Abstract
Fluoride contamination in water is a major problem across the globe, with health hazards such as dental and skeletal fluorosis. Most earlier studies are confined to local or regional scales. As the problem has serious socioeconomic implications, there is a need for a global perspective. Thus, here we review worldwide research for nearly a century on fluoride contamination in water. We investigated the distribution of fluoride contamination in water, its sources, mobilization and association. The major findings are: (1) Anomalous fluoride concentration in groundwater is mainly confined to arid and semiarid regions of Asia and North Africa. (2) The geogenic sources of fluoride in water are mainly fluorine-bearing minerals in rocks and sediments, whereas anthropogenic sources of fluoride in water are mainly pesticides and industrial waste. (3) Fluoride mobilization from geogenic sources is mainly controlled by alkalinity and temperature. (4) Fluoride occurrence in water is associated with ions such as sodium, arsenic chloride and bicarbonate. There are few associations of fluoride in water with calcium and magnesium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride contamination in water is the main concern across the globe since few decades. Many studies have reported fluoride-related health problems such as dental and skeletal fluorosis in humans which has a serious socioeconomic implication. Fluorine is the lightest and most electronegative element in the halogen group. It is abundant (625 mg/kg) in the earth’s crust and mobile at a higher temperature (Edmunds and Smedley 2001). In nature, fluorine occurs as negatively charged fluoride ion (F−) in water (Hem 1985). Fluorine-bearing minerals are usually associated with acidic igneous rocks, mineralized veins and sedimentary formations (Edmunds and Smedley 2001). Its presence in felsic rocks is significantly greater than mafic and metasedimentary rocks (Young et al. 2011).
To understand the mechanisms, it is very important to know the sources of fluoride in the water. The main sources of fluoride include fluorine-bearing minerals such as fluorite (CaF2), cryolite (Na3AlF6), topaz [Al2SiO4(F,OH2)], apatite [Ca5(Cl,F,OH)(PO4)3], amphiboles [A0–1B2C5T8O22(OH,F,Cl)], micas [AB2–3(X, Si)4O10(O, F, OH)2] and sellaite (MgF2) (Hem 1985; Pickering 1985; Datta et al. 1996; Jadhav et al. 2015). Weathering of these fluorine rich minerals is the most important geogenic source of fluoride enrichment in water. Anthropogenic sources also contribute fluoride in the water. This includes activities such as mining, usage of pesticides and brick kilns (Datta et al. 1996). Excess fluoride intake leads to dental fluorosis and at even higher intake could cause skeletal fluorosis. Hence, various national and international agencies have set standard permissible limits for fluoride in drinking water. The permissible limit set by WHO (2004) as well as Bureau of Indian Standards (BIS 2012) for fluoride in drinking water is 1.5 mg/L.
The studies conducted so far are mostly confined to local and regional scale. In this context, it is desired to have a global perspective of this problem. Hence, the article reviews worldwide research for nearly a century on fluoride contamination in water. The global distribution of fluoride contamination in water has been highlighted. In addition, the mechanism of fluoride release into water and its association with other ions has also been discussed. It is observed that semiarid and arid regions of world have greater probability of fluoride contamination in water. The fluoride-related health issues have also been elaborated in the article.
Sources of fluoride
The sources could be both geogenic such as the presence of fluorine-bearing minerals in rocks and sediments as well as anthropogenic such as use of pesticides and industrial waste. The details of both of the sources are discussed below.
Geogenic sources
Major geogenic sources of fluoride include fluorine-bearing minerals such as fluorite, apatite, amphiboles and micas that are found in various rocks and sediments (Handa 1975; Hem 1985). Pickering (1985) had reported fragments of minerals such as apatite, cryolite, fluorite or fluorspar and topaz as the main source of fluoride in soil. Coal also contains around 295 ppm of fluorine (Churchill et al. 1948). Further, Jacks et al. (1993) have also observed the occurrence of few percent of fluorine in calcrete and dolocrete. Another geogenic source could be atmospheric air and precipitation which contain 0.00001–0.0004 mg/L and below detection limit 0.089 mg/L of fluoride (Gupta et al. 2005).
Anthropogenic
The major anthropogenic sources of fluoride contamination in groundwater are unscientific use of phosphatic fertilizers (Kundu and Mandal 2009). This is very common in developing countries such as India. In addition, aluminum smelting, glass, phosphatic fertilizer, brick industries and coal-based power stations also contribute fluoride into the environment (Pickering 1985). Irrigation by fluoride-enriched water also contributes fluoride into groundwater (Pettenati et al. 2013). It is estimated that up to 0.34 mg/L of fluoride can be contributed by the use of superphosphate fertilizers in agricultural land (Rao 1997).
Areas near brick kiln industries also show a higher concentration of fluoride in groundwater (Datta et al. 1996). Clay used in the manufacture of bricks contains several hundred ppm of fluoride (MacDonald 1969). A research in the Republic of South Africa has shown that underground mine waters may contain high fluoride concentration of levels beyond 3 mg/L (Thole 2013).
Fluoride in surface water
The presence of fluoride has also been reported from several surface water bodies such as lakes and rivers throughout the globe (Table 1).
Fluoride concentrations in most of the rivers are generally within the permissible limit (1.5 mg/L). On the other hand, geothermal spring shows fluoride concentration as high as 2800 mg/L (Table 1).
Fluoride in food stuff
The concentration of fluoride in food stuff is reported by several researchers (Table 2).
Messaïtfa (2008) reported around 2.9 mg/kg of fluoride in dates, while Cao et al. (1996) reported variation in levels of fluoride in different varieties of tea (Table 3).
The “brick tea-type fluorosis” is the third type of fluorosis reported (Cao et al. 1996) after dental and skeletal fluorosis. This brick tea-type fluorosis is most common in China, especially in Tibet.
Mobilization of fluoride
The concentration of fluoride in natural water depends on many factors. This includes temperature, pH (Genxu and Guodong 2001), solubility of fluorine-bearing minerals, anion exchange between hydroxyl and fluoride ions, water residence time and the geological formations (Apambire et al. 1997). The process of mobilization is still unclear, but the most common mechanism for fluoride mobilization is displacement of fluoride ions (F−) by hydroxyl ions (OH−) (Hem 1985; Edmunds and Smedley 2001). Temperature and residence time speed up the dissolution of fluorine-bearing minerals present in the rocks (Saxena and Ahmed 2003).
Minerals such as illite, mica and amphiboles are known to displace fluoride ion with hydroxyl ion at higher alkalinity in groundwater (Hem 1985; Edmunds and Smedley 2001). The process of replacement of hydroxyl ions with fluoride from the muscovite is shown below
In general high alkalinity, bicarbonate concentration and moderate electrical conductivity are important factors responsible for fluoride dissolution in groundwater (Saxena and Ahmed 2001). Tirumalesh et al. (2007) had also reported that factors such as alkalinity, evaporation and high bicarbonate content of the groundwater promote dissolution of fluorine rich minerals. Clay content also plays an important role in fluoride dissolution because clay decreases hydraulic conductivity and thus increases the residence time of water in the aquifer (Rao et al. 2015). Dissolution of fluorite in groundwater in the presence of high HCO3 contents will be achieved by the following reaction (Guo et al. 2007):
In addition, removal of calcium also facilitates fluoride dissolution in groundwater as it raises the fluorite saturation ceiling conditions (Frengstad et al. 2001). A change from calcium-rich to sodium-rich groundwater has more chance of dissolution of fluoride from the mineral into the aqueous solution (Gao et al. 2007). Maina and Gaciri (1984) have also linked low calcium and magnesium concentrations with high fluoride values. Earlier, Handa (1975) observed that sodium bicarbonate-type facies are more prone of releasing fluoride from fluorite mineral.
The dissolution of fluorite takes place as
Conditions such as semiaridity and high temperature promote effective chemical weathering of fluorine-bearing rocks such as granite, gneiss, sandstone and shale (Su et al. 2015). Chemical weathering is most effective under arid to semiarid climatic conditions and, thus, may lead to higher fluoride and salinity content. Thus, most of the high fluoride regions are confined to the arid and semiarid zones. A successful relation between recharge and fluoride concentration in groundwater shows that the water near recharge zone is less contaminated than discharge zones (Gaciri and Davies 1993; Li et al. 2015). Further, studies also show that the concentration of fluoride varies with groundwater level fluctuation (Brindha et al. 2011).
Association
In this work, correlation of fluoride with other ions has been highlighted on the basis of observations by many researchers worldwide (Table 4).
It can be seen that many researchers (Das et al. 2003; Hudak and Sanmanee 2003; Kim and Jeong 2005; Vasquez et al. 2006; Edmunds and Smedley 2001) have reported a positive correlation of fluoride with depth. However, a few also reported high fluoride at shallower depth (Apambire et al. 1997; Gupta et al. 2005), while the linkage between precipitation and fluoride enrichment in groundwater was reported by Beg et al. (2011).
In addition, calcium bicarbonate-type facies usually have lower concentration of fluoride, while higher fluoride concentration in groundwater has been reported from sodium bicarbonate-type facies (Fantong et al. 2010; Table 4). The positive correlation between fluoride and chloride in groundwater has also been observed (Jacks et al. 1993; Kundu et al. 2001) (Table 4). Simultaneous occurrence of anomalous concentration of fluoride with arsenic in groundwater was also reported (Razo et al. 1993; Gomez et al. 2009; Buchhamer et al. 2012). It is also observed that high fluoride zones are usually associated with waters enriched in heavy oxygen isotope 18O (Datta et al. 1996; Tirumalesh et al. 2007). The linkage among evapotranspiration, heavy oxygen isotope 18O and fluoride concentration in groundwater was established by Datta et al. (1999).
Worldwide fluoride distribution in groundwater
Fluoride in groundwater shows both spatial and temporal variation. We have observed that in most countries, the fluoride-related health hazards are under control. Though, some dental fluorosis cases are reported (Fig. 1).
Worldwide distribution of fluoride; red bars highly affected areas; yellow bars moderately affected areas; green bars least affected areas; blue bars insufficient data (based on worldwide dataset, Table 5). Note the highly affected areas are in general arid and semiarid regions
In countries such as India, China, Ethiopia, Kenya and Argentina, fluoride contamination in groundwater is a serious issue (Table 5), while Mexico is moderately affected country (Table 5). The details of worldwide distributions of fluoride in groundwater are discussed below.
South Asia
The South Asia can be considered as the epicenter of fluoride contamination in groundwater. All countries of South Asia such as India, Pakistan and Sri Lanka are highly affected. The details of each of the regions are described below.
India
In India, almost all the states are known to have fluoride contamination in water (Fig. 2; Table 5).
Approximately 62 million people of India are affected by dental and skeletal fluorosis (Susheela 1999). This population is scattered through 17 states (Fig. 2). The first case of fluorosis was reported in Prakasam district of Andhra Pradesh (Fig. 2) by Shortt et al. (1937). However, the effect of fluoride contamination in India is most profound in the state of Rajasthan (Fig. 2).
Rajasthan
In Rajasthan, about 18 districts are at risk of fluorosis, where roughly 11 million people are affected (Hussain et al. 2010). The saline lakes in Jaisalmer, situated in the monsoon deprived Thar Desert, show good correlation between pH and fluoride concentration. This is attributed to leaching of the fluoride from fluorine-bearing minerals (Singh and Mukherjee 2014). Here, fluoride also shows positive correlation with bicarbonates. This could be attributed to low rainfall and high temperature in this area, which enhances calcite precipitation, thereby leading to deficiency of calcium ions in water. Further, the alkaline environment facilitates dissolution of fluoride (Singh and Mukherjee 2014). Hence in Jaisalmer, fluoride contamination is mainly geogenic, which is facilitated by factors such as high evaporation rate and high temperature (Singh and Mukherjee 2014). In Pokhran (Rajasthan), weathering of fluorine-bearing minerals in granitic rocks results in leaching of fluoride in groundwater. High alkaline conditions along with low rainfall and high temperature are responsible for the dissolution of fluoride (Singh et al. 2011).
Andhra Pradesh and Telengana
Andhra Pradesh and Telengana is the second most affected state in India after Rajasthan (Fig. 2). The Nalgonda district of Andhra Pradesh is most extensively studied part in India for fluoride problem. Fluoride concentration in groundwater up to 20 mg/L has been reported in Nalgonda (Rao et al. 1993). Weathering of fluorine-bearing granite and granitic gneisses is the major source of fluoride in Nalgonda district (Brindha et al. 2011). The granitic aquifers of Wailapally watershed also were reported to have fluoride concentration as high as 7.6 mg/L (Reddy et al. 2010; Table 5).
Sujatha and Reddy (2003) studied fluoride contamination in Ranga Reddy district and observed fluoride levels in the range of 0.5–4.5 mg/L in groundwater. The presence of fluoro-apatite minerals and extensive use of phosphatic fertilizers in the southeastern part of the district are the main causes of fluoride contamination.
In Anantapur district, fluoride as high as 5.80 mg/L (Table 5) was reported from groundwater and it has been linked to fluorine-bearing minerals in the strata (Rao and Devadas 2003). Sreedevi et al. (2006) have reported high fluoride concentration in Maheshwaram. They observed high values of fluoride in the post-monsoon season as compared to pre-monsoon season. This was attributed to high evapotranspiration during pre-monsoon seasons, which leads to the precipitation of fluoride-rich salts. During monsoon time, these salts present in the subsurface leached into the groundwater and increased the level of fluoride in post-monsoon season and hence increase fluoride concentration in the groundwater (Rao 2003).
In Guntur district, fluoride is contributed into groundwater from fluorine-bearing minerals. Another minor contribution is from fertilizer used in the field (Rao 2003). The fluoride concentration in this district is also high in the post-monsoon period as observed earlier in Maheshwaram. Similarly, in Varaha River basin higher fluoride concentration has been observed during post-monsoon season (Rao 2009). The fluoride concentration here ranges from 0.6 to 2.1 mg/L (Table 5).
Uttar Pradesh
The report of fluoride contamination in groundwater is mainly confined to aquifers of Gangetic plains in Uttar Pradesh (Fig. 2). Misra et al. (2006) studied Saidabad Tehsil of Mathura and observed that shallow aquifers of Ganga alluvial plains are more contaminated (0.6–2.5 mg/L) as compared to deep aquifers (0.1–1.5 mg/L). The source of fluoride in this part is geogenic, mostly attributed to weathering of sedimentary rocks such as shale. In addition to this, fluoride in the range of 0.1–2.5 mg/L (Table 5) has been reported in Mat Tehsil of Mathura district (Misra and Mishra 2007). The deeper aquifers have been reported to be more saline but less contaminated. Further, it has been reported that fluoride concentration has increased with time.
In Sonbhadra district, Raju et al. (2009) observed that fluorine-bearing minerals such as apatite and biotitic mica are the major sources of fluoride in groundwater. Scanty rainfall, intensive irrigation, heavy use of fertilizers and high evapotranspiration lead to fluoride enrichment in the groundwater. The longer residence time of groundwater in the weathered aquifer zone and low rate of dilution are favorable factors for the dissolution of fluorine-bearing minerals (Raju et al. 2009). Fluoride in the range of 0.1–14.8 mg/L was reported by Sharma et al. (2011) in Agra district, while Gupta et al. (1999) reported fluoride in the range of 0.11–12.80 mg/L (Table 5) in the same district. High fluoride contamination here was accounted by weathering of rocks accompanied by evaporation of groundwater (Sharma et al. 2011).
Karnataka and Kerala
In Karnataka (Fig. 2), high fluoride areas are found in granites and gneissic terrain (Latha et al. 1999). The arid climate coupled with lithology and lack of fresh water exchange in Pededavankahalla basin of Bellary district enhanced fluoride contamination (Wodeyar and Sreenivasan 1996).
In Kerala (Fig. 2), hornblende biotitic gneiss present in Palghat district is a major source of fluoride. Here, fluoride in groundwater shows a negative correlation with calcium and a positive correlation with pH (Shaji et al. 2007; Table 4).
Tamil Nadu
Chidambaram et al. (2012) studied Dindigul district of Tamil Nadu (Fig. 2) and observed fluoride value in the range of 0.02–3.4 mg/L (Table 5). The groundwater samples from an area adjacent to Cooum River (Chennai) show fluoride concentration in the range of 0.11–2.5 mg/L (Table 5) (Giridharan et al. 2008). It was observed that in Neyveli district, fluoride concentration in groundwater was higher in pre-monsoon season with respect to post-monsoon season (Jayaprakash et al. 2008), while in Mettur region, the higher concentration of fluoride was observed in post-monsoon season as compared to pre-monsoon season (Srinivasamoorthy et al. 2012).
Assam, Manipur, Chhattisgarh and Delhi
Das et al. (2003) identified Precambrian granite as a source of fluoride in Guwahati, Assam. In Manipur (Fig. 2), fluoride concentration around 1.78 mg/L in groundwater has been reported (Oinam et al. 2012). Chhattisgarh (Fig. 2) has higher fluoride in Barakar formation, which is coal bearing strata with mica and clays (Beg et al. 2011). The maximum concentration of fluoride (7.14 mg/L) in Delhi has been reported in Roopnagar locality (Table 5) in north Delhi (Shekhar and Sarkar 2013; Fig. 2). Here, fluoride contamination was observed to be higher in deeper aquifers.
Maharashtra
In shallow aquifers of the Yavatmal district of Maharashtra (Fig. 2), high fluoride concentration was observed. This was linked to leaching of fluoride from the weathered zone (Madhnure and Malpe 2007). Kodate et al. (2013) identified two main sources of fluoride in shallow aquifers: (1) limestone with fluorite, apatite and clays in Lameta Formation and (2) Shales and clays in Gondwana sediments.
Pakistan
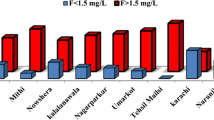
In East Punjab area of Pakistan, around 75 % of groundwater samples were reported to exceed WHO standard of fluoride (Farooqi et al. 2007a). In this area, groundwater exhibits a positive correlation between fluoride and sodium as well as bicarbonate ions (Table 4), while fluoride shows a negative correlation with calcium and magnesium ions (Table 4). Further, the concentration of fluoride is higher in shallower aquifer than deeper one (Farooqi et al. 2007b). Groundwater near Nagar Parkar Town (South East Pakistan) has also been reported to have fluoride concentrations up to 7.85 mg/L (Table 5). Dental and skeleton fluorosis cases are commonly reported from people living in Thar region (Rafique et al. 2008).
Sri Lanka
In Sri Lanka, high fluoride concentrations were observed mainly in eastern and southeastern part of the country (Fig. 3).
Fluoride distribution in Sri Lanka (modified after Dharmagunawardhane and Dissanayake 1993)
Most of the high concentrations of fluoride in groundwater samples were observed in boreholes located on charnockitic gneiss, calc gneiss, biotite gneiss and granulite (Dharmagunawardhane and Dissanayake 1993). Boreholes drilled in marble rarely reported values of fluoride in groundwater above 2.0 mg/L. Similarly, boreholes drilled in quartzite rarely reported fluoride in groundwater above 1.5 mg/L (Dharmagunawardhane and Dissanayake 1993). Dental fluorosis has been reported from Walawe River basin in southern Sri Lanka. Here, dry and hot climate is the major factor responsible for high fluoride concentrations in groundwater (Van Der Hoek et al. 2003).
A recent study in Sri Lanka reports that weathering leading to dissolution of fluoride-bearing heavy minerals releases both immobile and mobile elements (Jayawardana et al. 2012). The immobile elements are retained in the soils, while the mobile elements such as fluorine are released into the groundwater (Jayawardana et al. 2012).
East Asia
In East Asia, China is the most affected country. Here, the groundwater in areas of low rainfall and high temperature is highly contaminated compared with other regions. The details of contaminated regions of East Asia are discussed below.
China
Fuhong and Shuqin (1988) classified the major areas of fluoride contamination in China into three categories (Fig. 4).
Distribution of high fluorine groundwater in China based on occurrences (modified after Fuhong and Shuqin 1988)
-
1.
Shallow groundwater areas characterized by dry climate and evaporation exceeding precipitation. In this category, high fluoride level is found in areas such as western part of Songliao Plain, central part of north China Plain and Nemonguhigh Plain basin in central part of Shanxi Province. Besides, high fluoride levels are also found in inland basins and piedmont plains of Northwest China.
-
2.
Deep groundwater areas characterized by semiarid conditions. High fluoride levels in this category include areas such as coastal plain of Bohaiwan, eastern part of Huang-Huai-Hai Plain and basins of the central part of Shanxi.
-
3.
Groundwater system associated with hot spring and mines. High fluoride here is mostly found in the areas such as Liaodong mountains, Liaoning Peninsula, hills of southeastern China and mountains in southern Xizang.
Fluoride contamination in water reported by other researches is discussed below.
The Yuncheng basin in the southern part of the Shanxi province is one of the most extensively studied areas in China for fluoride contamination in groundwater. Currell et al. (2011) have reported a high fluoride level of 6.6 mg/L in Yuncheng basin. Fluoride in groundwater in this basin originated from the dissolution of fluorine-bearing minerals and desorption of exchangeable fluoride from the loess (Li et al. 2015). The high fluoride concentration in groundwater of central parts of Yuncheng basin is linked to the long-term interaction of groundwater (about 1000 years for shallow groundwater) with fluorine-bearing minerals (Han et al. 2006). Li et al. (2015) identified two possible ways of fluoride enrichment in this basin:
-
1.
The first by dissolution of evaporite during leaching process. The dissolution of evaporite, i.e., gypsum and mirabilite along with fluorine-bearing minerals, could increase the aqueous fluoride content.
-
2.
The second mechanism of fluoride enrichment could be infiltration of fluoride-rich groundwater from shallow aquifers to deeper ones.
Another major region for fluoride contamination in China is confined to northern part of the country, where about 43 million cases of fluorosis have been reported (Luo et al. 2008). Su et al. (2015) have observed fluoride concentration as high as 8.26 mg/L in groundwater of Datong basin in northern China. In dry regions (characterized by high evaporation rate and low rainfall), high fluoride concentrations in groundwater are due to fluoride dissolved from fluorine-bearing minerals (Zeng 1997).
Fluoride content in groundwater is low in higher rainfall areas because of dilution (Zeng 1997). But in Yuanmou County, sparse vegetation cover and deforestation causes greater surface runoff and further resulting in accumulation of fluoride at the valley bottoms (Cai et al. 2010). Thus, climate is the major factor controlling the concentration of fluorine in groundwater of China.
Mongolia
In Mongolia, the area affected by fluoride contamination is located in Hetao basin (Guo et al. 2012). In this region, fluoride concentration is reported in the range of 0.30–2.57 mg/L (Table 5). They observed no significant correlation between fluoride and arsenic.
South East Asia
In South East Asia, studies on fluoride contamination in water are limited to certain parts of Indonesia.
Indonesia
In Asembagus, the coastal area of East Java, wells found in the vicinity of riverbed were characterized by highest fluoride concentrations. Moreover, the river water, which is used for irrigation, has fluoride concentration as high as 14.2 mg/L (Heikens et al. 2005). On the other hand, the range of fluoride in well water is found to be in the range of less than 0.1–4.2 mg/L (Heikens et al. 2005).
Africa
Groundwater is the main source of fresh water supply for most of the rural communities in Africa. This groundwater in east African rift valley area has high level of fluoride concentration. This rift valley is a part of Great Rift Valley of Africa extending from Jordan valley down through Sudan, Ethiopia, Uganda, Kenya to Tanzania. It seems that high fluoride concentration in groundwater is somehow linked to geology of rift valley. It may be the anomalous concentration of fluoride in groundwater could be found in other areas along the great rift valley.
Ethiopia
In Ethiopia, tectonics associated with rift valley and semiarid climate are two major factors responsible for the high concentration of fluoride in both surface and subsurface water system. Tekle-Haimanot et al. (2006) have reported the high fluoride concentration of 264 mg/L in hot springs. The alkaline lakes of Ethiopia are reported to have fluoride up to 384 mg/L (Rango et al. 2009; Table 1). Similarly, Gizaw (1996) reported 295 mg/L of fluoride in lake water of the Main Ethiopian valley.
The fluoride concentration in groundwater of Ethiopia is relatively lower than that of surface water bodies. Ayenew (2008) has observed fluoride levels of 64 mg/L in deeper aquifers, 250 mg/L in lakes and up to 67 mg/L in geothermal wells.
Tanzania
In Tanzania, weathering associated with rock–water interaction is a significant factor for fluoride contamination. Nanyaro et al. (1984) studied the lowland rivers of Tanzania and observed that fluoride is derived from soil and weathering of fluorine rich nephelinite and carbonatitic rocks. However, in regions around Mt. Meru crater, gaseous emanations through mineral springs are responsible for increased fluoride concentrations (Nanyaro et al. 1984). High concentrations of fluoride in Mt. Meru area in northern Tanzania are attributed to weathering of villiaumite (NaF) triggered by high temperatures coupled with high precipitation (Nanyaro et al. 1984).
Kenya
In Kenya, some areas such as lake Elementaita (1640 mg/L) and lake Nakuru (2800 mg/L) were reported to have extremely high level of fluoride (Nair et al. 1984; Table 1). A positive correlation of fluoride concentration with depth of borehole was observed in these areas. Later, Nair et al. (1984) had reported that the majority of the samples from different parts of Kenya have fluoride concentrations above 1.0 mg/L. In Nairobi and its adjoining areas, fluoride concentrations are reported to be above 8 mg/L (Nair et al. 1984). The volcanic rocks lying adjacent to the rift valley region of Kenya have highest fluoride contaminations in groundwater, while surface water in the rift valley also has fluoride concentration up to 180 mg/L (Clarke et al. 1990). Districts such as Tana River, Kisii and Kirinyaga had fluoride concentrations below 0.5 mg/L (Nair et al. 1984). In other parts of Kenya such as the north eastern, Coast and Nyanza Provinces, low fluoride concentrations in water are observed.
The chemical weathering of alkaline volcanic rocks rich in sodium and fluoride and their associates such as calcareous tufa, lahar and ash are responsible for such high concentrations of fluoride (Gaciri and Davies 1993). Injection of fluorine from magmatic sources is also considered to be the major source of the fluoride contamination in Kenyan waters (Gaciri and Davies 1993).
Malawi
In Malawi, the fluoride concentrations were analyzed by Msonda et al. (2007). They observed fluoride concentration ranging from 0.50 to 6.98 mg/L in the rainy season and between 2 and 7.02 mg/L in a dry season (Table 5). High fluoride concentration in groundwater is confined to the central part. Weathered basement complex containing biotite is the major source of fluorine here. Besides, dissolution of hornblende, fluorite and amphiboles is reported here, which could be further leading to fluoride enrichment in this area (Msonda et al. 2007).
Cameroon
In Cameroon, fluoro-apatite and micas in granites were identified as the main source of fluoride in the groundwater. Here, fluoride enrichment in groundwater is linked to water–rock interactions at higher pH (Fantong et al. 2010). Moreover, fluoride concentrations around 15 mg/L are reported in very shallow groundwater of Mayo Tsanaga River basin in Cameroon. This trend of high fluoride in water is commonly observed along valleys associated with granitic basins (Fantong et al. 2010).
South Africa
In the Republic of South Africa, researchers have reported that around 803 sites are affected by endemic fluorosis. These include locations in western and Karoo Regions of Cape Province, north western, northern, eastern and western areas of Transvaal, western and central Free State (Thole 2013). Fluoride levels as high as 6 mg/L were reported in groundwater of Madibeng local municipality in northwest Province of South Africa (Thole 2013).
Nigeria and Ghana
In Nigeria, Dibal et al. (2012) have reported the presence of fluoride content in basement aquifers (0.03–10.30 mg/L), sedimentary rocks (0–5 mg/L), younger granites (0–0.89 mg/L) and volcanic aquifers (0–0.78 mg/L). Apambire et al. (1997) found the fluoride concentration in the range of 0.11–4.60 mg/L in upper regions of Ghana.
Europe
In most part of Europe, fluoride concentration in groundwater is within the permissible limits. Thus, the research is limited in the European subcontinents. However, there are few localities where high fluoride concentrations have been reported.
Estonia
In Estonia, the source of high fluoride in drinking water is the aquifer of carbonaceous Silurian to Ordovician age (Karro et al. 2006). However, even in highly affected parts of the Estonia such as the Parnu, Tartu, Jarva and Laae countries, rarely health hazards have been reported (Indermitte et al. 2009).
Germany
In Germany, Queste et al. (2001) have reported fluoride in Muenster region. Up to 8.8 mg/L of fluoride is reported from private wells (Table 5). Geological processes are responsible for high fluoride in the Muenster region.
North America
Mexico
In Northern Mexico, fluoride is found to be in the range of 0.5–3.7 mg/L (Razo et al. 1993). They also reported a positive correlation of fluoride with arsenic (Table 4). Vasquez et al. (2006) observed fluoride concentration in groundwater up to 7.59 mg/L in La Victoria area, Hermosillo City (Table 5). Here, higher values of fluoride are observed in deeper aquifers compared with shallower ones. 5.6 mg/L of fluoride is reported in the drinking water of Durango (Ortiz et al. 1998).
USA
In the USA, fluoride in groundwater ranges from less than 0.2 to 3.58 mg/L in Ohio (EPA Report 2012). Robertson (1985) observed up to 13 mg/L of fluoride in groundwater of Arizona. Up to 7.60 mg/L of fluoride was also reported from crystalline bedrock aquifers of Marathon County, Wisconsin (Ozsvath 2006). In Wisconsin, felsic igneous and equivalent rocks are considered to be the source rock for fluoride in groundwater.
Canada
In Canada, up to 15.1 mg/L of fluoride (Table 5) was reported in Lake St. Martin region of Manitoba (Desbarats 2009). In the Gaspe region (Quebec), some skeletal fluorosis cases are also reported (Boyle and Chagnon 1995).
South America
Argentina
In South America, Argentina is the most affected country as observed by various researchers. Smedley et al. (2002) studied La Pampa area of Argentina and found fluoride in the range of 2.9–25.7 mg/L in shallow aquifer (9–10 mbgl), while they reported higher concentration of fluoride in deeper aquifer beyond depth of 17 m in Talleres Norte borehole. Nicolli et al. (2012) studied Chaco-Pampean area and also reported fluoride up to 3.7 mg/L in the shallow aquifer and 7.34 mg/L in deeper aquifers. Kruse and Ainchil (2003) studied Buenos Aires Province and found fluoride content in the range of 0.2–5 mg/L. Further, Paoloni et al. (2003) observed fluoride concentrations in the range of 0.9–18.2 mg/L in south east sub-humid region of Pampa. Cabrera et al. (2001) also observed fluoride in the range of 1.4–10.6 mg/L in Pampian plain, whereas Cid et al. (2011) reported fluoride content in the range of 0.15–0.56 mg/L in Midwest of Argentina (Table 5).
In central Argentina, fluoride concentration in groundwater varies from 0.5 to 12 mg/L (Gomez et al. 2009; Table 5), and there is good correlation between fluoride and arsenic here (Table 4). This correlation is also observed by Buchhamer et al. (2012) in Chaco province (Table 4).
Medical perspective
Fluorine is one of the essential elements for human body. A daily dose of 0.5 mg/L of fluoride is required for the formation of enamel and bone mineralization (Table 6). Fluoride like any other trace elements can be considered as harmful or beneficial to humans depending on the amount ingested daily (Apambire et al. 1997). Deficiency of fluoride leads to the formation of dental caries, lack of enamel formation and bone fragility (Ayenew 2008; Edmunds and Smedley 2001). On the other hand, long-term intake of fluoride beyond permissible limit of 1.5 mg/L may result in skeletal fluorosis, manifested by chronic joint pain and osteosclerosis. This is generally observed in adults.
The most severe form of fluorosis is the crippling skeletal fluorosis. Further, high doses of fluoride have also been linked to cancer (Marshall 1990). Other experiments carried out in Japan (Tohyama 1996), USA (Takahashi et al. 2001) and Taiwan (Yang et al. 2000) proved that various types of cancers are associated with fluoride. Further, studies also show that in animals, kidney damage occurs even at lower levels of fluoride exposure over a long time (Manocha et al. 1975).
Symptoms such as pricking and tingling in the limbs, sporadic pain, back stiffness, burning-like sensation, muscle weakness, abnormal calcium deposits in bones and ligaments are seen in the human body. The deposited areas in the body are joints of neck, knee, pelvic and shoulder (Meenakshi and Maheshwari 2006). Grimaldo et al. (1995) observed that fluoride concentration is increased by boiling water.
Intake of fluoride beyond the limit may lead to dental and skeletal fluorosis up to the age of 12 years in the children (Apambire et al. 1997). However, dental fluorosis occurs generally in the age of 6–8 years. Susheela et al. (1993) reported the major health problems such as dental and skeletal fluorosis, deformation of bones in children and adults caused by excessive intake of fluoride. The fluoride also has direct effects on children’s intelligence (Lu et al. 2000). Chen et al. (2012) reported that with government support in China, the mitigation measures related to fluoride contamination were effective. The dental and skeletal fluorosis cases were minimized in China.
Fluoridation issue
Fluoridation, i.e., adding of fluoride in the water, is supported by one school of thought, while it is opposed by the other school of thought. In spite of this debate, there are parts of the world (Australia, New Zealand and Canada) where fluoridation in necessarily implemented. However, some countries in Scandinavian region use alternative sources of fluoride like fluoride-rich toothpaste (Lennon et al. 2004).
Conclusion
The study establishes that fluoride contamination in water is a worldwide phenomenon. The fluoride contamination in water is mostly observed in countries like India, China, Argentina, Mexico and Ethiopia. The arid to semiarid regions of these countries have prevalence of fluoride contamination in water. Beside climatic conditions, the primary control on fluoride contamination of water is the mineralogy of the host formation. Granite, gneiss, sandstone, etc., having more fluorine-bearing minerals favor geogenic enrichment of fluoride. In such formations, fluoride mobilization in water is facilitated by low rainfall, high temperature and high alkalinity. Fluoride contamination in water is associated with ions such as sodium, arsenic, bicarbonate and chloride. However, it is rarely associated with ions such as calcium and magnesium.
This study will help in framing policy for mitigation of fluoride contamination in water. Institutional intervention by way of identifying and isolating fluoride-contaminated sources may be an ideal measure. However, in case alternative drinking water sources are not available, proper treatment is mandatory. In long run, it would be advisable to adopt artificial recharge and rain water harvesting for dilution of fluoride-contaminated water.
References
Abdulrahman AI (1997) Fluoride content in drinking water supplies of Riyadh, Saudi Arabia. Environ Monit Assess 48(3):261–272. doi:10.1023/A:1005795820508
Adhikary PP, Dash CJ, Sarangi A, Singh DK (2014) Hydrochemical characterization and spatial distribution of fluoride in groundwater of Delhi state, India. Indian J Soil Conserv 42(2):170–173
Al-Amry AS (2009) Hydrogeochemistry and origin of fluoride in groundwater of Hidhran & Alburayhi Basin, northwest Taiz City, Yemen. Delta J Sci 33:10–20
Apambire WB, Boyle DR, Michel FA (1997) Geochemistry, genesis and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environ Geol 33(1):13–24. doi:10.1007/s002540050221
Arif M, Hussain I, Hussain J, Sharma S, Kumar S (2012) Fluoride in the drinking water of Nagaur Tehsil of Nagaur district, Rajasthan, India. Bull Environ Contam Toxicol 88(6):870–875. doi:10.1007/s00128-012-0572-4
Arveti N, Sarma MRS, Aitkenhead-Peterson JA, Sunil K (2011) Fluoride incidence in groundwater: a case study from Talupula, Andhra Pradesh, India. Environ Monit Assess 172(1–4):427–443. doi:10.1007/s10661-010-1345-3
Avtar R, Kumar P, Surjan A, Gupta LN, Roychowdhury K (2013a) Geochemical processes regulating groundwater chemistry with special reference to nitrate and fluoride enrichment in Chhatarpur area, Madhya Pradesh, India. Environ Earth Sci 70(4):1699–1708. doi:10.1007/s12665-013-2257-7
Avtar R, Kumar P, Singh CK, Sahu N, Verma RL, Thakur JK, Mukherjee S (2013b) Hydrogeochemical assessment of groundwater quality of Bundelkhand, India using statistical approach. Water Qual Expo Health 5(3):105–115. doi:10.1007/s12403-013-0094-2
Ayenew T (2008) The distribution and hydrogeological controls of fluoride in the groundwater of central Ethiopian rift and adjacent highlands. Environ Geol 54(6):1313–1324. doi:10.1007/s00254-007-0914-4
Ayenew T, Demlie M, Wohnlich S (2008) Hydrogeological framework and occurrence of groundwater in the Ethiopian aquifers. J Afr Earth Sci 52(3):97–113. doi:10.1016/j.jafrearsci.2008.06.006
Barot VV (1998) Occurrence of endemic fluorosis in human population of North Gujarat, India: human health risk. Bull Environ Contam Toxicol 61(3):303–310. doi:10.1007/s001289900763
Beg MK, Srivastav SK, Carranza EJM, de Smeth JB (2011) High fluoride incidence in groundwater and its potential health effects in parts of Raigarh District, Chhattisgarh, India. Curr Sci 100(5):750–754
Bergmann R (1995) Fluorid in der Ernährung des Menschen. Biologische Bedeutung für den wachsenden organismus (Habilitationsschrif). Virchow-Klinikum der Humboldt-Universität, Berlin
BIS (2012) Indian Standard Specification for drinking water. B.S.10500
Boyle DR, Chagnon M (1995) An incidence of skeletal fluorosis associated with groundwaters of the maritime carboniferous basin, Gaspé region, Quebec, Canada. Environ Geochem Health 17(1):5–12. doi:10.1007/BF00188625
Brindha K, Rajesh R, Murugan R, Elango L (2011) Fluoride contamination in groundwater in parts of Nalgonda district, Andhra Pradesh, India. Environ Monit Assess 172(1–4):481–492. doi:10.1007/s10661-010-1348-0
Buchhamer EE, Blanes PS, Osicka RM, Giménez MC (2012) Environmental risk assessment of arsenic and fluoride in the Chaco province, Argentina: research advances. J Toxicol Environ Health Part A 75(22–23):1437–1450. doi:10.1080/15287394.2012.721178
Cabrera A, Blarasin M, Villalba G (2001) Groundwater contaminated with arsenic and fluoride in the Argentine Pampean plain. J Environ Hydrol 9(6):1–9. http://www.hydroweb.com/jeh/jeh2001/blara.pdf
Cai H, Wang CQ, Zhang MG, Li XG, Guo CL (2010) Geological environment characteristics of potable water endemic fluorosis areas in Northeast China and the prevention and control measures. Geol China 37:645–650; In Chen H, Yan M, Yang X et al (2012) Spatial distribution and temporal variation of high fluoride contents in groundwater and prevalence of fluorosis in humans in Yuanmou County, Southwest China. J Hazard Mat 235:201–209. doi:10.1016/j.jhazmat.2012.07.042
Camargo JA (1996) Comparing levels of pollutants in regulated rivers with safe concentrations of pollutants for fishes: a case study. Chemosphere 33(1):81–90
Camargo JA, Ward JV, Martin KL (1992) The relative sensitivity of competing hydropsychid species to fluoride toxicity in the Cache la Poudre River (Colorado). Arch Environ Contam Toxicol 22:107
Cao J, Bai X, Zhao Y, Liu J, Zhou D, Fang S, Jia M, Wu J (1996) Fluorosis induced by drinking brick tea. Fluoride 29:139–143
Cao J, Zhao Y, Liu JW (2000) Fluoride in the environment and brick-tea-type fluorosis in Tibet. J Fluor Chem 106:93–97
Chae GT, Yun ST, Mayer B et al (2007) Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ 385(1):272–283. doi:10.1016/j.scitotenv.2007.06.038
Chakrabarti S, Bhattacharya HN (2013) Inferring the hydro-geochemistry of fluoride contamination in Bankura district, West Bengal: a case study. J Geol Soc India 82(4):379–391. doi:10.1007/s12594-013-0165-9
Chakraborti D, Chanda CR, Samanta G et al (2000) Fluorosis in Assam, India. Curr Sci 78(12):1421–1423
Chatterjee MK, Mohabey NK (1998) Potential fluorosis problems around Chandidongri, Madhya Pradesh, India. Environ Geochem Health 20(1):1–4. doi:10.1023/A:1006529925395
Chen YX, Lin MQ, He ZL et al (1996) Relationship between total fluoride intake and dental fluorosis in areas polluted by airborne fluoride. Fluoride 29:7–12
Chen H, Yan M, Yang X et al (2012) Spatial distribution and temporal variation of high fluoride contents in groundwater and prevalence of fluorosis in humans in Yuanmou County, Southwest China. J Hazard Mater 235:201–209. doi:10.1016/j.jhazmat.2012.07.042
Chidambaram S, Prasad MBK, Manivannan R, Karmegam U, Singaraja C, Anandhan P, Manikandan S (2012) Environmental hydrogeochemistry and genesis of fluoride in groundwaters of Dindigul district, Tamil Nadu (India). Environ Earth Sci 68(2):333–342. doi:10.1007/s12665-012-1741-9
Chuckpaiwong S, Nakornchai S, Surarit R, Soo-ampon S, Kasetsuwan R (2000) Fluoride in water consumed by children in remote areas of Thailand. Southeast Asian J Trop Med Public Health 31(2):319–324
Churchill HV, Rowley RJ, Martin LN (1948) Fluorine content of certain vegetation in Western Pennsylvania Area. Anal Chem 20(1):69–71. doi:10.1021/ac60013a018
Cid FD, Antón RI, Pardo R, Vega M, Caviedes-Vidal E (2011) Modelling spatial and temporal variations in the water quality of an artificial water reservoir in the semiarid Midwest of Argentina. Anal Acta 705(1):243–252. doi:10.1016/j.aca.2011.06.013
Clarke MCG, Woodhall DG, Allen D, Darling G (1990) Geological, volcanological and hydrogeological controls on the occurrence of geothermal activity in the area surrounding Lake Naivasha, Kenya. Ministry of Energy, Nairobi, Kenya; In Alemayehu T (2000) Water pollution by natural inorganic chemicals in the central part of the main Ethiopian rift. Ethiop J Sci 23(2):197–214. doi:10.4314/sinet.v23i2.18166
Currell M, Cartwright I, Raveggi M, Han D (2011) Controls on elevated fluoride and arsenic concentrations in groundwater from the Yuncheng Basin, China. Appl Geochem 26(4):540–552. doi:10.1016/j.apgeochem.2011.01.012
Czarnowski W, Wrześniowska K, Krechniak J (1996) Fluoride in drinking water and human urine in Northern and Central Poland. Sci Total Environ 191(1):177–184. doi:10.1016/0048-9697(96)05259-X
Dabeka RW, McKenzie AD (1995) Survey of lead, cadmium, fluoride, nickel and cobalt in food composites and estimation of dietary intakes of these elements by Canadians in 1986–1988. J Assoc Off Anal Chem Int 78:897–909
Dar MA, Sankar K, Dar IA (2011) Fluorine contamination in groundwater: a major challenge. Environ Monit Assess 173(1–4):955–968. doi:10.1007/s10661-010-1437-0
Das S, Mehta BC, Samanta SK, Das PK, Srivastava SK (2000) Fluoride hazards in ground water of Orissa, India. Indian J Environ Health 42(1):40–46
Das B, Talukdar J, Sarma S, Gohain B, Dutta RK, Das HB, Das SC (2003) Fluoride and other inorganic constituents in groundwater of Guwahati, Assam, India. Curr Sci 85(5):657–660
Datta PS, Deb DL, Tyagi SK (1996) Stable isotope (18O) investigations on the processes controlling fluoride contamination of groundwater. J Contam Hydrol 24(1):85–96. doi:10.1016/0169-7722(96)00004-6
Datta PS, Tyagi SK, Mookerjee P, Bhattacharya SK, Gupta N, Bhatnagar PD (1999) Groundwater NO3 and F contamination processes in Pushkar Valley, Rajasthan as reflected from 18O isotopic signature and 3H recharge studies. Environ Monit Assess 56(2):209–219. doi:10.1023/A:1005903619718
Datta DK, Gupta LP, Subramanian V (2000) Dissolved fluoride in the lower Ganges–Brahmaputra–Meghna river system in the Bengal Basin, Bangladesh. Environ Geol 39(10):1163–1168. doi:10.1007/s002549900094
Davraz A, Sener E, Sener S (2008) Temporal variations of fluoride concentration in Isparta public water system and health impact assessment (SW-Turkey). Environ Geol 56(1):159–170. doi:10.1007/s00254-007-1148-1
Deng Y, Wang Y, Ma T (2009) Isotope and minor element geochemistry of high arsenic groundwater from Hangjinhouqi, the Hetao Plain, Inner Mongolia. Appl Geochem 24(4):587–599. doi:10.1016/j.apgeochem.2008.12.018
Deng Y, Nordstrom DK, McCleskey RB (2011) Fluoride geochemistry of thermal waters in Yellowstone National Park: I. Aqueous fluoride speciation. Geochim Cosmochim Acta 75(16):4476–4489. doi:10.1016/j.gca.2011.05.028
Derakhshani R, Tavallaie M, Raoof M, Mohammadi TM, Abbasnejad A, Haghdoostf AA (2014) Occurrence of fluoride in groundwater of Zarand region, Kerman province, Iran. Fluoride 47(2):133–138
Desbarats AJ (2009) On elevated fluoride and boron concentrations in groundwaters associated with the Lake Saint-Martin impact structure, Manitoba. Appl Geochem 24(5):915–927. doi:10.1016/j.apgeochem.2009.02.016
Devadas DJ, Rao NS, Rao BT, Rao KS, Subrahmanyam A (2007) Hydrogeochemistry of the Sarada river basin, Visakhapatnam district, Andhra Pradesh, India. Environ Geol 52(7):1331–1342. doi:10.1007/s00254-006-0577-6
Dey RK, Swain SK, Mishra S et al (2012) Hydrogeochemical processes controlling the high fluoride concentration in groundwater: a case study at the Boden block area, Orissa, India. Environ Monit Assess 184(5):3279–3291. doi:10.1007/s10661-011-2188-2
Dharmagunawardhane HA, Dissanayake CB (1993) Fluoride problems in Sri Lanka. Environ Manag Health 4(2):9–16. doi:10.1108/09566169310033422
Dibal HU, Schoeneich K, Garba I, Lar UA, Bala EA (2012) Overview of fluoride distribution in major aquifer units of northern Nigeria. Health 4:1287–1294. doi:10.4236/health.2012.412189
Dissanayake CB (1991) The fluoride problem in the ground water of Sri Lanka—environmental management and health. Int J Environ Stud 38(2–3):137–155. doi:10.1080/00207239108710658
Edmunds WM, Smedley PL (2001) Fluoride in natural waters. In: Selinus O (ed) Essentials of medical geology. Springer, Netherlands, pp 311–336
EPA (2012) Report, Environmental Protection Agency, Ohio. www.epa.state.oh.us/Portals/28/documents/gwqcp/fluoride_ts.pdf. Assessed 30 March 2016
Fantong WY, Satake H, Ayonghe SN et al (2010) Geochemical provenance and spatial distribution of fluoride in groundwater of Mayo Tsanaga River Basin, Far North Region, Cameroon: implications for incidence of fluorosis and optimal consumption dose. Environ Geochem Health 32(2):147–163. doi:10.1007/s10653-009-9271-4
Farooqi A, Masuda H, Kusakabe M, Naseem M, Firdous N (2007a) Distribution of highly arsenic and fluoride contaminated groundwater from east Punjab, Pakistan, and the controlling role of anthropogenic pollutants in the natural hydrological cycle. Geochem J 41:213-234
Farooqi A, Masuda H, Firdous N (2007b) Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ Pollut 145(3):839–849. doi:10.1016/j.envpol.2006.05.007
Fawell J, Bailey J, Chilton J, Dahi E, Fewtrell, Magara Y (2006) Fluoride in drinking water. WHO report. IWA, London, UK
Frengstad B, Banks D, Siewers U (2001) The chemistry of Norwegian groundwaters: IV. The pH-dependence of element concentrations in crystalline bedrock groundwaters. Sci Total Environ 277:101–117
Fuge R, Andrews MJ (1988) Fluorine in the UK environment. Environ Geochem Health 10(3/4):96–104. doi:10.1007/BF01758677
Fuhong R, Shuqin J (1988) Distribution and formation of high-fluorine groundwater in China. Environ Geol Water Sci 12(1):3–10. doi:10.1007/BF02574820
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143(3):395–412. doi:10.1016/0022-1694(93)90201-J
Gao X, Wang Y, Li Y, Guo Q (2007) Enrichment of fluoride in groundwater under the impact of saline water intrusion at the salt lake area of Yuncheng basin, northern China. Environ Geol 53(4):795–803. doi:10.1007/s00254-007-0692-z
Garg VK, Suthar S, Singh S, Sheoran A, Jain S (2009) Drinking water quality in villages of southwestern Haryana, India: assessing human health risks associated with hydrochemistry. Environ Geol 58(6):1329–1340. doi:10.1007/s00254-008-1636-y
Genxu W, Guodong C (2001) Fluoride distribution in water and the governing factors of environment in arid north–west China. J Arid Environ 49(3):601–614. doi:10.1006/jare.2001.0810
Giri DK, Ghosh RC, Dey S, Mondal M, Kashyap DK, Dewanagan G (2013) Incidence of hydrofluorosis and its adverse effects on animal health in Durg district, Chhattisgarh. Curr Sci 105(11):1477
Giridharan L, Venugopal T, Jayaprakash M (2008) Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India. Environ Monit Assess 143(1–3):161–178. doi:10.1007/s10661-007-9965-y
Gizaw B (1996) The origin of high bicarbonate and fluoride concentrations in waters of the Main Ethiopian Rift Valley, East African Rift system. J Afr Earth Sci 22(4):391–402. doi:10.1016/0899-5362(96)00029-2
Gomez ML, Blarasin MT, Martínez DE (2009) Arsenic and fluoride in a loess aquifer in the central area of Argentina. Environ Geol 57(1):143–155. doi:10.1007/s00254-008-1290-4
Gopalakrishnan SB, Viswanathan G, Ilango SS (2012) Prevalence of fluorosis and identification of fluoride endemic areas in Manur block of Tirunelveli District, Tamil Nadu, South India. Appl Water Sci 2(4):235–243. doi:10.1007/s13201-012-0043-4
Grimaldo M, Borjaaburto VH, Ramirez AL, Ponce M, Rosas M, Diazbarriga F (1995) Endemic fluorosis in San-Luis-Potosi, Mexico. 1. Identification of risk-factors associated with human exposure to fluoride. Environ Res 68(1):25–30. doi:10.1006/enrs.1995.1004
Guo Q, Wang Y, Ma T, Ma R (2007) Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, Northern China. J Geochem Explor 93(1):1–12. doi:10.1016/j.gexplo.2006.07.001
Guo H, Yang S, Tang X, Li Y, Shen Z (2008) Groundwater geochemistry and its implications for arsenic mobilization in shallow aquifers of the Hetao Basin, Inner Mongolia. Sci Total Environ 393(1):131–144. doi:10.1016/j.scitotenv.2007.12.025
Guo H, Zhang Y, Xing L, Jia Y (2012) Spatial variation in arsenic and fluoride concentrations of shallow groundwater from the town of Shahai in the Hetao basin, Inner Mongolia. Appl Geochem 27(11):2187–2196. doi:10.1016/j.apgeochem.2012.01.016
Gupta MK, Singh V, Rajwanshi P et al (1999) Groundwater quality assessment of Tehsil Kheragarh, Agra (India) with special reference to fluoride. Environ Monit Assess 59(3):275–285. doi:10.1023/A:1006117604763
Gupta SK, Deshpande RD, Agarwal M, Raval BR (2005) Origin of high fluoride in groundwater in the North Gujarat-Cambay region, India. Hydrogeol J 13(4):596–605. doi:10.1007/s10040-004-0389-2
Gupta S, Banerjee S, Saha R, Datta JK, Mondal N (2006) Fluoride geochemistry of groundwater in Nalhati-1 block of the Birbhum district, West Bengal, India. Fluoride 39(4):318
Han Y, Yan SL, Ma HT et al (2006) Groundwater resources and environmental issues assessment in the six major basins of Shanxi province. Geological Publishing House, Beijing (in Chinese). In Li C, Gao X, Wang Y (2015) Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci Total Environ 508:155–165. doi:10.1016/j.scitotenv.2014.11.045
Handa BK (1975) Geochemistry and genesis of fluoride-containing ground waters in India. Groundwater 13(3):275–281
Heikens A, Sumarti S, Van Bergen M, Widianarko B, Fokkert L, Van Leeuwen K, Seinen W (2005) The impact of the hyperacid Ijen Crater Lake: risks of excess fluoride to human health. Sci Total Environ 346(1):56–69. doi:10.1016/j.scitotenv.2004.12.007
Heilman JR, Kiritsy MC, Levy SM, Wefel JS (1999) Assessing fluoride levels of carbonated soft drinks. J Am Dent Assoc 130:1593–1599. doi:10.14219/jada.archive.1999.0098
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, vol 2254. Department of the Interior, US Geological Survey, Virginia
Hudak PF, Sanmanee S (2003) Spatial patterns of nitrate, chloride, sulfate, and fluoride concentrations in the woodbine aquifer of North-Central Texas. Environ Monit Assess 82(3):311–320. doi:10.1023/A:1021946402095
Hussain J, Hussain I, Sharma KC (2010) Fluoride and health hazards: community perception in a fluorotic area of central Rajasthan (India): an arid environment. Environ Monit Assess 162(1–4):1–14. doi:10.1007/s10661-009-0771-6
Hussain I, Arif M, Hussain J (2012) Fluoride contamination in drinking water in rural habitations of Central Rajasthan, India. Environ Monit Assess 184(8):5151–5158. doi:10.1007/s10661-011-2329-7
Hussain J, Husain I, Arif M (2013) Fluoride contamination in groundwater of central Rajasthan, India and its toxicity in rural habitants. Toxicol Environ Chem 95(6):1048–1055. doi:10.1080/02772248.2013.832545
Ibrahim Y, Affan AA, Bjorvatn K (1995) Fluoride and fluorosis in the Sudan. First international workshop on fluorosis prevention and defluoridation of water. Ngurdoto, Tanzania, Oct 18-22. http://www.de-fluoride.net/1stproceedings/29-32.pdf. Accessed 18 May 2016
Indermitte E, Saava A, Karro E (2009) Exposure to high fluoride drinking water and risk of dental fluorosis in Estonia. Int J Environ Res Public Health 6(2):710–721. doi:10.3390/ijerph6020710
Jabal MS, Abustan I, Rozaimy MR, Al-Najar H (2014) Fluoride enrichment in groundwater of semi-arid urban area: Khan Younis City, southern Gaza Strip (Palestine). J Afr Earth Sci 100:259–266. doi:10.1016/j.jafrearsci.2014.07.002
Jacks G, Rajagopalan K, Alveteg T, Jönsson M (1993) Genesis of high-F groundwaters, southern India. Appl Geochem 8:241–244. doi:10.1016/S0883-2927(09)80043-7
Jadhav SV, Bringas E, Yadav GD, Rathod VK, Ortiz I, Marathe KV (2015) Arsenic and fluoride contaminated groundwaters: a review of current technologies for contaminants removal. J Environ Manag 162:306–325. doi:10.1016/j.jenvman.2015.07.020
Jayaprakash M, Giridharan L, Venugopal T, Kumar SK, Periakali P (2008) Characterization and evaluation of the factors affecting the geochemistry of groundwater in Neyveli, Tamil Nadu, India. Environ Geol 54(4):855–867. doi:10.1007/s00254-007-0868-6
Jayawardana DT, Pitawala HM, Ishiga H (2012) Geochemical assessment of soils in districts of fluoride-rich and fluoride-poor groundwater, north-central Sri Lanka. J Geochem Explor 114:118–125. doi:10.1016/j.gexplo.2012.01.004
Jha SK, Nayak AK, Sharma YK (2010) Potential fluoride contamination in the drinking water of Marks Nagar, Unnao district, Uttar Pradesh, India. Environ Geochem Health 32(3):217–226. doi:10.1007/s10653-009-9277-y
Jirsa F, Gruber M, Stojanovic A et al (2013) Major and trace element geochemistry of Lake Bogoria and Lake Nakuru, Kenya, during extreme draught. Chem Erde 73:275–282. doi:10.1016/j.chemer.2012.09.001
Kantharaja DC, Lakkundi TK, Basavanna M, Manjappa S (2012) Spatial analysis of fluoride concentration in groundwaters of Shivani watershed area, Karnataka state, South India, through geospatial information system. Environ Earth Sci 65(1):67–76. doi:10.1007/s12665-011-1065-1
Karro E, Indermitte E, Saava A, Haamer K, Marandi A (2006) Fluoride occurrence in publicly supplied drinking water in Estonia. Environ Geol 50(3):389–396. doi:10.1007/s00254-006-0217-1
Karthikeyan K, Nanthakumar K, Velmurugan P, Tamilarasi S, Lakshmanaperumalsamy P (2010) Prevalence of certain inorganic constituents in groundwater samples of Erode district, Tamilnadu, India, with special emphasis on fluoride, fluorosis and its remedial measures. Environ Monit Assess 160(1–4):141–155. doi:10.1007/s10661-008-0664-0
Keshavarzi B, Moore F, Esmaeili A, Rastmanesh F (2010) The source of fluoride toxicity in Muteh area, Isfahan, Iran. Environ Earth Sci 61(4):777–786. doi:10.1007/s12665-009-0390-0
Kilham P, Hecky RE (1973) Fluoride: geochemical and ecological significance in east African waters and sediments. Limnol Oceanogr 18:932–945
Kim K, Jeong GY (2005) Factors influencing natural occurrence of fluoride-rich groundwaters: a case study in the southeastern part of the Korean Peninsula. Chemosphere 58(10):1399–1408. doi:10.1016/j.chemosphere.2004.10.002
Kim Y, Kim JY, Kim K (2011) Geochemical characteristics of fluoride in groundwater of Gimcheon, Korea: lithogenic and agricultural origins. Environ Earth Sci 63(5):1139–1148. doi:10.1007/s12665-010-0789-7
Kiritsy MC, Levy SM, Warren JJ, Chowdhury GNU, Heilman JR, Marshall T (1996) Assessing fluoride concentrations of juices and juice-flavored drinks. J Am Dent Assoc 127(7):895–902. doi:10.14219/jada.archive.1996.0347
Kitano Y, Furukawa Y (1972) Distribution of fluoride in waters of Tokyo Bay. J Oceanogr Soc Japan 28(3):121–125. doi:10.1007/BF02109773
Kodate J, Pophare A, Gajbhiye R (2013) Hydrogeological impact on fluoride distribution in groundwater of western part of Bhadravati Tehsil, district Chandrapur, Maharashtra. In: Proceeding National conference on watershed management for sustainable development—WMSD
Kruse E, Ainchil J (2003) Fluoride variations in groundwater of an area in Buenos Aires Province, Argentina. Environ Geol 44(1):86–89. doi:10.1007/s00254-002-0702-0
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50(7):1025–1039. doi:10.1007/s00254-006-0275-4
Kundu MC, Mandal B (2009) Assessment of potential hazards of fluoride contamination in drinking groundwater of an intensively cultivated district in West Bengal, India. Environ Monit Assess 152(1–4):97–103. doi:10.1007/s10661-008-0299-1
Kundu N, Panigrahi M, Tripathy S, Munshi S, Powell MA, Hart B (2001) Geochemical appraisal of fluoride contamination of groundwater in the Nayagarh District of Orissa, India. Environ Geol 41(3–4):451–460. doi:10.1007/s002540100414
Lahermo P, Sandström H, Malisa E (1991) The occurrence and geochemistry of fluorides in natural waters in Finland and East Africa with reference to their geomedical implications. J Geochem Explor 41(1):65–79. doi:10.1016/0375-6742(91)90075-6
Latha SS, Ambika SR, Prasad SJ (1999) Fluoride contamination status of groundwater in Karnataka. Curr Sci 76(6):730–734
Lennon MA, Whelton H, Mullane DO, Ekstrand J (2004) Rolling revision of the WHO guidelines for drinking-water quality: fluoride. World Health Organization, Geneva, pp 1–12
Li C, Gao X, Wang Y (2015) Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci Total Environ 508:155–165. doi:10.1016/j.scitotenv.2014.11.045
Looie SB, Moore F, Malde MK, Jacks G (2013) Fluoride in groundwater, dates and wheat: estimated exposure dose in the population of Bushehr, Iran. J Food Compos Anal 29(2):94–99. doi:10.1016/j.jfca.2012.08.001
Lu Y, Sun ZR, Wu LN, Wang X, Lu W, Liu SS (2000) Effect of high-fluoride water on intelligence in children. Fluoride 33(2):74–78
Luo K, Feng F, Li H, Chou CL, Feng Z, Yunshe D (2008) Studies on geological background and source of fluorine in drinking water in the North China Plate fluorosis areas. Toxicol Environ Chem 90(2):237–246. doi:10.1080/02772240701456091
MacDonald HE (1969) Fluoride as air pollutant. Fluoride Quarterly Report 2(1):4–12. http://www.fluorideresearch.org/21/files/214-12.pdf
Madhavan N, Subramanian V (2002) Fluoride in fractionated soil samples of Ajmer district, Rajasthan. J Environ Monit 4(6):821–822. doi:10.1039/B208151B
Madhnure P, Malpe DB (2007) Fluoride contamination of groundwaters in rural parts of Yavatmal District, Maharashtra—causes and remedies. Gondwana Geol Mag 11:127–135
Madhnure P, Sirsikar DY, Tiwari AN, Ranjan HB, Malpe DB (2007) Occurrence of fluoride in the groundwaters of Pandharkawada area, Yavatmal district, Maharashtra, India. Curr Sci 92(5):675–679
Maina JW, Gaciri SJ (1984) Contributions to the hydrogeochemistry of the area to the immediate north of Nairobi Conservation Area, Kenya. J Afr Earth Sci 2(3):227–232. doi:10.1016/S0731-7247(84)80017-8
Manikandan S, Chidambaram S, Ramanathan AL et al (2014) A study on the high fluoride concentration in the magnesium-rich waters of hard rock aquifer in Krishnagiri district, Tamilnadu, India. Arab J Geosci 7(1):273–285. doi:10.1007/s12517-012-0752-x
Manocha SL, Warner H, Olkowski ZL (1975) Cytochemical response of kidney, liver and nervous system to fluoride ions in drinking water. Histochem J 7(4):343–355. doi:10.1007/BF01007019
Marshall E (1990) The fluoride debate: one more time. Science 247:276–277. doi:10.1126/science.2296717
Martin JM, Salvadori F (1983) Fluoride pollution in French rivers and estuaries. Estuar Coast Shelf Sci 17:231–242. doi:10.1016/0272-7714(83)90020-3
Meenakshi, Maheshwari RC (2006) Fluoride in drinking water and its removal. J Hazard Mater 13:456–463. doi:10.1016/j.jhazmat.2006.02.024
Meenakshi, Garg VK, Renuka K, Malik A (2004) Groundwater quality in some villages of Haryana, India: focus on fluoride and fluorosis. J Hazard Mater 106(1):85–97. doi:10.1016/j.jhazmat.2003.09.007
Messaïtfa A (2008) Fluoride contents in groundwaters and the main consumed foods (dates and tea) in Southern Algeria region. Environ Geol 55(2):377–383. doi:10.1007/s00254-007-0983-4
Misra AK, Mishra A (2007) Study of quaternary aquifers in Ganga Plain, India: focus on groundwater salinity, fluoride and fluorosis. J Hazard Mater 144(1):438–448. doi:10.1016/j.jhazmat.2006.10.057
Misra AK, Mishra A, Premraj (2006) Escalation of groundwater fluoride in the Ganga alluvial plain of India. Fluoride 39(1):35–38
Moghaddam AA, Fijani E (2008) Distribution of fluoride in groundwater of Maku area, northwest of Iran. Environ Geol 56(2):281–287. doi:10.1007/s00254-007-1163-2
Mondal NC, Prasad RK, Saxena VK, Singh Y, Singh VS (2009) Appraisal of highly fluoride zones in groundwater of Kurmapalli watershed, Nalgonda district, Andhra Pradesh (India). Environ Earth Sci 59(1):63–73. doi:10.1007/s12665-009-0004-x
Mondal D, Gupta S, Reddy DV, Nagabhushanam P (2014) Geochemical controls on fluoride concentrations in groundwater from alluvial aquifers of the Birbhum district, West Bengal, India. J Geochem Explor 145:190–206. doi:10.1016/j.gexplo.2014.06.005
Mor S, Singh S, Yadav P et al (2009) Appraisal of salinity and fluoride in a semi-arid region of India using statistical and multivariate techniques. Environ Geochem Health 31(6):643–655. doi:10.1007/s10653-008-9222-5
Msonda KWM, Masamba WRL, Fabiano E (2007) A study of fluoride groundwater occurrence in Nathenje, Lilongwe, Malawi. Phys Chem Earth Parts A/B/C 32(15):1178–1184. doi:10.1016/j.pce.2007.07.050
Mumtaz N, Pandey G, Labhasetwar PK (2015) Global fluoride occurrence, available technologies for fluoride removal and electrolytic defluoridation: a review. Crit Rev Environ Sci Technol. doi:10.1080/10643389.2015.1025638
Nair KR, Manji F, Gitonga JN (1984) The occurrence and distribution of fluoride in groundwaters of Kenya. East Afr Med J 61(7):503–512
Nanyaro JT, Aswathanarayana U, Mungure JS, Lahermo PW (1984) A geochemical model for the abnormal fluoride concentrations in waters in parts of northern Tanzania. J Afr Earth Sci 2(2):129–140. doi:10.1016/S0731-7247(84)80007-5
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 78(11):1313–1321. doi:10.1016/j.chemosphere.2010.01.010
Neal C, Smith CJ, Walls J, Billingham P, Hill S, Neal M (1990) Comments on the hydrological regulation of the halogen elements in rainfall, stemflow, throughfall and stream waters at an acidic forested area in mid-Wales. Sci Total Environ 91:1–11. doi:10.1016/0048-9697(90)90284-2
Neuhold JM, Sigler WF (1960) Effects of sodium fluoride on crap and rainbow trout. Trans Am Fish Soc 89:358–370
Nicolli HB, Bundschuh J, Blanco MDC, Tujchneider OC, Panarello HO, Dapena C, Rusansky JE (2012) Arsenic and associated trace-elements in groundwater from the Chaco-Pampean plain, Argentina: results from 100 years of research. Sci Total Environ 429:36–56. doi:10.1016/j.scitotenv.2012.04.048
Nriagu JO (1986) Chemistry of the River Niger I. Major ions. Sci Total Environ 58:81–88. doi:10.1016/0048-9697(86)90078-1
Oinam JD, Ramanathan AL, Singh G (2012) Geochemical and statistical evaluation of groundwater in Imphal and Thoubal district of Manipur, India. J Asian Earth Sci 48:136–149. doi:10.1016/j.jseaes.2011.11.017
Ortiz D, Castro L, Turrubiartes F, Milan J, Diaz-Barriga F (1998) Assessment of the exposure to fluoride from drinking water in Durango, Mexico, using a geographic information system. Fluoride 31(4):183–187
Ozsvath DL (2006) Fluoride concentrations in a crystalline bedrock aquifer Marathon County, Wisconsin. Environ Geol 50(1):132–138. doi:10.1007/s00254-006-0192-6
Pal B (1983) Potential hazards of nitrate and fluoride in underground waters. Water Res 17(3):353–354. doi:10.1016/0043-1354(83)90190-2
Paoloni JD, Fiorentino CE, Sequeira ME (2003) Fluoride contamination of aquifers in the southeast subhumid pampa, Argentina. Environ Toxicol 18(5):317–320. doi:10.1002/tox.10131
Periakali P, Subramanian S, Eswaramoorthi S, Arul B, Rao NR, Sridhar SGD (2001) Distribution of fluoride in the ground water of Salem and Namakkal Districts, Tamil Nadu. J Appl Geochem 3(2):120–132
Pettenati M, Perrin J, Pauwels H, Ahmed S (2013) Simulating fluoride evolution in groundwater using a reactive multicomponent transient transport model: application to a crystalline aquifer of Southern India. Appl Geochem 29:102–116. doi:10.1016/j.apgeochem.2012.11.001
Pickering WF (1985) The mobility of soluble fluoride in soils. Environ Pollut Ser B Chem Phys 9(4):281–308. doi:10.1016/0143-148X(85)90004-7
Queste A, Lacombe M, Hellmeier W, Hillermann F, Bortulussi B, Kaup M, Ott K, Mathys W (2001) High concentrations of fluoride and boron in drinking water wells in the Muenster region—results of a preliminary investigation. Int J Hyg Environ Health 203(3):221–224. doi:10.1078/S1438-4639(04)70032-2
Rafique T, Naseem S, Bhanger MI, Usmani TH (2008) Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert, Pakistan. Environ Geol 56(2):317–326. doi:10.1007/s00254-007-1167-y
Raju NJ, Dey S, Das K (2009) Fluoride contamination in groundwaters of Sonbhadra district, Uttar Pradesh, India. Curr Sci 96(7):979–985
Ramanaiah SV, Mohan SV, Rajkumar B, Sarma PN (2006) Monitoring of fluoride concentration in ground water of Prakasham district in India: correlation with physico-chemical parameters. J Environ Sci Eng 48(2):129
Rango T, Bianchini G, Beccaluva L, Ayenew T, Colombani N (2009) Hydrogeochemical study in the Main Ethiopian Rift: new insights to the source and enrichment mechanism of fluoride. Environ Geol 58(1):109–118. doi:10.1007/s00254-008-1498-3
Rango T, Bianchini G, Beccaluva L, Tassinari R (2010) Geochemistry and water quality assessment of central Main Ethiopian Rift natural waters with emphasis on source and occurrence of fluoride and arsenic. J Afr Earth Sci 57(5):479–491. doi:10.1016/j.jafrearsci.2009.12.005
Rango T, Kravchenko J, Atlaw B, McCornick PG, Jeuland M, Merola B, Vengosh A (2012) Groundwater quality and its health impact: an assessment of dental fluorosis in rural inhabitants of the Main Ethiopian Rift. Environ Int 43:37–47. doi:10.1016/j.envint.2012.03.002
Rao NS (1997) The occurrence and behaviour of fluoride in the groundwater of the Lower Vamsadhara River basin, India. Hydrol Sci J 42(6):877–892. doi:10.1080/02626669709492085
Rao NS (2003) Groundwater quality: focus on fluoride concentration in rural parts of Guntur district, Andhra Pradesh, India. Hydrol Sci J 48(5):835–847. doi:10.1623/hysj.48.5.835.51449
Rao NS (2009) Fluoride in groundwater, Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 152(1–4):47–60. doi:10.1007/s10661-008-0295-5
Rao NS, Devadas DJ (2003) Fluoride incidence in groundwater in an area of Peninsular India. Environ Geol 45(2):243–251. doi:10.1007/s00254-003-0873-3
Rao NR, Rao N, Rao KSP, Schuiling RD (1993) Fluorine distribution in waters of Nalgonda district, Andhra Pradesh, India. Environ Geol 21(1–2):84–89. doi:10.1007/BF00775055
Rao NS, Rao PS, Dinakar A, Rao PVN, Marghade D (2015) Fluoride occurrence in the groundwater in a coastal region of Andhra Pradesh, India. Appl Water Sci. doi:10.1007/s13201-015-0338-3
Ravindra K, Garg VK (2006) Distribution of fluoride in groundwater and its suitability assessment for drinking purpose. Int J Environ Health Res 16(2):163–166. doi:10.1080/09603120500538283
Ray D, Rao RR, Bhoi AV, Biswas AK, Ganguly AK, Sanyal PB (2000) Physico-chemical quality of drinking water in Rohtas district of Bihar. Environ Monit Assess 61(3):387–398. doi:10.1023/A:1006165615097
Razo LMD, Corona JC, García-Vargas G, Albores A, Cebrián ME (1993) Fluoride levels in well-water from a chronic arsenicism area of northern Mexico. Environ Pollut 80(1):91–94. doi:10.1016/0269-7491(93)90015-G
Reddy DV, Nagabhushanam P, Sukhija BS, Reddy AGS, Smedley PL (2010) Fluoride dynamics in the granitic aquifer of the Wailapally watershed, Nalgonda District, India. Chem Geol 269(3):278–289. doi:10.1016/j.chemgeo.2009.10.003
Reyes-Gómez VM, Alarcón-Herrera MT, Gutiérrez M, López DN (2015) Arsenic and fluoride variations in groundwater of an endorheic basin undergoing land-use changes. Arch Environ Contam Toxicol 68(2):292–304. doi:10.1007/s00244-014-0082-y
Robertson FN (1985) Solubility controls of fluorine, barium and chromium in ground water in alluvial basins of Arizona. In: Practical applications of ground-water geochemistry conference, Banff, Canada, June 1984, conference proceedings, pp 96–102
Rosso JJ, Troncoso JJ, Cirelli AF (2011) Geographic distribution of arsenic and trace metals in lotic ecosystems of the Pampa Plain, Argentina. Bull Environ Contam Toxicol 86:129–132. doi:10.1007/s00128-010-0177-8
Salve PR, Maurya A, Kumbhare PS, Ramteke DS, Wate SR (2008) Assessment of groundwater quality with respect to fluoride. Bull Environ Contam Toxicol 81(3):289–293. doi:10.1007/s00128-008-9466-x
Saxena V, Ahmed S (2001) Dissolution of fluoride in groundwater: a water-rock interaction study. Environ Geol 40(9):1084–1087. doi:10.1007/s002540100290
Saxena V, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43(6):731–736. doi:10.1007/s00254-002-0672-2
Schamschula R, Duppenthaler J, Sugar E, Toth K, Barmes D (1988) Fluoride intake and utilization by Hungarian children: associations and interrelationships. Acta Physiol Hung 72:253–261
Senior LA, Sloto RA (2006) Arsenic, boron, and fluoride concentrations in ground water in and near diabase intrusions, Newark Basin, Southeastern Pennsylvania. Scientific investigation report, 2006-5261. US Department of the interior, USGS. http://pubs.usgs.gov/sir/2006/5261/pdf/sir2006-5261.pdf. Accessed 18 May 2016
Shah MT, Danishwar S (2003) Potential fluoride contamination in the drinking water of Naranji area, northwest frontier province, Pakistan. Environ Geochem Health 25(4):475–481. doi:10.1023/B:EGAH.0000004579.54947.e7
Shaji E, Bindu JV, Thambi DS (2007) High fluoride in groundwater of Palghat District, Kerala. Curr Sci 92(2):240–245
Sharma BS, Agrawal J, Gupta AK (2011) Emerging challenge: fluoride contamination in groundwater in Agra District, Uttar Pradesh. Asian J Exp Biol Sci 2(1):131–134
Shekhar S, Sarkar A (2013) Hydrogeological characterization and assessment of groundwater quality in shallow aquifers in vicinity of Najafgarh drain of NCT Delhi. J Earth Syst Sci 122(1):43–54. doi:10.1007/s12040-012-0256-9
Shortt HE, Pandit CG, Raghavachari TNS (1937) Endemic fluorosis in the Nellore District of South India. Indian Med Gaz 72:396–398. In Rao NS, Rao PS, Dinakar A, Rao PVN, Marghade D (2015) Fluoride occurrence in the groundwater in a coastal region of Andhra Pradesh, India. Appl Water Sci. doi:10.1007/s13201-015-0338-3
Sigler WF, Neuhold JM (1972) Fluoride intoxication in fish: a review. J Wildl Dis 8:252–254. doi:10.7589/0090-3558-8.3.252
Singh CK, Mukherjee S (2014) Aqueous geochemistry of fluoride enriched groundwater in arid part of Western India. Environ Sci Pollut Res 22(4):2668–2678. doi:10.1007/s11356-014-3504-5
Singh V, Narain R, Prakash C (1987) Fluoride in irrigation waters of Agra district, Uttar Pradesh. Water Res 21(8):889–890. doi:10.1016/S0043-1354(87)80004-0
Singh B, Gaur S, Garg VK (2007) Fluoride in drinking water and human urine in Southern Haryana, India. J Hazard Mater 144(1):147–151. doi:10.1016/j.jhazmat.2006.10.010
Singh AK, Mondal GC, Kumar S, Singh TB, Tewary BK, Sinha A (2008) Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ Geol 54(4):745–758. doi:10.1007/s00254-007-0860-1
Singh CK, Rina K, Singh RP, Shashtri S, Kamal V, Mukherjee S (2011) Geochemical modeling of high fluoride concentration in groundwater of Pokhran area of Rajasthan, India. Bull Environ Contam Toxicol 86(2):152–158. doi:10.1007/s00128-011-0192-4
Skjelkvåle BL (1994) Factors influencing fluoride concentrations in Norwegian lakes. Water Air Soil Pollut 77:151–167. doi:10.1007/BF00483055
Smedley PL, Nicolli HB, Macdonald DMJ, Barros AJ, Tullio JO (2002) Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl Geochem 17(3):259–284. doi:10.1016/S0883-2927(01)00082-8
Sracek O, Wanke H, Ndakunda NN, Mihaljevič M, Buzek F (2015) Geochemistry and fluoride levels of geothermal springs in Namibia. J Geochem Explor 148:96–104. doi:10.1016/j.gexplo.2014.08.012
Sreedevi PD, Ahmed S, Madé B, Ledoux E, Gandolfi JM (2006) Association of hydrogeological factors in temporal variations of fluoride concentration in a crystalline aquifer in India. Environ Geol 50(1):1–11. doi:10.1007/s00254-005-0167-z
Srikanth R, Viswanatham KS, Kahsai F, Fisahatsion A, Asmellash M (2002) Fluoride in groundwater in selected villages in Eritrea (North East Africa). Environ Monit Assess 75(2):169–177. doi:10.1023/A:1014491915537
Srinivasamoorthy K, Vijayaraghavan K, Vasanthavigar M, Sarma S, Chidambaram S, Anandhan P, Manivannan R (2012) Assessment of groundwater quality with special emphasis on fluoride contamination in crystalline bed rock aquifers of Mettur region, Tamilnadu, India. Arab J Geosci 5(1):83–94. doi:10.1007/s12517-010-0162-x
Srivastava SK, Gupta RK, Bhargav JS (2000) Distribution of high fluoride in groundwater of Puri District, Orissa. Pollut Res 19(2):279–284
Su C, Wang Y, Xie X, Zhu Y (2015) An isotope hydrochemical approach to understand fluoride release into groundwaters of the Datong Basin, Northern China. Environ Sci Process Impacts 17(4):791–801. doi:10.1039/C4EM00584H
Sujatha D (2003) Fluoride levels in the groundwater of the south-eastern part of Ranga Reddy district, Andhra Pradesh, India. Environ Geol 44(5):587–591. doi:10.1007/s00254-003-0795-0
Sujatha D, Reddy BR (2003) Quality characterization of groundwater in the south-eastern part of the Ranga Reddy district, Andhra Pradesh, India. Environ Geol 44(5):579–586. doi:10.1007/s00254-003-0794-1
Susheela AK (1999) Fluorosis management programme in India. Curr Sci 77(10):1250–1256
Susheela AK, Kumar A, Bhatnagar M, Bahudur R (1993) Prevalence of endemic fluorosis with gastro-intestinal manifestations in people living in some North-Indian villages. Fluoride 26(2):97–104
Suthar S, Garg VK, Jangir S, Kaur S, Goswami N, Singh S (2008) Fluoride contamination in drinking water in rural habitations of Northern Rajasthan, India. Envir Monit Assess 145(1–3):1–6. doi:10.1007/s10661-007-0011-x
Takahashi K, Akiniwa K, Narita K (2001) Regression analysis of cancer incidence rates and water fluoride in the USA based on IACR/IARC (WHO) data (1978–1992). J Epidemiol 11(4):170–179
Tekle-Haimanot R, Melaku Z, Kloos H, Reimann C, Fantaye W, Zerihun L, Bjorvatn K (2006) The geographic distribution of fluoride in surface and groundwater in Ethiopia with an emphasis on the Rift Valley. Sci Total Environ 367(1):182–190. doi:10.1016/j.scitotenv.2005.11.003
Thakur JK, Singh P, Singh SK, Bhaghel B (2013) Geochemical modelling of fluoride concentration in hard rock terrain of Madhya Pradesh, India. Acta Geol Sin (English Edition) 87(5):1421–1433
Thole B (2013) Ground water contamination with fluoride and potential fluoride removal technologies for East and Southern Africa. In Ahmad, Dar (ed) Perspective in water pollution. http://www.intechopen.com. Accessed 18 May 2016
Tirumalesh K, Shivanna K, Jalihal AA (2007) Isotope hydrochemical approach to understand fluoride release into groundwaters of Ilkal area, Bagalkot District, Karnataka, India. Hydrogeol J 15(3):589–598
Tohyama E (1996) Relationship between fluoride concentration in drinking water and mortality rate from uterine cancer in Okinawa Prefecture, Japan. J Epidemiol 6(4):184–191
Turiel JF, Pérez-Miranda C, Almada G, Medina M, Riviere C, Gordillo M (2003) Surface-water quality for the Salí River watershed in NW Argentina. Environ Geol 43(8):941–949
Van Craenenbroeck W, Marivoet J (1987) A comparison of simple methods for estimating the mass flow of fluoride discharged into rivers. Water Sci Technol 19:729–740
Van Der Hoek W, Ekanayake L, Rajasooriyar L, Karunaratne R (2003) Source of drinking water and other risk factors for dental fluorosis in Sri Lanka. Int J Environ Health Res 13(3):285–293. doi:10.1080/0960312031000122433
Vasquez LV, Ramirez-Hernandez J, Reyes-Lopez J, Sol-Uribe A, Lazaro-Mancilla O (2006) The origin of fluoride in groundwater supply to Hermosillo City, Sonora, Mexico. Environ Geol 51(1):17–27
Vikas C, Kushwaha RK, Pandit MK (2009) Hydrochemical status of groundwater in district Ajmer (NW India) with reference to fluoride distribution. J Geol Soc India 73(6):773–784
Viswanathan G, Jaswanth A, Gopalakrishnan S, Aditya G (2009) Determining the optimal fluoride concentration in drinking water for fluoride endemic regions in South India. Sci Total Environ 407(20):5298–5307
Wei SHY, Hattab FN, Mellberg JR (1989) Concentration of fluoride and other selected elements in teas. Nutrition 5:237–240
Whitford GM (1997) Determinants and mechanisms of enamel fluorosis. Ciba Found Symp 205:226–241
WHO (2004) Guidelines for drinking-water quality: recommendations, vol 1. World Health Organization, Geneva
Wodeyar BK, Sreenivasan G (1996) Occurrence of fluoride in the groundwaters and its impact in Peddavankahalla Basin, Bellary District, Karnataka, India—a preliminary study. Curr Sci 70:71–74
Xu F, Ma T, Shi L, Zhang JW, Wang YY, Dong YH (2013) The hydrogeochemical characteristics of high iodine and fluoride groundwater in the Hetao plain, Inner Mongolia. Procedia Earth Planet Sci 7:908–911
Yang CS, Chung JY, Yang GY, Chhabra SK, Lee MJ (2000) Tea and tea polyphenols in cancer prevention. J Nut 130(2):472S–478S
Yasmin S, Monterio S, Ligimol PA, D’Souza D (2011) Fluoride contamination and fluorosis in Gaya Region of Bihar, India. Curr Biot 5(2):232–236
Young SM, Pitawala A, Ishiga H (2011) Factors controlling fluoride contents of groundwater in north-central and northwestern Sri Lanka. Environ Earth Sci 63(6):1333–1342
Zeng Z (1997) The control factors of the f formation in ground-water and its distributive regularity [j]. jilin geolgy 4; In Chen H, Yan M, Yang X et al (2012) Spatial distribution and temporal variation of high fluoride contents in groundwater and prevalence of fluorosis in humans in Yuanmou County, Southwest China. J Hazard Mat 235:201–209. doi:10.1016/j.jhazmat.2012.07.042
Zingde MD, Mandalia AV (1988) Study of fluoride in polluted and unpolluted estuarine environments. Estuar Coast Shelf Sci 27:707–712
Acknowledgments
The first author (SA) acknowledges University of Delhi for financial support through Non-NET scholarship. The third author (AS) acknowledges CSIR for financial support through SRF. We thank Eric Lichtfouse, Editor in Chief and two anonymous reviewers for their critical comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, S., Thakur, S.K., Sarkar, A. et al. Worldwide contamination of water by fluoride. Environ Chem Lett 14, 291–315 (2016). https://doi.org/10.1007/s10311-016-0563-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-016-0563-5