Abstract
Morphological development, including fin and labyrinth organ, body proportions and pigmentation, in laboratory-reared larval and juvenile climbing perch Anabas testudineus was described and behavioral features under rearing condition were observed. Body lengths (BL) of larvae and juveniles were 1.9 ± 0.1 (mean ± SD) mm just after hatching (day-0), 8.7 ± 1.3 mm on day-19, reaching 18.4 ± 2.1 mm on day-35 after hatching. Aggregate fin ray numbers attained full complements in juveniles larger than 8.3 mm BL. Preflexion larvae started feeding on day-2 following formation of the upper and lower jaws, the yolk being completely absorbed by day-7 after hatching. Teeth appeared in flexion larvae larger than 5 mm BL on day-6, with cannibalism starting shortly after and continuing with further growth. Melanophores on the body increased with growth, a large dark spot developing on the lateral midline around caudal margin of the body in the postflexion and juvenile stages. The labyrinth organ differentiated in postflexion larvae larger than 7.2 mm BL on day-16, with air-breathing starting at the same time. Body proportions attained constant in postflexion larvae larger than 7.0 mm BL, and habitat of fish shifted from bottom to mid-layer. With the exception of fin ray numbers, the above morphological developments corresponded to behavioral shifts that occurred in the postflexion stage (ca. 7 mm BL), their subsequent continuity illustrating that the species possessed most juvenile-equivalent functions from ca. 7 mm BL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The climbing perch, Anabas testudineus, a carnivorous air-breathing species using a labyrinth organ (Munshi and Hughes 1981; Olson et al. 1986; Trieu and Long 2001), is one of the most common freshwater fish species in tropical and subtropical Asia, often occurring in rice paddies, ditches and streams with dense vegetation (Rainboth 1996; Iwata et al. 2003). The species is widely distributed, including India, the Indochina Peninsula, southern China, Taiwan, the Philippines and Indonesia (Rainboth 1996; Wang et al. 1999; Kottelat 2001; Tan and Lim 2004). Although A. testudineus is an important food fish for local inland communities, the species has recently come under considerable pressure and is nearly extinct in some areas due to environmental changes (including urbanization and increasing land use for cropping) and over-fishing (Sverdrup-Jensen 2002; Mijkherjee et al. 2003). In addition, because of the impact of invading alien species (Welcomme and Vidthayanon 2003; De Silva et al. 2006) and the aquacultural development of hybrid/alien fishes (Na-Nakorn et al. 2004; Senanan et al. 2004), there is increasing concern regarding the substantial risk of loss of regional biodiversity (Nguyen and De Silva 2006). This situation has led to the necessity for aquacultural development, stock assessment and multiplication of indigenous fish species in the Indochinese region during recent years (Phillips 2002; Welcomme and Vidthayanon 2003). Anabas testudineus is one of the targeted species due to its importance as a food resource and the current status of its natural stocks.
Fundamental aquaculture trials for A. testudineus are ongoing in several countries (e.g., India and Vietnam) on the basis of recent studies on artificial breeding, seed production and nursing technologies (Trieu and Long 2001; Tuan et al. 2002; Mijkherjee et al. 2003; Sarkar et al. 2005). However, morphological and ecological features of larval and juvenile stages, as key issues for improving seed productivity as well as stock assessment, have scarcely been investigated so far, not only for this species, but also for other species of the entire genus Anabas. Furthermore, early life stage information is also necessary for any consideration of evolutionary ecology and phylogeny in the Anabantoidei, to which A. testudineus belongs. Therefore, this study aims to describe morphological development and provide behavioral information as a part of ecology for larval and juvenile A. testudineus using laboratory-reared specimens.
Materials and methods
Parental fishes and egg collection. Broodstocks of Anabas testudineus were collected from fish markets in Vientiane City, Laos, on 21 May 2007, and from small water bodies in the Namxuang area, 60 km north of Vientiane City, Laos, on 25 May 2007. The broodstocks were reared in the Living Aquatic Resources Research Center, Vientiane City, Laos. An LH-RH-analogue hormone (Suprefact: HOECHST AG Inc., Germany) was injected in combination with a dopamine inhibitor (Motilium: OLIC Ltd., Thailand) at the rate of 2 mg/100 g FW (fish wet weight) for the former and 1 mg/100 g FW for the latter, into two males (25 and 22 g body weight) and one female (50 g) at 16:30–17:00 on 14 June 2007, prior to stocking them in a tank containing 100 l water. Water temperature during the induced breeding period was 27.6–28.2°C.
Rearing larvae and juveniles. Fertilized eggs were obtained through the induced breeding process, the newly hatched larvae being separated into two rectangular aquaria (60 cm length × 45 cm width × 50 cm depth containing 120 l of water each) and reared under an ambient water temperature ranging from 27.2 to 29.1°C. From day-2 after hatching, fish were fed cultured zooplankton (Brachionus spp.) at a density of 10 individuals/ml in the rearing tanks three times a day until day-15. From days-8 to 15 inclusive, Moina spp. and Artemia nauplii were also added at a density of 2–3 individuals/ml three times a day. Thereafter, Moina spp. and fish meal were fed from day-16 until day-35.
Observations and measurements. Fertilized eggs were collected ca. 3 h after spawning, and larvae and juveniles on days-0–8, 10, 13, 16, 19, 26, 30 and 35 after hatching were preserved in 5% formalin immediately after sampling. All surviving fish were harvested and counted for survival rate estimation (%) on day-35. Observations and measurements were made in the Fisheries Division of Japan International Research Center for Agricultural Sciences, Tsukuba, Japan, and the fertilized eggs and some fish samples were registered in the Museum, Tokyo University of Marine Science and Technology (MTUF). Measurements were made on ten eggs [MTUF-P(L)-22109] and 8–12 fish from each day of sampling, totaling 128 larvae [1.7–8.3 mm in body length (BL)] and 39 juveniles (8.3–22.1 mm BL). Of these, 12 were dissected for observation of the labyrinth organ [MTUF-P(L)-16299–16310], the remainder (n = 155) being used for observations on general morphology, number of myomeres, pigmentation, fin development and the following morphometric measurements in mm: BL, head length (HL), pre-anal length (PAL), maximum body depth (BD), eye diameter (ED) and snout length (SnL), the PAL being measured from day-1 after anus opening and the others from day-0. Some specimens were stained in alizarin red S and/or alcian blue 8GX for observations of fin ray formation and jaw development. Fourteen specimens were sketched [MTUF-P(L)-16283–16296]. Egg diameters after spawning and the yolk volume (mm3) of larvae after hatching were measured. The yolk volume was calculated using the formula Volume (V) = 4/3 × Length (L) × Height (H) × Width (W). Developmental stages followed Kendall et al. (1984), except for yolksac, preflexion and flexion larval stages, since the yolk is still present at the flexion larval stage in this species. Measurement methods followed Leis and Trnski (1989).
Results
Spawning and hatching. Spawning took place approximately 12 h after hormone injection at 05:00 on 15 June 2007, ca. 14,000 fertilized eggs being obtained with a fertilization rate of 100%. Eggs were isolated, epipelagic and almost spherical in shape, the egg diameter ranging from 0.86 to 0.92 (mean ± SD: 0.89 ± 0.02) mm (n = 10). Hatching took place on the same day 10.5–11.0 h after spawning, the hatching rate approaching mostly 100%.
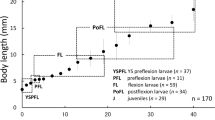
Larvae and juveniles. General morphology. The BL of newly hatched larvae (day-0) ranged from 1.7 to 2.0 (mean ± SD: 1.9 ± 0.1) mm (n = 9), reaching 6.0 ± 0.2 mm (n = 11) on day-10, 8.7 ± 1.3 mm (n = 12) on day-19, 11.4 ± 2.2 mm (n = 11) on day-26 and 18.4 ± 2.1 mm (n = 12) on day-35 (Fig. 1). BLs of larvae and juveniles at each developmental stage are shown in Table 1: Newly hatched larvae (n = 9) with large oval yolksac [vertical axis 0.66 ± 0.04 (mean ± SD) mm, horizontal axis 0.62 ± 0.04 mm]; yolksac length covering ca. 37% BL, its anterior margin bordering on ventral aspect of bent head (Fig. 2a); yolk rapidly decreasing during days-0–2 (Figs. 2a–c, 3), splitting into two portions in preflexion larvae on day-2 (3.1 ± 0.1 mm BL, n = 10); yolk moving dorsally to above abdominal cavity beside upper base of the pectoral fin (Fig. 2c), completely absorbed in flexion larvae by day-7 (5.1 ± 0.1 mm BL, n = 10; Figs. 2h, 3). The relationship between hours after hatching and yolk volume (mm3) was regressed by an exponential equation (Fig. 3). The head was initially bent with the ventral aspect bordering on the anterior margin of the yolksac in newly hatched larvae (Fig. 2a), subsequently separating from the anterior margin of the yolksac from day-2 (Fig. 2c). All myomeres were observable in larvae larger than 3.5 mm BL on day-3 (as 8–10 + 19–21 = 28–31; Fig. 2d). The ventral finfold was initially deeper than the dorsal one, originating on the anterior 35–40% BL, and the posterior half was deeper than the anterior half (Fig. 2a), divided into anterior and posterior portions of the anus in day-1 larvae (Fig. 2b), with both disappearing in juveniles (days-16–19; Fig. 2m); dorsal finfold initially originating on anterior 22–26% BL (Fig. 2a), disappearing in juveniles (days-16–19; Fig. 2m); caudal finfold initially round fan-shaped (Fig. 2a), distinctive notch in upper half of finfold near posterior tip of notochord appearing in flexion larvae larger than 4.9 mm BL (day-6; Fig. 2g), caudal finfold disappearing in juveniles (days-16–19; Fig. 2m).
Laboratory-reared larval and juvenile climbing perch Anabas testudineus. a Newly hatched larva [1.8 mm BL, MTUF-P(L)-16283]; b preflexion larva, day-1 [2.7 mm BL, MTUF-P(L)-16284]; c preflexion larva, day-2 [3.1 mm BL, MTUF-P(L)-16285]; d preflexion larva, day-3 [3.7 mm BL, MTUF-P(L)-16286]; e preflexion larva, day-4 [4.4 mm BL, MTUF-P(L)-16287]; f preflexion larva, day-5 [4.7 mm BL, MTUF-P(L)-16288]; g flexion larva, day-6 [4.9 mm BL, MTUF-P(L)-16289]; h flexion larva, day-7 [5.0 mm BL, MTUF-P(L)-16290]; i flexion larva, day-8 [5.5 mm BL, MTUF-P(L)-16291]; j flexion larva, day-10 [5.9 mm BL, MTUF-P(L)-16292]; k postflexion larva, day-13 [6.4 mm BL, MTUF-P(L)-16293]; l postflexion larva, day-16 [7.5 mm BL, MTUF-P(L)-16294]; m juvenile, day-19 [8.3 mm BL, MTUF-P(L)-16295]; n juvenile, day-26 [12.7 mm BL, MTUF-P(L)-16296]. YS Yolksac, GB gas bladder. 1 Lateral view, 2 dorsal view, 3 ventral view
Mouth and anus opened in day-1 larvae (Fig. 2b), mouth reaching a vertical through anterior ca. 30% of ED in all sizes from day-2 (Fig. 2c–n); upper and lower jaws formed in day-2 larvae (Fig. 2c) (upper jaw length 6–8% BL on day-2, 11–12% BL in flexion larvae larger than 5.4 mm BL on day-8); a few sharp, oblong conical teeth appearing on both jaws in flexion larvae larger than 5.0 mm BL (day-6), increasing in number with growth. Nostril appearing between tip of upper jaw and eye in day-2 larvae (Fig. 2c), dividing into two in juveniles (Fig. 2m), anterior nostril forming short tube and posterior one with a low rim in juveniles larger than 9.2 mm BL (day-26; Fig. 2n). Lateral line visible in day-1 larvae (Fig. 2b). Gas bladder appeared between abdominal cavity and notochord covering from upper base of pectoral fin to 5–6th myomere in day-3 larvae (3.7 ± 0.1 mm BL, n = 10; Fig. 2d), becoming invisible in juveniles (Fig. 2m).
Fin development. Dorsal fin ray anlagen appearing in flexion larvae larger than 4.9 mm BL (day-6; Fig. 2g), soft rays in flexion larvae larger than 5.9 mm BL (day-8; Figs. 2j, 4a), spines in flexion larvae larger than 6.2 mm BL (day-10), attaining full complement (XVI–XVII, 10–11) in postflexion larvae larger than 6.4 mm BL (day-13; Figs. 2k, 4a); soft ray segmentation initiated in postflexion larvae larger than 6.4 mm BL (day-13), completed (9–10 segmented rays) in juveniles larger than 14.0 mm BL (day-26). Anal fin ray anlagen appearing in flexion larvae larger than 4.9 mm BL (day-6; Fig. 2g), soft rays in flexion larvae larger than 5.9 mm BL (day-8; Figs, 2j, 4b), spines in flexion larvae larger than 6.2 mm BL (day-10), attaining full complement (IX–X, 10–11) in postflexion larvae larger than 6.4 mm BL (day-13; Figs. 2k, 4b); soft ray segmentation initiated in postflexion larvae larger than 6.4 mm BL (day-13), completed (9–10 segmented rays) in juveniles larger than 10.7 mm BL (day-26). Caudal fin ray anlagen appearing in flexion larvae larger than 4.1 mm BL (day-4; Fig. 2e), soft rays in flexion larvae larger than 4.7 mm BL (day-6; Figs. 2g, 4c), attaining full complement (9–10 + 9–10 = 19–20) in postflexion larvae larger than 6.8 mm BL (day-16; Figs. 2l, 4c); soft ray segmentation initiated in flexion larvae larger than 5.7 mm BL (day-10), completed (16 segmented rays) in postflexion larvae larger than 7.2 mm BL (day-16). Pectoral fin buds appearing in day-1 larvae (Fig. 2b), fin ray buds in flexion larvae larger than 4.8 mm BL (day-7; Fig. 2h), soft rays in postflexion larvae larger than 6.4 mm BL (day-13; Figs. 2k, 4d), attaining full complement (13–15) in juveniles (days-16–19; Figs. 2m, 4d); soft ray segmentation initiated in juveniles larger than 10.1 mm BL (day-19), completed (12–13 segmented rays) in juveniles larger than 11.3 mm BL (day-26). Pelvic fin buds appearing in flexion larvae larger than 5.4 mm BL (day-8; Fig. 2i), fin ray buds in postflexion larvae larger than 6.1 mm BL (day-13; Fig. 2k), soft rays in postflexion larvae larger than 6.6 mm BL (days-13–16; Figs. 2l, 4e), a spine in postflexion larvae larger than 7.5 mm BL (days-16–19; Fig. 2l), attaining full complement (I, 5) in postflexion larvae larger than 7.9 mm BL (days-16–19; Fig. 4e); all pelvic soft rays segmented and branched, former initiated in juveniles larger than 10.1 mm BL (day-19) and completed in juveniles larger than 14.0 mm BL (day-26), latter initiated in juveniles larger than 12.5 mm BL (day-26) and completed in juveniles larger than 16.5 mm BL (day-35). Branched rays except pelvic fin not present.
Proportions. Head length 15–25% BL in preflexion larval stage (Fig. 5a), proportion subsequently increasing with growth, reaching 39–47% BL in postflexion larvae larger than 7.0 mm BL (day-16; Fig. 5a). PAL initially 46–51% BL on day-1 (Fig. 5b), proportion rapidly decreasing to 38–47% BL with tail extension in preflexion larvae smaller than 3.2 mm BL (day-2), subsequently increasing to 68–74% BL in postflexion larvae larger than 7.0 mm BL (day-16; Fig. 5b). Maximum BD initially 32–40% BL (Fig. 5c), proportion rapidly decreasing with yolk absorption and body extension in preflexion larvae smaller than 3.7 mm BL (day-3), subsequently increasing to 38–43% BL in postflexion larvae larger than 7.0 mm BL (day-16; Fig. 5c). ED initially 10–15% BL (Fig. 5d), proportion rapidly decreasing to 8–9% with body extension in preflexion larvae smaller than 3.7 mm BL (day-3), proportion subsequently increasing to 11–14% BL in postflexion larvae larger than 6.4 mm BL (day-13; Fig. 5d). SnL 2–3% BL on day-2 (Fig. 5e), increasing to 6–8% BL in postflexion larvae larger than 7.0 mm BL (day-16; Fig. 5e).
Pigmentation. Melanophores absent on eyes in newly hatched larvae (Fig. 2a), deposition observed from day-1 (Fig. 2b). Dozens of stellate melanophores initially present on yolksac surface, particularly dense on ventral surface, decreasing in number with yolk absorption (Fig. 2a–g); small punctate melanophores covering dorsal surface of yolksac in day-1 larvae (Fig. 2b), disappearing in day-2 larvae (Fig. 2c). Small punctate melanophores initially scattered on snout (Fig. 2a), increasing in number and size with growth; several stellate or punctate melanophores appearing on upper jaw in preflexion larvae at 4.4 mm BL (day-4; Fig. 2e), on lower jaw in flexion larvae at 4.9 mm BL (day-6; Fig. 2g), increasing in number with growth. Several small stellate melanophores initially scattered on head and small punctate ones densely scattered on dorsal region of anterior body just behind head (Fig. 2a), former becoming punctate in day-1 larvae (Fig. 2b), increasing in number and size with growth. Several punctate or stellate melanophores appearing on throat in day-2 larvae (Fig. 2c), decreasing and completely lost in flexion larvae at 5.9 mm BL (day-10; Fig. 2j). Two or three stellate melanophores appearing on upper region of operculum in day-2 larvae (Fig. 2c), increasing and becoming punctate in postflexion larvae at 6.4 mm BL (day-13; Fig. 2k); several stellate melanophores appearing on circum-orbital section just behind eye in flexion larvae at 4.9 mm BL (day-6; Fig. 2g), increasing and extending below eye, and becoming punctate in postflexion larvae at 6.4 mm BL (day-13; Fig. 2k). Small dense punctate melanophores initially present on dorsal and ventral margins of body along finfold except posterior tip, particularly dense posteriorly notochord (Fig. 2a), ones on dorsal margin decreasing in number but increasing in size in day-1 larvae (Fig. 2b), becoming stellate in day-2 larvae (Fig. 2c), subsequently increasing in number with growth, becoming punctate in flexion larvae at 4.9 mm BL (day-6; Fig. 2g), covering entire dorsal region of body in postflexion larvae at 6.4 mm BL (day-13; Fig. 2k). Melanophores on ventral margin posterior to anus decreasing in number, increasing in size in day-1 larvae (Fig. 2b), stellate melanophores in addition to punctate ones appearing in day-2 larvae (Fig. 2c), both covering ventral margin along fin by juvenile stage (Fig. 2m). Melanophores on lateral body initially few (Fig. 2a), several stellate ones appearing in day-1 larvae (Fig. 2b), increasing and covering most of lateral body in flexion larvae at 5.9 mm BL (day-10; Fig. 2j), forming 7–8 horizontal bars in postflexion larvae at 6.4 mm BL (day-13; Fig. 2k), bars increasing to more than 10 and a large dark spot formed around caudal margin of body in late postflexion larvae larger than 6.9 mm BL (day-16; Fig. 2l), horizontal bars thereafter gradually fading (Fig. 2n). Small punctate melanophores on ventral region of gut just before anus appearing in day-1 larvae (Fig. 2b), becoming stellate in day-2 larvae (Fig. 2c), disappearing in flexion larvae at 5.0 mm BL (day-7; Fig. 2h), subsequently, several punctate melanophores re-appearing on the same region in postflexion larvae at 6.4 mm BL (day-13; Fig. 2k), increasing as ones on lateral body in juveniles at 8.3 mm BL (Fig. 2m), disappearing thereafter (Fig. 2n); many stellate melanophores appearing on ventral region of central trunk in day-2 larvae (Fig. 2c), fading with growth, disappearing in juveniles at 12.7 mm BL (day-26; Fig. 2n). Small dense melanophores present on dorsal surface of gas bladder in day-3 larvae (Fig. 2d), extending to ventral surface in flexion larvae at 5.0 mm BL (day-7; Fig. 2h). Several punctate melanophores appearing on caudal fin in flexion larvae at 4.9 mm BL (day-6; Fig. 2g), increasing in number with growth; dozens of punctate melanophores appearing on dorsal and anal fins in flexion larvae at 5.9 mm BL (day-10; Fig. 2j), increasing in number with growth, becoming more dense on lower half of dorsal fin in juveniles (days-19–26; Fig. 2m, n).
Labyrinth organ development. Labyrinth organ appearing in postflexion larvae larger than 7.2 mm BL (day-16) as primordial hypertrophy of upper portion of first gill arch (Fig. 6b), subsequently forming a spherical structure on that portion of gill arch in juveniles at 9.6 mm BL (day-19; Fig. 6c), and a fan-shaped membranous structure in juveniles at 12.6 mm BL (day-26; Fig. 6e) with further enlargement and structural complexity (triple-layered membranes) in juveniles larger than 21.9 mm BL (day-35; Fig. 6f, g).
Development of labyrinth organ in laboratory-reared larval and juvenile climbing perch Anabas testudineus. a Flexion larva, day-10 [5.8 mm BL, MTUF-P(L)-16299]; b postflexion larvae, day-16 [7.2 mm BL, MTUF-P(L)-16302]; c juvenile, day-19 [9.6 mm BL, MTUF-P(L)-16304]; d juvenile, day-26 [10.2 mm BL, MTUF-P(L)-16306]; e juvenile, day-26 [12.6 mm BL, MTUF-P(L)-16307]; f juvenile, day-30 [14.9 mm BL, MTUF-P(L)-16308]; g juvenile, day-35 [21.9 mm BL, MTUF-P(L)-16309]. LO Labyrinth organ, MS membranous structure(s), GR gill rakers. Bar 1 mm
Notes on behavioral aspects. Newly hatched larvae remained motionless, floating in the surface layer. On day-1 (mean ± SD: 2.6 ± 0.1 mm BL, n = 9), the majority still remained motionless in the surface layer, although some demonstrated jerking action. On day-2, larvae starting feeding, and a greater number demonstrated a jerking action for prey capture as well as escape, although still passively carried by water movements. On day-3 (3.7 ± 0.1 mm BL, n = 10), some larvae began spontaneously settling on the bottom of the aquarium, approximately 30% of larvae having settled by day-4. During days-5–7, larvae settling on the bottom increased in number, ca. 70% having settled by day-10 (6.0 ± 0.2 mm BL, n = 11). Cannibalism also started at this period, larger individuals biting the caudal portion of smaller larvae. Most prey individuals were observed dying with ragged tails. Subsequently, larvae moved to the mid-water layer from day-13 (6.4 ± 0.4 mm BL, n = 12), all having made this transition with continuing cannibalism by day-16 (7.2 ± 1.0 mm BL, n = 12). Air-breathing also started on day-16. The number of harvested fish on day-35 was 2,343, the survival rate being estimated as 16.7%. The behavioral transitions and associated morphological developments are illustrated in Fig. 7.
Discussion
Anabas testudineus has attained the juvenile stage by 8.3 mm SL (based on completion of aggregate fin ray number) (Table 1, Figs. 2m, 4). However, several morphological and associated behavioral events, the former including constancy in body proportions and differentiation of the labyrinth organ, and the latter, the beginning of air-breathing and habitat shift from bottom to mid-layer, were observed at ca. 7 mm BL, such phenomena continuing thereafter until day-35 (Figs. 5, 6, 7). These features suggested that this species possessed most juvenile-equivalent functions at ca. 7 mm BL, despite being much smaller than juvenile size.
According to Sarkar et al. (2005), Anabas testudineus collected from Punarbhaba River, West Bengal, India, spawned on average more than 100,000 eggs per female (average weight 67 g), very much more than the quantity obtained either in this study (ca. 14,000 eggs) from one female weighing 50 g or in another artificial breeding trial (ca. 42,500 eggs from five females of total weight 198 g) (S. Morioka, unpublished data). The egg diameter of 0.86–0.92 mm observed in the present study was greater than that reported by Sarkar et al. (2005) (0.60–0.84 mm). In addition, whereas Sarkar et al. (2005) reported larval length larger than 7 mm on day-5 and 22.4 mm a month after hatching, the BL (mean ± SD) observed in this study on days-5 and 30 were 4.8 ± 0.2 mm and 16.9 ± 2.3 mm, respectively (Fig. 1), despite similar water temperatures in both cases [average 28.4°C (in the present study) vs. 28.5°C]. These differing numbers and sizes of eggs, and growth rates may be attributable to inter-populational differences in two remote populations (Mekong River system in Laos vs. Punarbhaba River system in India) in reproductive features of this widely distributed species (Wang et al. 1999; Kottelat 2001; Tan and Lim 2004), although further investigations are to be made.
Yolk absorption was completed by day-7 in the present study, although it was more rapid during the initial 2 days after hatching (Fig. 3). This indicated that A. testudineus has a ca. 5-day preparatory period from days-2 (start of feeding) to 6 (just before complete yolk absorption) inclusive for the shift from endogenous to exogenous energy dependent periods (under the experimental temperature regime during this study). The shift from endogenous to exogenous energy-dependent periods has been examined in several species of marine fishes, covering aspects of development and survival during the larval stages (Kohno 1998; Moteki et al. 2001). However, similar investigations of freshwater fishes, including A. testudineus, are very few, despite being necessary for any improvement in seed productivity. In addition, the period between spawning to hatching and the duration of yolk absorption were considered the essential features in order to examine phylogenetical aspects in the Anabantoidei group since this fish group has long been under discussion concerning phylogenetics and evolutionary species diversification (Rüber et al. 2006).
The appearance and development of teeth and advanced swimming ability, indicated by notochord flexion and caudal fin development, were considered the essential factors in the manifestation of cannibalism in this species, such occurring after or during the processes of these morphological changes (Fig. 7). Although cannibalism-induced mortality was not monitored in this study, the survival rate obtained on day-35 (16.7%) was possibly lowered by cannibalism, considering the cannibalism-related survival losses recorded for other species, such as Clarias gariepinus of Clariidae (Hecht and Applebaum 1988) and Scomberomorus niphonius of Scombridae (Obata 2006). Previous studies have reported factors leading to cannibalism, including the high stocking densities and large size variations, as well as refuge availability and lighting conditions (Giles et al. 1986; Hecht and Applebaum 1988; Smith and Reay 1991; Hseu et al. 2003). Therefore, these factors need to be examined for A. testudineus so as to reduce the incidence of cannibalism and thus improve seed productivity.
Although the function and morphology of the labyrinth organ, and associated ecological aspects (Munshi et al. 1986; Olson et al. 1986) have been considered in adult A. testudineus as well as in other species of Anabantidae, e.g., Ctenopoma muriei (see Randle and Chapman 2004, 2005), ontogeny and developmental morphology in larval and juvenile stages have been limited to date. The differentiation of the labyrinth organ observed in this study coincided with the onset of air-breathing (Figs. 6, 7), a finding mostly concurring with that of Hughes et al. (1986), who reported that the labyrinth organ started functioning from the juvenile stage in this species. Additionally, in the air-breathing species Macropodus opercularis of Osphronemidae, a description of the developmental morphology of the labyrinth organ showed similarly that the occurrence of air-breathing followed the onset of primordial hypertrophy of the first gill arch and continued with the subsequent enlargement of the organ (Matayoshi and Shokita 1982). Because A. testudineus is an obligate air-breather (Sayer 2005), it is highly resistant to low levels of dissolved oxygen in the surrounding water. This characteristic suggests a considerable aptitude of the species for seed production and aquaculture under high stocking densities, although some treatments for reducing the cannibalism incidence are to be applied. In addition, organogenesis of the labyrinth organ in all species included in Anabantoidei should be necessarily studied in future investigations on phylogeny and evolutionary ecology, the latter being somewhat contentious issues in this group (Randle and Chapman 2004; Sayer 2005; Rüber et al. 2006).
Apart from the acquacultural viewpoint, the information on morphology and ecology during the early life stages is indispensable, particularly for investigating mechanisms of survival and recruitment of the species. Additionally, sympatric anabantoid species exhibiting similar larval and juvenile morphology should be similarly studied to enable more effective species identifications and stock assessment.
References
De Silva SS, Nguyen TTT, Abery NW, Amarasinghe US (2006) An evaluation of the role and impacts of alien finfish in Asian inland aquaculture. Aquacul Res 37:1–17
Giles N, Wright RM, Nord ME (1986) Cannibalism in pike fry. Esox lucius L.: some experiments with fry densities. J Fish Biol 29:107–113
Hecht T, Applebaum S (1988) Observations on intraspecific aggression and coeval sibling cannibalism by larval and juvenile Clarias gariepinus (Clariidae: Pisces) under controlled conditions. J Zool 214:21–44
Hseu JR, Chang HF, Ting YY (2003) Morphometric prediction of cannibalism in larviculture of orange-spotted grouper, Epinephelus coioides. Aquaculture 218:203–207
Hughes GM, Munshi JSD, Ojha J (1986) Post-embryonic development of water- and air-breathing organs of Anabas testudineus (Bloch). J Fish Biol 29:443–450
Iwata A, Ohnishi N, Kiguchi Y (2003) Habitat use of fishes and fishing activity in plain area of southern Laos. Asian Afr Area Stud 3:51–86
Kendall AW Jr, Ahlstrom EH, Moser HG (1984) Early life history stages of fishes and their characters. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richardson SL (eds) Ontogeny and systematics of fishes, Am Soc Ichthyol Herpetol Spec Publ No 1. Allen Press, Lawrence, pp 11–22
Kohno H (1998) Early life history features influencing larval survival of cultivated tropical finfish. In: De Silva SS (ed) Tropical mariculture. Academic Press, London, pp 71–111
Kottelat M (2001) Fishes of Laos. WHT Publications Ltd, Colombo
Leis JM, Trnski T (1989) The larvae of Indo-Pacific shorefishes. NSW University Press, Kensington
Matayoshi S, Shokita S (1982) Development of air-breathing organ in Paradicefish, Macropodus opercularis (Linnaeus) from Okinawa-jima, the Ryukyus. Bull Coll Sci Univ Ryukyus 33:61–67
Mijkherjee M, Praharaj A, Das S (2003) Conservation of endangered fish stocks through artificial propagation and larval rearing techniques in West Bengal, India. Aquaculture Asia 8:8–11
Moteki S, Yoseda K, Sahin T, Üstündağ C, Kohno H (2001) Transition from endogenous to exogenous nutritional sources in larval Black Sea turbot Psetta maxima. Fish Sci 67:571–578
Munshi JSD, Hughes GM (1981) Gross and fine structure of the pseudobranch of the climbing perch, Anabas testudineus (Bloch). J Fish Biol 19:427–438
Munshi JSD, Olson KR, Ojha J, Ghosh TK (1986) Morphology and vascular anatomy of the accessory respiratory organs of the air-breathing climbing perch, Anabas testudineus (Bloch). Amer J Anat 176:321–331
Na-Nakorn U, Kamonrat W, Ngamsiri T (2004) Genetic diversity of walking catfish, Clarias macrocephalus, in Thailand and evidence of genetic introgression from introduced farmed C. gariepinus. Aquaculture 240:145–163
Nguyen TTT, De Silva SS (2006) Freshwater finfish biodiversity and conservation: an Asian perspective. Biodiver Conserv 15:3543–3568
Obata Y (2006) Stock enhancement of Japanese Spanish mackerel Scomberomorus niphonius in the eastern Seto Inland Sea, Japan. Nippon Suisan Gakkaishi 72:459–462
Olson KR, Munshi JSD, Ghosh TK, Ojha J (1986) Gill microcirculation of the air-breathing climbing perch, Anabas testudineus (Bloch): relationships with the accessory respiratory organs and systematic circulation. Am J Anat 176:305–320
Phillips MJ (2002) Fresh water aquaculture in the Lower Mekong Basin. MRC Technical Paper No. 7, Mekong River Commission, Phnom Penh
Rainboth WJ (1996) Fishes of the Cambodian Mekong. FAO Species Identification Field Guide for Fishery Purposes. FAO, Rome
Randle AM, Chapman LJ (2004) Habitat use by the African anabantid fish Ctenopoma muriei: implications for costs of air breathing. Ecol Freshw Fish 13:37–45
Randle AM, Chapman LJ (2005) Air-breathing behaviour of the African anabantoid fish Ctenopoma muriei. J Fish Biol 67:292–298
Rüber L, Britz R, Zardoya R (2006) Molecular phylogenetics and evolutionary diversification of labyrinth fishes (Perciformes: Anabantoidei). Syst Biol 55:374–397
Sarkar UK, Deepak PK, Kapoor D, Negi RS, Paul SK, Singh S (2005) Captive breeding of climbing perch Anabas testudineus (Bloch, 1792) with Wova-FH for conservation and aquaculture. Aquacul Res 36:941–945
Sayer MDJ (2005) Adaptations of amphibious fish for surviving life out of water. Fish Fish 6:186–211
Senanan W, Kapuscinski AR, Na-Nakorn U, Miller LM (2004) Genetic impacts of hybrid catfish farming (Clarias macrocephalus × C. gariepinus) on native catfish populations in central Thailand. Aquaculture 235:167–184
Smith C, Reay P (1991) Cannibalism in teleost fish. Rev Fish Fish 1:41–64
Sverdrup-Jensen S (2002) Fisheries in the lower Mekong basin: status and perspectives. MRC Technical Paper No. 6, Mekong River Commission, Phnom Penh
Tan HH, Lim KKP (2004) Inland fishes from the Anambas and Natuna Islands, South China Sea, with description of a new species of Betta (Teleostei: Osphronemidae). Raffles Bull Zool Suppl 11:107–115
Trieu NV, Long DN (2001) Seed production technology of climbing perch (Anabas testudineus): a study of the larval rearing. In: Xuan VT, Buu BC, Hidaka T, Kobayashi H, Yamada R, Wilder MC, Yamasaki S, Watanabe T (eds) Proceedings of the 2001 annual workshop of JIRCAS Mekong Delta Project. Cantho University, Cantho, pp 199–202
Tuan NA, Hanh HM, Lan LM, Long DN, Tam DH, Lanh NV, Thanh LTN (2002) Preliminary results on rearing climbing perch (Anabas testudineus) in concrete tanks and earthen ponds. In: Xuan VT, Buu BC, Tsurumi K, Dung LV, Chau NM, Miyata S (eds) Proceedings of the 2002 annual workshop of JIRCAS Mekong Delta Project. Cantho University, Cantho, pp 227–230
Wang TY, Tzeng CS, Shen SC (1999) Conservation and phylogeography of Taiwan paradise fish, Macropodus opercularis Linnaeus. Acta Zool Taiwan 10:121–134
Welcomme RL, Vidthayanon C (2003) The impact of introductions and stocking of exotic species in the Mekong basin and policies for their control. Technical Paper No. 9, Mekong River Commission, Phnom Penh
Acknowledgments
We express our sincere gratitude to K. Sano, Laboratory of Global Fisheries Science, University of Tokyo, and the staff of the Aquaculture Improvement and Extension Project, Japan International Cooperation Agency, for their kind assistance with broodstock collection and to L. Khamsivilay, Director of the Living Aquatic Resources Research Center, Laos, for his logistical support. Our thanks also go to G. Hardy for his polite and constructive English revision.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Morioka, S., Ito, S., Kitamura, S. et al. Growth and morphological development of laboratory-reared larval and juvenile climbing perch Anabas testudineus . Ichthyol Res 56, 162–171 (2009). https://doi.org/10.1007/s10228-008-0081-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-008-0081-y