Abstract
The first days of exogenous feeding are crucial for larval recruitment. A correct first prey item and the determination of the optimal weaning age, can reduce labor cost and fish mortality to a great extent. This study was conducted to evaluate the use of small and regular Artemia fransiscana (AF and EG Artemia, Inve, Belgium) as first feed for jade perch Scortum barcoo, and to determine the minimum required duration of this life feed phase before transition to dry feed can occur. Therefore, we compared first feeding of 3 days AF nauplii instar I with 3 days of EG nauplii instar I and evaluated whether the optimal weaning age for jade perch larvae was at 7, 10, 13 or 16 days post-hatching (DPH). The study was performed in 25-L tanks in a recirculating system and lasted for 21 days (4–24 DPH). Growth, survival and tissue fatty acid composition of the larvae in the different treatments were analyzed. Results indicate that, after the start of exogenous feeding at 4 days DPH, jade perch larvae require a minimum of 9 days of live feed until 12 DPH. Co-feeding ideally starts on 10 DPH. Larvae fed AF Artemia for the first 3 days showed a significantly faster growth than larvae fed EG Artemia, although their survival was lower. Gape width of larvae at 3 and 6 DPH was measured, and implications for prey size are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there is an increasing interest for species diversification to support the development of sustainable aquaculture. A promising candidate for fresh and brackish water aquaculture is the fast-growing Australian jade perch Scortum barcoo, which can be stocked at high densities in recirculating aquaculture systems (RAS) and feeds on grow-out diets with very low levels of fishmeal and fish oil (Van Hoestenberghe et al. 2013). Although some aspects of its rearing cycle have been studied (Song et al. 2009; Gang et al. 2010; Van Hoestenberghe et al. 2013), information on larval rearing techniques remains scant. Due to their very small size and an initially poorly developed digestive system, larval rearing is for most fish species considered as the main bottleneck for successful cultivation. An appropriate larval feeding strategy is essential for developing a rearing protocol that ensures a reliable production of fry. The transition from yolk sac absorption to exogenous feed and the switch from live feed to dry feed are crucial phases (Baskerville-Bridges and Kling 2000; Sorgeloos et al. 2001). For most species, live feed (mostly rotifers Brachionus spp. and brine shrimp Artemia spp.) is applied as first exogenous feed as it stimulates larval feeding activity through their erratic swimming behavior, and the release of metabolic wastes and chemicals (Kolkovski et al. 1997a, b). The choice of which live feed should be applied is mainly based on the gape size of the larvae when exogenous feeding starts (Yufera and Darias 2007). The classic feeding sequence for fish larvae is to start with (1) rotifers, followed by (2) freshly hatched instar I Artemia nauplii and (3) instar II Artemia enriched with specific lipids and vitamins (Zambonino Infante and Cahu 1994; Dhert et al. 2001; Sorgeloos et al. 2001) before a gradual transition to dry feeds occurs. Live feed production is time-consuming, labor-intensive and subject to variability in quality as compared to formulated feeds which have a more stable quality and are readily available “off-the-shelf.” The switch from live to dry feed is described as the weaning phase. In function of the species and specific rearing conditions, there are many deviations from this generalized scheme. For some authors, it is absolutely necessary to first feed European sea bass Dicentrachus labrax with rotifers for a prolonged period before Artemia can be applied (Barnabe and Guissi 1994), while for others, it is possible to skip the rotifer period (Coves et al. 1991). Also in gilthead sea bream Sparus aurata, the period that rotifers should be applied before switching to Artemia, varies among authors (Fernandez-Diaz and Yufera 1997). This has also been verified for Cobia Rachycentron canadum larvae where the rotifer period could be shortened by 3 days when smaller AF Artemia type Vietnam strain are used (Nhu et al. 2009). Reducing the rotifer application time in European sea bass and sea bream is referred to as the “French technique,” where smaller sized Artemia are used to (partly) replace rotifers. Based on previous experiences, jade perch larvae clearly require at least some days of live feed before dry feeds can be applied, since nearly all larvae died when access to live feed was denied.
Since jade perch larvae are similar in size to European sea bass, we want to evaluate in this study, whether the smaller sized AF Artemia (195 × 499 µm) (Inve, Belgium) can improve larval performance as compared to first feeding with the larger (270 × 540 µm) Great Salt Lake A. fransiscana strain (EG Artemia, USA, Inve, Belgium). In addition, we want to determine the optimal weaning age, as a too short or too long period of live feed application can lead to poor larval growth (Person Le Ruyet et al. 1993; Holt 1993; Leu et al. 1991) and cost effectiveness.
Materials and methods
Experimental design
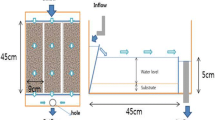
The experiment was performed at the Laboratory of Aquaculture & Artemia Reference Center (Ghent University, Belgium) following a randomized block design. Four independent recirculation units consisted each of six 25-L rectangular PVC tanks, a mechanical filter, a biological filter and a 100-L reservoir tank equipped with aeration, a heating element and submersed pump. Tap water was mixed with natural seawater to maintain salinity at 5 g L−1, and the pH was kept at 7.8–8.2. Water temperature, total ammonia nitrogen and nitrite-N were measured daily and maintained at, respectively 28 ± 1 °C, <0.5 and <0.5 mg L−1.
Water was delivered to each system through a constant head, and the water flow to each tank was regulated by a perforated cap, screwed to the individual water supply pipes and set at a constant water flow of 1 L min−1. Tank outlets were fitted with screens of different mesh sizes in function of the age of the larvae in order to retain larvae and live feed in the tank. The tank effluent was directed to a mechanical filter and a moving bed biological filter filled with plastic beads (type Kaldness), before entering the stock tank from where it was pumped back to the rearing tanks. Feces and dead larvae were siphoned out daily. Approximately 20 % of the total water volume in each block was changed daily. Photoperiod was set at 12-h light: 12-h dark with an illumination of 340 lux at the water surface through TL lights.
The larvae were purchased from a commercial hatchery in Queensland, Australia (transit time 46 h), and arrived 2-day post-hatching (DPH) when the larval yolk sac was almost resorbed. The larvae were stocked in a large buffer tank for 1 day to acclimatize to the laboratory conditions, and next day, they were distributed at 300 larvae per rearing tank (12 larvae L−1). The yolk sac was completely resorbed at the beginning of the first day of the trial (DPH 4). One replicate of each treatment was randomly assigned to one of the tanks in each recirculation unit, so six different feeding treatments with four replicates per treatment were compared (Table 1). The first 3 days larvae were either fed AF before switching to EG (treatments AF3EG9, AF3EG6 and AF3EG3) or fed only EG (treatments EG12, EG9 and EG6) Artemia nauplii instar I. From 7 DPH, only EG Artemia were used, either as freshly hatched nauplii or as enriched metanauplii. Depending on the treatment, larvae were then weaned on 7 (AF3EG3, EG6), 10 (AF3EG6, EG9) or 13 DPH (AF3EG9, EG12). The switch from live feed to dry feed (the weaning) was done gradually by introducing a 3-day co-feeding period of enriched EG Artemia with a dry feed, Orange 2/3 micro diet (200–300 µm) (Inve Aquaculture NV, Baasrode, Belgium). From 16 to 24 DPH, only Orange dry feed were used in all treatments, and shift in feed size (Orange 2/3 to Orange 3/5, 300–500 µm) was induced by fish size.
Feeds and feeding
Nauplii of AF Artemia Vietnam strain and EG 240 Salt Lake A. fransiscana (Inve Aquaculture NV, Baasrode, Belgium) were hatched as described by Van Stappen (1996). In brief, cysts were incubated at 2 g L−1 in 10-L-conical PVC tanks filled with natural seawater. Water temperature was maintained at 29 °C by a 100-W submersed heater, and continuous illumination and strong aeration were provided. Nauplii were harvested after 20 h on a 150-µm sieve, rinsed thoroughly with fresh seawater and either fed immediately to the fish larvae or concentrated and stored at 4 °C to be used later that day. To prepare enriched EG Artemia nauplii, the EG cysts were hatched and nauplii harvested as described above and subsequently resuspended in clean seawater at a density of 200 mL−1. The enrichment product (Easy DHA Selco, Inve, Belgium) was added in a single dose of 0.6 g L−1. Sanocare Hatch Controller (Inve, Belgium) was added at 100 mg L−1 in order to prevent bacterial proliferation. Temperature was similar as for hatching and moderate aeration was provided. Enriched nauplii were harvested after 24 h, rinsed thoroughly to remove all enrichment medium and either fed immediately or kept in cold storage as described above.
Artemia were supplied to the larvae twice a day at 3 Artemia mL−1 culture water when only Artemia were fed, and at 1.5 Artemia mL−1 when co-feeding occurred. The dry feed Orange was supplied 5 times a day at a total of 50 % of larval biomass day−1 when co-feeding with Artemia and 100 % of larval biomass day−1 when larvae were completely weaned to dry feed.
Sampling and measurements
Just before the start of the trial (DPH3), 150 larvae were sampled from the pooling group before larvae were distributed to the rearing tanks. Each 3 days (DHP6, DPH9, DPH12, DPH15, DPH18 and DPH24,) a minimum of 5 larvae were randomly sampled from each tank for measurement of wet weight (WW), dry weight (DW) and length. In total, from each tank, 34 larvae were sampled. At the end, larvae were also sampled for fatty acid (FA) analysis. The WW and DW of five fish from each tank were measured. The WW was determined on a Sartorius BP221S weighing scale, while DW was measured after putting the fish in an oven (Heraeus Instruments T 12) for 4 h at 114 °C. Survival % was calculated as the number of surviving larvae divided by the number of larvae initially stocked minus the number of larvae sampled (n = 34 for each tank). Specific growth rate was calculated as SGR (%d −1) = [(ln W 2−lnW 1)/(t 2−t 1)] × 100 where W 2 and W 1 are body wet weights (g) at time t 1 and t 2 (t 1 being the first day of the experiment and t2 the last day). For length measurement, larvae were deeply anesthetized using excessive (200 mg L−1) tricaine methane sulfonate (MS-222, Sigma-Aldrich, St Louis, MO, USA) and immediately fixated in Bouin solution with 1:20 (v:v) glacial acetic acid. After 24 h, specimens were transferred to 70 % ethanol to minimize the loss of bone mineral (Green and McCormick 2001). Standard length (SL) of the larvae was measured with a Olympus SZX7 stereomicroscope and Cell D software (Soft Imaging System, Olympus NV). The SL was determined as the length of a straight line between the tip of the snout and the most caudal point of the notochord. If the larva was bended, the length of the line from the tip of the snout to the bending point was added to the length of the line from this bending point to the most caudal point of the notochord. Strongly curved or badly preserved larvae were excluded from the measurements. As a result, 3–12 larvae per tank were measured. The gape width (GW, distance between the articular-quadrate joints) of 13 specimens of the initial larvae group (3 DPH) and three specimens of 6 DPH larvae was measured to the nearest µm in order to estimate the correlation between GW and prey size. The specimens used for determination of GW exhibited a mouth opening between 9 and 47° as is determined by the angle of the upper lip opposed to the lower lip.
Fatty acid composition
The larvae, Artemia and dry feed were subjected to direct transesterification (FAME analysis) without prior lipid extraction or purification (Lepage and Roy 1984). To determine the absolute quantity of the FA, 11,14 eicosadienoic acid (20:2 n-6, Nu Check prep. Inc) was added as an internal standard. Samples were homogenized and weighed prior to addition of chemicals and the internal standard (5 mg). The samples were diluted in solvents with methanolic acetyl chloride (20/1 methanol/HCl) for transmethylation of the FA. After 1 h incubation at 100 °C, samples were diluted with water and extracted thrice with hexane (+butyl hydroxy toluene). This hexane fraction was dried with anhydrous sodium sulfate and after isolation of the residue, and the hexane fraction was completely flushed with nitrogen and washed several times with 5 ml of hexane. The solvents were evaporated on a rotary evaporator at 35 °C and flushed to dryness with nitrogen. The dried FA methyl esters were dissolved in 0.5 ml iso-octane, and 0.25 µl of the dilution was injected on the gas chromatograph (Chrompack CP9001) equipped with a CP9010 liquid auto sampler and a temperature programmable on-column injector. The capillary column (BPX70, 50 m × 0.32 mm and film thickness 0.25 µm) was connected to a 2.5-m-long methyl deactivated pre-column with detection mode FID. A/D conversion of the FID signal and subsequent data capture to a PC was done with an Agilent 35900E A/D converter. Integration and calculations were performed on a MS Windows-based computer using Agilent GC Chemstation Rev.B.02.01.
Statistical analysis
Significant differences between larval weight, length, mortality and FA profile were determined by performing one-way analysis of variance (ANOVA) followed by a Tukey’s HSD multiple comparison of means. This includes the Levene’s test to check for homogeneity of variance. Statistical significance was accepted at a probability value of 5 % or less.
Results and discussion
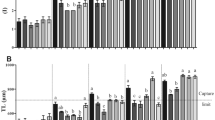
When their yolk sac is completely resorbed (4 DPH), jade perch larvae are small [0.43 ± 0.03 mg WW (Fig. 1); SL 3.85 ± 0.20 mm (Table 2)] compared to other freshwater fish like Macquarie perch Macquaria australasica (3.9 mg WW at 7 DPH) (Sheikh-Eldin et al. 1997), pikeperch Sander lucioperca (6.1 mm TL at 7 DPH) (Ostaszewska 2005), carp Cyprinus carpio (6.5 mm WW at 2 DPH) (Escaffre et al. 1997), Pangasius boucourti (3.7 mg WW at 2 DPH) (Hung et al. 2002) and African catfish Clarias gariepinus (3.5 mg WW at 2 DPH) (Chepkirui-boit et al. 2011). Jade perch larvae are similar in size to the marine European sea bass larvae (4–4.5 mm SL, Rekecki et al. 2009) but larger than most marine percids.
Based on the current results, it is clear that an Artemia supply of only 6 days (AF3EG3 and EG6) is not long enough to sustain optimal larval growth. At DPH 12, most larvae of these treatments were emaciated, which is an obvious sign of starvation, and nearly all larvae in these treatments died by 15 DPH (Fig. 2). When Artemia were supplied for at least 9 days (AF3EG9, AF3EG6, EG12 and EG9), larvae started to grow extremely fast from DPH 12 onwards. The best DW and WW gain over 21 days was obtained for treatment AF3EG9 with a total feeding of 12 days Artemia of which AF Artemia for the first 3 days. In contrast, when only EG Artemia were used, there was no difference in weight gain between larvae that received Artemia for 12 or 9 days (EG12 and EG9).
At 9 DPH, larvae that had been fed 3 days of Artemia and subsequently 3 days of EG Artemia nauplii instar I or 3 days of enriched Artemia had a similar WW. Since at this age there was no immediate effect on weight gain at DPH 9 between these treatments, we may conclude that there is no need to feed enriched Artemia for the first 6 days of exogenous feeding.
The trends in WW were also reflected in the DW (Table 3) and length of the larvae (Table 2). The SGR over 21 days for treatments AF3EG9, AF3EG6, EG12 and EG9 were between 24 and 27 % per day, which is extremely fast compared to most fish (Kestemont et al. 2007; Süzer et al. 2011; Andrade et al. 2012). Larvae of AF3EG9 weighed 140 mg at the end of the trial (24 DPH, Fig. 1).
Taking into account the extremely fast growth in the first days of exogenous feeding, it can be concluded that jade perch larvae can be weaned onto dry feeds at 12 DPH (10 mg WW and 7 mm SL) although co-feeding another 3 days with Artemia until 15 DPH (ca. 27 mg WW and 10 mm SL) is recommended. Co-feeding is useful as the metabolic products of the live feed are thought to stimulate uptake and ingestion of the dry diets (Hart and Purser 1996; Kolkovski et al. 1997b, c; Daniels and Hodson 1999; Koven et al. 2001; Cahu and Zambonino Infante 2001). The proposed weaning strategy is comparable with the strategy used for African catfish, which can be weaned after 8 days of Artemia feeding, at a WW of 13–14 mg (Chepkirui-boit et al. 2011). Although jade perch larvae are at the start of exogenous feeding smaller than other freshwater perches, their fast growth enables them to be weaned at a younger age then pike perch (8.1 mg at 19 DPH) (Kestemont et al. 2007) or Macquarie perch (9.3 mg at 22 DPH) (Sheikh-Eldin et al. 1997). By comparison, salt water perciformes such as black sea bass, European sea bass, sea bream and red porgy can only be weaned successfully at, respectively, 18–25 DPH (Bradley et al. 2000), 20–25 DPH (3.4 mg) (Cahu and Zambonino Infante 1994), 25 DPH (5.97 mm) (Süzer et al. 2011) and 20 DPH (Andrade et al. 2012). Although some jade perch larvae survived weaning at DPH 7 (five specimens from AF3EG3 and three specimens from EG6), these larvae were much smaller (10 mm) and had an extremely low survival rate at 24 DPH. It is therefore demonstrated that jade perch do require a certain period of live prey before dry feeds should be applied. To our knowledge, only commercial species from the tilapia family and common carp can be reared successfully without the use of live feeds (Escaffre et al. 1997).
In general, survival was low in all treatments (Fig. 2) with the best survival in EG12 (9.1 ± 4.8 %) and EG9 (8.6 ± 3.9 %). In these treatments, mortality occurred principally in the first 3 days (DPH 4–6) and to a lesser extent from DPH 7–9. From DPH 10 onwards, we noticed almost no mortality. When Artemia exposure was more than 6 days, survival was significantly higher in the treatments that received only EG Artemia and no AF Artemia. This was surprising since instar I AF Artemia are richer in EPA than EG Artemia. But EG Artemia are significantly bigger than AF Artemia and therefore contain more energy per specimen compared to AF Artemia. The hunt for an Artemia specimen, whether it is EG or AF, requires an equal amount of energy. Therefore, a swallowed EG Artemia could have resulted in a higher netto energy uptake compared to an AF Artemia, thereby leading to better survival.
Cox and Pankhurst (2000) described that a prior learning experience with prey can affect larval performance. Although their work concerned the switch from rotifers to Artemia, it could help to clarify the higher mortality that occurred in larvae that had to switch from AF to EG Artemia. The external morphological differences between AF and EG Artemia are small, but, nevertheless, larvae fed continuously EG Artemia could have benefited in DPH 7–9 from their prior experience with EG Artemia during DPH 4–6. The GW of the larvae at the start (3 DPH) was 321 ± 65 µm and of larvae at 6 DPH, 352 ± 103 µm. With EG Artemia measuring 270 µm in width, the prey size/GW ratio is, respectively, 84 % at start of exogenous feeding and 77 % after 3 days of exogenous feeding. For AF Artemia (195 µm width), the prey size/GW values were 61 and 55 %, respectively. Different authors state that the most appropriate prey/gape ratio is 25–50 % (Shirota 1970; Bremigan and Stein 1994; Fernández-Díaz et al. 1994; Østergaard et al. 2005). However, the higher survival of larvae fed EG compared to larvae fed AF Artemia, indicate that this most appropriate ratio may not be valid for jade perch. Therefore, a too large prey size at initial feeding may not have been the cause for low survival in the treatments where Artemia exposure was minimum 9 days. There was visual proof that larvae at 6 DPH contained good amounts of EG Artemia nauplii in their stomach (Fig. 3, 6 DPH). On the other hand, since mortality mainly occurred in the first 3 days of the trial, the overall low survival rate could be related to the stress of transport from Australia (46 h). This could have led to less vital larvae that lacked energy for swimming and predation. A relatively short starvation period during transport may further induce feeding and digestive problems that can seriously affect the surviving larvae beyond a point of no return (Gwak and Tanaka 2001; Dou et al. 2002). However, it cannot be excluded that jade perch larvae may benefit from much smaller prey like rotifers in the first 3–6 days of exogenous feeding and that a selection took place favoring the strongest larvae that were capable of gulping down Artemia, while the weaker larvae died. A further study on the use of rotifers as adequate first feeding (DPH 4–9) of jade perch is therefore advised.
There was no difference in FA composition between the larvae of different treatments (Table 4). As expected, the FA composition of the larvae was similar to the FA composition of the feeds, especially the Orange micro diet. This is similar to previous findings on the FA composition of juvenile jade perch (Van Hoestenberghe et al. 2013). Only n-6 HUFA were present at a much higher concentration in the larvae compared to the dry feed. What is remarkable, is that both n-3/n-6 and DHA/EPA ratios of the larvae were higher than the respective ratios in the dry feed, which indicates that jade perch larvae have a preference to store n-3 FA and DHA over, respectively, n-6 FA and EPA. Being a precursor for eicosanoids that assist in immune reactions, EPA may be well used to construct these hormones that are needed in the development of the immune system of young animals. Another possible explanation for the reduction of EPA is the elongation of EPA to DHA, since high amounts of DHA are present in the larvae. The lower survival of larvae fed EPA-rich AF Artemia as first feed can furthermore indicate that high inclusions of EPA in the larval diet for jade perch are not necessary, or can even be harmful, which is also valid for the larvae of the Eurasian perch Perca fluviatilis (Abi-Ayad et al. 2000). In addition, the fact that the enrichment of EG in the first 6 days of larval feeding did not have an immediate effect on larval performance, indicates that in the first feeding days, only a small inclusion of HUFA is sufficient to sustain optimal growth, as has been demonstrated for juvenile jade perch (Van Hoestenberghe et al. 2013). These low larval dietary HUFA requirements are comparable to those of African catfish (Verreth et al. 1994) and Tilapia (Isik et al. 1999) larvae that, respectively, require low or no amounts of dietary HUFA. In contrary, Eurasian perch (Abi-ayad et al. 2000), trout Oncorhynchus mykiss (Wirth et al. 1997) and anadromous fish such as striped bass morone saxatilis (Webster and Lovell 1990) and salmonids (Sargent et al. 1989) require substantial amounts of n-3 HUFA in their larval diet. Furthermore, the DHA/EPA ratio of jade perch larvae is similar to the ratio in pikeperch larvae (4.5) that were fed EG Artemia enriched with Easy DHA Selco and subsequently Orange (Sserwadda 2012), indicating a similar importance in storing DHA over EPA in both freshwater percids.
In conclusion, jade perch larvae grow very fast when fed with Artemia nauplii but are at the start of exogenous feeding more comparable in size to marine rather than to freshwater percid larvae. Life feed should be applied for a minimum of 9 days (until DPH 12), and the best results were obtained when life feed was applied for 12 days (until DPH 15). It is hypothesized that no rotifers are necessary as a start feed since the use of smaller AF Artemia reduced larval performance, although the possibility of selection for the bigger specimens in the first days may not be excluded. Further research on larval rearing of jade perch should focus on improving survival during the first days of exogenous feeding.
References
Abi-ayad SMEA, Kestemont P, Mélard C (2000) Dynamics of total lipids and fatty acids during embryogenesis and larval development of Eurasian perch (Perca fluviatilis). Fish Physiol Biochem 23:233–243
Andrade CAP, Nascimento F, Conceicao LEC, Linares F, Lacuisse M, Dinis MT (2012) Red porgy, Pagrus pagrus, larvae performance and nutritional condition in response to different weaning regimes. J World Aquacult Soc 43:321–334
Barnabe G, Guissi A (1994) Adaptation of the feeding behaviour of larvae of the sea bass, Dicentrarchus labrax, to an alternating live food/compound-food feeding regime. Aquacult Fish Manag 25:537–546
Baskerville-Bridges B, Kling LJ (2000) Early weaning of Atlantic cod (Gadus morhua) larvae onto a microparticulate diet. Aquaculture 189:109–117
Bradley TM, Nardi G, Berlinsky D, Watson M (2000) Investigations of selected parameters for growth of larval and juvenile Black sea bass Centropristis striata L. J world Aquacult Soc 31:426–435
Bremigan MT, Stein RA (1994) Gape-dependant larval foraging and zooplankton size: implications for fish recruitment across systems. Can J Fish Aquat Sci 51:913–922
Cahu CL, Zambonino Infante JL (1994) Early weaning of sea bass Dicentrarchus labrax. larvae with a compound diet: effect on digestive enzymes. Comp Biochem Physiol 109:213–222
Cahu CL, Zambonino Infante JL (2001) Substitution of live food by formulated in marine fish larvae. Aquaculture 200:161–180
Chepkirui-Boit V, Ngugi CC, Bowman J, Oyoo-Okoth E, Rasowo J, Mugo-Bundi J, Cherop L (2011) Growth performance, survival, feed utilization and nutrient utilization of African catfish (Clarias gariepinus) larvae co-fed Artemia and a micro-diet containing freshwater atyid shrimp (Caridina nilotica) during weaning. Aquacult Nutr 17:82–89
Coves D, Demavrin G, Breuil G, Devauchelle N (1991) Culture of sea bass (Dicentrarchus labrax). In: Mcvey JP (ed) CRC handbook of mariculture and finfish aquaculture, vol II., CRC PressBoca Raton, USA, pp 3–20
Cox ES, Pankhurst PM (2000) Feeding behavior of greenback flounder larvae Rhombosolea taparina (Günther) with different exposure histories to live prey. Aquaculture 183:285–297
Daniels HV, Hodson RG (1999) Weaning success of southern flounder juveniles: effects of changeover period and diet type on growth and survival. N Am J Aquacult 61:47–50
Dhert P, Rombout G, Suantika G, Sorgeloos P (2001) Advancement of rotifer culture and manipulation techniques in Europe. Aquaculture 200:129–146
Dou SZ, Masuda R, Tanaka M, Tsukamoto K (2002) Feeding resumption, morphological changes and mortality during starvation in Japanese flounder larvae. J Fish Biol 60:1361–1380
Escaffre AM, Zambonino Infante JL, Cahu CL, Mambrini M, Bergot P, Kaushik SJ (1997) Nutritional value of soy protein concentrate for larvae of common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Aquaculture 153:63–80
Fernandez-Diaz C, Yufera M (1997) Detecting growth in gilthead seabream Sparus aurata L. larvae fed microcapsules. Aquaculture 153:93–102
Fernández-Díaz C, Pascual E, Yúfera M (1994) Feeding behaviour and prey size selection of gilthead seabream, Sparus aurata L., larvae fed on inert and live food. Mar Biol 118:323–328
Gang L, Hongxin T, Guozhi L, Chuan SD (2010) Effect of density on Scortum barcoo (McCulloch & Waite) juvenile performance in circular tanks. Aquacult Res 41:1898–1904
Green BS, McCormick MI (2001) Ontogeny of the digestive and feeding systems in the anemonefish Amphiprion melanopus. Environ Biol Fish 61:73–83
Gwak WS, Tanaka M (2001) Development changes in RNA: DNA ratios of fed and starved laboratory-reared Japanese flounder larvae and juveniles, and its applications to assessment of nutritional conditions for wild fish. J Fish Biol 59:902–915
Hart PR, Purser GJ (1996) Weaning of hatchery reared greenback flounder (Rhombosolea tapirina Gunther) from live to artificial diets: effects of age and duration of the changeover period. Aquaculture 145:171–181
Holt J (1993) Feeding larval red drum on microparticulate diets in a closed recirculating water system. J World Aquacult Soc 24:225–230
Hung LT, Tuan NA, Cacot P, Lazard J (2002) Larval rearing of the Asian Catfish, Pangasius bocourti (Siluroidei, Pangasiidae): alternative feeds and weaning time. Aquaculture 212:115–127
Isik O, Sarihan E, Kusvuran E, Gul O, Erbatur O (1999) Comparison of the fatty acid composition of the freshwater fish larvae Tilapia zillii, the rotifer Brachionus calyciflorus, and the microalgae Scenedesmus abundans, Monoraphidium minitum and Chlorella vulgaris in the algae-rotifer-fish larvae food chain. Aquaculture 174:299–311
Kestemont P, Xueliang X, Hamza N, Maboudou J, Toko II (2007) Effect of weaning age and diet on pikeperch larviculture. Aquaculture 264:197–204
Kolkovski S, Arieli A, Tandler A (1997a) Visual and chemical cues stimulate microdiet ingestion in sea bream larvae. Aquacult Int 5:527–536
Kolkovski S, Koven W, Tandler A (1997b) The mode of action of Artemia in enhancing utilization of microdiet by gilthead seabream Sparus aurata larvae. Aquaculture 155:193–205
Kolkovski S, Tandler A, Izquierdo MS (1997c) Effects of live food and dietary digestive enzymes on the efficiency of microdiets for seabass (Dicentrarchus Zabrax) larvae. Aquaculture 148:313–322
Koven W, Kolkovski S, Hadas E, Gamsiz K, Tandler A (2001) Advances in the development of microdiets for gilthead seabream, Sparus aurata: a review. Aquaculture 194:107–121
Lepage G, Roy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25:1391–1396
Leu MY, Liou CH, Wu CH (1991) Feasibility of using micro-coated diet fed to larval yellow-finned black porgy, Acanthopagrus lam (Houttuyn). J Fish Sot Taiwan 18:287–294
Nhu VC, Dierckens K, Nguyen TH, Tran MT, Sorgeloos P (2009) Can umbrella-stage Artemia fransiscana substitute enriched rotifers for Cobia (Rachycentron canadum) fish larvae? Aquaculture 289:64–69
Ostaszewska T (2005) Development changes of digestive system structures in pikepech (Sander Lucioperca L.). Electr J Ichthyol 2:65–78
Østergaard P, Munk P, Janekarn V (2005) Contrasting feeding patterns among species of fish larvae from the tropical Andaman Sea. Mar Biol 146:595–606
Person Le Ruyet J, Alexandre JC, Thebaud L, Mugnier C (1993) Marine fish larvae feeding: formulated diets or live preys? J World Aquacult Soc 24:211–224
Rekecki A, Dierckens K, Laureau S, Boon N, Bossier P, Van den Broeck W (2009) Effect of germ-free rearing environment on gut development of larval sea bass (Dicentrarchus labrax L.). Aquaculture 293:8–15
Sargent JR, Henderson RJ, Tocher DR (1989) The lipids. In: Halver JE (ed) Fish Nutrition, 2nd edn. Academic Press, San Diego, p 153
Sheikh-Eldin M, De Silva SS, Ingram BA (1997) Effects of diets and feeding rate on the survival and growth of Macquarie perch (Macquaria australasica) larvae, a threatened Australian native fish. Aquaculture 157:35–50
Shirota A (1970) Studies on the mouth size of fish larvae. Bull Jpn Soc Sci Fish 36:353–368
Song LP, An L, Zhu ZA, Li X, Wang AY (2009) Effects of dietary lipids on growth and feed utilization of Jade Perch, Scortum barcoo. J World Aquacult Soc 40:266–273
Sorgeloos P, Dhert P, Candreva P (2001) Use of brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture 200:147–159
Sserwadda M (2012) Weaning of pikeperch (Sander lucioperca L.) larvae and the importance of highly unsaturated fatty acids. Thesis submitted in partial fulfillment of the requirements for the academic degree of Master of Science in Aquaculture, Laboratory for Aquaculture & Artemia Reference Center, Faculty of animal production, Ghent University, Belgium, p 41
Süzer C, Kamaci HO, Coban D, Saka S, Firat K, Karacaoglan A (2011) Early weaning of sea bass (D. labrax) larvae: effects on growth performance and digestive enzyme activities. Turk J Fish Aquat Sci 11:491–497
Van Hoestenberghe S, Roelants I, Vermeulen D, Goddeeris BM (2013) Total replacement of fish oil with vegetable oils in the diet of juvenile Jade perch scortum barcoo reared in recirculating aquaculture systems. J Agr Sci Tech B 3:385–398
Van Stappen G (1996) Artemia: use of cysts. In: Lavens P, Sorgeloos P (eds) Manual on the production and use of live food for aquaculture, FAO Fisheries technical paper no. 361, pp 132–170
Verreth J, Custers G, Melgur W (1994) The metabolism of neutral and polar lipids in eleuthero-embryos and starving larvae of the African catfish Clarias gariepinus. J Fish Biol 45:961–971
Webster CD, Lovell RT (1990) Response of striped bass larvae fed brine shrimp from different sources containing different fatty acid compositions. Aquaculture 90:49–61
Wirth M, Steffens W, Meinelt T, Steinberg C (1997) Significance of docosahexaenoic acid for rainbow trout (Oncorhynchus mykiss) larvae. Fett/Lipid 99:251–253
Yufera M, Darias MJ (2007) The onset of exogenous feeding in marine fish larvae. Aquaculture 268:53–63
Zambonino Infante JL, Cahu CL (1994) Development and response to a diet change of some digestive enzymes in sea bass Dicentrarchus labrax larvae. Comp Biochem Physiol 109A:213–222
Acknowledgments
This project was supported by the Interreg IVa Flanders–The Netherlands project Aquavlan with financial support of the European Regional Development Fund and with the help of the province of Vlaams-Brabant and East Flanders. The authors want to thank Jorg Desmyter (Laboratory of aquaculture, Ugent) for helping out with system setup and running the experiment, Dominique Adriaens (Evolutionary Morphology of Vertebrates, Department of Biology, Ugent) and Annemie Decostere and Wim Van den Broeck (Department of Morphology, UGent) for their advice in morphological measurements and interpretations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Hoestenberghe, S., Wille, M., De Swaef, E. et al. Effect of weaning age and the use of different sized Artemia nauplii as first feed for jade perch Scortum barcoo . Aquacult Int 23, 1539–1552 (2015). https://doi.org/10.1007/s10499-015-9903-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-015-9903-x