Abstract
The chemical characteristics of surface, groundwater and mine water of the upper catchment of the Damodar River basin were studied to evaluate the major ion chemistry, geochemical processes controlling water composition and suitability of water for domestic, industrial and irrigation uses. Water samples from ponds, lakes, rivers, reservoirs and groundwater were collected and analysed for pH, EC, TDS, F, Cl, HCO3, SO4, NO3, Ca, Mg, Na and K. In general, Ca, Na, Mg, HCO3 and Cl dominate, except in samples from mining areas which have higher concentration of SO4. Water chemistry of the area reflects continental weathering, aided by mining and other anthropogenic impacts. Limiting groundwater use for domestic purposes are contents of TDS, F, Cl, SO4, NO3 and TH that exceed the desirable limits in water collected from mining and urban areas. The calculated values of SAR, RSC and %Na indicate good to permissible use of water for irrigation. High salinity, %Na, Mg-hazard and RSC values at some sites limit use for agricultural purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is a natural resource to basic human need and a precious national asset. India accounts for 2.2% of the global land and 4% of the world water resources, but must support 16% of the world population. Water bodies are continuously in natural dynamic state and are sometimes upset by human activities. More than half of India’s water resources are either polluted or under pollution threat. Rapid growth in population, haphazard industrialization and sprawl of urban centers coupled with lack of adequate resources in rural areas, have created critical situations for water resources. Quality is of equal importance to quantity in water resource evaluation and pollution threatens to reduce the quantity of usable waters. One of the oldest records of water pollution is direct dumping of human wastes into water bodies. Mining is also a major activity causing water pollution (Allen et al. 1996; Choubey 1991; Galero et al. 1998; Ratha and Venkataraman 1997). Damage to quality and quantity of surface and groundwater due to mining and dumping of overburden and spoils; sometimes on the surface water body itself, spreading of overburden through rolling and washing causes chemical pollution. The Damodar River, one of the most polluted rivers of India and flows through the country’s richest coal mining belt (Tiwary and Dhar 1994; Dey 1981, 1985; Dey et al. 1987). Industries, thermal power plants, mining machineries and vehicular sources are added point and non-point sources of pollution.
Damodar River basin
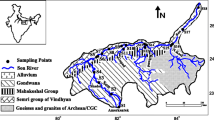
The Damodar River basin extends from 22°45′N to 24°30′N and 84°45′E to 88°00′E and circumscribes parts of Jharkhand and West Bengal. It is about 11.7 and 8.5% of the total area of these two states (Fig. 1). Damodar is a small rain-fed river (541 km long) flowing through the cities of Ramgarh, Bokaro, Dhanbad, Asansol, Durgapur, Bardwan and Hawrah, before joining the lower Ganga (Hooghly Estuary) at Shayampur 55 km downstream of Hawrah. The river basin is underlain by granites and granitic gneisses of Archeans, sandstones and shales of the Gondwanas and the Recent alluvials. The geology of the lower Damodar is quite distinct from the upper basin. The lithology of the upper catchment is quartzites, quartz-mica-schists, biotite gneisse, biotite-schist, garnetiferous-gneiss and schist, acid granulites with hornblend and amphibolites of Archean age (Ghose 1983). Gondwana rocks consisting of sandstones, shales and fire clays with coal seams also form the part of the upper catchment. Though not rich in metallic minerals, the Damodar basin is the storehouse of coal and contains 46% of the coal reserves of India. Other than coal, fire clay, bauxite, mica, limestones occur within the basin.
Groundwater in the upper catchment is circulated in secondary openings of joints, cracks and fissures. The weathered residuums of the hard rocks as well as other zones of discontinuity are the principle repositories of groundwater in the area. The weathered zone is usually of limited thickness and fractures and joints openings cease with depth. The thickness of weathered mantle in the hard rock zone of area is about 15–25 m in the topographic lows. Groundwater in the weathered and fracture zones of hard rocks occur under unconfined condition. Groundwater circulating through the fracture zone is sometimes held under pressure. Depth of the water table in the hard rock of the area generally ranges from 2.0 m to 10.0 m below ground level. The Gondwana sediments form the semi-consolidated formations and have better water bearing potential. The splintery shales of Talchir and basal pebbles bed, the variegated Barren Measure shales and the Barakar sandstones are the major lithounits of the Gondwana Formations. Gondwana sandstones in general are good aquifers; however, their yield at active mines is poor from dewatering. Groundwater occurs under unconfined condition in the weathered mantles at depths varying from 7 to 14 m and in semiconfined conditions in the deeper aquifers. Water levels in the pre-monsoon period vary from 3 to 5 m bgl around Jharia in a narrow stretch to deeper depths of 5–7 m in the outlying area and at depths of 7–9 m around Mahuda and along the Jamunia River in the west. The post-monsoon water levels rises become 1–2 m bgl near Jharia and 2–3 m in outlying area to a range of 4–5 m around Mahuda and along the Jamunia River.
Materials and methods
Representative 102 groundwater (60 tube wells + 42 dug wells) and 73 surface water samples (ponds, lakes, rivers and reservoirs) were collected from urban, rural, industrial and mining areas of the upper Damodar River basin in pre-monsoon months (Figs. 2, 3). Twelve mine water samples were also collected from opencast and underground coal mines of Jharia region for geochemical analysis. The samples were collected in 1-l narrow-mouth pre-washed polyethylene bottles. Electrical conductivity (EC) and pH values were measured in the field using a portable conductivity and pH meter. In the laboratory, the water samples were filtered through 0.45 μm Millipore membrane filters to separate suspended sediments. The acid titration method was used to determine the concentration of bicarbonate (APHA 1992). Major anions (F, Cl, NO3, SO4) were analysed by ion chromatograph (Dionex Dx-120) using anion AS12A/AG12 column coupled with an anion self-regenerating suppressor (ASRS) in recycle mode. A combination of Na2CO3 and NaHCO3 was used as an eluent maintained at flow rate of 1.15 ml min−1. Major cations (Ca, Mg, Na, K) were also measured by ion chromatograph by using cation column (CS12A/CS12G) and cation self-regenerating suppressor (CSRS) in recycle mode. The analytical precision was maintained by running a known standard after every ten samples. An overall precision, expressed as percent relative standard deviation (RSD), was obtained for the entire samples. Analytical precision for cations (Ca, Mg, Na, and K) and anions (F, Cl, NO3, HCO3 and SO4) was within 10%. Cationic and anionic charge balance (<10%) is an added proof of the precision of the data.
Results and discussion
Major ion chemistry
Major ion chemistry of groundwater, surface water and mine water are given in Table 1. The results of the physico-chemical analysis of groundwaters collected from different locations of Hazaribagh, Koderma, Giridih, Bokaro and Dhanbad districts of upper Damodar River catchment are summarized in Table 2. The salient features of the major ion chemistry of the area are as follows:
Groundwater
-
The pH of the analysed samples varies from 6.46 to 8.49 in tube wells and 6.69–8.87 in dug wells. In general, pH of water samples was slightly acidic to alkaline in nature and the mean value of pH of groundwater of the area was 7.71.
-
The EC values vary from 170 to 1,846 μS cm−1 for tube well water and 218–1,918 μS cm−1 for dug wells. The average conductivity value (892 μS cm−1) for dug wells was slightly higher as compared to tube wells (844 μS cm−1).
-
Total dissolved solids (TDS), varied from 123 to 1,323 mg l−1 (avg. 639 mg l−1) in tube wells and 144–1,552 mg l−1 (avg. 741) in dug wells.
-
Shallow aquifer (dug wells) water has higher concentration of dissolved ions as compared to the deep tube wells and surface water.
-
Spatial distribution of TDS concentration in the groundwater is illustrated in Fig. 4. Higher concentration of TDS was observed in the samples collected from active mining and industrial sites especially from the south of Dhanbad, Chas-Bokaro, Nirsa, Koderma and Giridih sites.
-
Figures 5, 6 and 7 show that water samples collected from mining and effluents disposal sites, especially around Jharia, Bokaro, Koderma and Giridih sites, have high concentration of SO4, Cl and total hardness (TH).
-
The large variations in the EC, TDS and ionic concentrations in the groundwater of the area may be attributed to variation in geochemical processes and the impact of mining and anthropogenic activities (Mohan et al. 2000; Singh and Hasnain 1999).
-
The cation chemistry is dominated by calcium, constituting 48 and 45% of the total cations (TZ+) respectively, in tube wells and dug wells. Calcium was followed by magnesium, sodium and potassium ions. The cation chemistry indicated that 42% of the groundwater samples are Ca > Mg > Na > K while 36% belong to Ca > Na > Mg > K and 15% of Na > Ca > Mg > K. Only 7% samples are Mg > Ca > Na > K type. Weathering and cation exchange processes normally control the levels of these cations.
-
HCO3 and Cl were the dominant ions in anion chemistry in tube well and dug well waters. Bicarbonate contributes an average of 53 and 50% to the total anions followed by chloride (26 and 27%) and sulphate (17 and 19%) respectively, in tube well and dug well with minor contribution (<5%) from nitrate and fluoride.
-
The chloride concentration varied between 3.6 and 341 mg l−1 in tube wells and between 2.1 and 392 mg l−1 in dug wells. The mean concentration of chloride in tube wells and dug wells water samples was 97 and 102 mg l−1, respectively.
-
Concentration of nitrate ranged from 0.09 to 232 mg l−1 (avg. 23.8) in tube wells and 0.02–217 mg l−1 (avg. 26.6 mg l−1) in dug well water. The concentration of nitrate was relatively high in some dug well and tube well water samples. However, in majority of the samples, nitrate was below the threshold value of WHO (1997) and BIS (1991).
-
Concentration of fluoride was low in major part of the river basin, except in some samples where it exceeded 1.5 mg l−1 limit. Concentration of fluoride was high in the water from Koderma and some wells of north-eastern part of Giridih districts (Fig. 8). Low concentration of fluoride in the major part of the study area indicated limited lithogenic input of this ion.
Surface water
For physico-chemical characterization of surface water, 28 samples from ponds/lakes, 30 from Tilaiya, Konar, Tenughat, Panchet and Mainthon reservoirs and 15 from Damodar River and its tributaries were analysed in Table 1. The major features of the major ion chemistry of surface water are as follows:
-
Surface water is alkaline with a pH between 7.30 and 8.62 in pond water and 7.60–8.23 in river/reservoir water.
-
EC, TDS and ionic concentration of dissolved species are relatively higher in ponds/lake water as compared to the river and reservoir water.
-
Calcium was the dominant cation, contributing 37 and 45%, respectively, in water from ponds/lakes and river/reservoir. The relative contribution of sodium was higher in pond water (30%) as compared to river/reservoir water (26%). Potassium was the least dominant cation (4 and 5%) in water of ponds and river/reservoirs.
-
Anion chemistry was dominated by HCO3 constituting 47 and 75% of the total anions, respectively, in ponds/lake and river/reservoir. SO4 was the dominant anion in the pond water samples collected near the mining and industrial sites and its concentration varied between 2.1 and 624 mg l−1. SO4 constitutes 24 and 13% of the total anions in the ponds and river/reservoirs. Contribution of chloride in anionic budget is high (28%) in ponds/lakes as compared to the river/reservoirs (10%).
-
Concentration of NO3 and F was low in the analysed surface water of the area, except in some of the ponds.
Mine water
-
pH of the mine water of the area was alkaline in nature (7.60–8.50) and conductivity varied between 372 and 1,642 μS cm−1. The average conductivity (1,009 μS cm−1) and TDS (839 mg l−1) values for mine water are higher as compared to the groundwater (864 μS cm−1 and 669 mg l−1) and surface water (411 μS cm−1 and 303 mg l−1).
-
SO4 was the most dominant ion in the mine water, constituting 30% of the TDS and contributing 69% to total anionic balance in equivalent unit.
-
Contribution of HCO3 and Cl to the total anionic balance (TZ−) was low as compared to the ground and surface water, indicating different geochemical processes controlling the water composition.
-
Calcium and magnesium are the dominant cations in the mine water contributing 48 and 32% of the total cations (TZ+) followed by sodium (18%) and potassium (2%).
Weathering and solute acquisition processes
The major ion chemistry of the aquatic system was mainly controlled by weathering of rock forming minerals with minor contribution from atmospheric and anthropogenic sources (Berner and Berner 1987; Sarin et al. 1989; Singh and Hasnain 2002). The dissolution of CO2 and oxidation of sulphides are the two major reactions providing the bulk of the protons used to chemically weather carbonates, silicates and aluminosilicate minerals. The dissolution of carbonate rocks proceeds more rapidly than silicate breakdown and was the likely mechanism of solute acquisition in aquatic systems. However, the solution products of silicate weathering are difficult to quantify because the degradation of silicates incongruently generates a variety of solid phases (mostly clays) along with dissolved species. The relative proportions of the various ions in solution depend on their relative abundance in the host rock as well as on their solubility (Sarin et al. 1989).
The abundance of various ions can be modeled in terms of weathering of various rock forming minerals (Sarin et al. 1989; Singh and Hasnain 1998; Pandey et al. 1999). The proportion of HCO3 and SO4 in the water reflects the relative dominance of the two major sources of protons, i.e., carbonation and sulphide oxidation, during chemical weathering. The ternary anion diagram relating HCO3, SO4 and Cl shows that most of the samples of groundwater and surface water contain a high amount of HCO3 and plotted points cluster towards the alkalinity apex with secondary trends towards Cl and SO4 (Fig. 9a). The relative high ratio of HCO3/(HCO3 + SO4) in most of the surface and sub-surface water (>0.5) signified that carbonic acid weathering was the major proton producer in these waters (Pandey et al. 2001). The bicarbonates are derived mainly from the soil zone CO2 and weathering of parent minerals. The soil zone in the subsurface contains elevated CO2 pressure (produced by decay of organic matter and root respiration), which in turn combines with rainwater to form bicarbonate (Drever 1988).
Bicarbonate may also be derived from the dissolution of carbonates and/or silicate minerals by the carbonic acid:
The plotted points in the ternary anion diagram for mine water and surface and subsurface water collected near the mining sites cluster towards the SO4 apex with minor contribution from HCO3 and Cl (Fig. 9a). The low ratio of HCO3/(HCO3 + SO4) for mine water (0.22) and water collected near coal mining areas (<0.5) suggests that either sulphide oxidation and/or coupled reactions (involving carbonic acid weathering and sulphide oxidation) control the solute acquisition process in the affected mining areas. On a global basis, one third of the SO4 in aquatic systems is derived from rock weathering and about 60% from fossil fuel burning with minor amounts from volcanism (5%) and cycling salts (2%). Rock weathering sources typically contribute two major forms of sulphur in sedimentary rocks, pyrite—FeS2 and gypsum—CaSO4 (Berner and Berner 1987). Pyrite (FeS2) occurs as a secondary mineral in the Gondwana coals and associated sediments. The observed high values of sulphate in waters of the affected mining area may be attributed to the oxidative weathering of pyrites by reaction sequence (Lowson et al. 1993):
High concentration of sulphate in ground and mine water from the mining belt of Jharia and other coal mining regions of India are also reported by other workers (Choubey 1991; Tiwary 2001).
The bulk of the chloride in water is assumed to be either from atmospheric sources or from sea water. Abnormal concentrations of chloride may also result from pollution by sewage wastes and leaching of saline residues in the soil (Appelo and Postma 1993). A rough estimate of the atmospheric contribution of Cl to the aquatic system can be assessed by comparing the chemical composition with the local rainwater chemistry (Pandey et al. 1994; Sarin et al. 1989). The low concentration of Cl in the rainwater of the study area indicates limited atmospheric contribution (Singh et al. 2005, 2007). Further the large lateral variations and high concentrations of chloride in some surface and groundwater may be attributed to the local recharge and contamination by anthropogenic sources such as domestic waste effluents and untreated industrial discharge.
There are three major sources of nitrogen in the water—biological fixation, precipitation, and the application of fertilizers (Berner and Berner 1987). Human activities have also influenced the nitrogen load considerably. Anthropogenic sources of nitrogen include (1) point source including industrial sewage, refuse dumps etc, discharged directly into the surface water, (2) diffuse source including runoff and leaching from rural and urban land and (3) precipitation (Kumar and Anderson 1993). The concentration of nitrate was relatively high in some dug well and surface water samples attributed to the anthropogenic sources. The fluoride was usually derived from the weathering of fluoride bearing rock forming minerals like muscovite, biotite, fluorite, fluoro-apatite, etc. Koderma district is rich in mineral deposits and was famous for its mica production worldwide. The Great Mica-Belt of Jharkhand and Bihar extends for a distance of 160 km with an average width of 25 km passing through this and adjoining districts of Hazaribagh and Giridih. The observed high values of fluoride around Koderma may be attributed to the weathering product of the fluorite bearing minerals associated with the rock formation of the area.
The cation diagram (Fig. 9b) relating Ca, Mg and (Na + K) shows that although in the majority of samples (Ca + Mg) exceeds (Na + K); however in some samples contribution of alkalies (Na + K) exceeds alkaline earths (Ca + Mg). Further, the observed low ratio of (Ca + Mg)/(Na + K) i.e., 4.12 and relatively high contribution of alkalies (30%) towards the total cations suggest that coupled reactions involving carbonate and silicate weathering, control the solute acquisition process. High concentration of Ca and Mg is attributed to the weathering of crystalline dolomitic limestones and Ca–Mg silicates (amphiboles, pyroxenes, olivine, biotite). The dolomitic limestones usually occur as isolated bands and lenticles of varying dimensions in the study area, while the basic rocks occur as Archean lavas and are intrusive at some places (Ghosh 1983). The sodium and potassium in the aquatic system is derived from the atmospheric deposition, evaporite dissolution and silicate weathering. The weathering of Na and K silicate minerals like albite, anorthite, orthoclase and microcline are the major possible sources of Na and K. In addition, dissolution of Na/K salts developed in the drainage basin due to cycling wetting and drying phases during high and low flow regimes of Damodar River may also contribute sodium and potassium (Sarin et al. 1989; Singh et al. 2005). The high concentration of K in some groundwater and pond water may be attributed to anthropogenic activities.
Saturation indices and dissolution/precipitation of carbonates
The potential for a chemical reaction can be determined by calculating the chemical equilibrium of the water with the mineral phase. The equilibrium state of the water with respect to a mineral phase can be determined by calculating a saturation index (SI) using analytical data (Garrels and Mackenzie 1971; Stumm and Morgan 1981). The SI is defined as the logarithm of the ratio of ion activity product (IAP) to the mineral equilibrium constant at a given temperature and given as:
A positive SI specifies that the water being supersaturated with respect to the particular mineral phase and therefore incapable of dissolving more of the mineral and under suitable physico-chemical condition, the mineral phase in equilibrium may precipitate. A negative index (SI) indicates undersaturation condition and dissolution of mineral phase, while neutral SI is in equilibrium state with the mineral phase. The plot of saturation indices of calcite (SIc) versus dolomite (SId) demonstrate that most of the waters are supersaturated with respect to dolomite and calcite and the SId values are higher than the SIc values (Fig. 10). The supersaturation indicates the precipitation of calcium as Ca and/or Ca–Mg carbonate. This may signify the presence of calcareous nodules (kankar), which contain a mixture of calcite and dolomite in the area. About 25% of the analysed water samples are undersaturated with calcite and dolomite. An analysis plotting in this field represents water that has come from an environment where calcite and dolomite are impoverished or where Ca and Mg exist in other forms. Waters of this type will dissolve calcite and/or dolomite if the water comes in contact with source rocks.
Quality assessment
The data obtained by chemical analyses were evaluated in terms of suitability for drinking and general domestic use, irrigation, livestock and industrial use.
Suitability for drinking and general domestic use
To assess the suitability for drinking and public health, the hydrochemical parameters of the groundwater of the study area were compared with the prescribed specification of WHO (1997) and Indian standard for drinking water (BIS 1991). Table 3 shows that most of the parameters exceed the desirable limits of WHO (1997) and BIS (1991), though it is within the maximum permissible limits. The EC and concentration of TDS is more than the desirable limits of 750 μS cm−1 and 500 mg l−1, respectively, in 55 and 63% of the total groundwater samples. The higher EC and TDS values may cause a gastrointestinal irritation in the consumers. The TH of the analysed water samples varies between 60 and 768 mg l−1 (avg. 324 mg l−1) in tube wells, 42–949 mg l−1 (avg. 365 mg l−1) in dug wells indicating soft to very hard types of water. Hardness of the water is attributable to the presence of alkaline earths, i.e., Ca and Mg. The data indicate that 52% of the total samples have TH beyond the safe limit of 300 mg l−1 for drinking water (BIS 1991). Hardness has no known adverse effect on health but it can prevent formation of lather with soap and increases the boiling point of water. The high TH may cause encrustation on water supply distribution systems. There is some suggestive evidence that long term consumption of extremely hard water might lead to an increased incidence of urolithiasis, anecephaly, parental mortality, some types of cancer and cardio-vascular disorders (Agrawal and Jagetia 1997; Durvey et al. 1991). The recommended limit for sodium concentration in drinking water is 200 mg l−1. A higher sodium intake may cause hypertension, congenial heart diseases and kidney problems. Concentrations of sodium are within the prescribed limit of 200 mg l−1 in 99% of the analysed groundwater samples. Concentration of Ca, Mg and K is also found within the highest permissible limits with few exceptions.
The contents of HCO3 and Cl have no known adverse health effects; however it should not exceed the safe limits of 300 and 250 mg l−1, respectively, in drinking water. The analytical data show that HCO3 exceeds the safe limits in about 40% and Cl in 6% of the samples. Higher concentration of Cl in drinking water causes a salty taste and has a laxative effect in people not accustomed to it. Concentration of sulphate exceeds the desirable limits of 200 mg l−1 in about 9% of the samples, collected near active and abandoned coal mining areas. Higher concentration of sulphate in drinking water is associated with respiratory problems (Maiti 1982; Subba 1993). High sulphate concentration may have a laxative effect with excess of Mg in water. Sulphate may also cause corrosion of metals in the distribution system, particularly in waters having low alkalinity.
Nitrate has undesirable effects when present in drinking water. High concentrations of nitrates can cause methanemoglobinaemia, gastric cancer, goiter, birth malformations and hypertension (Majumdar and Gupta 2000). About 14% of the groundwater samples have NO3 concentration higher than the recommended level of 45 mg l−1 (BIS 1991). Fluoride is an essential element for maintaining normal development of teeth and bones. The concentration of fluoride exceeds the permissible limits of 1.5 mg l−1 in about 18% of the groundwater samples.
Suitability for livestock
Water for livestock should be of high quality to prevent livestock diseases, salt imbalance, or poisoning by toxic constituents. Most of the water quality variables for livestock are the same as for human drinking-water resources although the total permissible levels of total suspended solids and salinity may be higher. Irrigation canals, ponds, rivers, reservoirs and groundwater may serve as water supplies for livestock. The data in Ayers and Wescot (1985) and Shuval et al. (1986) indicate that water having salinity <1,500 mg l−1 and Mg <250 mg l−1 is suitable for drinking by most livestock. Surface and groundwater of the area meet these standards and are suitable for livestock with some exceptions.
Suitability for irrigation uses
On irrigated lands, salinization is the major cause of loss of production and is one of the most prolific adverse environmental impacts associated with irrigation. Saline conditions severely limit the choice of crops, adversely affect crop germination and yields, and can cause soils to be difficult to work. Careful management can reduce the rate of salinity build up and minimize the effects on crops. Management strategies include: leaching, altering irrigation methods and schedules, installing sub-surface drainage, changing tillage techniques, adjusting crop patterns and incorporating soil ameliorates. All such actions, which may be very costly, would require careful study to determine their local suitability. It is important that all evaluation regarding irrigation water quality is linked to the evaluation of the soils to be irrigated (Ayers and Wescot 1985). Low quality irrigation waters might be hazardous on heavy, clayey soils, while the same water could be used satisfactorily on sandy and/or permeable soils. The important hydrochemical properties of ground, surface and mine water used to determine its suitability for irrigation are:
EC and sodium percentage (Na%)
EC and sodium concentration are very important in classifying irrigation water. The salts, besides affecting the growth of the plants directly, also affect soil structure, permeability and aeration, which indirectly affect plant growth. The sodium percentage (Na%) in the water samples is calculated by the equation:
The sodium percentage (%Na) in the area ranges between 8.8 and 88.8% (avg. 26%) in tube wells, 11.6–88.7% (avg. 28%) in dug wells water and 11.4–70% (avg. 34%) in surface water. A high sodium percent causes deflocculation and impairment of the tilth and permeability of soils (Karanth 1987). As per the Indian Standard (BIS 1991), maximum sodium of 60% is recommended for irrigation water. The plot of analytical data on the Wilcox (1955) diagram relating EC and sodium percent shows that the Damodar River basin waters are excellent to good and good to permissible quality and may be used for irrigation purposes (Fig. 11).
Plot of sodium percent versus electrical conductivity (after Wilcox 1955)
Alkali and salinity hazard (SAR)
The total concentration of soluble salts in irrigation water can be categorized as low (EC = <250 μS cm−1), medium (250–750 μS cm−1), high (750–2,250 μS cm−1) and very high (2,250–5,000 μS cm−1). While a high salt concentration in water leads to formation of saline soil, a high sodium concentration leads to development of an alkaline soil. Excessive solutes in irrigation water are a common problem in semi-arid areas where water loss through evaporation is maximal. Salinity problems encountered in irrigated agriculture are most likely to arise where drainage is poor, which allows the water table to rise close to the root zone of plants, causing the accumulation of sodium salts in the soil solution through capillary rise following surface evaporation. The sodium or alkali hazard in the use of water for irrigation is determined by the absolute and relative concentration of cations and is expressed in terms of sodium adsorption ratio (SAR). It can be estimated by the formula:
There is a significant relationship between SAR values of irrigation water and the extent to which sodium is adsorbed by the soils. If water used for irrigation is high in sodium and low in calcium, the cation-exchange complex may become saturated with sodium. This can destroy the soil structure due to dispersion of clay particles. The calculated value of SAR in the study area ranges from 0.37–3.56 in surface water, 0.23–14.1 in groundwater and 0.35–3.33 in mine waters. The plot of data on the US salinity diagram, in which the EC is taken as salinity hazard and SAR as alkalinity hazard, shows that most of the water samples fall in the category C1S1 and C2S1, indicating low to medium salinity and low alkali water, and it can be used for irrigation in most soil and crops with little danger (Fig. 12). However, about 30% of the water samples of the area fall in C3S1 class and show high salinity hazard and low alkali hazards (Richards 1954). High salinity water cannot be used on soils with restricted drainage and requires special management for salinity control. Plants with good salt tolerance should be selected for such areas.
US salinity diagram for classification of irrigation waters (after Richards 1954)
Residual sodium carbonate (RSC)
The quantity of bicarbonate and carbonate in excess of alkaline earths (Ca + Mg) also influence the suitability of water for irrigation purposes. When the sum of carbonates and bicarbonates is in excess of calcium and magnesium, there may be possibility of complete precipitation Ca and Mg (Raghunath 1987). To quantify the effects of carbonate and bicarbonate, residual sodium carbonate (RSC) has been computed by the equation:
A high value of RSC in water leads to an increase in the adsorption of sodium in soil (Eaton 1950). Waters having RSC values greater than 5 meq l−1 are considered harmful to the growth of plants, while waters with RSC values above 2.5 meq l−1 are not considered suitable for irrigation purpose. In most of the analysed water samples, RSC values are below 2.5 meq l−1; only three samples exceed 5.0 meq l−1 limits. This indicates that water is suitable for irrigation uses.
Permeability index (PI)
Doneen (1964) classified irrigation waters based on the permeability index (PI). PI is defined by
Sixty-six percent of the water samples fall in Class-I and 32% in Class-II in the Doneen’s chart (Domenico and Schwartz 1990), implying that the water is of good quality for irrigation purposes with 75% or more of maximum permeability. However, 35 surface water and 8 groundwater samples belong to Class-III, i.e., unsuitable category (Fig. 13).
Doneen (1964) classification of irrigation water based on the permeability index
Magnesium hazard (MH)
Szabolcs and Darab (1964) proposed magnesium hazard (MH) value for irrigation water as given below
MH > 50 are considered harmful and unsuitable for irrigation use. In the analysed waters, 8 groundwater, 12 surface water and 2 mine water have MH > 50.
Suitability for industrial use
The water quality requirement for industries varies considerably between areas, kind of industries and processes. One useful parameter to assess quality for industrial purposes is the SI of minerals (Rhades and Bernstein 1971). The SI of the present study water is positive for calcite and dolomite in most samples, indicating the possibility of precipitation (Fig. 11). The supersaturation restricts the safe use of water for industrial purpose, particularly in electrical power stations, industrial boiler houses, etc. The high TDS, hardness and sulphate concentration in some samples also make this water unsafe for textiles, paper and allied industries. Food industries such as dairying, brewing and carbonated beverage canning must comply with drinking water standards with disinfections and treatment before use.
Conclusions
Weathering of rock-forming minerals and anthropogenic contribution from domestic and coal-based industries are the major controlling factors for water chemistry. High concentration of TDS, SO4 and Cl in surface and sub-surface water samples collected from the mining and effluents disposal sites indicated the impact of mining and allied activities on water quality of the area. Excavation and dumping processes associated with the mining activities expose fresh surfaces for weathering and accelerated the dissolution processes and rate of release of ions in solution, causing increase in concentration of TDS and other dissolved ions. The calculated saturation indices for calcite (SIc) and dolomite (SId) demonstrate that most of the waters are supersaturated, signifying the presence of calcareous nodules (kankar). The quality assessment shows that in general, the water is suitable for domestic purposes. However, high values of EC, TDS, TH, NO3 and SO4 at some sites make it unsafe for drinking and demand detailed regional groundwater investigations. The assessment of water for irrigation use shows that the water is of good to permissible quality. However, high values of salinity, Mg-hazard and permeability index (PI) at certain sites restrict suitability for agriculture.
References
Agrawal V, Jagetia M (1997) Hydrogeochemical assessment of groundwater quality in Udaipur city, Rajasthan, India. Proceedings of National conference on dimension of environmental stress in India, Department of Geology, MS University, Baroda, India, pp 151–154
Allen SK, Allen JM, Lucas S (1996) Concentration of contaminants in surface water samples collected in west-central Indiana impacted by acid mine drainage. Environ Geol 27:34–37
APHA, AWWA, WPCF (1992) Standard methods for the examination of water and waste water, 16th edn. APHA, Washington DC
Appelo CAJ, Postma D (1993) Geochemistry, groundwater and pollution. AA Balkema, Rotterdam
Ayers RS, Wescot DW (1985) Water quality for irrigation. FAO Irrigation and Drainage Paper No. 20, Rev 1, FAO, Rome
Berner EK, Berner RA (1987) The global water cycle: geochemistry and environment. Prentice-Hall, Englewood Cliffs
BIS (1991) Bureau of Indian Standards—Indian standard specification for drinking water IS: 10500
Choubey VD (1991) Hydrological and environmental impact of coal mining, Jharia coalfield, India. Environ Geol 17:185–194
Dey AK (1981) The Damodar River water quality—upstream and down stream of Durgapur barrage. J IPHE India 3:57–60
Dey AK (1985) The saga of the Damodar River. J Ind Chem Soc IXII:1038–1042
Dey AK, Sen AK, Modak DP, Karim MR (1987) Some industrial waste effluents in Asanasol-Ranging region and their impact on the Damodar River water quality. J Geol Soc India 30:386–392
Domenico PA, Schwartz FW (1990) Physical and chemical hydrologeology. Wiley, New York
Doneen LD (1964) Notes on water quality in agriculture. Water science and engineering paper 4001, Department of Water Sciences and Engineering, University of California, California
Drever JI (1988) The geochemistry of natural waters. Prentice Hall, Englewood Cliffs
Durvey VS, Sharma LL, Saini VP, Sharma BK (1991) Handbook on the methodology of water quality assessment. Rajasthan Agriculture University, India
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 39:123–133
Galero DM, Pesci HE, Depetris PJ (1998) Effects of quarry mining and of other environmental impacts in the mountainous Chicam-Toctina drainage basin (Cordoba, Argentina). Environ Geol 34:159–166
Garrels RM, Mackenzie FT (1971) Gregor’s denudation of the continents. Nature 231:382–383
Ghose NC (1983) Geology, tectonics and evolution of the Chotanagpur granite–gneiss complex, eastern India. Recent Res Geol Hindustan Publ Corp Delhi 10:211–247
Karanth KR (1987) Groundwater assessment, development and management, Tata McGraw-Hill Publ. Com. Ltd. New Delhi, India
Kumar SC, Anderson HW (1993) Nitrogen isotopes as indicators of nitrate sources in Minnesota sand plane aquifers. Groundwater 31:260–271
Lowson RT, Reedy BJ, Beattie JK (1993) The chemistry of acid mine drainage. Chem Aust 60:389–391
Maiti TC (1982) The dangerous acid rain. Sci Rep 9:360–363
Majumdar D, Gupta N (2000) Nitrate pollution of groundwater and associated human health disorders. Indian J Environ Health 42:28–39
Mohan R, Singh AK, Tripathi JK, Chaudhary GC (2000) Hydrochemistry and quality assessment of groundwater in Naini industrial area, District Allahabad, Uttar Pradesh. J Geol Soc India 55:77–89
Pandey K, Sarin MM, Trivedi JR, Krishnaswami S, Sharma KK (1994) The Indus River system (India–Pakistan): major ion chemistry, uranium and strontium isotopes. Chem Geol 116:245–259
Pandey SK, Singh AK, Hasnain SI (1999) Weathering and geochemical processes controlling solute acquisition in Ganga headwater-Bhagirathi River, Garhwal Himalaya, India. Aquat Geochem 5:357–379
Pandey SK, Singh AK, Hasnain SI (2001) Hydrochemical characteristics of meltwater draining from Pindari glacier, Kumon Himalaya. J Geol Soc India 57:519–527
Raghunath HM (1987) Groundwater. Wiley Eastern Ltd., Delhi
Ratha DS, Venkataraman G (1997) Application of statistical methods to study seasonal variation in the mine contaminants in soil and groundwater of Goa, India. Environ Geol 29:253–262
Rhades JD, Berstein L (1971) Chemical physical and biological characteristics of irrigation and soil water. In: Ciaccio LL (ed) Water and water pollution. Marcel Dekker Inc., New York
Richards LA (1954) Diagnosis and improvement of saline and alkali soils, US Department of Agri. Hand Book, No. 60
Sarin MM, Krishnaswamy S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga–Brahmaputra river system: weathering processes and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009
Shuval HI, Adin A, Fiatal B, Raawitz E, Yekuterl P (1986) Wastewater irrigation in developing countries. Health effects and technological solutions. World Bank Technical Paper 52. Washington DC
Singh AK, Hasnain SI (1998) Major ion chemistry and control of weathering in a high altitude basin, Alaknanda, Garhwal Himalaya, India. Hydrol Sci J 43:825–845
Singh AK, Hasnain SI (1999) Environmental geochemistry of Damodar River basin, east coast of India. Environ Geol 37:124–136
Singh AK, Hasnain SI (2002) Aspects of weathering and solute acquisition processes controlling chemistry of sub-alpine proglacial streams of Garhwal Himalaya, India. Hydrol Process 16:835–849
Singh AK, Mondal GC, Singh PK, Singh S, Singh TB, Tewary BK (2005) Hydrochemistry of reservoirs of Damodar River basin, India: weathering processes and water quality assessment. Environ Geol 8:1014–1028
Singh AK, Mondal GC, Kumar S, Singh KK, Kamal KP, Sinha A (2007) Precipitation chemistry and occurrence of acid rain over Dhanbad, coal city of India. Environ Monit Assess 125:99–110
Stumm W, Morgan JJ (1981) Aquatic chemistry. Wiley Interscience, New York
Subba Rao N (1993) Environmental impact of industrial effluents in groundwater regions of Visakhapatnam Industrial Complex. Indian J Geol 65:35–43
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th International Congress of Isss, Trans, vol II, pp 803–812
Tiwary RK (2001) Environmental impact of coal mining on water regime and its management. Water Air Soil Pollut 132:185–199
Tiwary RK, Dhar BB (1994) Effects of coal mining and coal based industrial activities on water quality of the river Damodar with specific reference to heavy metals. Int J Surf Mining Reclam Environ 8:111–115
WHO (1997) Guidelines for drinking-water quality, V.1, recommendations. World Health Organisation, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. US Dept of Agricul Cir 969, Washington DC
Acknowledgments
Dr. Abhay Kumar Singh is thankful to Department Science and Technology, Government of India for financial assistance in the form of Fast Tract Project (SR/FTP/ES-99/2001). Support extended by Dr. P. K. Singh, Dr. S. Singh, Mr A. Barat and Mr K. K. Singh is gratefully acknowledged. Thanks are due the anonymous referee of the journal, who helped immensely in rewriting the paper in terms of content and language.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.K., Mondal, G.C., Kumar, S. et al. Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ Geol 54, 745–758 (2008). https://doi.org/10.1007/s00254-007-0860-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-0860-1