Zhang N, Wang L, Jiang S, Qin JG, Zhang D (2015) Effect of weaning time on the performance of Trachinotus ovatus (Linnaeus 1758) larvae. Aquac Nutr 21:670–678. https://doi.org/10.1111/anu.12183
Ma Z, Qin J, Nie Z (2012) Morphological changes of marine fish larvae and their nutrition need. Nov Sci Pub 1–20
Mejri SC, Tremblay R, Audet C, Wills PS, Riche M (2021) Essential fatty acid requirements in tropical and cold-water marine fish larvae and juveniles. Front Mar Sci 8:680003. https://doi.org/10.3389/fmars.2021.680003
Mir IN, Srivastava PP, Bhat IA, Muralidhar AP, Varghese T, Gireesh-Babu P, Jain KK (2018) Expression and activity of trypsin and pepsin during larval development of Indian walking catfish (Clarias magur). Aquaculture 491:266–272. https://doi.org/10.1016/j.aquaculture.2018.03.049
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175. https://doi.org/10.1016/s0021-9258(19)45228-9
Mondal S, Roy T, Sen SK, Ray AK (2008) Distribution of enzyme-producing bacteria in the digestive tracts of some freshwater fish. Acta Ichthyol Piscat 38:1–8. https://doi.org/10.3750/aip2008.38.1.01
Morales AE, Pérez-Jiménez A, Hidalgo MC, Abellán E, Cardenete G (2004) Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp Biochem Physiol Part C Toxicol Pharmacol 139:153–161. https://doi.org/10.1016/j.cca.2004.10.008
Morioka S, Ito S, Kitamura S, Vongvichith B (2009) Growth and morphological development of laboratory-reared larval and juvenile climbing perch Anabas testudineus. Ichthyol Res 56:162–171. https://doi.org/10.1007/s10228-008-0081-y
Moyano FJ, Diaz M, Alarcón FJ, Sarasquete MC (1996) Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol Biochem 15:121–130. https://doi.org/10.1007/bf01875591
Mozanzadeh MT, Bahabadi MN, Morshedi V, Azodi M, Agh N, Gisbert E (2021) Weaning strategies affect larval performance in yellowfin seabream (Acanthopagrus latus). Aquaculture 539:1–51. https://doi.org/10.1016/j.aquaculture.2021.736673
Ochoa S (1955) malic dehydrogenase from pig heart: l-malate+ DPN+⇆ Oxalacetate+ DPNH+ H+. https://doi.org/10.1016/0076-6879(55)01128-2
Pan C-H, Chien Y-H, Hunter B (2003) The resistance to ammonia stress of Penaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin. J Exp Mar Biol Ecol 297:107–118. https://doi.org/10.1016/j.jembe.2003.07.002
Panepucci L, Fernandes MN, Sanches JR, Rantin FT (2000) Changes in lactate dehydrogenase and malate dehydrogenase activities during hypoxia and after temperature acclimation in the armored fish, Rhinelepis strigosa (Siluriformes, Loricariidae). Rev Bras Biol 60:353–360. https://doi.org/10.1590/s0034-71082000000200021
Pérez-Jiménez A, Guedes MJ, Morales AE, Oliva-Teles A (2007) Metabolic responses to short starvation and refeeding in Dicentrarchus labrax. Effect of dietary composition. Aquaculture 265:325–335. https://doi.org/10.1016/j.aquaculture.2007.01.021
Pittman K, Yúfera M, Pavlidis M, Geffen AJ, Koven W, Ribeiro L, Zambonino-Infante JL, Tandler A (2013) Fantastically plastic: fish larvae equipped for a new world. Rev Aquac 5:S224–S267. https://doi.org/10.1111/raq.12034
Pradhan PK, Jena J, Mitra G, Sood N, Gisbert E (2014) Effects of different weaning strategies on survival, growth and digestive system development in butter catfish Ompok bimaculatus (Bloch) larvae. Aquaculture 424:120–130. https://doi.org/10.1016/j.aquaculture.2013.12.041
Rao TR (2003) Ecological and ethological perspectives in larval fish feeding. J Appl Aquac 13:145–178. https://doi.org/10.1300/j028v13n01_06
Ribeiro L, Couto A, Olmedo M, Álvarez-Blázquez B, Linares F, Valente LM (2008) Digestive enzyme activity at different developmental stages of blackspot seabream, Pagellus bogaraveo (Brunnich 1768). Aquac Res 39:339–346. https://doi.org/10.1111/j.1365-2109.2007.01684.x
Ribeiro L, Zambonino-Infante JL, Cahu C, Dinis MT (1999) Development of digestive enzymes in larvae of Solea senegalensis, Kaup 1858. Aquaculture 179:465–473. https://doi.org/10.1016/s0044-8486(99)00180-5
Rick W, Stegbauer HP (1974) α-Amylase measurement of reducing groups, in: Methods of enzymatic analysis. Elsevier, pp 885–890. https://doi.org/10.1016/b978-0-12-091302-2.50074-8
Rønnestad I, Yúfera M, Ueberschär B, Ribeiro L, Sæle Ø, Boglione C (2013) Feeding behaviour and digestive physiology in larval fish: current knowledge, and gaps and bottlenecks in research. Rev Aquac 5:S59–S98. https://doi.org/10.1111/raq.12010
Salhi M, Bessonart M (2012) Growth, survival and fatty acid composition of Rhamdia quelen (Quoy and Gaimard, 1824) larvae fed on artificial diet alone or in combination with A rtemia nauplii. Aquac Res 44:41–49. https://doi.org/10.1111/j.1365-2109.2011.03004.x
Sarma K, Pal AK, Ayyappan S, Das T, Manush SM, Debnath D, Baruah K (2010) Acclimation of Anabas testudineus (Bloch) to three test temperatures influences thermal tolerance and oxygen consumption. Fish Physiol Biochem 36:85–90. https://doi.org/10.1007/s10695-008-9293-3
Segner H, Witt U (1990) Weaning experiments with turbot (Scophthalmus maximus): electron microscopic study of liver. Mar Biol 105:353–361. https://doi.org/10.1007/bf01316306
Siddaiah GM, Kumar R, Kumari R, Damle DK, Rasal KD, Manohar V, Sundaray JK, Pillai BR (2022) Dietary supplementation of fish protein hydrolysate improves growth, feed efficiency and immune response in freshwater carnivore fish, Channa striata fingerlings. Aquacult Res. https://doi.org/10.1111/are.15848
Singh K, Munilkumar S, Sahu NP, Das A, Devi GA (2019) Feeding HUFA and vitamin C-enriched Moina micrura enhances growth and survival of Anabas testudineus (Bloch, 1792) larvae. Aquaculture 500:378–384. https://doi.org/10.1016/j.aquaculture.2018.09.049
Singh PK, Munilkumar S, Sundaray JK, Santhanam P, Sharma A, Haque R, Satheesh M (2023) Effect of selenium, vitamin C and highly unsaturated fatty acids-enriched Brachionus calyciflorus on growth, survival, physio-metabolic and anti-oxidative responses in Anabas testudineus (Bloch, 1792) larvae. Aquaculture 568:739293. https://doi.org/10.1016/j.aquaculture.2023.739293
Singha K, Munilkumara S, Sahub NP, Dasc A (2015) Food type preference and size in relation to mouth gape of larval stages of climbing perch Anabas testudineus. Ecol Env Conserv 21:2039–2045
Srichanun M, Tantikitti C, Kortner TM, Krogdahl Å, Chotikachinda R (2014) Effects of different protein hydrolysate products and levels on growth, survival rate and digestive capacity in Asian seabass (Lates calcarifer Bloch) larvae. Aquaculture 428:195–202. https://doi.org/10.1016/j.aquaculture.2014.03.004
Stadler M, Nuyens V, Seidel L, Albert A, Boogaerts JG (2005) Effect of nutritional status on oxidative stress in an ex vivo perfused rat liver. J Am Soc Anesthesiol 103:978–986. https://doi.org/10.1097/00000542-200511000-00012
Takahara S, Hamilton HB, Neel JV, Kobara TY, Ogura Y, Nishimura ET (1960) Hypocatalasemia: a new genetic carrier state. J Clin Invest 39:610–619. https://doi.org/10.1172/jci104075
Tan HH, Lim KK (2004) Inland fishes from the Anambas and Natuna Islands, South China Sea, with description of a new species of Betta (Teleostei: Osphronemidae). Raffles Bull Zool 11:107–115
Thompson EF (2013) Towards the development of a larval feeding strategy for the white-margined sole (Dagetichthys Marginatus). Dissertation, Rhodes University
Thompson KL, Faulk CK, Fuiman LA (2019) Applying the ontogeny of digestive enzyme activity to guide early weaning of pigfish, Orthopristis chrysoptera (L.). Aquac Res 50:1404–1410. https://doi.org/10.1111/are.14015
Tseng HC, Grendell JH, Rothman SS (1982) Food, duodenal extracts, and enzyme secretion by the pancreas. Am J Physiol-Gastrointest Liver Physiol 243:G304–G312. https://doi.org/10.1152/ajpgi.1982.243.4.g304
Valente LM, Moutou KA, Conceicao LE, Engrola S, Fernandes JM, Johnston IA (2013) What determines growth potential and juvenile quality of farmed fish species? Rev Aquac 5:S168–S193. https://doi.org/10.1111/raq.12020
Van Trieu N, Long DN (2001) Seed production technology of Climbing perch (Anabas testudineus): a study on the larval rearing. https://doi.org/10.1079/ac.64965.20203483891
Verreth J, Van Tongeren M (1989) Weaning time in Clarias gariepinus (Burchell) larvae. Aquaculture 83:81–88. https://doi.org/10.1016/0044-8486(89)90062-8
Verreth JA, Torreele E, Spazier E, Van der Sluiszen A, Rombout JH, Booms R, Segner H (1992) The development of a functional digestive system in the African catfish Clarias gariepinus (Burchell). J World Aquac Soc 23:286–298. https://doi.org/10.1111/j.1749-7345.1992.tb00792.x
Walford J, Lam TJ (1993) Development of digestive tract and proteolytic enzyme activity in seabass (Lates calcarifer) larvae and juveniles. Aquaculture 109:187–205. https://doi.org/10.1016/0044-8486(93)90215-k
Washington IM and Van Hoosier G (2012) Clinical biochemistry and hematology. In The laboratory rabbit, guinea pig, hamster, and other rodents (pp 57–116). Academic Press. https://doi.org/10.1016/B978-0-12-380920-9.00003-1
Wedemeyer GA (1981) Methods for determining the tolerance of fishes to environmental stressors. J Str Fis 1:247–276
Wei LS, Wee W, Abdullah Z (2021) Climbing perch farming, Anabas testudineus (BLOCH, 1792) and its health management in malaysia. UMK Press, Malaysia
Williot P, Rouault T, Brun R, Pelard M, Mercier D (2011) Preliminary results on larval rearing the European sturgeon, Acipenser sturio, in: Biology and conservation of the European sturgeon Acipenser sturio L. 1758. Springer, pp 395–405. https://doi.org/10.1007/978-3-642-20611-5_28
Wootton IDP (1964) Micro-analysis in medical biochemistry. Britain, United Kingdom
Wróblewski F, Ladue JS (1955) Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med 90:210–213. https://doi.org/10.3181/00379727-90-21985
Yu X, Wu Y, Deng M, Liu Y, Wang S, He X, Allaire-Leung M, Wan J, Zou Y, Yang C (2019) Tetracycline antibiotics as PI3K inhibitors in the Nrf2-mediated regulation of antioxidative stress in zebrafish larvae. Chemosphere 226:696–703. https://doi.org/10.1016/j.chemosphere.2019.04.001
Yufera M, Moyano FJ, Astola A, Pousao-Ferreira P, Martinez-Rodriguez G (2012) Acidic digestion in a teleost: postprandial and circadian pattern of gastric pH, pepsin activity, and pepsinogen and proton pump mRNAs expression. PLoS ONE 7:33687. https://doi.org/10.1371/journal.pone.0033687
Zalina I, Saad CR, Rahim AA, Christianus A, Harmin SA (2011) Breeding performance and the effect of stocking density on the growth and survival of climbing perch, Anabas testudineus. J Fish Aquat Sci 6:834. https://doi.org/10.3923/jfas.2011.834.839
Zambonino Infante JL, Cahu CL, Peres A (1997) Partial substitution of di-and tripeptides for native proteins in sea bass diet improves Dicentrarchus labrax larval development. J Nutr 127:608–614. https://doi.org/10.1093/jn/127.4.608
Acknowledgements
All authors are thankful to the Vice-Chancellor, ICAR- Central Institute of Fisheries Education, Mumbai, for supplying the required infrastructure to carry out the present research. The first author duly acknowledges ICAR-CIFE for the fellowship during the research period.
Author information
Authors and Affiliations
Contributions
Patekar Prakash: Data curation, Investigation, Software, Writing -original draft. Sikendra Kumar: Conceptualization, Supervision, Writing—review & editing. Parimal Sardar: Supervision, Writing – review and editing. Sukham Munilkumar: Practical supervision, Writing – review and editing. Sujata Sahoo: Chemical analysis, Data curation. M. Satheesh: Writing – review & editing, Formal analysis. Halpati Reena: Editing, Formal analysis. Vijayakumar Mannur: Formal analysis, Software implementation. Anusha Patel: Data curation and editing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was carried out in accordance with the recommendations made by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) of government of India. These recommendations ensure the welfare and maintenance of fish used in laboratory studies for biomedical research and product testing.
Consent for publication
I, Dr. Sikendra Kumar, give my consent for information about a research paper to be published in Fish Physiology and Biochemistry journal.
Competing interests
The authors affirm that they have no known financial or interpersonal conflicts that would have seemed to have an impact on the research presented in this study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prakash , P., Kumar, S., Sardar, P. et al. Optimization of weaning strategy in the climbing perch (Anabas testudineus, Bloch 1792) larvae on growth, survival, digestive, metabolic and stress responses. Fish Physiol Biochem 49, 1151–1169 (2023). https://doi.org/10.1007/s10695-023-01248-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01248-8